Abstract
We demonstrate that the addition of bed bug, Cimex lectularius, alarm pheromone to desiccant formulations greatly enhances their effectiveness during short-term exposure. Two desiccant formulations, diatomaceous earth (DE) and Dri-die (silica gel), were applied at the label rate with and without bed bug alarm pheromone components, (E)-2-hexenal, (E)-2-octenal, and a (E)-2-hexenal:(E)-2-octenal blend. First-instar nymphs and adult females were subjected to 10-min exposures, and water loss rates were used to evaluate the response. Optimal effectiveness was achieved with a pheromone concentration of 0.01 M. With Dri-die alone, the water loss was 21% higher than in untreated controls, and water loss increased nearly two times with (E)-2-hexenal and (E)-2-octenal and three times with the (E)-2-hexenal: (E)-2-octenal blend. This shortened survival of first-instar nymphs from 4 to 1 d, with a similar reduction noted in adult females. DE was effective only if supplemented with pheromone, resulting in a 50% increase in water loss over controls with the (E)-2-hexenal:(E)-2-octenal blend, and a survival decrease from 4 to 2 d in first-instar nymphs. Consistently, the addition of the pheromone blend to desiccant dust was more effective than adding either component by itself or by using Dri-die or DE alone. Based on observations in a small microhabitat, the addition of alarm pheromone components prompted bed bugs to leave their protective harborages and to move through the desiccant, improving the use of desiccants for control. We concluded that short exposure to Dri-die is a more effective treatment against bed bugs than DE and that the effectiveness of the desiccants can be further enhanced by incorporation of alarm pheromone. Presumably, the addition of alarm pheromone elevates excited crawling activity, thereby promoting cuticular changes that increase water loss.
Keywords: bed bug control, alarm pheromone, desiccation, Cimex lectularius
Alarm pheromones typically prompt a frenzied, rapid dispersal reaction of nearby conspecifics (Blum 1985). In the bed bug, Cimex lectularius, the active ingredients of the alarm pheromone are (E)-2-hexenal and (E)-2-octenal, present as a 75:25 blend in adults and a 30:70 blend for first-instar nymphs (Levinson et al. 1971, 1974). At low concentrations, (E)-2-hexenal and (E)-2-octenal, along with other chemicals, modify bed bug behavior by promoting an aggregation response (Siljander et al. 2008), which is responsible for retaining bugs of mixed stages in protected cracks and crevices (Usinger 1966), facilitating water balance (Benoit et al. 2007), and enhancing reproduction by serving as “brood centers” (Reinhardt and Siva-Jothy 2007). An alarm response elicited by mechanical disturbance or agitation also prompts the release of (E)-2-hexenal and (E)-2-octenal, but in this case, higher concentrations are released. In addition to a role of alarm pheromone in defense and alarm (Usinger 1966), our recent results suggest that these same chemicals break quiescence and prompt the bed bugs to depart from their aggregations. The bed bugs are subsequently guided to their hosts by body temperature and other, albeit undefined, kairomones (Usinger 1966). The available evidence thus indicates that (E)-2-hexenal and (E)-2-octenal in high concentration function as a general excitant but at low levels function as part of the aggregation pheromone.
Historically, one of the most widespread tools for insect control are desiccant dusts, diatomaceous earths, and silica gels (Ebeling 1971). Although controversial (Korunic 1998, Subramanyam and Roesli 2000), most workers agree that these dusts cause damage to water proofing cuticular lipids, resulting in death by rapid desiccation (Allan and Patrican 1994, Appel et al. 1999). Diatomaceous earth (DE) is usually regarded as an abrasive that scratches the cuticular surface, and Dri-die (silica aerogel) is a nonabrasive and sorptive agent that adsorbs the cuticular lipids from the cuticular surface. The net effect of both agents is the same: cuticular lipids are removed and the water-proofing capacity of the cuticle is diminished (Appel et al. 1999). Desiccant dusts tend to lose their effectiveness at high relative humidities (>81%) or in the presence of free water; dry human comfort standards of 30 – 50% RH and 22–24°C (Reinikainen et al. 1992, Ferng and Lee 2002) are thus compatible with the use of desiccant dusts for bed bug control. Elevating temperatures is one effective strategy to increase insect movement and thereby increase contact with a desiccant (Athanassiou et al. 2005). In this study, we show that another tool for increasing contact with diatomaceous earth and Dri-die (abrasive versus sorptive, respectively) is to augment these agents with the addition of bed bug alarm pheromones.
Materials and Methods
Bed Bug Culture
Cimex lectularius originated from specimens collected in Columbus, OH (Dublin Strain), and from the National Pest Control Association (NPA strain). Cultures were maintained at 85% RH (using saturated salt solutions; Winston and Bates 1960), 15 h:9 h L:D, and 25°C (±1°C) and were reared on chicken blood by membrane feeding (Montes et al. 2002). All experiments were performed on first-instar nymphs, 1 wk after hatching, and on females 1 wk after adult eclosion. All observations and experiments were conducted in the dark in red light. Bugs were handled with aspirators and felt-tipped forceps.
