Abstract
Work from multiple groups continues to provide additional evidence for the powerful and highly diverse roles, both protective and pathogenic, that B cells play in autoimmune diseases. Similarly, it has become abundantly clear that antibody-independent functions may account for the opposing influences that B cells exercise over other arms of the immune response and ultimately over autoimmunity itself. Finally, it is becoming apparent that the clinical impact of B cell depletion therapy may be to a large extent determined by the functional balance between different B cell subsets that may be generated by this therapeutic intervention. In this review, we postulate that our perspective of B cell tolerance and our experimental approach to its understanding are fundamentally changed by this view of B cells. Accordingly, we shall first discuss current knowledge of B cell tolerance conventionally defined as the censoring of autoantibody-producing B cells (with an emphasis on human B cells). Therefore, we shall discuss a different model that contemplates B cells not only as targets of tolerance but also as mediators of tolerance. This model is based on the notion that the onset of clinical autoimmune disease may require a B cell gain-of-pathogenic function (or a B cell loss-of-regulatory-function) and that accordingly, disease remission may depend on the restoration of the physiological balance between B cell pathogenic and protective functions.
Introduction
It has been amply demonstrated that B cells play critical initiating and/or amplifying pathogenic roles in a wide variety of autoimmune diseases through both antibody-dependent and antibody-independent mechanisms (1–3). A large body of experimental evidence to that effect has been recently validated by the observed beneficial effect of B cell depletion therapies in multiple autoimmune diseases both in humans and mice (3–9). Of note, conditions that improve with B cell depletion therapy (BCDT) include both diseases typically considered of B cell origin (SLE, idiopathic autoimmune thrombocytopenia, dermatomyositis and autoimmune blistering diseases) as well as diseases for which B cells are not viewed as prime movers (including rheumatoid arthritis, multiple sclerosis and type 1 diabetes) (4, 5, 8–13). These observations strongly suggest that the pathogenic functions of B cells must be undoubtedly diverse and, while it is likely that they may also include the action of non-autoreactive B cells, it seems unquestionable that autoreactive B cells need to be censored in order to avoid or ameliorate autoimmune diseases. Given that B cells with at least some degree of autoreactivity are extremely common in the primary, pre-antigenic repertoire of mice and humans it is apparent that effective censoring mechanisms must be operative during early B cell development (central tolerance) (14–17). Moreover, regulation must also be enforced at later stages of B cell differentiation to censor autoreactive cells that either escape earlier checkpoints or are generated anew from mature non-autoreactive B cells (peripheral tolerance). Indeed, multiple checkpoints enforced by different mechanisms have been defined throughout B cell development (from immature B cells to pre-plasma cells) mostly in animal models (18–22).
While our knowledge of the mechanisms of human B cell tolerance is continuously expanding (17, 23–27) several critical questions remain to be addressed. They include: i) the precise definition of the censoring mechanisms that enforce physiological tolerance at different checkpoints during B cell development; 2) the mechanisms of breakdown of physiological tolerance in autoimmunity (bearing in mind that they may differ from disease to disease and that that even within a single disease they may be different for separate autoantigens); c) the relative contribution to immuno-pathogenesis of antigen-specific (autoreactive) versus antigen non-specific (non-autoreactive) B cells; and d) the critical and largely unexplored question of whether the main objective of B cell tolerance is merely to prevent the accumulation of autoantibody-secreting B cells or to block autoreactive B cells from becoming pathogenic by antibody-independent mechanisms (gain-of-function model).
Here we will try and put what’s known about human B cell tolerance and its breakdown in autoimmune diseases in the context of these models. In doing so, we shall discuss B cells as both targets and mediators of immunological tolerance.
B cells as targets of immunological tolerance
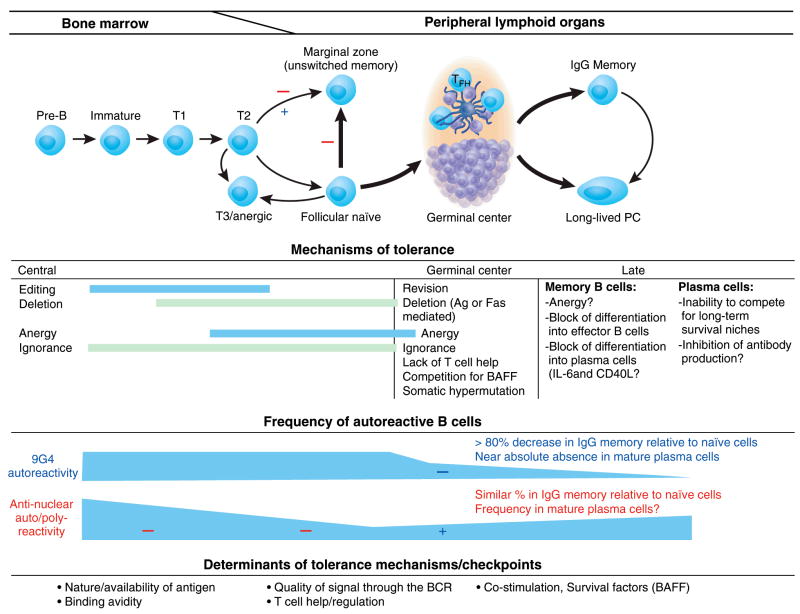
In animal models, B cell tolerance is established through multiple mechanisms both intrinsic and extrinsic to the autoreactive B cells in question (Figure 1). These mechanisms have been elegantly discussed in detail in several recent reviews (19, 21, 22, 28). Their relative participation and effectiveness in censoring autoreactive cells depend on a combination of self-antigen availability, its avidity and ability to cross-link the BCR and to engage innate immune receptors (TLR), the abundance of survival factors (in particular BAFF/B-cell-activating factor) and the ability of the autoreactive B cell to compete for them and the balance between activating Th cells and inhibitory Treg cells. In addition, autoreactive B cells can also be inhibited by macrophages and dendritic cells at least in part through the production of IL-6 and CD40L (29, 30). Ultimately, the combination of these factors determines whether an immature autoreactive B cell is censored by one or more of the following mechanisms: a) maturational arrest and follicular exclusion leading to premature death and clonal deletion (31, 32); b) receptor editing (eliminating the original autoreactivity by the secondary rearrangement of a new light chain) (33); c) receptor dilution due to the co-expression of two light chains as a result of allelic inclusion. This process may decrease the density of surface autoantibody expression partly or completely, in the latter case owing to intracellular sequestration of the autoreactive receptor rather than preferential pairing of the light chain editor (34, 35); or c) anergy (defined as the inability of chronically stimulated autoreactive cells to respond to further antigenic stimulation and spanning a wide spectrum of other phenotypic and functional characteristics that vary with the experimental system used to study anergy) (22, 31, 36–40). Of note, transgenic autoreactive anergic B cells have been typically considered maturationally arrested on the basis of a transitional phenotype. However, important recent data contributed by Merrell et al have demonstrated that chronic B cell receptor stimulation may also induce an anergic late transitional (T3) phenotype in mature, non-self reactive wild-type cells (41). These data suggest that autoreactive B cells may progress to the mature compartment yet regress back into the T3 compartment upon stimulation by self antigen. This observation raises important questions regarding the locale and type of stimulation that would induce anergy in mature autoreactive cells that escaped that fate at an earlier stage of differentiation.
