We have reported several times during the last decade that venous blood returning from the splanchnic viscera has liver-supporting qualities not found to the same degree in other kinds of arterial or venous blood (20, 21, 22, 33, 34, 35, 36). The so-called hepatotrophic effects of portal blood have been noted under several experimental conditions to include hypertrophy, glycogen storage, hyperplasia and increase of several synthetic functions.
In recent publications, the stages in the development of the hepatotrophic concept were summarized (32, 33, 34). The multifactorial nature of splanchnic hepatotrophic influences was emphasized, but with evidence that the main splanchnic venous hepatotrophic factors were endogenous hormones of which the single most important seemed to be insulin.
The work herein reported provides further information about splanchnic hepatotrophic actors in dogs. Many of the test dogs were made chronically diabetic with alloxan or by total pancreatectomy. The results have reemphasized the primal hepatotrophic role played by endogenous insulin while, at the same time, pointing out other complex and contributory hormonal and nutritional interrelationships.
METHODS
Animal Groups
Fifty-three mongrel dogs, weighing 11 to 29 kilograms, contributed to the data of finished experiments. Approximately 70 additional dogs were used but discarded because of failure to bring their study to completion. In order of frequency, the experimental losses were caused by the inability to produce diabetes with alloxan, thrombosis of the venous anastomoses or grafts, postoperative intussusception, duodenal infarction after total pancreatectomy, intra-abdominal or wound infection, distemper and fatal insulin reactions. All further descriptions apply only to the 53 definitive dogs.
The various operations, biopsies and sacrifice procedures were performed under anesthesia with pentobarbital sodium supplemented with phencyclidine hydrochloride (Sernylan®) and succinylcholine chloride (Anectine®). Two hours before sacrifice, 47 of the dogs were given intravenously 0.07 to 0.41 millicurie of [CH3-3H] thymidine per kilogram of body weight. The specific activity of the [CH33-3H] thymidine was 6.4 curies per milli-mole. The end point of all experiments and controls was a comparison of the structure, biochemical analyses and deoxyribonucleic acid synthesis in the right versus the left liver lobar complexes.
Group 1
Eleven normal dogs were anesthetized. After obtaining several grams of tissue from one of the right and one of the left liver lobes for the various analyses, the dogs were sacrificed. The two right liver lobes supplied by the right branch of the portal vein and the five left lobes supplied by the left portal branch were weighed. In our past experience, the ratio of the right to the left lobes has been about 30:70 (20, 33).
Five of the unaltered dogs were studied without a prior period of known good nutrition. The other six were first conditioned for at least three weeks with a standard kennel diet.
Group 2
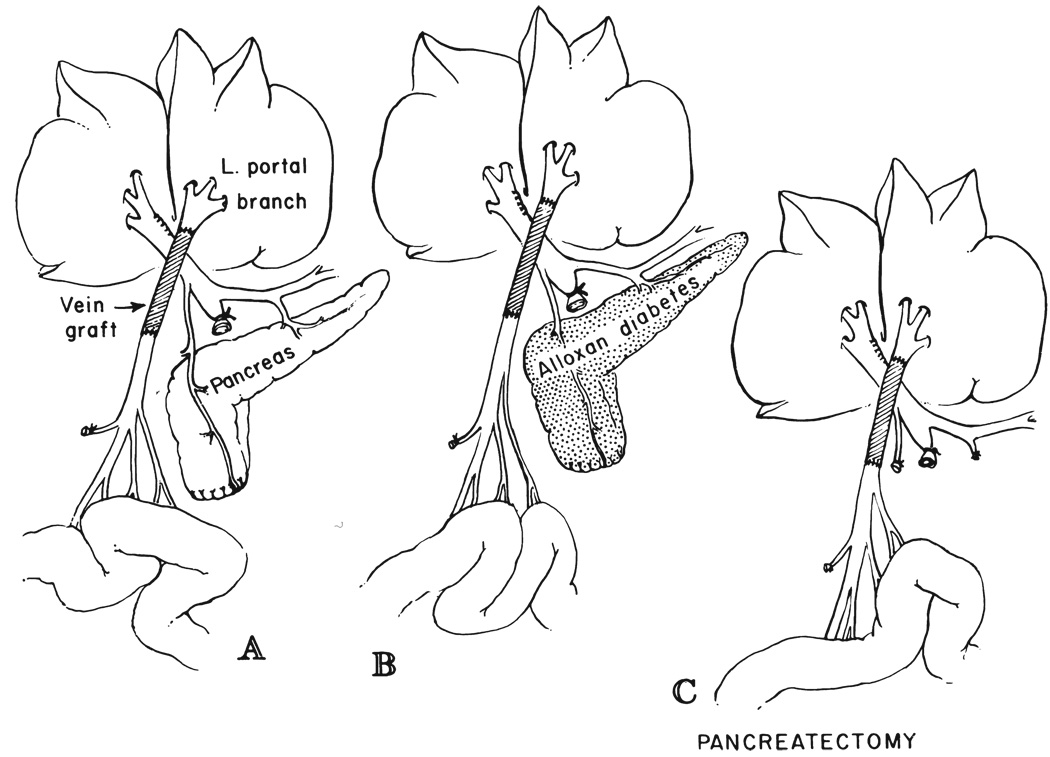
Ten dogs had a previously described splanchnic venous division procedure (33) which diverts the nutrient rich intestinal venous blood into the left liver lobes through a reversed external jugular vein graft, whereas the right liver lobes were perfused by the hormone rich pancreaticogastroduodenosplenic blood (Fig. 1A). The tail of the inferior lobe of the pancreas was resected (Fig. 1A), since venous blood from this pancreatic tissue often drains independently into the intestinal vein. By removing it, pancreatic drainage into the left liver lobes was prevented. The dogs were sacrificed 55 to 66 days later, for a mean follow-up period of 59.4 days.
