Abstract
We present an enzyme-linked immunosorbent assay (ELISA) system for the simultaneous determination of immunoglobulin G antibodies directed against several viruses. Antibodies to up to eight different viruses could be determined for three different sera on one microtitration plate. After subtraction of the absorbance values obtained with the control antigens, the viral antigen absorbancies were expressed as percentages of the absorbance obtained with a pooled immunoglobulin standard. This value, the relative antibody activity, was rapidly calculated by means of a computer directly connected to the ELISA photometer and was stored on magnetic disks, thereby facilitating seroepidemiological studies. The reproducibility of the relative antibody activity was calculated to at best +/- 3.6% (standard deviation) in an intraassay test and to at worst +/- 20.4% (standard deviation) in an interassay test. Each serum was analyzed only at a dilution of 1/75. The sensitivity of this single-dilution ELISA (SD-ELISA) method for the detection of titer rises was compared with those of conventional methods, mostly complement fixation but also hemagglutination inhibition. A total of 142 of 155 (92%) paired sera showing fourfold complement fixation or hemagglutination inhibition rises also showed significant results in SD-ELISA. A total of 22 of 57 (39%) significant relative antibody activity rises were significant in complement fixation or hemagglutination inhibition. Overall, up to twice as many significant titer rises could be detected with SD-ELISA. Most of these seemed to have a sound correlation with clinical data. The specificity of SD-ELISA was found to be similar to that of complement fixation, with some cross-reactions occurring between herpes simplex and varicella-zoster virus antigens and between parainfluenza viruses. We have found SD-ELISA to be a valuable clinical virological tool that supplements conventional serology.
Full text
PDF


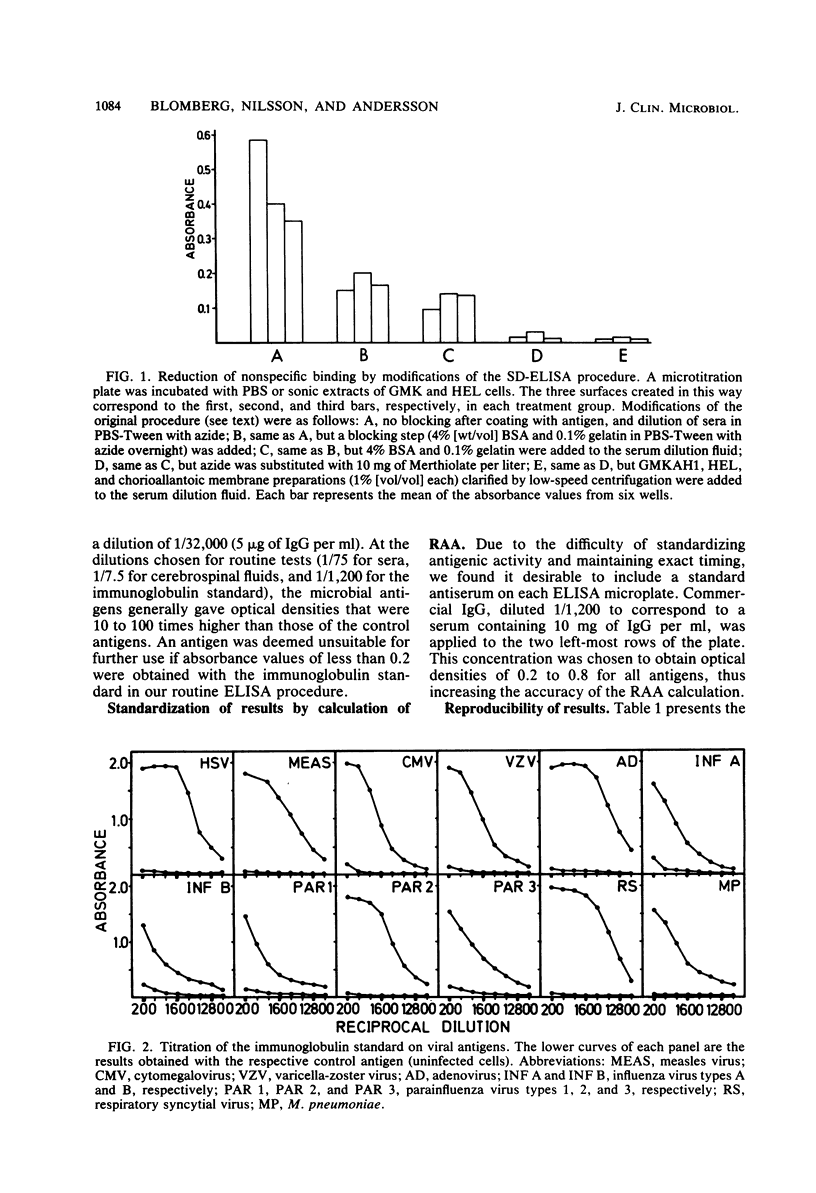
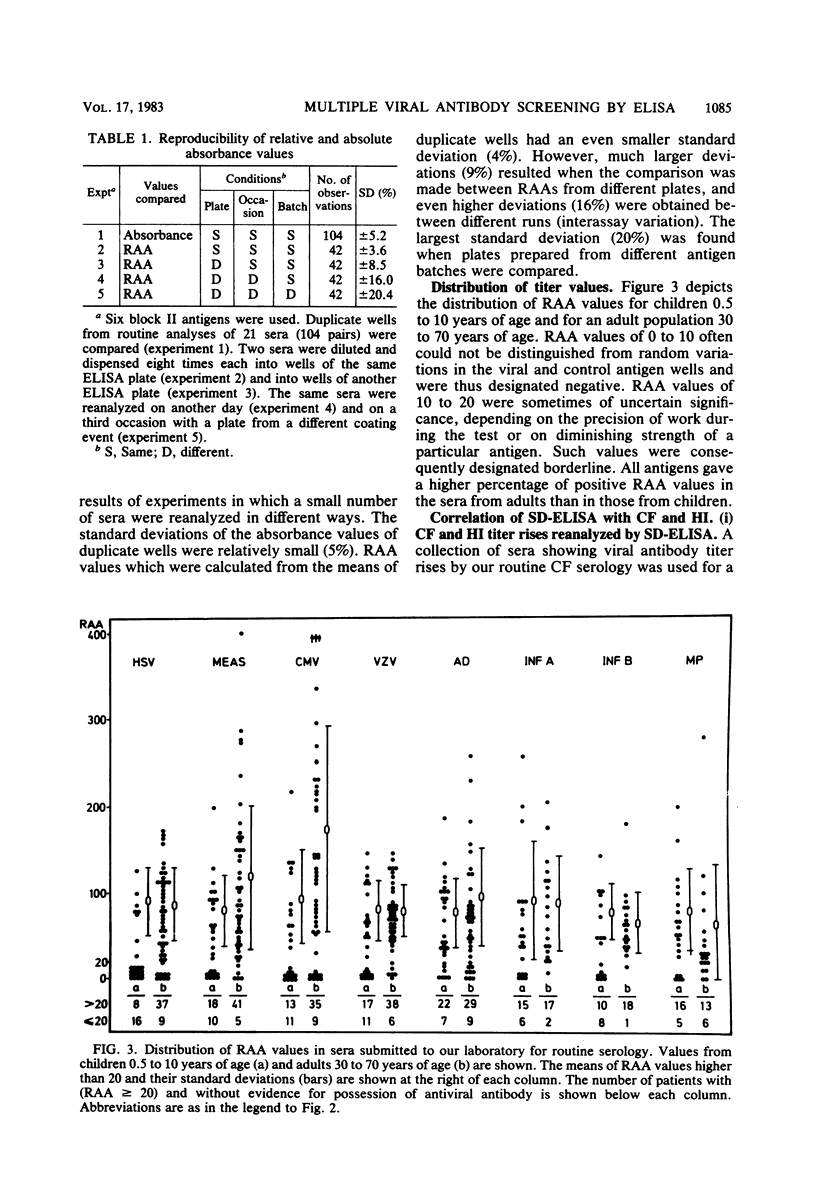

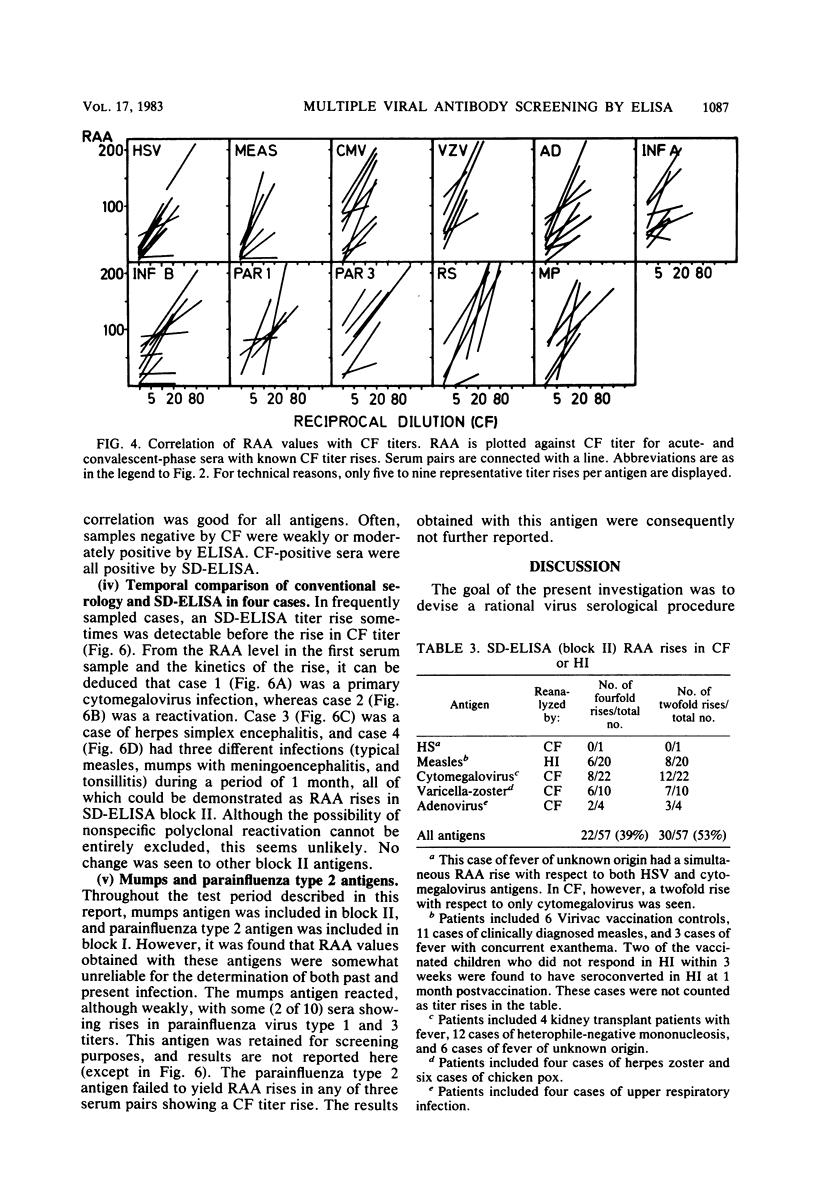
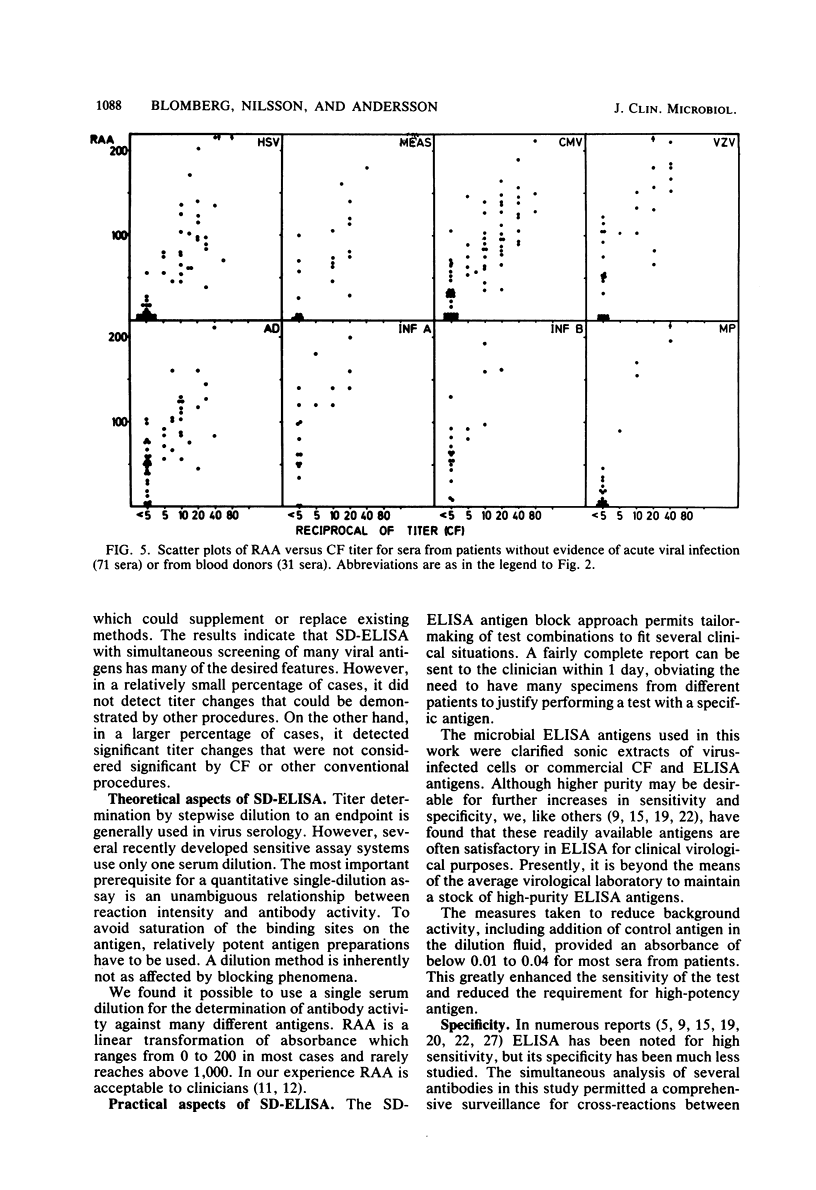


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankerst J., Christensen P., Kjellén L., Kronvall G. A rountine diagnostic test for IgA and IgM antibodies to rubella virus: absorption of IgG with Staphylococcus aureus. J Infect Dis. 1974 Sep;130(3):268–273. doi: 10.1093/infdis/130.3.268. [DOI] [PubMed] [Google Scholar]
- Blomberg J., Mattson J., Börjesson B., Hast S., Andersson P., Holm K. VIRUS--a laboratory information system for clinical virology. Methods Inf Med. 1979 Oct;18(4):207–214. [PubMed] [Google Scholar]
- Booth J. C., Hannington G., Aziz T. A., Stern H. Comparison of enzyme-linked immunosorbent assay (ELISA) technique and complement-fixation test for estimation of cytomegalovirus IgG antibody. J Clin Pathol. 1979 Feb;32(2):122–127. doi: 10.1136/jcp.32.