Figure 1. Checkpoints and mechanisms of B cell tolerance with emphasis on human models.
Schematic representation of the multiple checkpoints, mechanisms and determinants of tolerance during early and late B cell development. By and large, the information summarized is derived from transgenic mouse models. The two main human experimental models of B cell tolerance are depicted superimposed on the figure (checkpoints for 9G4 cells are shown with blue signs and ANA reactivity is shown in red; +: positive selection; ×: negative selection). This figure ignores the B1 compartment since no clear and unequivocal definition or even definitive proof of its existence is available for humans. The model also tries to incorporate existing questions regarding the identity of human recirculating MZ cells and the nature of the MZ precursor population (either transitional or follicular naïve B cells). For the purpose of this review, peripheral blood IgD+ (unswitched) CD27+ memory cells are considered a circulating MZ equivalent and represent the IgM+ memory population studied in terms of ANA autoreactivity. Such autoreactivity has been shown to decrease significantly when either the transitional or naïve follicular compartment is compared with unswitched memory cells. Accordingly, a red negative selection sign has been assigned to these potential checkpoints. T: transitional cells; PC: plasma cells; FTH: follicular T helper cells. A table summarizing the CD antigens that permit classification of human B cell populations is also included in the figure (112). In addition to these subsets, germinal center B cells are characterized as IgD−, CD38++, CD10+ and generally CD27+ (54). Spleen marginal zone B cells are typically CD27+, CD21++, CD23+/−, CD1c+ and IgD− (although an outer marginal zone population is also observed in human spleen) (112). Peripheral blood unswitched CD27+ memory B cells may represent a recirculating marginal zone population also bearing the CD1c marker (113). Finally, long-lived plasma cells found in human bone marrow as well as spleen and tonsils express CD138 in addition to high levels of CD38 (114).
Of interest, anergy can also contribute to censoring mature autoreactive B cells in some transgenic systems (38, 42). Autoreactivity at the mature stage can also be enforced by additional mechanisms during antigen-induced, T cell-dependent GC reactions (19, 21, 22). GC mechanisms of tolerance include: cell death induced either by FasL on activated T cells or by self-antigen induced BCR signaling; prevention of autoreactive plasma cell differentiation by BCR signaling; lack of productive interaction with follicular T helper cells; attenuation of autoreactivity by BCR modification either through somatic hypermutation or receptor revision. Finally, additional mechanisms of tolerance may also be operative during the late stages of B cell differentiation and antibody production as reviewed elsewhere (21). Late mechanisms and the specific locales where they may take place are less well understood although checkpoints for anti-Smith pre-plasma cells (CD138+ non-secreting plasma cells) are operative in the bone marrow and in the spleen of normal mice) (20).
Essentially, all the previous mechanisms amount to negative selection of autoreactive B cells. It should be noted however that positive selection may shift cells into the B1a and marginal zone (MZ) compartments (43). At least in the mouse, both B1 cells (defined as IgMhigh, IgDlow, CD5+) and MZ B cells (defined as IgMhigh, CD21high, CD23low, IgDlow) represent innate-like B cell populations that may contribute to the regulation of autoreactive B cells through a compartmentalization process (in the peritoneal and pleural cavities for B1 cells and in the splenic marginal zone for MZ cells) that prevents them from either encountering antigen and/or undergoing further maturation and diversification (in GC for instance) (44–47). Of particular interest is this context, partially autoreactive B cells as a result of light chain allelic inclusion have been shown to accumulate in the MZ (47). Of note however, in other models, positive selection or expansion of autoreactive marginal zone B cells can also induce clinical autoimmunity (48).
Finally, on other animal models, the activation of some types of autoreactive B cells (including rheumatoid factor, anti-DNA and anti-La B cells) appears to be prevented by clonal ignorance or antigenic indifference (49–52). These mechanisms however permit the maturation and accumulation of such autoreactive cells and therefore, the censoring achieved is rather tenuous. Indeed, the danger of the persistence of ignorant cells is illustrated by the AM14 transgenic model in which autoreactive rheumatoid factor B cells normally develop in the follicular and MZ compartments without generating autoantibodies in normal mice but not in an autoimmune background (53).
Despite tremendous progress in our understanding of B cell tolerance, much less is know about the specific defects in different checkpoints underlying tolerance breakdown in autoimmune diseases. In particular, as discussed elsewhere, the importance of breakdown of mechanisms of central tolerance is unclear in many animal systems (22). These aspects are discussed for human disease below. An in-depth discussion of other experimental systems is outside the scope of this review and can be found in recent publications by other authors (19, 22).
