Abstract
The ability to rapidly identify immune cell subsets such as CD4 cells, which became possible around the same time as the onset of the HIV/AIDS pandemic, was one of the greatest advances in clinical and diagnostic immunology. The evolution of this global pandemic and the subsequent development of treatment strategies to prolong the life of infected individuals mean that it is now more crucial than ever that we develop affordable, reliable and accurate methods for the enumeration of CD4 cells. Here, we provide an overview of the historical developments in CD4 enumeration technologies that are related to HIV infection, and summarize the current technological challenges that must be overcome to meet the needs of those living with HIV infection.
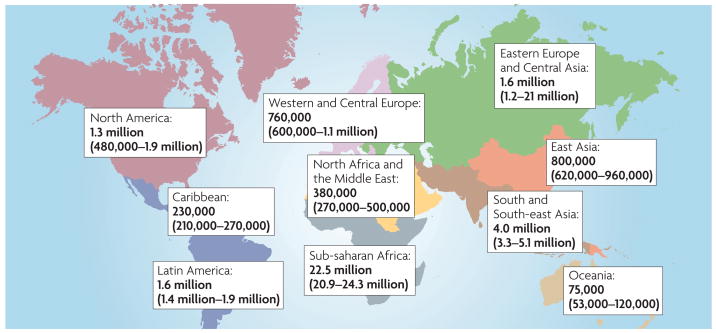
Recent figures from the World Health Organization (WHO) indicate that almost 33.2 million people are living with HIV infection worldwide, and over two-thirds of infected individuals are present in developing countries with limited resources (FIG. 1). In the region that has been hardest hit by AIDS — sub-Saharan Africa — the prevalence rate of HIV infection is 39%, yet only 17% of the HIV-infected individuals in this region had access to life-saving antiretroviral (ARV) therapy in 2007. Globally, more than 50% of those infected with HIV are women. Furthermore, recent reports from the WHO state that 1,800 babies are born with HIV every day because their mothers cannot access the necessary drugs or healthcare treatment. It is predicted that between 2008 and 2010 there will be 45 million new infections in resource-constrained and middle-income countries if no additional preventative methods are introduced.
Figure 1. Global distribution of HiV-1 infection, December 2007.
Data from ReF. 88. The total number of adults and children estimated to be living with HIV infection is 33.2 million.
It is now well recognized that, in the majority of cases, the development of AIDS parallels the decline in CD4+ T cells, which play a vital role in the regulation of the immune response. HIV specifically targets and binds to the CD4 antigen and chemokine receptor 5 (CCR5) or CXC chemokine receptor 4 (CXCR4) on CD4+ T cells to replicate, ultimately causing the destruction and deterioration of the immune system. The monitoring of CD4+ T cells during the course of HIV infection is therefore a crucial component in the monitoring of disease progression and the response to ARV therapy.
Over the past 25 years there have been major technological advances in the ways that CD4+ T cells are enumerated, with flow cytometry now generally regarded as the predicate technique. During this period, flow cytometry has progressed from large, expensive and complex fluorescence-activated cell sorting (FACS) machines that require highly specialized operating personnel, to smaller, more affordable bench-top instruments that can be used by individuals with minimal training. This shift was made possible in part by including multicolour analysis coupled with simplified gating technologies. More recently, several low-cost, point of care, micro-diagnostic technologies have been developed for use in rural settings, however, full validation of these technologies is still awaited.
In many resource-constrained countries, including countries in sub-Saharan Africa, the cost of flow cytometers has prohibited the routine use of CD4 monitoring. However, the pressure of the AIDS pandemic has stimulated significant advances in the development of lower-cost methods for flow cytometry. This article will focus on flow cytometry as it relates to CD4 enumeration to determine the initiation and monitoring of ARV therapy.
The early years of the HIV epidemic
In 1981, independent research groups in New York and California published reports of a high incidence of Pneumocystis carinii pneumonia (PCP) and the development of Kaposi’s sarcoma (a relatively rare skin carcinoma) in men who have sex with men1–3. It was soon ascertained that affected individuals were immunosuppressed and that this immunosuppression resulted from a reduced percentage and absolute number of helper T cells (now defined as CD4+ T cells). These findings stimulated scientific teams around the world to search for the causative agent1–5. Two years later, it was determined that this immunosuppressive disorder was caused by a retrovirus that is now known as HIV6,7. HIV was found to specifically target and destroy CD4+ T cells8 and the destruction of these cells correlated with the onset of AIDS. The development of PCP, the most common opportunistic infection associated with AIDS, usually occurred at a CD4 count <200 cells per μl9. It thus became obvious that the development of methods to monitor this decline would be extremely important.
It is interesting to note that the emergence of the need to monitor the percentage of CD4 cells and the CD4 count coincided with improvements in computer miniaturization and processing power and the development of monoclonal antibodies10,11. In the early 1980s, monoclonal antibodies were being introduced into clinical laboratory practice as tools for use in leukaemia diagnosis12. The use of antibodies that were specific for T-cell antigens was crucial for monitoring of HIV/AIDS patients. The proliferation of computer usage in industry and science, coupled with the simultaneous development of smaller bench-top flow cytometers, meant that this crucial combination of technology and medical science allowed the identification of individuals who had reduced or declining numbers of CD4+ T cells, and the enumeration of these cells quickly became one of the cornerstones of HIV/AIDS monitoring13,14.
Flow cytometry
In 1954, Wallace Coulter developed an instrument that could measure cell size and count the absolute number of cells15, and thus the discipline of flow cytometry was born. Further developments enabled the production of instruments that could measure cell size and nucleic acid content using a two-dimensional approach that compared light scatter and light absorption16,17. These instruments were cumbersome and required specialist operators, but immunologists began to use them to investigate the functions of immune cells. The HIV pandemic, it could be argued, greatly influenced the way these instruments were viewed, as they made the transition from being used primarily as research tools to being used in diagnostic testing at clinical facilities. By the mid-1980s, bench-top flow cytometers were available and as the technology advanced the instruments became progressively smaller. Coupled with the availability of monoclonal antibodies, the increasing number of available fluorochromes (compounds that emit light at a greater wavelength than the light source they are excited with) and computer improvements, flow cytometers are now accessible for almost every clinical laboratory.