Petri Plate Tests for Short-Term Exposure
Exposure methods and experimental design were based on procedures described by Allan and Patrican (1994), Arlian and Vyszenski-Moher (1995), and Appel et al. (1999). (E)-2-hexenal and (E)-2-octenal were from Sigma (>99.9% purity; Sigma, St. Louis, MO) and were diluted in high-performance liquid chromatography (HPLC)-grade acetone to 0.1, 0.01, and 0.001 M. A blend of (E)-2-hexenal:(E)-2-octenal (75:25 for adult and 30:70 for first-instar nymphs based on glandular contents; Schildknecht 1964, Levinson and Bar Ilan 1970) was also tested at those same concentrations. Acetone served as a control. All solution preparations and applications were done in glass. Filter paper used in the experiments was No. 3 Whatman (Whatman, Hillsboro, OR). Diatomaceous earth (6.1 g/m2 label rate; 90% silica; Celite 545; Fisher, Pittsburgh, PA) and Dri-die (6.1 g/m2 label rate; 95% amorphous silica aerogel, 2% ammonium fluosilicate; Roussel Environmental Health, Frenchtown, NJ) were used as the desiccant dust formulations and were used at the label rate. Treatment exposures were conducted in 100 by 15-mm sterile disposable petri dishes with perforated lids (Fisher).
Briefly, a 9-cm-diameter filter paper disc was sprayed with antistatic cling spray (Static Guard; Proctor and Gamble, Cincinnati, OH), the dust was weighed and applied to the disc to produce a consistent and even distribution, and the filter paper disc was enclosed in a 9-cm petri dish. The antistatic spray had no effect on survival of the bugs (data not shown). A 20-μl spot (not exceeding 2 cm diameter) of (E)-2-hexenal or (E)-2-octenal in acetone was applied to opposite sides of the filter paper and 2 cm from the edge of the dish; applications were air dried before adding the desiccant dust. Bugs were placed, five at a time, in the center of the filter paper disc and allowed to roam freely through the desiccant dust for 10 min. This was conducted at 40% RH and 25°C, conditions consistent with human comfort standards (Reinikainen et al. 1992, Ferng and Lee 2002). After a 10-min exposure in the petri dish, bugs were removed, dust was removed using a stream of air, and each bug was placed into a 1-cm3 mesh-covered chamber so they could be monitored individually for determinations of water balance characteristics.
Harborage-Evacuating Experiments
To determine whether these chemicals can be used to expel bed bugs from their off-host harborages, quiescent bed bugs were examined. A 20 by 40-cm plastic container without a cover (to allow pheromone dispersal) was divided into four parallel sections of 10 cm. The relative humidity was maintained at 33% throughout the experiments using saturated MgCl2 (Winston and Bates 1960). The first section remained empty, and the second was treated with Dri-die. The third remained empty, and the fourth was the location for a 1 by 1-cm section of paper folded at 45° to offer a harborage for the bed bugs. Bed bugs (N = 10, replicated five times) were introduced into the arena and quarantined until all 10 bugs were quiescent under the harborage. After the bed bugs ceased moving, the desiccant was applied, followed by the alarm pheromone. Survival of the bed bugs was monitored until all 10 bed bugs died or 40 d had elapsed for the first-instar nymphs and 80 d for the adult females. Concentrations of the chemicals applied and the type of desiccant were based on those most effective in the short-range experiments. Additionally, three more replicates with 15 individuals each were conducted after 24 h to determine the water loss rates of first-instar nymphs and females.
Determination of Water Balance Characteristics
Bugs were weighed using an electrobalance (CAHN; ±0.2-μg precision and ±6-μg accuracy at 1 mg; Ventron, Cerritos, CA). Each bug was weighed individually, within <1 min, without enclosure or anesthesia; bugs were released from the aspirator tip and permitted to walk onto the weighing pan of the balance and were returned to their chambers. After being weighed, the bugs were placed at 0% RH (Drierite; Toolson 1978) in a sealed 3,000-cm3 glass desiccator; the desiccator contained a perforated porcelain plate that provided a platform for the bugs above the Drierite located at the base of the desiccator. Although 0% is not a relative humidity to which bed bugs are exposed in their natural environment, it is the only RH where there is no interference from the effects of passive sorption, i.e., the rate is not masked by sorption, as described by Wharton (1985). Bugs were weighed daily, and each net transpiration rate was based on five consecutive mass measurements. Bugs were placed in a 90°C (0% RH) drying oven and weighed until mass remained constant, which was defined as the dry mass (d). The dry mass was subtracted from each mass measurement to convert the values into water mass (m) values. The initial water mass was expressed as a proportion of the fresh mass × 100 to calculate the percentage body water content. To determine net transpiration rates (integumental plus respiratory water loss), each consecutive mass measurement was converted into a water mass value (m0 = initial water mass; mt = water mass at any time t) and fit to the equation mt = m0e−kt, which permits the net transpiration rate (−k) to be derived from the slope of a regression on a plot of ln mt/m0 against time. This equation for net transpiration rate determination only applies if mass measurements are obtained at 0% RH because it is only under these conditions that water loss is exponential (Wharton 1985). Survivorship was based on 50% mortality. Observations were used to verify the following criteria for death: unable to right and crawl and failure to respond to prodding or bright light.