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins S. C., Ingwer I., Zeckel M. L., White A. C. Parameters affecting the enzyme-linked immunosorbent assay of immunoglobulin G antibody to a rough mutant of Salmonella minnesota. Infect Immun. 1978 Sep;21(3):721–728. doi: 10.1128/iai.21.3.721-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Feldbush T. L., McGivern P. L., Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978 Feb;15(2):131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- CASEY H. L. STANDARDIZED DIAGNOSTIC COMPLEMENT FIXATION METHOD AND ADAPTATION TO MICRO TEST. I. LABORATORY BRANCH COMPLEMENT FIXATION METHOD BY LABORATORY BRANCH TASK FORCE. II. ADAPTATION OF LBCF METHOD TO MICRO TECHNIQUE. Public Health Monogr. 1965;74:1–34. [PubMed] [Google Scholar]
- Chia W. K., Spence L. Quantitative determination of cytomegalovirus IgG antibody by enzyme-linked immunosorbent assay (ELISA). Can J Microbiol. 1979 Sep;25(9):1082–1086. doi: 10.1139/m79-165. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J. Antigen requirements, sensitivity, and specificity of enzyme immunoassays for measles and rubella viral antibodies. J Clin Microbiol. 1979 Jun;9(6):657–664. doi: 10.1128/jcm.9.6.657-664.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUENALP A. GROWTH AND CYTOPATHIC EFFECT OF RUBELLA VIRUS IN A LINE OF GREEN MONKEY KIDNEY CELLS. Proc Soc Exp Biol Med. 1965 Jan;118:85–90. [PubMed] [Google Scholar]