Mechanisms of B cell tolerance in humans
For obvious reasons, experimental approaches to the study of B cell tolerance are more limited in humans than in the mouse. Essentially, two major experimental systems have been employed to assess the frequency of autoreactive B cells in different developmental compartments and to identify the checkpoints and mechanisms that limit their access to compartments that presumably hold the most pathogenic potential (i.e., the long-lived IgG memory and plasma cell compartments) (23, 24) (Figures 2 and 3). Our laboratory has concentrated on the study of a particular subset of autoreactive human B cells identified by the expression of an idiotype recognized by the 9G4 rat anti-human Ig monoclonal antibody (for convenience, we designate these cells as 9G4+ cells and the antibodies they produce as 9G4 antibodies) (23, 54). This system has the advantage that cells can be identified using flow cytometry thereby allowing the analysis of millions of cells that are homogeneous at least for the expression of this autoreactivity-associated marker. Moreover, in healthy subjects, IgM 9G4 antibodies are universally autoreactive at a minimum against the N-acetyllactosamine residues expressed by the blood group Ii antigens and many other self-glycoproteins including CD45/B220. The latter autoreactivity is responsible for the ability of 9G4 antibodies to bind to B cells and may contribute to B cell lymphopenia in active SLE (55, 56). At least some IgM 9G4 antibodies are also able to recognize other self antigens such as DNA, gangliosides, IgG (rheumatoid factor activity), and neutrophil cytoplasmic antigens (ANCA activity) and may in some cases cross-react with the lipid A moiety of LPS (57–60). The combination of these properties together with the high frequency of 9G4 B cells in the primary repertoire of healthy subjects (5–10% of all immature and naïve B cells) and the absolute absence of VH4-34 genetic polymorphism in the human population create a situation as close as one can get in humans to experimental transgenic systems where homogeneous autoreactive B cells have to co-exist with competing non-autoreactive B cells. Of significant importance, 9G4-associated autoreactivity is owed to the utilization by these B cells and antibodies of heavy chains encoded by VH4-34 genes that preserve the germline-encoded expression of the 9G4 idiotype (61). Therefore, one should bear in mind when trying to understand the regulation of the autoreactivity of 9G4 B cells that while 9G4+ cells may be universally autoreactive that may not be the case for all VH4-34+ B cells since somatic hypermutation may abolish both the expression of the 9G4 idiotype as well as the associated autoreactivity.
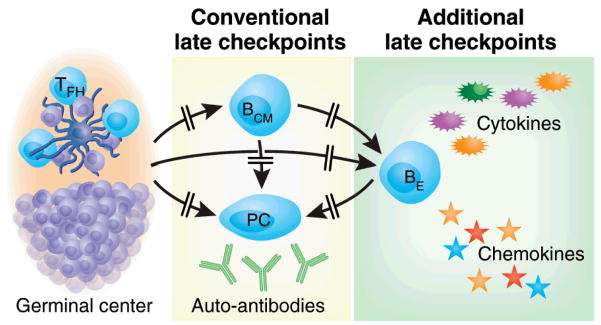
Figure 2. A broader view of B cell tolerance. Additional late checkpoints.
Conventional late checkpoints are viewed as preventing the generation of autoreactive long-lived memory B cells and plasma cells in order to avoid autoantibody accumulation. Additional checkpoint could be envisioned that block the generation of autoreactive effector B cells (BE) from either newly recruited GC cells or pre-existing central memory cells (BCM) (85, 86). Such hypothetical checkpoints are indicated in this figure (Whether effector B cells may represent a distinct subset of memory B cells and whether they can also generate plasma cells remains to be elucidated.
Figure 3. The dual nature of B cells in autoimmunity.
B cells are endowed with a Janus-like quality that enables them to perform functions that either promote or suppress autoimmunity. While division of labor is likely to exist between different B cell populations it is also possible that the function of a given B cell subset could be induced or modulated by the dominant cytokine environment characteristic of different autoimmune diseases.
Taken together, our studies of the 9G4 system have overcome two major obstacles that traditionally hamper the understanding of human B cell tolerance: 1) the identification of a population of well defined autoreactive B-cells which are relevant and specific for a given autoimmune disease (SLE) and whose frequency is large enough to permit accurate measurements (62); 2) the analysis of autoimmune secondary lymphoid tissue in a systematic fashion (54). As it regards disease relevance and specificity, elevated serum titers of 9G4 IgG antibodies, while very rare in healthy subjects, are found in approximately 70% of patients with active SLE, are highly specific for this disease where they correlate with disease activity and organ involvement (kidney and CNS) and participate in anti-DNA antibody responses (including antibodies obtained from kidney eluates) and anti-Smith responses (60, 63, 64). At a cellular level, despite the high frequency of 9G4 naïve B-cells, in healthy subjects 9G4 cells are markedly diminished (80–90%) in the IgG memory compartment and 9G4+ plasma cells are very hard to find (54, 65). The absence of 9G4+ plasma cell described in our laboratory using intracellular staining of magnetically purified CD138+ plasma cells from healthy bone marrow and tonsils is indeed striking and consistent with the virtual absence of 9G4 antibodies in more than 200 multiple myeloma tumors thus far analyzed by different groups (65–67).
These observations led us to postulate that autoreactive 9G4 cells should be strictly censored in the peripheral compartment of healthy subjects and more specifically in the germinal centers (GC) since these functional and anatomical structures are the primary, albeit not the only, generators of isotype switched memory cells and long-lived plasma cells (68, 69). Indeed, our analysis of normal tonsil and spleen specimens demonstrated that naïve 9G4 cells are normally prevented from establishing GC reactions as demonstrated by the absence of 9G4+ GCs in more than 700 follicles (from 12 tonsils and 5 spleens) thus far analyzed in our laboratory. Such strict censoring appears to be largely accomplished in the early phases of the GC reaction (23, 54, 62) (Figure 1). The actual mechanism(s) responsible for the censoring remain to be elucidated in more detail. However, despite the fact that 9G4 cells do become mature follicular cells (in contrast to some but not all mouse models of anergy), these cells are anergic in their response to BCR stimulation ex vivo as indicated by Ca++ flux analysis. More recent results using intracellular phospho-flow cytometry techniques also indicate BCR hyporesponsiveness in 9G4 cells (Jenks et al, manuscript in preparation). Of note, as also reported for mouse anti-HEL anergic B cells, healthy 9G4 B cells hypo-proliferate under BCR plus CpG DNA stimulatory conditions indicating that co-stimulation through TLR9 fails to overcome their anergic state (Manjarrez-Orduño et al, manuscript in preparation) (40). Finally, we have shown that 9G4 B cells accumulate in substantial numbers in the splenic marginal zone (MZ). While the actual origin of MZ B cells remain controversial, there is strong evidence that at least in part they derive from a transitional/MZ precursor population through a developmental pathway separate from follicular cells (70). Accordingly, our observations of the presence of high frequency of 9G4 B cells within both the follicular and MZ compartments strongly suggest that immature/transitional 9G4 B cells may be heterogeneous in terms of their autoreactivity and contain distinct subpopulations that are differentially selected. Current investigation in our laboratory is aimed at the elucidation of the types of autoreactivity present in early 9G4 cells and their preferential selection into the follicular and MZ compartments. We are also investigating the role of receptor editing in this early selection phase.
As in the mouse, understanding which and how physiological mechanisms of tolerance go awry in human autoimmune diseases remains a formidable challenge. In the 9G4 system, all the serological observations generated over almost 15 years by different groups, strongly indicated that subversion of these mechanisms should occur in SLE in a disease specific fashion (54, 63).