Sample Sizes and Statistics
A total of 45 individuals (three replicates of 15 bugs each) was used to determine each water balance characteristic. Each replicate was from a separate rearing batch of bugs. Data are presented as mean ± SE. Comparisons were made using an analysis of variance (ANOVA) and Bonferfoni-Dunn tests, and percentage data were arcsine transformed before analysis (Sokal and Rohlf 1995). Characteristics derived from regression lines were compared using a test for the equality of several slopes (Sokal and Rohlf 1995). Survivorship estimates were compared using t-statistics.
Results
Body Size of Bed Bugs
Fresh (initial) mass, water mass, and percentage body water content were nearly identical for all first-instar nymphs: an average of 0.13 mg fresh mass, 0.037 mg dry mass, 0.093 mg water mass, and 71.54% body water content, with no significant differences between groups that were used in Dri-die and diatomaceous earth exposure experiments (Tables 1 and 2; ANOVA, P > 0.05). For the females, the average fresh mass was 5.51 mg, dry mass was 1.82 mg, water mass was 3.69 mg, and body water content was 66.97%; there were no significant differences between groups in this study. In all cases, water mass was a positive correlate of dry mass, with R ≥ 0.94 – 0.96 (ANOVA, P < 0.001) for bugs that were exposed to Dri-die (Table 1) and R ≥ 0.94 – 0.98 (ANOVA, P < 0.001) for bugs that were exposed to diatomaceous earth (Table 2).
Table 1.
Comparison of net transpiration rates (integumental plus respiratory water loss) in first instar nymphs and female adults of Cimex lectularius after 10 min of crawling through Dri-die (label rate) with and without the addition of bed bug alarm pheromone
| Treatment | Water balance characteristic |
|||
|---|---|---|---|---|
| f (mg) | m (mg) | % | NTR (%/h) | |
| First-instar nymphs | ||||
| Acetone-only control | 0.12 ± 0.05a | 0.09 ± 0.02a | 75.0 ± 1.8a | 0.461 ± 0.014a |
| DD + Acetone | 0.13 ± 0.04a | 0.09 ± 0.02a | 69.2 ± 2.2a | 0.582 ± 0.017b |
| DD + (E)-2-hexenal | ||||
| 0.001 M | 0.12 ± 0.03a | 0.08 ± 0.02a | 66.7 ± 1.5a | 0.563 ± 0.017b |
| 0.01 M | 0.15 ± 0.02a | 0.11 ± 0.02a | 73.3 ± 1.7a | 0.913 ± 0.023b |
| 0.1 M | 0.13 ± 0.03a | 0.09 ± 0.01a | 69.2 ± 1.2a | 0.624 ± 0.013c |
| DD + (E)-2-octenal | ||||
| 0.001M | 0.13 ± 0.04a | 0.10 ± 0.01a | 76.9 ± 2.4a | 0.592 ± 0.014b |
| 0.01M | 0.11 ± 0.05a | 0.10 ± 0.03a | 71.4 ± 1.6a | 0.845 ± 0.014d |
| 0.1M | 0.14 ± 0.04a | 0.08 ± 0.02a | 72.7 ± 1.2a | 0.598 ± 0.018c |
| DD + Blend | ||||
| 0.001 M | 0.14 ± 0.04a | 0.10 ± 0.03a | 71.4 ± 1.7a | 0.501 ± 0.012b |
| 0.01 M | 0.12 ± 0.02a | 0.08 ± 0.01a | 66.7 ± 2.0a | 1.289 ± 0.015e |
| 0.1 M | 0.15 ± 0.02a | 0.10 ± 0.01a | 66.7 ± 1.7a | 0.706 ± 0.021d |
| Adult females | ||||
| Acetone-only control | 5.49 ± 0.05a | 3.62 ± 0.02a | 65.9 ± 1.8a | 0.104 ± 0.018a |
| DE + Acetone | 5.41 ± 0.04a | 3.59 ± 0.02a | 66.4 ± 2.2a | 0.162 ± 0.012b |
| DD + (E)-2-hexenal | ||||
| 0.001 M | 5.43 ± 0.03a | 3.64 ± 0.02a | 67.0 ± 1.5a | 0.171 ± 0.017b |
| 0.01 M | 5.55 ± 0.02a | 3.57 ± 0.02a | 64.3 ± 1.7a | 0.251 ± 0.023c |
| 0.1 M | 5.52 ± 0.03a | 3.60 ± 0.01a | 65.2 ± 1.2a | 0.184 ± 0.013b |
| DD + (E)-2-octenal | ||||
| 0.001M | 5.54 ± 0.04a | 3.60 ± 0.01a | 65.0 ± 2.4a | 0.192 ± 0.014c |
| 0.01M | 5.57 ± 0.05a | 3.66 ± 0.03a | 65.7 ± 1.6a | 0.231 ± 0.014c |
| 0.1M | 5.61 ± 0.04a | 3.71 ± 0.02a | 66.1 ± 1.2a | 0.179 ± 0.011b |
| DD + Blend | ||||
| 0.001 M | 5.53 ± 0.04a | 3.71 ± 0.03a | 67.1 ± 1.7a | 0.221 ± 0.012c |
| 0.01 M | 5.48 ± 0.02a | 3.58 ± 0.01a | 65.3 ± 2.0a | 0.411 ± 0.015d |
| 0.1 M | 5.41 ± 0.02a | 3.58 ± 0.01a | 66.2 ± 1.7a | 0.241 ± 0.021c |
Data shown are mean ± SE, N = 45. The same lowercase letters within a column denote no significant difference (ANOVA; P > 0.05). DD, Dri-die; f, fresh (initial) mass; m, initial water mass; %, initial percentage water content; NTR, net transpiration rate following treatment; Blend, 30:70 for larvae and 75:25 for adults, vol:vol (E)-2-hexenal: (E)-2-octenal.