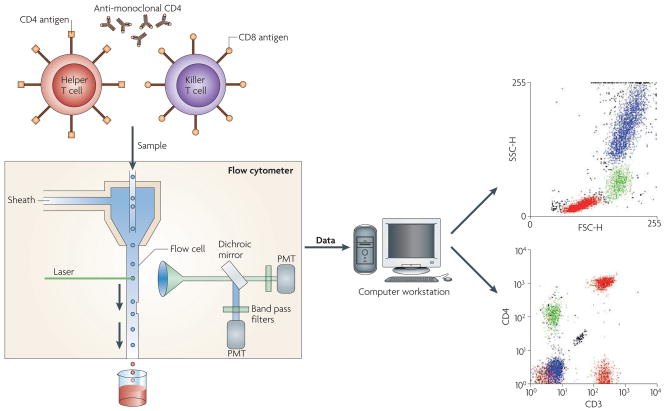
Flow cytometry enables the examination of microscopic particles (such as cells) that are suspended in a stream of fluid which is termed sheath fluid. This fluid is focused hydrodynamically such that the cells flow in single file through a flow cell in which a beam of light (usually a laser) is focused. As the cells pass through the laser beam they scatter the light so that forward scatter (FSC) and side scatter (SSC) light is captured; this enables the size and granularity of the cells to be determined. In addition, if a cell is labelled with antibodies that carry a fluorochrome, as the cell passes in front of the laser beam the fluorochrome emits light at a wavelength that is higher than the single wavelength light source and which can be detected by fluorescent detectors. The flow cytometers that are in clinical use can analyse at least four fluorochromes simultaneously, in addition to the FSC and SSC. This is known as multiparametric analysis. The information that is generated is computer analysed so that specific analysis regions (known as gates) can be created, which allows the user to build up a profile of the size, granularity and antigen profile of the target cell population (FIG. 2).
Figure 2.
Schematic representation of flow cytometry.
The fundamental basis of CD4 counting and identification relies on the specific use of electronic gating approaches. One of the earliest and most popular approaches that was used from the mid-1980s to early-1990s relied on using light scatter and anti-CD45 and anti-CD14 monoclonal antibodies. This gating approach was termed the two-colour approach. Using the FSC and SSC characteristics of leukocytes as they passed through the laser beam enabled distinct leukocyte populations to be identified. An appropriate lymphocyte analysis region was defined when these parameters were combined with the use of anti-CD45 antibodies while the monocyte contamination within the analysis region could be calculated when these parameters were combined with the use of anti-CD14 antibodies. A second tube contained two antibody classes and isotype controls, matched to the test antibodies that were in the subsequent tubes (anti-CD3 and anti-CD19, anti-CD3 and anti-CD4, anti-CD3 and anti-CD8 and finally anti-CD19 and anti-CD16/CD56)18,19. This panel of antibodies formed the basis for the Centers for Disease Control and Prevention (CDC) consensus panel that, at the time, was believed to provide the optimum approach for quality monitoring purposes, but by today’s standards is overly complex and expensive. A second major problem was that this approach relied on the use of either the total leukocyte count or the total lymphocyte count to determine the absolute lymphocyte subset count, an approach which has inbuilt error20,21. One major disadvantage of such a gating strategy is that as the sample deteriorates over time, the level of non-lymphocyte contamination increases and thus samples that are more than 36 hours old are often difficult to analyse22. More recently, techniques such as PanLeucogating technology (see later) have overcome the issue of sample aging23. This is particularly relevant in regions of the world where there are delays in transporting the sample from the collecting site to the central or higher-level reference laboratory where the CD4 assay is carried out.
In the early-1990s researchers realized that because of these logistical problems, new technologies would need to be developed to reduce processing time while providing accurate CD4 counts. Two new gating strategies were developed almost simultaneously, one termed CD45 gating and the other T gating, both of which helped to more accurately identify the lymphocyte analysis regions24,25. A unique development was the introduction of an anchor marker, which is a marker that remains consistent through every analysis tube to enable the operator to obtain an accurate and consistent gate check between all analysis tubes24,25. Researchers soon realized that a further advantage of the anchor marker was that it also enabled the analysis of blood samples for longer periods post-phlebotomy than previous gating strategies. A recent study has shown that using CD45 anchor gating, samples can be analysed for up to five days post-phlebotomy24, thus enabling samples to be transported over longer distances before analysis22.
CD4+ T-cell monitoring and HIV
ARV drug regimens suppress HIV replication. The timing and initiation of such therapy is therefore important because if left untreated, HIV infection is ultimately fatal in nearly all cases. Currently, monitoring of CD4+ T-cell counts has become a crucial indicator of when to initiate ARV therapy, but no universal consensus has yet emerged, with some groups recommending that treatment should be initiated when the count is <200 CD4+ T cells per μl, others between 200–350 CD4+ T cells per μl and still others suggest starting highly active anti-retroviral therapy (HAART) at counts of >350 CD4+ T cells per μl26–34. However, it should be stressed that a low CD4+ T cell count should not be used as an indicator of HIV infection, and therefore an indicator for the commencement of ARV therapy, without evidence of HIV infection. Currently, nearly 3 million people are receiving ARV therapy in low- and middle-income countries, according to a new report from WHO, the Joint united Nations Programme on HIV/AIDS (UNAIDS) and the united Nations Children’s Fund (UNICEF)35. More significantly, the price of ARV therapies has fallen by up to 53% in developing countries in recent years. The average annual cost of a two-combination approach (a fixed-dose combination of Zidovudine and Lamivudine plus a single dose of Efavirenz), was US$403 per person in 2007, according to Médecins Sans Frontières36.
Although the cost of ARVs has fallen, increased access to treatment has raised the question of how the therapeutic efficacy should be monitored. While a patient is receiving ARVs, it is important that they are monitored to identify drug toxicities and to assess their degree of adherence. Patients who fail to adhere to prescribed ARV doses are at risk for developing antiretroviral resistance37,38. Problems with adherence are detected by self-reporting, pill counts and by therapeutic drug monitoring or by careful monitoring of the viral load and the general CD4 trend. It is crucial to monitor CD4 levels in HIV-infected patients over the course of the disease (with 3–6 monthly intervals generally being within accepted good practice guidelines in the developed world) to determine when to initiate ARV therapy and prophylaxis against opportunistic infections. However, although access to treatment for HIV-infected individuals is headline news, the regular laboratory monitoring of such treatment receives little or no attention.