Proving this point required access to secondary lymphoid tissue in autoimmune patients. This limitation was overcome through the pioneering use of tonsil biopsies for such purpose (54). With this approach we have demonstrated that indeed faulty GC censoring of 9G4 B-cells occurs specifically in SLE and is not shared by RA patients (54). Thus, approximately 25% of all productive GC in SLE tonsils are 9G4+ as compared to virtually none in healthy subjects or in RA tonsils. This defect results in a 10- to 25-fold expansion of 9G4+ B-cells within the IgG memory compartment of secondary lymphoid tissue. Moreover, 9G4 antibodies are expressed in up to 30% of PBL plasmablasts in active SLE which represents a pronounced contrast with the remarkable absence of 9G4 plasma cells in normal subjects(71, 72). Together with previous demonstrations of the high specificity of 9G4 serum antibodies for SLE (>95% and comparable to anti-ds DNA antibodies) and of the association of serum 9G4 antibody levels with disease activity and organ involvement in SLE (63, 71, 72), our results clearly establish the relevance of studying the fate of 9G4 B-cells to assess B-cell tolerance in SLE. Still, a precise definition of the actual defects involved in the breach of 9G4 B cell tolerance in SLE remains to be established. Studies of B cell signaling, BAFF-mediated survival, antigen-availability in the GC, collaboration with follicular T helper cells and suppression by Treg cells are currently underway. Of note, our initial studies of HIV patients have identified a subset of patients with serum IgG levels of 9G4 antibodies comparable to those previously found only in active SLE. These observations provide an opportunity to identify common factors between the two conditions, such as potential defects in Treg cell function, that could influence the regulation of the 9G4-associated autoreactivity.
Another highly informative experimental system has employed single cell PCR technology to isolate the antibodies expressed by B cells and determine the fate of B cells with autoreactivity against nuclear antigens (either global ANA reactivity or anti-DNA reactivity) both in healthy subjects and in autoimmune patients with SLE and RA (17, 25–27, 73). Several striking findings have been derived from these studies. First, immature B cells in the primary bone marrow repertoire of healthy subjects display a very high level of autoreactivity (~75%) and a significant degree of polyreactivity. Secondly, several early checkpoints operative in the bone marrow as well as peripherally appear to be effective in healthy subjects as this initial level of nuclear autoreactivity diminishes progressively as immature B cells differentiate into mature naïve B cell (20% autoreactive and 5% polyreactive) and then into unswitched IgM memory cells which are essentially devoid of the autoreactivity and polyreactivity (when the antibodies are expressed as IgG molecules) present in earlier B cells. In this system, clonal deletion and possibly receptor editing appear to account for the censoring observed at these early checkpoints. Of great interest, additional work by the same investigators has demonstrated that these early censoring check points seem to malfunction in SLE and RA patients leading to significantly higher levels of anti-nuclear reactivity in the naïve B cells than healthy controls (73, 74). Somewhat unexpectedly however, recent results have shown that the level and type of nuclear autoreactivity eliminated in the early checkpoints in healthy subjects are restored during the GC reaction by somatic hypermutation and antigen selection resulting in high levels of ANA reactivity in the post-GC IgG memory compartment (27). These studies raise important questions. Perhaps most critical is whether GC do not effectively censor pathogenic autoreactivity in humans. Several explanations could be invoked to reconcile these findings with the absence of clinical autoimmunity in healthy subjects and with the description in mouse models of multiple GC-based tolerance mechanisms as well as our own results in the 9G4 system (19, 23, 54). Thus, it is possible that the low-affinity autoreactivity detected in the IgG compartment might not be pathogenic and consequently there would be no need for further censoring. Alternatively, the autoreactivity detected could be pathogenic or at least have the potential to become pathogenic but it would be regulated at later checkpoints thereby blocking their differentiation into antibody-producing plasma cells, an scenario consistent with the description in mouse models of pre-plasma cell autoreactivity checkpoints (20, 21). It should be noted however that the GC are the site of differentiation of long-lived plasma cells from high-affinity B cells and therefore, it seems likely that GC should be operative in the implementation of late tolerance checkpoints (75, 76). It could therefore be postulated that while GC might allow and/or promote the generation of autoreactive memory cells of low affinity, they would censor the different ion of high affinity autoreactive B cells.
A multi-dimensional view of B cell tolerance beyond the censoring of autoantibody production
Multiple challenges remain to be overcome in order to understand and manipulate B cell tolerance. It seems indisputable that effective tolerance mechanisms should prevent the expansion and activation of pathogenic autoreactive B cells. Typically, as previously discussed in this review, the process of tolerance is defined as the elimination, editing or silencing of self-reactive B cells in order to ultimately prevent the excessive production of pathogenic autoantibodies. Hence, by and large, the investigation of B cell tolerance deals with pathogenic B cells defined as autoantibody-producing B cells and conventional readouts of tolerance and evaluation of tolerogenic checkpoints consist of the frequency of autoreactive B cells and the level of serum autoantibodies.
It is obvious therefore, that a critical challenge is to be able to discriminate pathogenic from merely self-reactive B cells. Moreover, as the evidence continues to accumulate for antibody-independent pathogenic roles in multiple autoimmune diseases, it becomes essential to understand the mechanisms and consequences of B cell tolerance from a wider angle. Are all autoreactive B cells created equal? How can we identify pathogenic B cells? What determines the pathogenic function(s) of an autoreactive B cell? How is this function acquired and can it be reversed? What’s the relative pathogenic contribution of antibody-mediated and antibody-independent functions? A detailed understanding of these questions would lead to better diagnostic, prognostic and therapeutic tools for the prevention of autoimmunity in high-risk subjects and for the management of disease in autoimmune patients.
Hence, it should be useful to discuss a more encompassing model which postulates that at least in some autoimmune conditions or in some of their phases (such as pre-autoimmunity or during spontaneous or treatment-induced disease remission), the presence of autoantibody-producing cells is not sufficient for B cell-mediated autoimmunity (Figure 2). The need for this model is powerfully illustrated by the consistent observation that even isotype-switched autoantibodies may precede disease onset by several years (as in SLE and type 1 diabetes) thereby suggesting that additional pathogenic functions are required for disease induction (77, 78). Such a gain-of-pathogenic function model would require that autoreactive B cells acquire other pathogenic properties such as the ability to activate pathogenic T cells, the production of pro-inflammatory cytokines and/or the promotion of tertiary lymphoid tissue in target organs (79–81). While these additional functions may also be B cell extrinsic and also include the generation of additional autoantibody species and the modification of pre-existing ones (such as modified glycosylation leading to increased pathogenic activity) (82, 83), the therapeutic benefit of B cell depletion and, at least in several diseases, the antibody-independent benefit of this intervention, strongly point to B cells as critical recipients of gain-of-pathogenic function (3, 4, 10, 84). While a detailed discussion of the multiple mechanisms that could account for this process is outside the scope of this review, suffice it to say here that a main corollary of this model would be that effective B cell tolerance should preclude the generation of effector B cells with pathogenic, antibody-independent function as we and others have proposed (85–87) (Figure 2). Whether such effector cells share a common precursor with antibody-secreting cells or are the progeny of a different precursor population also remains to be elucidated.