Table 2.
Comparison of net transpiration rates (integumental plus respiratory water loss) in first-instar nymphs and adult females of C. lectularius after 10 min of crawling through diatomaceous earth (label rate) with addition of bed bug alarm pheromone
| Treatment | Water balance characteristic |
|||
|---|---|---|---|---|
| f (mg) | m (mg) | % | NTR (%/h) | |
| First-instar nymphs | ||||
| Acetone-only control | 0.14 ± 0.03a | 0.11 ± 0.03a | 73.3 ± 2.3a | 0.395 ± 0.022a |
| DE + acetone | 0.11 ± 0.02a | 0.08 ± 0.01a | 72.7 ± 2.1a | 0.421 ± 0.021a |
| DE + (E)-2-hexenal | ||||
| 0.001 M | 0.15 ± 0.04a | 0.09 ± 0.02a | 66.7 ± 1.3a | 0.441 ± 0.020a |
| 0.01 M | 0.12 ± 0.04a | 0.08 ± 0.02a | 75.0 ± 1.8a | 0.582 ± 0.016c |
| 0.1 M | 0.12 ± 0.05a | 0.08 ± 0.01a | 66.7 ± 1.2a | 0.495 ± 0.018b |
| DE + (E)-2-octenal | ||||
| 0.001M | 0.14 ± 0.04a | 0.10 ± 0.01a | 71.4 ± 2.0a | 0.413 ± 0.019a |
| 0.01M | 0.13 ± 0.02a | 0.09 ± 0.02a | 69.2 ± 2.3a | 0.574 ± 0.012c |
| 0.1M | 0.10 ± 0.03a | 0.07 ± 0.01a | 70.0 ± 1.5a | 0.479 ± 0.013b |
| DE + blend | ||||
| 0.001 M | 0.11 ± 0.03a | 0.08 ± 0.02a | 72.7 ± 1.9a | 0.477 ± 0.020a |
| 0.01 M | 0.14 ± 0.02a | 0.11 ± 0.02a | 78.6 ± 1.8a | 0.796 ± 0.025d |
| 0.1 M | 0.15 ± 0.04a | 0.11 ± 0.03a | 73.3 ± 1.3a | 0.517 ± 0.011c |
| Adult females | ||||
| Acetone-only control | 5.49 ± 0.15a | 3.70 ± 0.13a | 67.3 ± 3.5a | 0.104 ± 0.011a |
| DE + acetone | 5.51 ± 0.17a | 3.76 ± 0.19a | 68.2 ± 1.5a | 0.109 ± 0.018a |
| DE + (E)-2-hexenal | ||||
| 0.001 M | 5.42 ± 0.21a | 3.66 ± 0.17a | 67.5 ± 3.1a | 0.114 ± 0.017a |
| 0.01 M | 5.58 ± 0.13a | 3.74 ± 0.11a | 67.0 ± 2.7a | 0.191 ± 0.015a |
| 0.1 M | 5.48 ± 0.19a | 3.71 ± 0.22a | 67.7 ± 2.2a | 0.121 ± 0.015a |
| DE + (E)-2-octenal | ||||
| 0.001M | 5.62 ± 0.09a | 3.80 ± 0.18a | 67.6 ± 2.1a | 0.133 ± 0.024a |
| 0.01M | 5.43 ± 0.15a | 3.60 ± 0.16a | 66.3 ± 2.6a | 0.186 ± 0.012b |
| 0.1M | 5.61 ± 0.13a | 3.77 ± 0.09a | 67.2 ± 1.9a | 0.173 ± 0.023b |
| DE + blend | ||||
| 0.001 M | 5.53 ± 0.14a | 3.69 ± 0.14a | 66.7 ± 3.7a | 0.133 ± 0.012a |
| 0.01 M | 5.59 ± 0.19a | 3.75 ± 0.12a | 67.1 ± 1.8a | 0.263 ± 0.012c |
| 0.1 M | 5.64 ± 0.16a | 3.79 ± 0.20a | 67.2 ± 2.7a | 0.173 ± 0.012b |
Data shown are mean ± SE, N = 45. The same lowercase letters within a column denote no significant difference (ANOVA; P > 0.05). DE, diatomaceous earth; f, fresh (initial) mass; m, initial water mass; %, initial percentage water content; NTR, net transpiration rate following treatment; Blend, 30:70 for larvae and 75:25 for adults, vol:vol (E)-2-hexenal: (E)-2-octenal.