The effect of ARV therapy on the CD4+ T cell population is now well documented, with current regimens significantly increasing CD4 counts, as well as increasing the life expectancy of infected individuals by several years39–47. The usual monitoring approach is to measure viral load (HIV RNA levels) and/or the absolute number of CD4+ T cells. It is this monitoring phase that is problematic in resource-constrained settings, particularly in under-developed countries where few laboratories are equipped with the appropriate technology and the transport infrastructure is poor. Patients often have to travel long distances for blood tests for monitoring because viral load measurement and, until recently, flow cytometric analysis to provide CD4 counts could only be effectively undertaken at specialist centres. Therefore, the HIV pandemic has raised a number of significant issues that, although less relevant in developed countries, are major issues in countries with limited resources. As stated earlier, increasing the availability of ARVs in countries with limited resources has been given primary consideration by many organizations. However, relevant planning and implementation of the laboratory monitoring phase must also be taken into consideration.
One important aspect of CD4 monitoring is that not only do the reference ranges within a country or population need to be determined, but the cut-off points for the initiation of ARVs and opportunistic infection therapy also need to be defined. Age-adjusted variations in lymphocyte populations have been demonstrated in several studies48–50 and have been described in detail by Richard Stiehm51 (TABLE 1). In the united States, the normal level of CD4+ T cells in adults averages around 1,000 cells per μl, but can range from approximately 450 to >1500 cells per μl52. When an individual’s CD4 level is 200 cells per μl, a patient is diagnosed as having AIDS19,53. At this point, there is a high probability that AIDS-related opportunistic infections and neoplasms will manifest5,54,55. The CDC recommends initiating prophylaxis against opportunistic infections when CD4 levels are <200 cells per μl56 (TABLE 2). However, there is variation in the reference ranges between more-developed and less-developed countries, with this variation reflecting several issues, including the HIV disease burden and spectrum in the population under study; the prevalence of endemic co-infections (such as tuberculosis); nutritional status; and, equally as important, the capacity and infrastructure of the health systems concerned57. Developing countries might choose to adopt a universal option to initiate prophylaxis against opportunistic infections depending on the level of access to healthcare. A universal option recommends primary initiation against opportunistic infections when an HIV-infected individual’s CD4 count falls below 350 cells per μl in any WHO clinical stage57. When a CD4 count is not available, the WHO clinical staging system can be used to determine when prophylaxis against opportunistic infections should be initiated (BOX 1). expanded access to CD4 testing is encouraged to guide the initiation of ARV therapy and monitor its progress57.
Table 1.
Normal human blood lymphocyte subpopulations at various ages*
| Cord blood | 2–3 months old |
4–8 months old |
12–23 months old |
2–5 years old |
7–17 years old |
Adult | |
|---|---|---|---|---|---|---|---|
| Total lymphocytes (cells per μI) | |||||||
| Median | 5,400 (41%) | 5,680 (66%) | 5,990 (64%) | 5,160 (59%) | 4,060 (50%) | 2,400 (40%) | 2,100 (32%) |
| Confidence intervals‡ | 4,200 (35%)–6,900 (47%)§ | 2,920 (55%)–8,840 (78%) | 3,610 (45%)–8,840 (79%) | 2,180 (44%)–8,270 (72%) | 2,400 (38%)–5,810 (64%) | 2,000 (36%)–2,700 (43%)§ | 1,600 (28%)–2,400 (39%)§ |
| CD3 T cells (cells per μI) | |||||||
| Median | 3,100 (55%) | 4,030 (72%) | 4,270 (71%) | 3,300 (66%) | 3,040 (72%) | 1,800 (70%) | 1,600 (73%) |
| Confidence intervals‡ | 2,400 (49%)–3,700 (62%)§ | 2,070 (55%)–6,540 (78%) | 2,280 (45%)–6,450 (79%) | 1,460 (53%)–5,440 (81%) | 1,610 (62%)–4,230 (80%) | 1,400 (66%)–2,000 (76%)§ | 960 (61%)–2,600 (84%)§ |
| CD4 T cells (cells per μI) | |||||||
| Median | 1,900 (35%) | 2,830 (52%) | 2,950 (49%) | 2,070 (43%) | 1,800 (42%) | 800 (37%) | 940 (46%) |
| Confidence intervals‡ | 1,500 (28%)–2,400 (42%)§ | 1,460 (41%)–5,116 (64%) | 1,690 (36%)–4,600 (61%) | 1,020 (31%)–3,600 (54%) | 900 (35%)–2,860 (51%) | 700 (33%)–1,100 (41%) | 540 (32%)–1,660 (60%)§ |
| CD8 T cells (cells per μI) | |||||||
| Median | 1,500 (29%) | 1,410 (25%) | 1,450 (24%) | 1,320 (25%) | 1,180 | 800 (30%) | 520 (27%) |
| Confidence intervals‡ | 1,200 (26%)–2,000 (33%)§ | 650 (16%)–2,450 (35%) | 720 (16%)–2,490 (34%) | 570 (16%)–2,230 (38%) | 630 (22%)–1,910 (38%) | 600 (27%)– 900(35%)§ | 270 (13%)– 930(40%)§ |
| B cells (CD19 or CD20; cells per μI)|| | |||||||
| Median | 1,000 (20%)¶ | 900 (23%) | 900 (23%) | 900 (23%) | 900 (24%) | 400 (16%)¶ | 246 (13%)¶ |
| Confidence intervals‡ | 200 (14%)– 1,500 (23%)§ | 500 (19%)–1,500 (31%) | 500 (19%)–1,500 (31%) | 500 (19%)–1,500 (31%) | 700 (21%)–1,300 (28%) | 300 (12%)– 500 (22%)§ | 122 (10%)– 632 (31%) |
| CD4 to CD8 ratio | |||||||
| Median | 1.2 | 2.2 | 2.1 | 1.6 | 1.4 | 1.3 | 1.7 |
| Confidence intervals‡ | 0.8–1.8 | 1.3–3.5 | 1.2–3.5 | 1.0–3.0 | 1.0–2.1 | 1.1–1.4 | 0.9–4.5 |
The lymphocyte percentage represents the percentage of total leukocytes, whereas the CD3, CD4, CD8 and B cell (CD19 or CD20) percentages represent the percentage of total lymphocytes. Each subgroup contains at least 22 normal subjects.