- Grillner L., Blomberg J. Hemolysis-in-gel and neutralization tests for determination of antibodies to mumps virus. J Clin Microbiol. 1976 Jul;4(1):11–15. doi: 10.1128/jcm.4.1.11-15.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman M. B., Blackburn C. K., Zimmerman S. E., French M. L. Comparison of enzyme-linked immunosorbent assay for acute measles with hemagglutination inhibition complement fixation, and fluorescent-antibody methods. J Clin Microbiol. 1981 Aug;14(2):147–152. doi: 10.1128/jcm.14.2.147-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNETTE E. H., JENSEN F. W., GUENTHER R. W., MAGOFFIN R. L. Serologic responses to para-influenza viruses in patients with mumps virus injection. J Lab Clin Med. 1963 May;61:780–788. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- SEVER J. L., HUEBNER R. J., CASTELLANO G. A., BELL J. A. SEROLOGIC DIAGNOSIS "EN MASSE" WITH MULTIPLE ANTIGENS. Am Rev Respir Dis. 1963 Sep;88:SUPPL–359. doi: 10.1164/arrd.1963.88.3P2.342. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Magoffin R. L. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J Gen Virol. 1969 Apr;4(3):321–328. doi: 10.1099/0022-1317-4-3-321. [DOI] [PubMed] [Google Scholar]
- de Savigny D., Voller A. The communication of ELISA data from laboratory to clinician. J Immunoassay. 1980;1(1):105–128. doi: 10.1080/01971528008055779. [DOI] [PubMed] [Google Scholar]
- van Loon A. M., van der Logt J. T., van der Veen J. Enzyme-linked immunosorbent assay for measurement of antibody against cytomegalovirus and rubella virus in a single serum dilution. J Clin Pathol. 1981 Jun;34(6):665–669. doi: 10.1136/jcp.34.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]