Dri-die Treatment in Petri Dish Assays
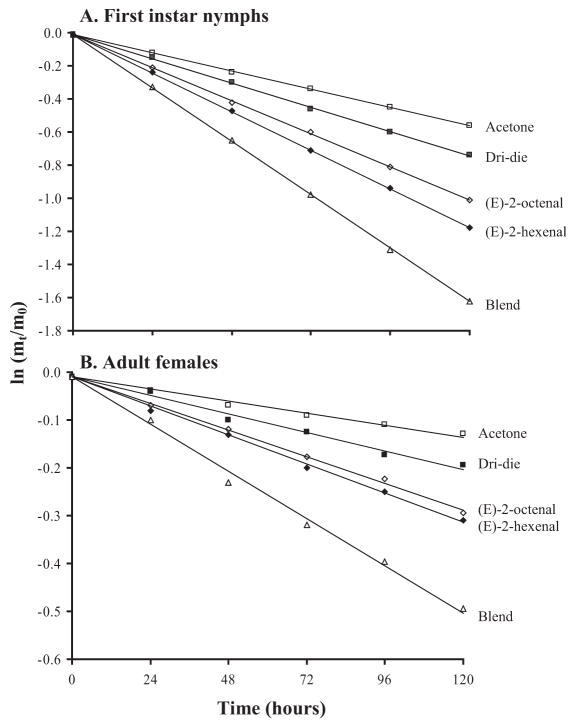
First-instar nymphs and adult females exhibited a consistent exponential pattern of water loss at 0% RH, thus permitting the net transpiration rate to be derived from the slope of the line (Fig. 1A and B). All treatments involving a 10-min crawling exposure to Dri-die resulted in an increase in net transpiration rate compared with controls that crawled on filter paper treated only with acetone (Fig. 1a and b). Exposure that combined Dri-die with 0.01 M (E)-2-hexenal:(E)-2-octenal blend resulted in a nearly three-fold increase in the net transpiration rate (Fig. 1a and b). An approximate two-fold increase in net transpiration rate was observed when 0.01 M (E)-2-hexenal or 0.01 M (E)-2-octenal was tested individually (Fig. 1a and b). Concentrations of 0.001 M (E)-2-hexenal, (E)-2-octenal, or the (E)-2-hexenal:(E)-2-octenal blend had no effect on increasing the net transpiration rate of the bugs and produced results not significantly different from results obtained by exposure to only Dri-die (Table 1; P > 0.05). A concentration of 0.1 M (E)-2-hexenal, (E)-2-octenal, or the (E)-2-hexenal:(E)-2-octenal blend increased the net transpiration rate, but the increase was not as pronounced as the effect at 0.01 M (Table 1). Consistently, the effect of the (E)-2-hexenal:(E)-2-octenal blend was greater than the effect of either component tested separately. Thus, bed bugs that were exposed to Dri-die in combination with a synthetic blend of alarm pheromone components lost water at a much faster rate than those treated with Dri-die alone.
Fig. 1.
Proportion of water mass lost at 0% RH, 25°C by (A) first-instar nymphs and (B) adult females of C. lectularius after 10-min exposure to Dri-die (label rate) with and without alarm pheromone added. At 0% RH conditions, the slope of the regression through the points on the semilog plot is the net transpiration rate (integumental plus respiratory water loss). mt, water mass at any time t; m0, initial water mass. Each point is the mean of 45 bugs, and the SEs lie within the confines of symbols used on the graph. Data shown are for 0.01 M of alarm pheromone, the concentration showing the greatest effect.
Survivorship of 50% of first-instar nymphs at 0% RH (25°C) was 4.1 ± 0.4 d for acetone-only controls (no Dri-die) and 2.6 ± 0.8 d when exposed to Dri-die (t-statistics; P < 0.05). Survivorship dropped to 1.2 ± 0.4 d after exposure to Dri-die plus the 0.01 M (E)-2-hexenal:(E)-2-octenal blend, 3 d earlier than in the controls (t-tests; P < 0.05), 2 d earlier (1.6 ± 0.7 d; t-tests; P < 0.05) for (E)-2-hexenal, and 1.8 ± 0.9 d earlier for (E)-2-octenal alone (no significant difference between these two compounds; t-test; P > 0.05) when these components were tested individually. Females exposed to only Dri-die survived 17.0 ± 1.1 d at 0% RH; those exposed to either (E)-2-hexenal or (E)-2-octenal survived ≈9 d, and adult females treated with Dri-die and the chemical blend survived 6.5 ± 1.9 d. The higher net transpiration rates for the bed bugs consistently translated into higher rates of mortality.