Confidence intervals given are at the fifth to ninety-fifth percentiles unless otherwise indicated.
At the twenty-fifth to seventy-fifth percentiles.
B cells use the CD20 antigen unless otherwise indicated.
Use the CD19 antigen. Table modified, with permission, from ReF. 51 © Elsevier (1996).
Table 2.
Criteria for starting and stopping prophylaxis of opportunistic infections in HIV-infected adults*
| Opportunistic infection | Criteria for initiating primary prophylaxis | Criteria for stopping primary prophylaxis |
|---|---|---|
| Pneumocystis carinii pneumonia | CD4+ count of <200 cells per μl or oropharyngeal Candida infection (AI) | Not applicable |
| CD4+ count of >200 cells per μl for≥3 months (AI) | Not applicable | |
| Toxoplasmosis | Immunoglobulin G antibody to Toxoplasma and CD4+ count of <100 cells per μl (AI) | CD4+ count of >200 cells per μl for≥3 months (AI) |
| Disseminated Mycobacterium avium complex | CD4+ count of <50 cells per μl (AI) | CD4+ count of >100 cells per μl for≥3 months (AI) |
Table modified, with permission, from ReF. 56.
Box 1. WHO clinical staging of HIV disease among adults and adolescents*‡.
Clinical Stage 1
|
Clinical Stage 4
|
Clinical Stage 2
| |
Clinical Stage 3
|
Data from REF. 45.
Some additional specific conditions can also be included in regional classifications, such as the reactivation of American trypanosomiasis (meningoencephalitis and/or myocarditis) in the WHO Region of the Americas, and penicilliosis in Asia.
Unexplained is used when the condition is not explained by other conditions.
Assessment of body weight among pregnant woman needs to consider the expected weight gain of pregnancy.
It should be noted that the decline in the CD4 count varies between patients and that the plasma viral load is a strong predictor of the rate of CD4 decline and progression to AIDS or death58. Some patients, from the point of infection, have a very rapid decline in the CD4 count to <200 cells per μl in less than two years (rapid progressors) whereas in other individuals in whom the decline is slower (slow progressors), the CD4 count can take as long as 15 years to fall below this level13,59,60. More recently, the rate of CD4 decline in the cohort analysed by the Multicenter AIDS Cohort Study shows a slope of -0.010 cells per μl per year when measured three years after seroconversion61. A reduction in the CD4 count can also be idiopathic with no determinable cause; therefore a low CD4 count does not necessarily indicate the onset of AIDS62,63.
The need for low-cost CD4 monitoring
The HIV pandemic affects most of the world, but it has a significantly greater adverse effect in sub-Saharan Africa where healthcare resources are limited and many individuals cannot afford treatment. In addition, as already mentioned, patients might have to travel long distances to receive treatment. These factors affect the frequency of CD4+ T-cell monitoring and can delay or even prevent ARV initiation. Timely monitoring of the CD4 count in HIV-infected patients in resource-constrained settings demands the development of simple, low-cost, accessible, reliable and accurate CD4 enumeration techniques. These techniques can be categorized in three ways: gating and counting strategies that can be applied to existing bench-top flow cytometers within centralized laboratories that can drastically reduce costs; small and less technically demanding flow cytometers that can be installed in clinics64–66; and non-flow cytometric methods that can provide rapid results without the need for expensive equipment or even electricity67–69. Although unproven as yet, some of these advances might permit point-of-care CD4 testing where the results can be obtained in a rural clinic setting.
Some fundamental issues are central to the implementation of these technologies. First, they must identify and monitor the CD4 count precisely and accurately. The traditional approach of calculating absolute CD4 counts is to use the total leukocyte (or lymphocyte) count obtained from a haematology analyzer and then use the percentage of CD4+ T cells (as a percentage of total leukocytes or lymphocytes) from a flow cytometer to calculate the absolute values — the so-called ‘dual platform’ (DP) approach55. Often, however, two separate samples that are collected in the same anticoagulant are used — one is used to obtain the total leukocyte or lymphocyte count and the other is used to obtain the percentage of total lymphocytes from the flow cytometer, and each has its own in-built variation. Thus, when the results from each sample are combined to determine the absolute CD4 count the variation is compounded such that inter-laboratory variation between two centres can be as high as 40%20,21. The effect that haematology analyzers have on the resulting CD4 count was illustrated by Robinson and colleagues21, with these findings being confirmed by external quality assessment (EQA) studies undertaken by the united Kingdom National external Quality Assessment Scheme (UK NEQAS) for Leukocyte Immunophenotyping20,70. This could effectively lead to the introduction of different treatment decisions between centres. Thus, the need to derive accurate and precise absolute CD4 counts has led to the development of instruments that can produce both percentage and absolute values, termed the ‘single platform’ (SP) approach71.
Two SP approaches are in widespread use today: volumetric and bead-based. The principle of the volumetric approach is that a known volume of sample, usually delivered by precision fluidics (for example, by a Hamilton syringe), is passed through the flow cell and laser beam for analysis. In the bead-based approach, a known number of beads is added to a known volume of sample, thus allowing the bead to cell ratio, and subsequently the absolute cell count (in this instance the CD4 count), to be calculated53,71–75. However, an important feature of any absolute counting system is pipetting accuracy and minimum sample manipulation. As such, a lyse no-wash approach in which red blood cells are selectively lysed is used in most absolute counting methods, but a no-lyse no-wash approach is the preferred option71,76. It is the latter approach that forms the basis of one such system that has been specifically designed for use in resource-constrained countries64.