FIG. 1.
Splanchnic division experiments in which the right liver lobes received venous return from the pancreaticogastroduodenosplenic region and the left liver lobes received venous blood from the intestines. Note resection of the inferior pole of the pancreas, which often drains into the mesenteric vein. A, Nondiabetic dogs of group 2. B, Alloxan-induced diabetic dogs of group 3. C, Dogs of group 4 with total pancreatectomy.
Group 3
Four dogs had the same splanchnic division procedure as those in group 2 from three to eight weeks after stable diabetes mellitus was induced with a single injection of 70 to 80 milligrams of alloxan—mesoxalyl urea—per kilogram of body weight (Fig. 1B). The diabetes mellitus was managed by subcutaneous injections of neutral protamine Hagedorn insulin, mainly with the aid of frequent fasting blood sugar determinations. The dogs were sacrificed 60 to 64 days following splanchnic division after a mean follow-up period of 61.6 days.
Group 4
Six dogs had splanchnic venous division as in group 2, but in addition, total pancreatectomy with preservation of the duodenum and common duct (Fig. 1C) was performed at the same operation. The resulting diabetes mellitus was managed with insulin, as in group 3. Fifty-three to 64 days later, the dogs were sacrificed after a mean follow-up period of 60.8 days.
Group 5
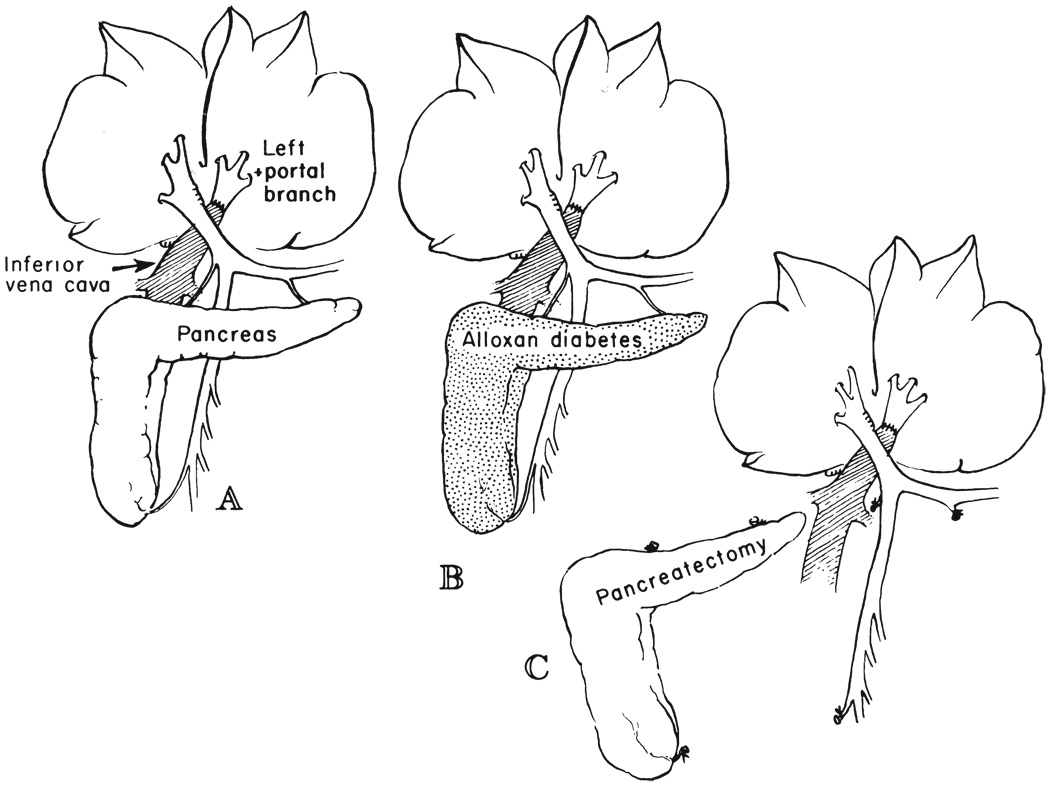
Twelve dogs had a partial portacaval transposition (Fig. 2A). The left portal branch was detached and anastomosed end-to-end to the suprarenal inferior vena cava. Thus, the left lobes were perfused with systemic venous blood, including the renal and adrenal effluent, whereas the right lobes were exposed to the total nonhepatic splanchnic venous return. The dogs were sacrificed 61 to 71 days after operation for a mean follow-up period of 63.6 days.