Diatomaceous Earth Treatment in Petri Dish Assays
A 10-min exposure to diatomaceous earth had no appreciable effect on net transpiration rates of first-instar nymphs or adult females (Table 2). However, net transpiration rates of the bugs increased 1.5-fold when diatomaceous earth was combined with (E)-2-hexenal or (E)-2-octenal and 2.0-fold when the (E)-2-hexenal:(E)-2-octenal blend was used at a concentration of 0.01 M. At a lower concentration, 0.001 M, no appreciable effect on net transpiration rate was noted for either the single compounds or the blend. Exposure to diatomaceous earth with 0.1 M (E)-2-hexenal, (E)-2-octenal, or the blend caused a slight, but significant, increase in net transpiration rate (Table 2). The synthetic alarm pheromone blend had a greater impact on increasing net transpiration rate than did the individual components at all concentrations (Table 2).
Net transpiration rates of bugs after exposure to Dri-die were higher than rates after exposure to diatomaceous earth (Table 1; ANOVA, P < 0.05). This was reflected in longer survival in dry air (25°C) for diatomaceous earth treatments than for Dri-die treatments. Survival time of bed bugs treated with diatomaceous earth (4.3 ± 0.4 d) did not differ significantly from the acetone-only treated controls (3.7 ± 0.9 d, no significant difference; t-statistics; P > 0.05). With a pheromonal additive, survivorship dropped to 2.6 ± 0.3 d with 0.01 M (E)-2-hexenal, 2.4 ± 0.5 d with (E)-2-octenal (t-statistics; P>0.05), and 1.9 ± 0.7 d for the (E)-2-hexenal:(E)-2-octenal blend (Table 2; t-statistics; P < 0.05). Results were similar for adult females: diatomaceous earth only reducing survival in the presence of alarm pheromone components. Thus, Dri-die consistently generated a greater increase in the net transpiration rate than did diatomaceous earth, and the difference resulted in reduced survival time.
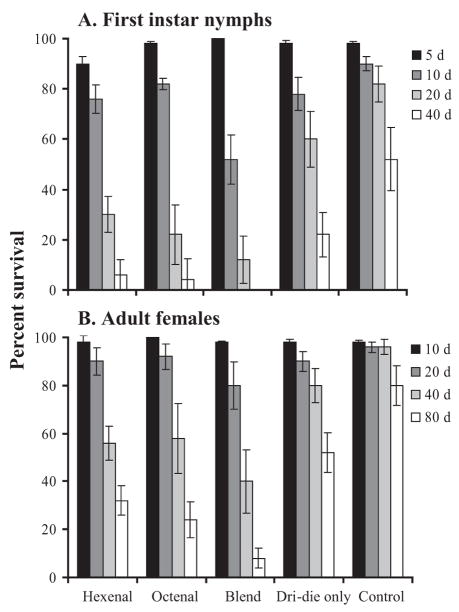
Termination of Quiescence and Departure From Harborage
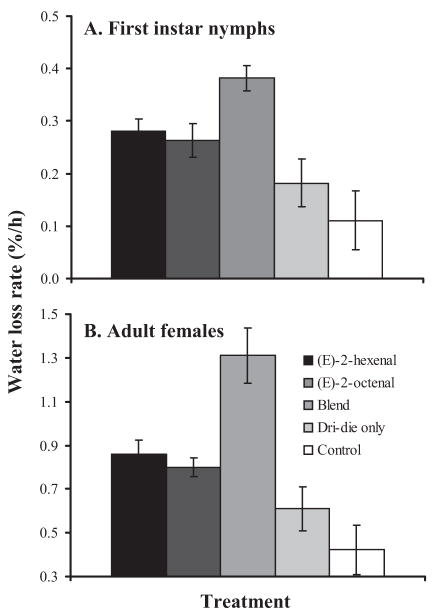
Based on the above results, only Dri-die and concentrations of alarm pheromone components or blends of 0.01 M were evaluated for termination of quiescence and departure from their harborages. Our results suggest that application of alarm pheromone components caused the immediate dispersal of bed bugs from their harborages onto areas where Dri-die was present Application of the alarm pheromone resulted in a significant reduction, by at least 50%, in survival of first-instar nymphs and adult females when used in combination with Dri-die (Fig. 2). All the bed bugs evacuated their harborage in <5 min once alarm pheromone components [(E)-2-hexenal and (E)-2-octenal applied individually or as a blend] were applied, and this time was significantly less than bed bugs held under control (acetone-only or no applied chemicals) conditions (>20 min; ANOVA, P < 0.05). As before, application of the alarm pheromone resulted in a higher water loss rate than when Dri-die was used alone (Fig. 3), and this is likely to be the factor that reduced survival. We conclude that application of the alarm pheromones, (E)-2-hexenal and (E)-2-octenal, can be used to evoke dispersal of bed bugs from their harborages and, in combination with a desiccant, may enhance control.
Fig. 2.