The introduction of SP technologies has been beneficial and has lowered inter-laboratory variation in comparison with dual platform (DP) approaches. UK NEQAS for Leukocyte Immunophenotyping, an immune monitoring proficiency testing programme, monitored the inter-laboratory variation of participating laboratories over an 11-year period. The data showed that SP technology gave a lower inter-laboratory coefficient of variation (CV) than the DP approach. It is this variation that will potentially impact on patient treatment decisions, and thus its reduction through the introduction of improved technologies will in turn benefit patient care. This analysis also provided data on the error that is encountered in clinical testing77. For example, when a CD4 count of 200 cells per μl was expected, the actual reportable range was 167–233 cells per μl, a relative error of 16%, which increased to 29% at 47 cells per μl but decreased to 14% at approximately 500 cells per μl78. UK NEQAS for Leukocyte Immunophenotyping therefore reasoned that if SP techniques have lower inter-laboratory variation, the error should be lower. This was confirmed when results from 48 consecutive EQA samples (equating to 10,626 data sets) from 180 international laboratories were analysed using SP and DP techniques. This revealed that at every CD4+ T-cell level, SP techniques had a lower measurement error, proving that SP techniques are more accurate and precise than the DP approach79. This should, in turn, result in better patient management. Furthermore, it has also been demonstrated that participation in an EQA programme improves inter-laboratory variation80, and that DP technologies are up to 2.4 times more likely to fail an EQA exercise for absolute counts than SP technologies70.
As well as developing precise and accurate absolute CD4+ T cell counting strategies, there has been a need to develop improved gating strategies. It is well established that CD45 gating or double-anchor gating strategies are the techniques of choice in the developed world17,18. However, the panel of antibodies used with such strategies encompasses the identification of all lymphocyte subsets (b, T and natural killer cells) and can be expensive. The rationale behind using such a comprehensive panel has been questioned in countries with limited resources where the CD4 count is the only lymphocyte subset parameter that is needed to monitor when to initiate treatment and treatment efficacy. In response to this controversy, an integrated primary gating strategy that was both robust and low-cost was developed23,72,81. This technique, known as PanLeucogating technology, offers a significant number of advantages for use in resource-constrained settings: it is low cost as only two monoclonal antibodies are used; technical errors are minimized owing to its low complexity; and samples up to five days old can still be analysed by excluding non-relevant events such as debris platelets and monocytes82. The UK NEQAS for Leukocyte Immunophenotyping has shown that a gating technique such as PanLeucogating reduces the probability of failing their EQA programme by as much as 7.4 times when compared to other gating strategies such as FSC/SSC and CD14/CD45 gating70.
Quality control and proficiency testing
Training, education and quality control (QC), both internal QC (IQC) and EQA, are necessary to ensure accurate and precise CD4 counts. Proficiency testing programmes can regularly monitor the performance of laboratories that provide T-cell subset enumeration. evidence from EQA programmes now indicates that CD4 counts can be obtained with high precision, but the question of accuracy has always remained. The absence of any suitable reference preparation with a predefined percentage and absolute number of CD4+ T cells has hindered the identification of a reference technique. However, a potential solution to this problem might now be available. In 2000, a three-year European initiative was established that attempted to develop stabilized reference preparations for several flow cytometric applications that included CD4+ T cells83. The study concluded however, that because there is no internationally recognized reference analysis technique, the development of a reference preparation might be more problematic than first envisioned. Thus, only control preparations could be manufactured until an internationally agreed reference analysis technique is assigned. The fundamental difference between a reference preparation and control preparation is that the former contains an exact, predefined number of cells whereas the latter contains an expected range of cells. However, one key factor that emerged from the study was that a panel of stabilized whole-blood preparations could be manufactured that contained various levels of T-cell subsets. In 2004, UK NEQAS for Leukocyte Immunophenotyping and the Immunology Quality Assessment proficiency testing programme, which is based at Duke university, Durham, North Carolina, USA, began a collaboration to develop a training panel of 20 stabilized whole-blood samples (containing single, duplicate and triplicate samples of defined CD4 counts) that could be used as a training tool. This approach will enable laboratories that are new to T-cell subset enumeration to evaluate their performance before testing patient material. In addition, it will help them detect where errors are occurring and act as a pre-qualification exercise before embarking on multicentre EQA exercises.
The future of CD4 testing
ARVs are now becoming increasingly available in many countries and their use must be monitored effectively to determine the point at which to initiate therapy and also to identify those patients at risk of treatment failure because of drug resistance. The CD4 count and viral load testing are used for monitoring in developed countries. These tests provide crucial and independent evidence of disease progression and response to therapy. However, in resource-constrained countries it is difficult to provide viral load testing because of cost and other considerations. It follows, therefore, that the current practical solution to providing accurate and effective monitoring is by using the CD4 count. Ideally, because of potential specimen transportation issues, the newer CD4 counting technologies should be capable of being used at the point of care. Thus, in the past three or four years several new small flow cytometers have been developed. One key feature of these machines is that highly trained operators are not required, and the small size of the instruments generally enables their transportation and placement in lower-level laboratories, of particular value in resource-constrained countries65. Furthermore, because electricity can also be a problem in such areas, the newer platforms should also be capable of running on direct current or battery as well as alternating current. They should also be resistant to, or tolerant of, dust and high heat and humidity.
Both existing and new instruments must be assessed and validated. To achieve this, and enhance the results obtained from clinical trials, standard guidelines should be implemented. There are two aspects to such guidelines: an outline of the strategies used to validate instruments (validation) and an outline of the procedures (standard operating procedures) that should be used by personnel when undertaking laboratory testing and CD4 counting. With the latter, national guidance documents are already in existence for developed countries such as those published by the CDC, the british Committee for Standardization in Haematology, and the Clinical and Laboratory Standards Institute84–87. These documents outline the gating strategies, appropriate staining panels and the procedures that should be employed for enumerating lymphocyte subsets. However, it should be considered that such guidance documents were specifically written for developed countries and for the instruments that were available at the time of writing. More recently, the National Laboratory Standards Institute published guidelines that incorporate the use of the PanLeucogating approach but it does not specifically address the needs of resource-constrained countries. Although such documents can be easily adapted to address the needs of these countries, the bigger issue of having protocols and procedures that ensure existing and new instruments and technologies can be successfully validated must be urgently addressed. At this time, the lack of any internationally accepted reference preparation means that any validation process has to be undertaken at multiple international sites and thus might need to incorporate a tiered approach. For example, validation would begin at reference laboratories and then at lower-level laboratories where the test will be used. Validation protocols will need to address issues such as temperature stability, specimen stability for testing, transportation issues and their performance in EQA programmes. The latter is of particular importance because as most EQA programmes now use stabilized peripheral blood for performance monitoring it is important that new and existing instruments are capable of being effectively quality-assessed using such material70,76,80,82. EQA validation protocols will need to be developed for the current technologies that are not compatible with stabilized whole-blood products.