FIG. 2.
Partial portacaval transposition experiments in which the right liver lobes received all the splanchnic venous return and the left liver lobes received portal venous inflow from the suprarenal inferior vena cava. A, Nondiabetic dogs of group 5. B, Alloxan-induced diabetic dogs of group 6. C, Dogs of group 7 with total pancreatectomy.
Group 6
Four dogs had partial portacaval transposition at one and one-half to seven weeks after the induction of diabetes mellitus with a single injection of 55 to 70 milligrams of alloxan per kilogram of body weight (Fig. 2B) and after a stable daily dosage of neutral protamine Hagedorn insulin had been established. Forty-one, 60, 62 and 64 days after the partial transposition, with a mean follow-up period of 56.5 days, the dogs were sacrificed.
Group 7
Six dogs had partial portacaval transposition and total pancreatectomy at the same operation (Fig. 2C). Postoperatively, they were treated daily with subcutaneously injected insulin. One dog died after 51 days, and the other five were sacrificed after 56, 68, 68, 70, and 79 days. The mean survival time for all six dogs was 65.3 days.
Biochemical Studies
Liver biopsies were quick-frozen and kept at minus 20 degrees C. until the analyses were completed. The method of Bloom and his associates (4) was used to separate the trichloroacetic acid soluble glycogen fraction from the insoluble one. The anthrone method of Seifter and his colleagues (29) was used to quantitate both fractions.
The protein content of the liver was determined on the tissue sample used for the determination of labile glycogen. After extraction of the labile glycogen fraction with ice-cold trichloroacetic acid, the insoluble precipitate was dissolved in 3 milliliters of 3 per cent alkaline deoxycholate and assayed for protein with the biuret method of Gornall and his colleagues (14).
The extraction of lipids from 4 to 5 grams of the liver tissue was carried out as described by Bligh and Dyer (3) with minor modifications. Total cholesterol value, triglycerides and phospholipids were determined according to the method of Zlatkis and his associates (40), Fletcher (13) and Sunderman and Sunderman (37), respectively.
Extraction and purification of deoxyribonucleic acid from liver tissue were carried out according to the method of Schneider and Greco (28) with minor modifications. About 300 milligrams of frozen liver tissue were homogenized in 10 volumes of 0.3 molar perchloric acid at zero degree C. The homogenate was centrifuged at zero degree C., and the supernatant was discarded. The pellet was suspended in 4 milliliters of 1.0 molar sodium hydroxide and heated at 37 degrees C. for one hour. After adding 1.5 milliliters of 3.0 molar ice-cold perchloric acid, the precipitate was centrifuged and washed once again with the same perchloric acid. The sediment, which was now free of ribonucleic acid and the acid soluble fraction, was suspended in 4 milliliters of 0.3 molar perchloric acid and heated for 15 minutes at 90 degrees C. After centrifugation, the supernatant was removed and saved. The pellet was again extracted with hot perchloric acid, and after centrifugation, the two supernatants were combined. The content of deoxyribonucleic acid was determined with diphenylamine reagent, as described by Burton (7), using calf thymus deoxyribonucleic acid as the standard.
The in vivo incorporation of [CH3-3H] thymidine into deoxyribonucleic acid was estimated by measuring the specific activity of the deoxyribonucleic acid obtained by extraction. A 0.4 milliliter aliquot of the deoxyribonucleic acid extract was placed in a counting vial, dissolved in 2 milliliters of a solubilizer, and mixed with 10 milliliters of toluene-2, 5-diphenyloxazole scintillation fluid. The radioactivity was measured in a liquid scintillation counter. A correction was made for the dilution factor, and the results were expressed as counts per minute per 10 micrograms of deoxyribonucleic acid.