Survival of (A) first-instar nymphs and (B) adult females of C. lectularius within a microhabitat to test Dri-die effectiveness with and without alarm pheromone components. Times denote when bed bug survival was measured. Each measurement represents the results form 50 individuals. Hexenal, (E)-2-hexenal; Octenal, (E)-2-octenal; Blend, combination of (E)-2-hexenal and (E)-2-octenal in a ratio of 30:70 for larvae and 75:25 for adults.
Fig. 3.
Water loss rates of (A) first-instar nymphs and (B) adult females of C. lectularius after 24 h in a microhabitat containing Dri-die. Each value is the mean ± SE of 45 bugs.
Discussion
In response to 0.01 M (E)-2-hexenal and (E)-2-octenal, bed bugs showed an excited, continuous searching behavior, crawling rapidly over the bottom of the petri dish and failing to settle into clusters at the edge of the dish. Response to 0.1 M was not as high in most cases and may result from the complete saturation of pheromones, possibly leading to a lower response. This reduced or varying response to high concentration of chemical cues has been shown in many other arthropods, including bed bugs (Benoit et al. 2008, Siljander et al. 2008). Thus, these bed bugs exhibited a classic alarm response. Pheromones are often mixtures of compounds that are concentration and proportion specific (Blum 1985), and this seems to be the case for the bed bug alarm pheromone. The alarm pheromone consists of two major active ingredients, (E)-2-hexenal and (E)-2-octenal, with the (E)-2-hexenal predominating in the mixture when it is released naturally by adults (Levinson et al. 1971, 1974). Consistent with the predominance of (E)-2-hexenal in the blend, the response to (E)-2-hexenal observed in this study was more pronounced than the response to (E)-2-octenal, and the response to either component alone was lower than the response to the natural (E)-2-hexenal:(E)-2-octenal blend. The most useful application of the bed bug alarm pheromone may be to cause dispersal. Applying a desiccant such as Dri-die and using the alarm pheromone to evoke increased bed bug movement may be a useful technique for controlling bed bugs, including pesticide-resistant strains. It is important to note that, even though the desiccating conditions in this study seem to be severe, possibly because the bugs are adapted to dry human comfort standards (30–50% RH), these experimental conditions are similar to the environment in their habitat.
Species that can be effectively controlled by desiccant dusts include a variety of arthropod pests, but most noteably ticks (Allan and Patrican 1994), cowpea weevils (Appel et al. 1999), cockroaches and silverfish (Faulde et al. 2006a, b), ants (Brinkmann and Gardner 2001), and numerous pests of grain (Akbar et al. 2004, Athanassiou et al. 2005). We now extend this list to include bed bugs, with Dri-die but not diatomaceous earth. The efficacy of diatomaceous earth seems to depend somewhat on the formulation; sometimes it works and sometimes it does not (Allan and Patrican 1994). Resistance also seems to be an issue with diatomaceous earth (Korunic and Ormesher 2000, Rigaux et al. 2001). Previous studies concluded that Dri-die seems to be superior to diatomaceous earths (Allan and Patrican 1994, Appel et al. 1999), and that is what we observed in this study during short-term exposure. Two key points that may alter the effectiveness of Dri-die and DE are the duration of bed bug exposure and the residual effects. Indeed, future studies are needed to test these two aspects for C. lectularius. Even so, this would not change our interpretations that short-term exposure to Dri-die, particularly in the presence of alarm pheromones, is more effective than DE for altering bed bug water balance characteristics. Last, how well the approach of using Dri-die with an alarm pheromone additive will work under large-scale field conditions, using human comfort standards, remains to be tested.
Acknowledgments
We thank G. Keeney, Ohio State University, for providing the bed bugs used in this study. We also appreciate the thoughtful comments provided by the reviewers. This research was supported in part by NIH-NIAID Grant R01-AI058279 and an OARDC Director’s Fellowship to J.B.B.