The Bill and Melinda Gates Foundation recently provided US$8.6 million for an initiative based at Imperial College, London, UK that aims to develop a simple, affordable, robust and rapid test to measure CD4+ T cells in HIV/AIDS patients in resource-constrained countries. The initiative attempts to co-ordinate the collaboration of academia, industry and other institutions to develop such a test over a four-year period. It is such initiatives that are clearly urgently required to enable the routine monitoring of CD4+ T cells in individuals that do not have access to sophisticated western style health care. However, it should also be considered that with the introduction of any new technique, whether based on flow cytometry or simplified counting approaches, there should be appropriate guidance and quality monitoring systems in place. These should encompass national guidelines and incorporate internal QC and external QA measures to ensure that whatever the technique used to assess CD4+ T cells, the patient receives the most appropriate and timely treatment.
References
- 1.Gottlieb MS, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 2.Masur H, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981;305:1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 3.Siegal FP, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305:1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 4.Ammann AJ, et al. Acquired immune dysfunction in homosexual men: immunologic profiles. Clin Immunol Immunopath. 1983;27:315–325. doi: 10.1016/0090-1229(83)90084-3. [DOI] [PubMed] [Google Scholar]
- 5.Schroff RW, Gottlieb MS, Prince HE, Chai LL, Fahey JL. Immunological studies of homosexual men with immunodeficiency and Kaposi’s sarcoma. Clin Immunol Immunopathol. 1983;27:300–314. doi: 10.1016/0090-1229(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 6.Barré-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 7.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 8.McDougal JS, et al. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986;231:382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- 9.Margolick JB, et al. Decline in total T cell count is associated with onset of AIDS, independent of CD4(+) lymphocyte count: implications for AIDS pathogenesis. Clin Immunol Immunopathol. 1998;88:256–263. doi: 10.1006/clin.1998.4577. [DOI] [PubMed] [Google Scholar]
- 10.Kohler G, Howe SC, Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976;6:292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 11.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 12.Janossy G. In: Methods in Hematology The Leukemic Cell. Catovsky D, editor. Churchill Livingstone; London: 1981. pp. 129–183. [Google Scholar]
- 13.Giorgi JV. Characterization of T lymphocyte subset alterations by flow cytometry in HIV disease. Ann New York Acad Sci. 1993;677:126–137. doi: 10.1111/j.1749-6632.1993.tb38771.x. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi JV, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopath. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 15.Coulter WH. High speed automatic blood cell counter and analyser. Proc Soc Exp Biol Med. 1954;12:1034–1035. [Google Scholar]
- 16.Kamentsky LA, Melamed MR, Derman H. Spectrophotometer: new instrument for ultrarapid cell analysis. Science. 1965;150:630–631. doi: 10.1126/science.150.3696.630. [DOI] [PubMed] [Google Scholar]
- 17.Kamentsky LA, Melamed MR. Spectrophotometric cell sorter. Science. 1967;156:1364–1365. doi: 10.1126/science.156.3780.1364. [DOI] [PubMed] [Google Scholar]
- 18.Loken MR, Brosnan JM, Bach BA, Ault KA. Quality control in flow cytometry: 1: Establishing optimal lymphocyte gates for immunophenotyping by flow cytometry. Cytometry. 1990;11:453–459. doi: 10.1002/cyto.990110402. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus infection. MMWR. 1992;41:1–19. [PubMed] [Google Scholar]
- 20.Barnett D, Granger V, Whitby L, Storie I, Reilly JT. Absolute CD4+ T-lymphocyte and CD34+ stem cell counts by single platform flow cytometry: The way forward. Br J Haematol. 1999;106:1059–1062. doi: 10.1046/j.1365-2141.1999.01632.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson G, et al. Effect of type of haematology analyser on CD4 count. Lancet. 1992;340:485. doi: 10.1016/0140-6736(92)91807-k. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron M, et al. Selection of lymphocyte gating protocol has an impact on the level of reliability of T-cell subsets in aging specimens. Cytometry. 2002;50:53–61. doi: 10.1002/cyto.10092. [DOI] [PubMed] [Google Scholar]
- 23.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 24.Mandy FF, Bergeron M, Recktenwald D, Izaguirre CA. A simultaneous three-color T cell subsets analysis with single laser flow cytometers using T cell gating protocol. Comparison with conventional two-color immunophenotyping method. J Immunol Meth. 1992;156:151–162. doi: 10.1016/0022-1759(92)90021-k. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson JK, Jones BM, Hubbard M. CD4 T-lymphocyte determinations on whole blood specimens using a single-tube three-color assay. Cytometry. 1993;14:685–689. doi: 10.1002/cyto.990140614. [DOI] [PubMed] [Google Scholar]
- 26.Antiretroviral Therapy Cohort Collaboration. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46:607–615. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein KA, et al. Initiation of antiretroviral therapy at CD4 cell counts >/=350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47:27–35. doi: 10.1097/QAI.0b013e31815acacc. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SB, et al. AIDS Society of India Guidelines Development Committee. API consensus guidelines or use of antiretroviral therapy in adults (API-ART guidelines). Endorsed by the AIDS Society of India. J Assoc Physicians India. 2006;54:57–74. [PubMed] [Google Scholar]
- 29.Dybul M, et al. Panel on Clinical Practices for the Treatment of HIV. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep. 2002;51 (RR7):1–55. [PubMed] [Google Scholar]
- 30.Iribarren JA, et al. Spanish GESIDA/Nacional AIDS Plan Recommendations for antiretroviral therapy in HIV-infected Adults (October 2004) Enferm Infecc Microbiol Clin. 2004;22:561–563. doi: 10.1157/13069520. [DOI] [PubMed] [Google Scholar]
- 31.Hammer SM, et al. International AIDS Society-USA panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 32.Beck EJ, et al. National adult antiretroviral therapy guidelines in resource-limited countries: concordance with 2003 WHO guidelines? AIDS. 2006;20:1497–1502. doi: 10.1097/01.aids.0000237365.18747.13. [DOI] [PubMed] [Google Scholar]
- 33.Gazzard B on behalf of the BHIVA Writing Committee. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2006) HIV Med. 2006;7:487–503. doi: 10.1111/j.1468-1293.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 34.Opravil M, et al. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count >350 × 106/l. AIDS. 2002;16:1371–1381. doi: 10.1097/00002030-200207050-00009. [DOI] [PubMed] [Google Scholar]
- 35.UNAIDS. 2008 report on the global AIDS epidemic [online] UNAIDS; Geneva, Switzerland: 2008. < http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp>. [Google Scholar]
- 36.Médecins Sans Frontières. Untangling the web of price reductions: a pricing guide for the purchase of ARVs for developing countries [online] 2007 < http://www.accessmed-msf.org/resources/key-publications/key-publication-detail/article/untangling-the-web-10th-version-english/>(MSF.