B cells as agents of immunological tolerance
B cells are powerful mediators of antigen presentation, cytokine and chemokine production and multiple other antibody-independent functions (summarized in Table 1) (2, 3). Through these functions, B cells exert critical influence over lymphoid architecture, T cell and dendritic cell regulation and possibly (although this aspect is less well understood), over the regulation of other B cell subsets. Through a poorly understood balance between these functions B cells can play either protective or pathogenic roles in autoimmunity (Figure 3). Such Janus-like behavior of B cells in autoimmunity is illustrated by their duplicitous influence over T regulatory cells. On the one hand, B cells mediate or enable auto-inflammatory disease by inhibiting Tregs at least in some cases after being recruited by effector CD4 T cells (88, 89). On the other hand, the combined evidence from several studies strongly suggests the existence of protective regulatory B cells capable of preventing or suppressing autoimmunity. This beneficial effect can be mediated either by the production of cytokines such as IL-10 or TGFβ, the induction of T cell anergy and/or by the ability of at least some B cell populations to induce Treg cells (90–98). Of significant interest, B cells may also be essential for the induction of cardiac allograft tolerance that can be induced by anti-CD45RB antibodies (99). In this case, it has been postulated that activated B cells might enhance the responsiveness of responder T cells to Tregs.
Table 1.
Antibody-independent functions of B-cells (3)
| Lymphotoxin-dependent | Formation of the T cell zone | |
| Formation of the marginal zone | ||
| Maturation of FDC networks | ||
| Homeostasis of dendritic cells | ||
| Recruitment of germinal center DC cells | ||
| Lymphoid neogenesis | ||
|
| ||
| Lymphotoxin- independent | T cell functions | Priming/expansion of naïve CD4 cells |
| Priming of naïve CD8 cells | ||
| T cell anergy-Tolerance | ||
| Activation of autoreactive CD4/CD8 cells | ||
| Promotion of Th2 differentiation | ||
| Induction of Th1 differentiation | ||
| Inhibition of Th1 and Th17 cells (TLR-mediated) (101) | ||
| Recruitment of FTH (follicular helper) T cells | ||
| Inhibition of Tregulatory cells | ||
| Expansion of Tregulatory cells | ||
|
| ||
| Dendritic cells functions | Maduration and migration of DCs | |
| Activation of DCs | ||
| Inhibition of DC-induced T cell immunity | ||
| Attenuation of IL-12-induced Th1 differentiation | ||
|
| ||
| Cytokine production | IL-1, IL-4, IL-6, IL-8, IL-7, G-CSF, GM-CSF, IL-10 IL-12, TNFα, LTα, TGFβ, BMP-6/7, VEGF-A | |
|
| ||
| Chemokine | MIP-1α, MIP-1β, IL-16, CXCL13 (81) | |
|
| ||
| Lymphangiogenesis and lymph node expansion | ||
These tantalizing observations raise a number of important questions regarding the nature, timing and mechanism of action of protective, regulatory B cells. Indeed, different studies suggest that the disease suppressing activity of B cells may only occur in specific situations depending on either: 1) their absolute numbers; 2) their relative frequency and dominance over pathogenic B cells; and/or 3) a gain-of-inhibitory function that was previously absent. Thus, in CIA (collagen-induced arthritis) models a protective population of IL-10 producing B cells with a phenotype of transitional2/MZ precursors (T2-MZP) is capable of inducing disease remission upon adaptive transfer even if the same phenotypical endogenous population is incapable of preventing disease in CIA-immunized animals (100). In this model, the adoptive transfer of T2-MZP B cells at an early stage of disease renders autoreactive T cells unresponsive to CII and unable to produce cytokines essential for the pathogenesis of arthritis. T2-MZP B cell transfer significantly suppressed the CII-specific IgG response and induced a switch away from a “pathogenic” Th1 IgG2a toward a more “protective Th2 like” IgG1 response. Of great interest, these effects were mediated by inhibition of CII-specific IFNγ-producing Th1 cytokines. The data indicate that the suppression induced by T2-MZP B cellsis, at least in part, specific for the antigen to which these B cells had been exposed. This would suggest that antigen-specific autoreactive B cells gained a protective ability upon transfer that they did not initially have. Similarly, a gain-of-inhibitory function can also be gleaned from a report published during the writing of this manuscript showing that TLR-activated B cells can suppress Th1 and Th17 responses through the production of IL-10 and induce clinical remission in EAE (101).
The significance of these results is enhanced by elegant results in NOD mice and by our own observations in human SLE. Thus, Hu et al. have shown that upon B cell depletion and reconstitution, the expanding B cells (with a predominance of transitional B cells) mediate dominant suppression of diabetogenic T cells (8). Similarly, our own data in SLE patients treated with Rituximab-induced B cell depletion therapy have shown a strong correlation between long-term clinical responses and a B cell reconstitution profile dominated for more than 2 years by large numbers of transitional B cells and a scarcity of memory B cells (97, 102).
It should be noted however that the actual mechanism of action of these protective B cells remains to be elucidated. Thus, in accord with the observations of Merrell and colleagues previously discussed (41), it is likely that phenotypically T2-MZP cells would include anergic T3 B cells with high T cell tolerogenic potential due to lack of expression of CD86 and other co-stimulatory molecules (103). As we have previously discussed in our Rituximab studies in SLE, this mechanism could contribute to explain the beneficial effect of repopulating B cells (97, 102, 104). Of particular interest, our unpublished observations strongly indicate that cells with a T3 phenotype are over-represented in the repopulating repertoire of Rituximab-treated patients with good clinical and immunological responses (Anolik et al, Submitted). It is also important to bear in mind that IL-10 itself might contribute to the down-regulation of B7 co-stimulatory molecules perhaps providing a link between the two proposed mechanisms (105).