References Cited
- Akbar W, Lord JC, Nechols JR, Howard RW. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases conidia attachment. J Econ Entomol. 2004;97:273–280. doi: 10.1603/0022-0493-97.2.273. [DOI] [PubMed] [Google Scholar]
- Allan SA, Patrican LA. Susceptibility of immature Ixodes scapularis (Acari: Ixodidae) to desiccants and an insecticidal soap. Exp Appl Acarol. 1994;18:691–702. doi: 10.1007/BF00051536. [DOI] [PubMed] [Google Scholar]
- Appel AG, Moar WJ, Tanley MJ. Water loss and mortality of adult cowpea weevils (Coleoptera: Bruchidae) exposed to desiccants and desiccating environments. Environ Entomol. 1999;28:979–982. [Google Scholar]
- Arlian LG, I, Ekstrand A. Water balance in Drosophilia pseudoobscura, and its ecological implications. Ann Entomol Soc Am. 1975;68:827–832. [Google Scholar]
- Athanassiou CG, Vayias BJ, Dimizas CB, Kavallieratos NG, Papagregoriou AS, Buchelos CTh. Insecticidal efficacy of diatomaceous earth against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Tribolium confuses du Val (Coleoptera: Tenebrionidae) on stored wheat: influence of dose rate, temperature and exposure interval. J Stored Products Res. 2005;41:47–55. [Google Scholar]
- Benoit JB, Yoder JA, Rellinger EJ, Ark JT, Keeney GD. Prolonged maintenance of water balance by adult females of the American spider beetle, Mezium affine Boieldieu, in the absence of food and water resources. J Insect Physiol. 2005;51:565–573. doi: 10.1016/j.jinsphys.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Del Grosso NA, Yoder JA, Denlinger DL. Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. Am J Trop Med Hyg. 2007;76:987–993. [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Phillips SA, Elnitsky MA, Yoder JA, Lee RE, Jr, Denlinger DL. The seabird tick, Ixodes uriae, uses uric acid in penguin feces as a kairomone and guanine in tick feces as an assembly pheromone on the Antarctica Peninsula. Polar Biol. 2008;31:1445–1451. [Google Scholar]
- Blum MS. Exocrine systems. In: Blum SM, editor. Fundamentals of insect physiology. Wiley; New York: 1985. pp. 535–579. [Google Scholar]
- Brinkman MA, Gardner WA. Use of diatomaceous earth and entomopathogen combinations against the red imported fire ant (Hymenoptera: Fomicidae) Fla Entomol. 2001;84:740–741. [Google Scholar]
- Ebeling W. Sorptive dusts for pest control. Annu Rev Entomol. 1971;16:123–158. doi: 10.1146/annurev.en.16.010171.001011. [DOI] [PubMed] [Google Scholar]
- Faulde MK, Scharninghausen JJ, Cavaljuga S. Toxic and behavioral effect of different modified diatomaceous earths on the German cockroach, Blattella germanica (L.) (Orthoptera: Blattellidae) under simulated field conditions. J Stored Prod Res. 2006a;42:253–263. [Google Scholar]
- Faulde MK, Tisch M, Scharninghausen JJ. Efficacy of modified diatomaceous earth on different cockroach species (Orthroptera, Blattellidae) and silverfish (Thysanura, Lepismatidae) J Pest Sci. 2006b;79:155–161. [Google Scholar]
- Ferng SF, Lee LW. Indoor air quality assessment of daycare facilities with carbon dioxide, temperature, and humidity as indicators. J Environ Health. 2002;65:14–18. [PubMed] [Google Scholar]
- Korunic Z. Diatomaceous earths, a group of natural insecticides. J Stored Prod Res. 1998;34:87–97. [Google Scholar]
- Levinson HZ, Bar Ilan AR. Assembling and alerting scents produced by the bed bug Cimex lectularius L. Experientia. 1971;27:102–103. doi: 10.1007/BF02137766. [DOI] [PubMed] [Google Scholar]
- Levinson HZ, Levinson AR, Maschwitz U. Action and composition of the alarm pheromone of the bed bug Cimex lectularius L. Naturwissenschaften. 1974;12:684–6895. doi: 10.1007/BF00606522. [DOI] [PubMed] [Google Scholar]
- Levinson HZ, Levinson AR, Müller B, Steinbrecht RA. Structure of sensilla, olfactory perception, and behavior of the bedbug, Cimexlectularius, in response to its alarm pheromone. J Insect Physiol. 1974;20:1231–1248. doi: 10.1016/0022-1910(74)90229-7. [DOI] [PubMed] [Google Scholar]
- Montes C, Cuadrillero C, Vilella D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J Med Entomol. 2002;39:675–679. doi: 10.1603/0022-2585-39.4.675. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Siva-Jothy MT. Biology of the bed bugs. (Cimicidae) Annu Rev Entomol. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- Reinikainen LM, Jaakkola JJ, Seppanen O. The effect of air humidification on symptoms and perception of indoor air quality in office workers: a six-period cross-over trial. Arch Environ Health. 1992;1:8–15. doi: 10.1080/00039896.1992.9935938. [DOI] [PubMed] [Google Scholar]
- Rigaux M, Haubrugeand E, Fields PG. Mechanisms for tolerance to diatomaceous earth between strains of Tribolium castaneum. Entomol Exp Appl. 2001;101:33–39. [Google Scholar]
- Siljander E, Gries R, Khaskin G, Gries G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. J Chem Ecol. 2008;34:708–718. doi: 10.1007/s10886-008-9446-y. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. W. H. Freeman; San Francisco, CA: 1995. [Google Scholar]
- Subramanyam Bh, Roesli R. Inert dusts. In: Subramanyam Bh, Hagstrum DW., editors. Alternatives to pesticides in stored-product IPM. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 321–380. [Google Scholar]
- Toolson EC. Diffusion of water through the arthropod cuticle: thermodynamic consideration of the transition phenomenon. J Thermal Biol. 1978;3:69–73. [Google Scholar]
- Usinger RL. Thomas Say Foundation. Vol. 7. Entomological Society of America; College Park, MD: 1966. Monograph of Cimicidae. [Google Scholar]
- Wharton GW. Water balance of insects. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Vol. 4. Pergamon; Oxford, United Kingdom: 1985. pp. 565–603. [Google Scholar]
- Winston PW, Bates DS. Saturated salt solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]