- 37.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antiviral Ther. 2008;13:25–36. [PubMed] [Google Scholar]
- 38.Huang KH, Loutfy MR, Tsoukas CM, Bernard NF. Immune correlates of CD4 decline in HIV-infected patients experiencing virologic failure before undergoing treatment interruption. BMC Infect Dis. 2008;8:59. doi: 10.1186/1471-2334-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bushman F, Landau NR, Emini EA. New developments in the biology and treatment of HIV. Proc Natl Acad Sci USA. 1998;95:11041–11042. doi: 10.1073/pnas.95.19.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casado JL, et al. Predictors of long-term response to protease inhibitor therapy in a cohort of HIV-infected patients. AIDS. 1998;12:131–135. doi: 10.1097/00002030-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Dieye TN, et al. Spontaneous apoptosis and highly active antiretroviral therapy (HAART) Biomed Pharmacother. 2000;54:16–20. doi: 10.1016/s0753-3322(00)88636-9. [DOI] [PubMed] [Google Scholar]
- 42.Dyrhol-Riise AM, Voltersvik P, Rosok BI, Olofsson J, Asjo B. Normalization of CD4+ cell numbers and reduced levels of memory CD8+ cells in blood and tonsillar tissue after highly active antiretroviral therapy in early HIV type-1 infection. AIDS Res Hum Retrovir. 2000;16:191–201. doi: 10.1089/088922200309287. [DOI] [PubMed] [Google Scholar]
- 43.Evans TG, et al. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res. 1998;39:163–173. doi: 10.1016/s0166-3542(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 44.Fessel WJ, et al. Dissociation of immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:314–320. doi: 10.1097/00126334-200004010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson LP, et al. Changes in survival after acquired immunodeficiency syndrome (AIDS): 1984–1991. Am J Epidemiol. 1993;138:952–964. doi: 10.1093/oxfordjournals.aje.a116815. [DOI] [PubMed] [Google Scholar]
- 46.Pandolfi F, et al. The Italian quality control study for evaluation of CD4 cells in centres involved in the treatment of HIV-1 patients. Clin Exp Immunol. 1998;111:564–573. doi: 10.1046/j.1365-2249.1998.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peter K, Gambertoglio JG. Zidovudine phosphorylation after short-term and long-term therapy with zidovudine in patients infected with the human immunodeficiency virus. Clin Pharmacol Ther. 1996;60:168–176. doi: 10.1016/S0009-9236(96)90132-0. [DOI] [PubMed] [Google Scholar]
- 48.Erkellor-Yuksel FM, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–222. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 49.Denny T, et al. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA. 1992;267:1484–1488. [PubMed] [Google Scholar]
- 50.Giorgi JV, et al. In: Manual of Clinical Laboratory Immunology. 4. Rose NR, et al., editors. ASM Press; Washington, D.C., USA: 1992. pp. 174–181. [Google Scholar]
- 51.Stiehm ER. In: Immunologic Disorders in Infants and Children. 4. Stiehm ER, Ochs H, Winkelstein J, editors. W.B. Saunders Company; Philadelphia: 1996. p. 217. [Google Scholar]
- 52.Bofill M, et al. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol. 1992;88:243–252. doi: 10.1111/j.1365-2249.1992.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CDC. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1993;41:1–35. [PubMed] [Google Scholar]
- 54.Fahey JL, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson JKA. Immunophenotyping specimens from HIV-infected persons: laboratory guidelines from the Centers for Disease Control and Prevention. Cytometry. 1994;18:55–59. doi: 10.1002/cyto.990180111. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JE, Masur H, Holmes King KK. Criteria for starting, discontinuing, and restarting opportunistic infection prophylaxis for adults with human immunodeficiency virus infection, Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51(RR08):1–46. [PubMed] [Google Scholar]
- 57.WHO. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents, and adults: Recommendations for a public health approach. WHO; Geneva, Switzerland: 2006. [online], < http://www.who.int/hiv/pub/guidelines/ctx/en/index.html>. [Google Scholar]
- 58.Moss AR, Bacchetti P. Natural history of HIV infection. AIDS. 1989;3:55–61. doi: 10.1097/00002030-198902000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Mellors JW, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Int Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 60.Moss AR, et al. Risk factors for AIDS and HIV seropositivity in homosexual men. Am J Epidemiol. 1987;125:1035–1047. doi: 10.1093/oxfordjournals.aje.a114619. [DOI] [PubMed] [Google Scholar]
- 61.Herbeck JT, et al. Lack of evidence for changing virulence of HIV-1 in North America. PLoS One. 2008;3:e1525. doi: 10.1371/journal.pone.0001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spira TJ, et al. Unexplained opportunistic infections and CD4 T lymphocytopenia: an analysis of five cases. N Engl J Med. 1993;328:386–392. doi: 10.1056/NEJM199302113280603. [DOI] [PubMed] [Google Scholar]
- 63.Smith DK, et al. CDC Idiopathic CD4 T Lymphocytopenia (ICL) Task Force. Unexplained opportunistic infections and CD4 T lymphocytopenia in persons without HIV infection, United States. N Engl J Med. 1993;328:373–379. doi: 10.1056/NEJM199302113280601. [DOI] [PubMed] [Google Scholar]
- 64.Strauss K, et al. Performance evaluation of the FACSCount System: a dedicated system for clinical cellular analysis. Cytometry. 1996;26:52–59. doi: 10.1002/(SICI)1097-0320(19960315)26:1<52::AID-CYTO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 65.Spacek LA, et al. Evaluation of a low-cost method, the Guava EasyCD4 assay, to enumerate CD4-positive lymphocyte counts in HIV-infected patients in the United States and Uganda. J Acquir Immune Defic Syndr. 2006;41:607–610. doi: 10.1097/01.qai.0000214807.98465.a2. [DOI] [PubMed] [Google Scholar]