Combined, the available evidence in mice and humans strongly indicates that a disturbed balance between protective and pathogenic B cell activity may strongly contribute to active autoimmune disease and that a favorable balance appears to be restored by B cell depletion therapy in patients with good clinical response (Figure 4). Of significant interest, this model is also supported by the observation that the ability of B cells to produce anti-inflammatory IL-10 is restored in patients with Multiple Sclerosis after treatment with Rituximab (106). In these studies, the IL-10 producing cells were characterized as naïve on the basis of their expression of IgD and CD27. It should be noted however that, as shown by our laboratory, these markers would not discriminate between naïve and transitional cells and therefore, the actual phenotype of the protective B cells remains open to question (86, 97, 107).
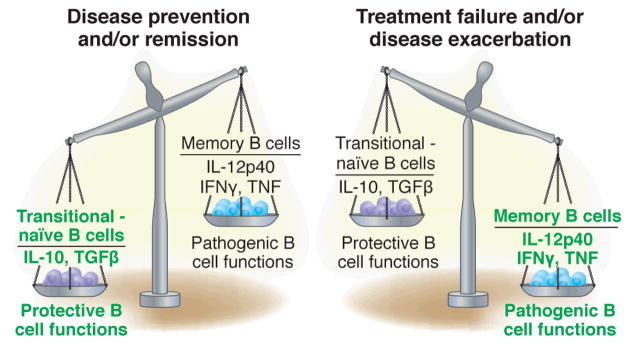
Figure 4. Functional balance between protective and pathogenic B cell functions.
This figure is predicated on the notion that pro-inflammatory pathogenic functions may be concentrated on memory B cells whereas transitional and possibly naïve B cells might provide protection at least in part by the secretion of cytokines such as IL-10 and TGFβ. Consequently, the relative balance achieved between these two opposing populations/activities would determine disease course and response to treatment.
These important observations and the models proposed to explain them raise more questions than they answer including the nature of the regulatory B cells, the mechanisms whereby they may gain protective function and the triggers of such gain-of-function. It seems likely however that the function of a given B cell subset will be altered by the cytokine milieu of the autoimmune disease in question. Consistent with this concept is the observation that IL-10 may have pro-inflammatory effects when primed by type I IFN (108). This scenario may help explain the seemingly paradoxical pathogenic role of B cell-produced IL-10 in SLE (109, 110). It can also account for our observation that a dominance of transitional B cells is not necessarily protective in patients with SLE (Anolik et al, manuscript in preparation).
Finally, it also remains to be determined whether the transitional/naïve-dominated B cell environment that can be induced by B cell depletion can restore B and T cell tolerance. Our own results however strongly indicate that this is indeed the case as we have found that physiological censoring of autoreactive 9G4 B cells is restored in SLE patients with long-term responses whose peripheral B cell compartment is dominated by transitional cells during reconstitution (111).
Acknowledgments
Supported in part by grants R01 AI049660-05, U19 Autoimmunity Center of Excellence AI56390, and Center for Biodefense of Immuno-Compromised Populations N01-AI50029 (IS);
References
- 1.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunological Reviews. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Chan AC. B Cell Immunobiology in Disease: Evolving Concepts from the Clinic. Annual Review of Immunology. 2006:24. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 3.Sanz I, Anolik JH, Looney RJ. B cell depletion therapy in autoimmune diseases. Front Biosci. 2007;12:2546–2567. doi: 10.2741/2254. [DOI] [PubMed] [Google Scholar]
- 4.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand J, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JCW, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-Cell-Targeted Therapy with Rituximab in Patients with Rheumatoid Arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, et al. B-Lymphocyte Depletion Reduces Skin Fibrosis and Autoimmunity in the Tight-Skin Mouse Model for Systemic Sclerosis. Am J Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K, et al. Yin Yang 1 is a critical regulator of B-cell development. Genes & Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B Lymphocyte Depletion by CD20 Monoclonal Antibody Prevents Diabetes in Nonobese Diabetic Mice despite Isotype-Specific Differences in Fc{gamma}R Effector Functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 10.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-Cell Depletion with Rituximab in Relapsing-Remitting Multiple Sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt E, Hunzelmann N, Zillikens D, Brocker EB, Goebeler M. Rituximab in refractory autoimmune bullous diseases. Clinical and Experimental Dermatology. 2006;31:503–508. doi: 10.1111/j.1365-2230.2006.02151.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of Pemphigus Vulgaris with Rituximab and Intravenous Immune Globulin. N Engl J Med. 2006;355:1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 13.Todd DL. Rituximab in the treatment of dermatomyositis: An open-label pilot study. Arthritis & Rheumatism. 2005;52:601–607. doi: 10.1002/art.20849. [DOI] [PubMed] [Google Scholar]
- 14.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- 15.Souroujon M, White-Scharf ME, Andreschwartz J, Gefter ML, Schwartz RS. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988;140:4173–4179. [PubMed] [Google Scholar]
- 16.McHeyzer-Williams MG, Nossal GJ. Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. J Immunol. 1988;141:4118–4123. [PubMed] [Google Scholar]
- 17.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant Autoantibody Production by Early Human B Cell Precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 18.Goodnow C. Pathways for self-tolerance and the treatment of autoimmune diseases. The Lancet. 2001;357:2115–2121. doi: 10.1016/s0140-6736(00)05185-0. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC, Sprent J, de St Groth BF, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 20.Culton DA, O’Conner BP, Conway KL, Diz R, Rutan J, Vilen BJ, Clarke SH. Early Preplasma Cells Define a Tolerance Checkpoint for Autoreactive B Cells. J Immunol. 2006;176:790–802. doi: 10.4049/jimmunol.176.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodnow CC, Campbell JA, Rui L, Vinuesa CG. Tolerance mechanisms in the late phase of the antibody response. Adv Exp Med Biol. 2007;596:163–168. doi: 10.1007/0-387-46530-8_15. [DOI] [PubMed] [Google Scholar]
- 22.Shlomchik MJ. Sites and Stages of Autoreactive B Cell Activation and Regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardemann H, Nussenzweig MC. Advances in Immunology. Academic Press; 2007. B-Cell Self-Tolerance in Humans; pp. 83–110. [DOI] [PubMed] [Google Scholar]
- 25.Wardemann H, Hammersen J, Nussenzweig MC. Human Autoantibody Silencing by Immunoglobulin Light Chains. J Exp Med. 2004;200:191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006 doi: 10.1084/jem.20052033. jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in Human IgG+ Memory B Cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar KR, Mohan C. Understanding B-cell tolerance through the use of immunoglobulin transgenic models. Immunol Res. 2007 doi: 10.1007/s12026-007-8008-7. [DOI] [PubMed] [Google Scholar]