- 66.Thakar MR, Kumar BK, Mahajan BA, Mehendale SM, Paranjape RS. Comparison of capillary based microflurometric assay for CD4+ T cell count estimation with dual platform Flow cytometry. AIDS Res Ther. 2006;3:26. doi: 10.1186/1742-6405-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balakrishnan P, et al. An inexpensive, simple, and manual method of CD4 T-cell quantitation in HIV-infected individuals for use in developing countries. J Acquir Immune Defic Syndr. 2004;36:1006–1010. doi: 10.1097/00126334-200408150-00002. [DOI] [PubMed] [Google Scholar]
- 68.Yari A, et al. SMARThivCD4mos: A complexity-free and cost effective model technology for monitoring HIV patients CD4 number in resource-poor settings. Bioinformation. 2008;2:257–259. doi: 10.6026/97320630002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lutwama F, et al. Evaluation of Dynabeads and Cytospheres compared with flow cytometry to enumerate CD4+ T cells in HIV-infected Ugandans on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48:297–303. doi: 10.1097/QAI.0b013e31817bbc3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitby L, et al. Quality control of CD4+ T-lymphocyte enumeration: results from the last nine years of the United Kingdom External Quality Assurance Scheme for Immune Monitoring (1993–2001) Cytometry. 2002;50:102–110. doi: 10.1002/cyto.10094. [DOI] [PubMed] [Google Scholar]
- 71.Brando B, et al. Cytoflourometric methods for assessing absolute numbers of cells subsets in blood. Cytometry. 2000;42:327–346. doi: 10.1002/1097-0320(20001215)42:6<327::aid-cyto1000>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 72.O’Gorman MR, Nicholson JKA. Adoption of single-platform technologies for enumeration of absolute T-lymphocyte subsets in peripheral blood. Clin Diag Lab Immunol. 2000;7:333–335. doi: 10.1128/cdli.7.3.333-335.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schnizlein-Bick CT, et al. DAIDS/NIAID New Technology Evaluation Group. Evaluation of TruCount absolute count tubes for determining CD4 and CD8 cell numbers in Human Immunodeficiency Virus-positive adults. Clin Diag Lab Immunol. 2000;7:336–343. doi: 10.1128/cdli.7.3.336-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Storie I, et al. Flow rate calibration I: a novel approach for performing absolute cell counts. Cytometry. 2003;55:1–7. doi: 10.1002/cyto.b.10051. [DOI] [PubMed] [Google Scholar]
- 75.Storie I, et al. Perfect count: a novel approach for the single platform enumeration of absolute CD4+ T-lymphocytes. Cytometry. 2004;57:47–52. doi: 10.1002/cyto.b.10065. [DOI] [PubMed] [Google Scholar]
- 76.Barnett D, et al. Reduction of intra- and interlaboratory variation in CD34+ stem cell enumeration using stable test material, standard protocols and targeted training. DK34 Task Force of the European Working Group of Clinical Cell Analysis (EWGCCA) Br J Haematol. 2000;108:784–792. doi: 10.1046/j.1365-2141.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 77.Kunkl A, et al. Grading of laboratories on CD4+ T-lymphocyte evaluations based on acceptable data boundaries defined by the measurement error. Cytometry. 2002;50:117–126. doi: 10.1002/cyto.10069. [DOI] [PubMed] [Google Scholar]
- 78.Barnett D, Denny TN. Lymphocyte immunophenotyping in Human Immunodeficiency virus infection: for richer, for poorer. Flow Cytometry. 2007:1–16. [Google Scholar]
- 79.Whitby L, Granger V, Walker C, Reilly JT, Barnett D. The relative error of dual versus single platform technology for CD4+ T lymphocyte enumeration: the impact on clinical decision making (Abstract 42) Cytometry B Clin Cytom. 2005;68B:69. [Google Scholar]
- 80.Mandy F, Bergeron M, Houle G, Bradley J, Fahey J. Impact of the international program for quality assessment and standardization for immunological measures relevant to HIV/AIDS: QASI. Cytometry. 2002;50:111–116. doi: 10.1002/cyto.10088. [DOI] [PubMed] [Google Scholar]
- 81.Denny TN, et al. A North American multilaboratory study of CD4 counts using flow cytometric panLeukogating (PLG): a NIAID-DAIDS Immunology Quality Assessment Program Study. Cytometry B Clin Cytom. 2008;74B (Suppl 1):52–64. doi: 10.1002/cyto.b.20417. [DOI] [PubMed] [Google Scholar]
- 82.Glencross DK, et al. Large-scale affordable PanLeucogated CD4+ testing with proactive internal and external quality assessment: in support of the South African national comprehensive care, treatment and management programme for HIV and AIDS. Cytometry B Clin Cytom. 2008;74B (Suppl 1):40–51. doi: 10.1002/cyto.b.20384. [DOI] [PubMed] [Google Scholar]
- 83.Barnett D. In: Generic RTD Activities and Research Infrastructures FP5 1998–2002: Consolidated Report. Baig SS, editor. European Commission — Office for Official Publications of the European Community; Luxembourg: 2003. pp. 510–511. [Google Scholar]
- 84.Barnett D, et al. Guidelines for the determination of CD4+ T lymphocytes in Immunosuppressed Individuals. Clin Lab Haematol. 1997;19:231–241. doi: 10.1046/j.1365-2257.1997.00091.x. [DOI] [PubMed] [Google Scholar]
- 85.CDC. 1994 revised guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus (HIV) infections. MMWR Recomm Rep. 1994;43(RR3):1–21. [PubMed] [Google Scholar]
- 86.CDC. 1997 revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV) MMWR Recomm Rep. 1997;46(RR2):1–29. [PubMed] [Google Scholar]
- 87.CDC. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. MMWR Recomm Rep. 2003;52(RR2):1–13. [PubMed] [Google Scholar]
- 88.UNAIDS/WHO. AIDS epidemic update [online] UNAIDS/WHO; Geneva, Switzerland: 2007. < http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf>. [Google Scholar]