- 29.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, Vilen BJ. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Cutting Edge: Low-Affinity, Smith Antigen-Specific B Cells Are Tolerized by Dendritic Cells and Macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodnow CC. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 32.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. Journal of Experimental Medicine. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813 –1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Receptor Editing Can Lead to Allelic Inclusion and Development of B Cells That Retain Antibodies Reacting with High Avidity Autoantigens. J Immunol. 2005;175:5067–5076. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- 36.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito T, Wang Y-H, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX-F, Yao Z, Cao W, Liu Y-J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acevedo-Suarez CA, Kilkenny DM, Reich MB, Thomas JW. Impaired Intracellular Calcium Mobilization and NFATc1 Availability in Tolerant Anti-Insulin B Cells. J Immunol. 2006;177:2234–2241. doi: 10.4049/jimmunol.177.4.2234. [DOI] [PubMed] [Google Scholar]
- 39.Noorchashm H, Bui A, Li HL, Eaton A, Mandik-Nayak L, Sokol C, Potts KM, Pure E, Erikson J. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. International Immunology. 1999;11:765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- 40.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 41.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and Anergy in Bone Marrow B Cells of a Novel Immunoglobulin Transgenic Mouse that Is Both Hapten Specific and Autoreactive. Immunity. 2001;14:34–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 43.Cancro MP, Kearney JF. B Cell Positive Selection: Road Map to the Primary Repertoire? J Immunol. 2004;173:15–19. doi: 10.4049/jimmunol.173.1.15. [DOI] [PubMed] [Google Scholar]
- 44.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 45.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 46.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Li H, Weigert M. Autoreactive B Cells in the Marginal Zone that Express Dual Receptors. J Exp Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone b cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 49.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. Journal of Experimental Medicine. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig-Marrony S, Soulas P, Julien S, Knapp AM, Garaud JC, Martin T, Pasquali JL. Natural Autoreactive B Cells in Transgenic Mice Reproduce an Apparent Paradox to the Clonal Tolerance Theory. J Immunol. 2001;166:1463–1470. doi: 10.4049/jimmunol.166.3.1463. [DOI] [PubMed] [Google Scholar]
- 51.Aplin BD, Keech CL, de Kauwe AL, Gordon TP, Cavill D, McCluskey J. Tolerance through Indifference: Autoreactive B Cells to the Nuclear Antigen La Show No Evidence of Tolerance in a Transgenic Model. J Immunol. 2003;171:5890–5900. doi: 10.4049/jimmunol.171.11.5890. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Manser T. Antinuclear Antigen B Cells That Down-Regulate Surface B Cell Receptor during Development to Mature, Follicular Phenotype Do Not Display Features of Anergy In Vitro. J Immunol. 2005;174:4505–4515. doi: 10.4049/jimmunol.174.8.4505. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Shlomchik MJ. Autoantigen-specific B cell activation in Fas-deficient rheumatoid factor immunoglobulin transgenic mice. Journal of Experimental Medicine. 1999;190:639–649. doi: 10.1084/jem.190.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cappione A, III, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cappione AJ, Pugh-Bernard AE, Anolik JH, Sanz I. Lupus IgG VH4.34 Antibodies Bind to a 220-kDa Glycoform of CD45/B220 on the Surface of Human B Lymphocytes. J Immunol. 2004;172:4298–4307. doi: 10.4049/jimmunol.172.7.4298. [DOI] [PubMed] [Google Scholar]
- 56.Bhat NM, Bieber MM, Hsu FJ, Chapman CJ, Spellerberg M, Stevenson FK, Teng NN. Rapid cytotoxicity of human B lymphocytes induced by VH4-34 (VH4.21) gene-encoded monoclonal antibodies, II. Clinical & Experimental Immunology. 1997;108:151–159. doi: 10.1046/j.1365-2249.1997.d01-976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorpe Turner, Stevenson Spellerberg, Thorpe Natvig, Thompson Human monoclonal antibodies encoded by the V4-34 gene segment show cold agglutinin activity and variable multireactivity which correlates with the predicted charge of the heavy-chain variable region. Immunology. 1998;93:129–136. doi: 10.1046/j.1365-2567.1998.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spellerberg MB, Chapman CJ, Mockridge CI, Isenberg DA, Stevenson FK. Dual recognition of lipid A and DNA by human antibodies encoded by the VH4-21 gene: a possible link between infection and lupus. Human Antibodies & Hybridomas. 1995;6:52–56. [PubMed] [Google Scholar]
- 59.Bhat NM, Bieber MM, Spellerberg MB, Stevenson FK, Teng NN. Recognition of auto- and exoantigens by V4-34 gene encoded antibodies. Scandinavian Journal of Immunology. 2000;51:134–140. doi: 10.1046/j.1365-3083.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 60.Longhurst C, Ehrenstein MR, Leaker B, Stevenson FK, Spellerberg M, Chapman C, Latchmen D, Isenberg DA, Cambridge G. Analysis of immunoglobulin variable region genes of a human IgM anti-myeloperoxidase antibody derived from a patient with vasculitis. Immunology. 1996;87:334–338. doi: 10.1046/j.1365-2567.1996.463529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potter KN, Li Y, Pascual V, Williams RC, Jr, Byres LC, Spellerberg M, Stevenson FK, Capra JD. Molecular characterization of a cross-reactive idiotope on human immunoglobulins utilizing the VH4-21 gene segment. Journal of Experimental Medicine. 1993;178:1419–1428. doi: 10.1084/jem.178.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pugh-Bernard AE. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Vollenhoven RF, Bieber MM, Powell MJ, Gupta PK, Bhat NM, Richards KL, Albano SA, Teng NN. VH4-34 encoded antibodies in systemic lupus erythematosus: a specific diagnostic marker that correlates with clinical disease characteristics. Journal of Rheumatology. 1999;26:1727–1733. [PubMed] [Google Scholar]
- 64.del Rincon I, Zeidel M, Rey E, Harley JB, James JA, Fischbach M, Sanz I. Delineation of the human systemic lupus erythematosus anti-smith antibody response using phage-display combinatorial libraries. J Immunol. 2000;165:7011–7016. doi: 10.4049/jimmunol.165.12.7011. [DOI] [PubMed] [Google Scholar]
- 65.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rettig MB, Vescio RA, Cao J, Wu CH, Lee JC, Han E, DerDanielian M, Newman R, Hong C, Lichtenstein AK, et al. VH gene usage in multiple myeloma: complete absence of the VH4.21 (VH4-34) gene. Blood. 1996;87:2846–2852. [PubMed] [Google Scholar]
- 67.Froyland M, Thompson KM, Thorpe SJ, Sahota SS, Gedde-Dahl T, Bogen B. A VH4-34+ myeloma protein with weak autoreactivity. Haematologica. 2007;92:690–693. doi: 10.3324/haematol.10850. [DOI] [PubMed] [Google Scholar]
- 68.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of Autoantibody Responses via Somatic Hypermutation Outside of Germinal Centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 70.Meyer-Bahlburg A, Andrews SF, Yu KOA, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isenberg D, Spellerberg M, Williams W, Griffiths M, Stevenson F. Identification of the 9G4 idiotope in systemic lupus erythematosus. British Journal of Rheumatology. 1993;32:876–882. doi: 10.1093/rheumatology/32.10.876. [DOI] [PubMed] [Google Scholar]
- 72.Isenberg DA, McClure C, Farewell V, Spellerberg M, Williams W, Cambridge G, Stevenson F. Correlation of 9G4 idiotope with disease activity in patients with systemic lupus erythematosus. Annals of the Rheumatic Diseases. 1998;57:566–570. doi: 10.1136/ard.57.9.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samuels J, Ng Y-S, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KGC, Light A, O’Reilly LA, Ang S-M, Strasser A, Tarlinton D. bcl-2 Transgene Expression Inhibits Apoptosis in the Germinal Center and Reveals Differences in the Selection of Memory B Cells and Bone Marrow Antibody-forming Cells. J Exp Med. 2000;191:475–484. doi: 10.1084/jem.191.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol. 2008 doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- 77.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes. 1997;46:1701–1710. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 78.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of Autoantibodies before the Clinical Onset of Systemic Lupus Erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 79.Vinuesa CG, Cook MC. The molecular basis of lymphoid architecture and B cell responses: implications for immunodeficiency and immunopathology. Curr Mol Med. 2001;1:689–725. doi: 10.2174/1566524013363276. [DOI] [PubMed] [Google Scholar]
- 80.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 81.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM. Lymphoid Neogenesis in Rheumatoid Synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 82.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 83.Collin M, Shannon O, Bjorck L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proceedings of the National Academy of Sciences. 2008;105:4265–4270. doi: 10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McFarland HF. The B Cell -- Old Player, New Position on the Team. N Engl J Med. 2008;358:664–665. doi: 10.1056/NEJMp0708143. [DOI] [PubMed] [Google Scholar]
- 85.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunology. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 86.Sanz I, Wei C, Lee E-H, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Seminars in Immunology. 2008 doi: 10.1016/j.smim.2007.12.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner ECB, Sanz I. A New Population of Cells Lacking Expression of CD27 Represents a Notable Component of the B Cell Memory Compartment in Systemic Lupus Erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 88.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, Morris MA, Pizarro TT, Ernst PB, Cominelli F, et al. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114:389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, Gunzer M. Naive B-cells generate regulatory T-cells in the presence of a mature immunological synapse. Blood. 2007 doi: 10.1182/blood-2006-10-053793. blood-2006-2010-053793. [DOI] [PubMed] [Google Scholar]
- 91.Chen X, Jensen PE. Cutting Edge: Primary B Lymphocytes Preferentially Expand Allogeneic FoxP3+ CD4 T Cells. J Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 92.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of Arthritis by Interleukin 10-producing B Cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic Intestinal Inflammatory Condition Generates IL-10-Producing Regulatory B Cell Subset Characterized by CD1d Upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 94.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proceedings of the National Academy of Sciences. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, Gunzer M. Naive B-cells generate regulatory T-cells in the presence of a mature immunological synapse. Blood. 2007 doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 96.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. PNAS. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anolik JH, Friedberg JW, Barnard J, Owen T, Zheng B, Quach T, Wei C, Cushing E, Kelly J, Milner EC, et al. B Cell Reconstitution After Rituximab Recapitulates B Cell Ontogeny with a Preponderance of Transitional B cells and a Paucity of Memory B Cells. Arthritis & Rheumatism. 2006;54:S776. [Google Scholar]
- 98.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and Treg by B1 and B2 B cells. European Journal of Immunology. 2007:9999. doi: 10.1002/eji.200737296. NA. [DOI] [PubMed] [Google Scholar]
- 99.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee MKIV, Sonawane S, Kim J, Wang J, et al. Cutting Edge: Transplant Tolerance Induced by Anti-CD45RB Requires B Lymphocytes. J Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 100.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel Suppressive Function of Transitional 2 B Cells in Experimental Arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 101.Lampropoulou V, Hoehlig K, Roch T, Neves P, Gomez EC, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, et al. TLR-Activated B Cells Suppress T Cell-Mediated Autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 102.Anolik J, Barnard J, Owen T, Zheng B, Kemshett S, Looney J, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis & Rheumatism. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 103.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anolik J, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis & Rheumatism. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 105.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 106.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct Effector Cytokine Profiles of Memory and Naive Human B Cell Subsets and Implication in Multiple Sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 107.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner ECB, Fisher RI, Sanz I. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clinical Immunology. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Sharif MN, Tassiulas I, Hu Y, Mecklenbrauker I, Tarakhovsky A, Ivashkiv LB. IFN-{alpha} Priming Results in a Gain of Proinflammatory Function by IL-10: Implications for Systemic Lupus Erythematosus Pathogenesis. J Immunol. 2004;172:6476–6481. doi: 10.4049/jimmunol.172.10.6476. [DOI] [PubMed] [Google Scholar]
- 109.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. Journal of Experimental Medicine. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcon-Segovia D, Wijdenes J, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis & Rheumatism. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 111.Anolik J, Barnard J, Owen T, Dutcher P, Hadley J, Miller C, Looney J, Sanz I. Restoration of Proper Germinal Center Regulation of Autoreactive B Cells in Human SLE After B Cell Depletion Therapy. arthritis & Rheumatism. 2006;54:S806. [Google Scholar]
- 112.Sanz I, Wei C, Lee FEH, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Seminars in Immunology. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a pre-diversified immunoglobulin repertoire. Blood. 2004 doi: 10.1182/blood-2004-01-0346. 2004-2001-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Medina F, Segundo C, Campos-Caro A, Gonzalez-Garcia I, Brieva JA. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002;99:2154–2161. doi: 10.1182/blood.v99.6.2154. [DOI] [PubMed] [Google Scholar]