Pathologic Studies
Samples of liver tissue were fixed in 10 per cent normal buffered Formalin® (aqueous solution of formaldehyde). Frozen sections were cut and stained with Sudan IV for fat, and then the remainder was processed and the paraffin sections were stained with hematoxylin and eosin, Gordon and Sweet's silver impregnation method for reticulin fibers, Perls’ Prussian blue method for iron, trichrome for collagen and fibrin, periodic acid-Schiff method for glycogen, and Pearse’s method for ceroid and lipofuscin.
Other paraffin sections were covered by stripping film. The autoradiographs were exposed for 28 to 64 days, developed and then stained through the emulsion with Ehrlich’s hematoxylin and eosin by Pelc’s method.
Additional small hepatic samples were initially fixed in glutaraldehyde solution, then postfixed in osmic acid and embedded in Epon® (synthetic embedding medium). Half micron and ultrathin sections were cut. The former were stained with azure II for examination in the light microscope, while the latter were stained with lead citrate and examined in an electron microscope.
The size of the hepatocytes was determined on hematoxylin and eosin-stained sections by a method previously described (33). In essence, the technique consists of tracing out large numbers of hepatocytes on standard thickness paper, cutting out the silhouettes and weighing them. Midzonal hepatocytes were also used for measuring rough endoplasmic reticulum length per area of cytoplasm by the morphometric method of Loud (19).
RESULTS
Splanchnic Division
Gross findings
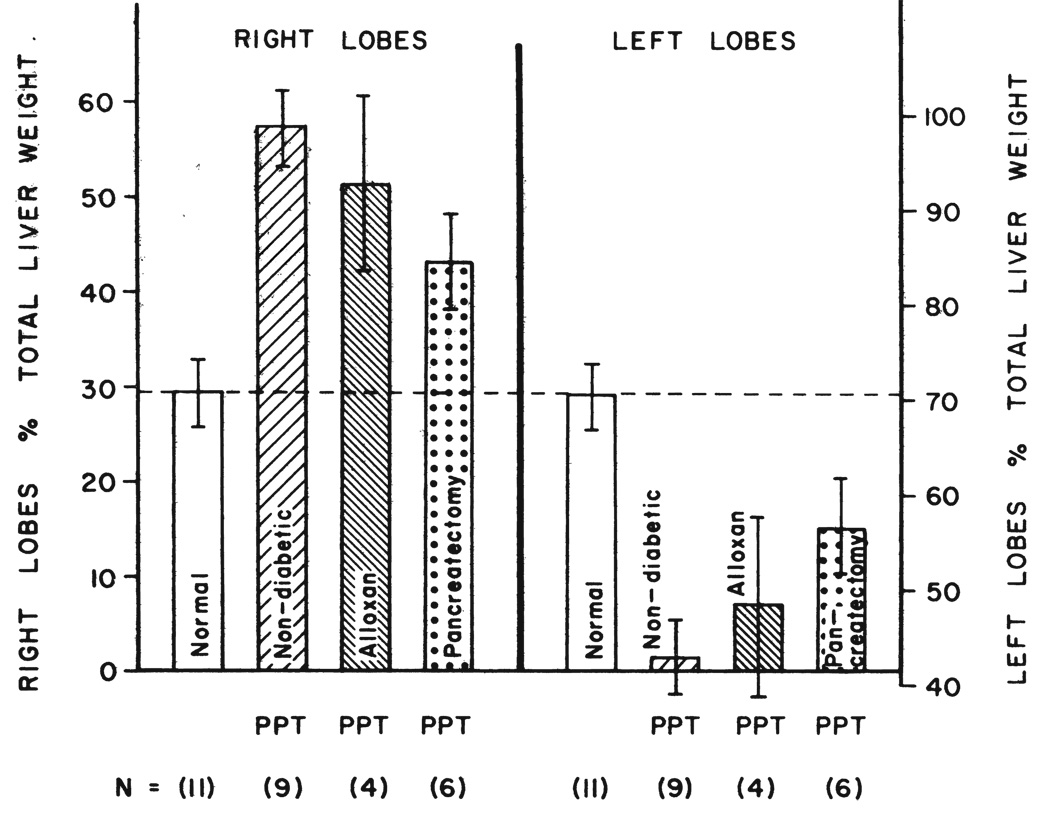
The ratio of weights of the right to left liver lobes in the 11 normal unaltered dogs of group 1 was 29.4:70.6 ± 3.4 (S.D.) per cent (Fig. 3). Two months after the splanchnic division operations, these ratios in the ten dogs of group 2 were 45.2:54.8 ± 5.9 (S.D.) per cent (Fig. 3), a change which was statistically significant, p < 0.001.
FIG. 3.
The effect of chronic splanchnic division, CD, upon the weights of the right and left liver lobes, which normally have a 30:70 ratio as shown. Note that, in nondiabetic dogs, the right lobes given pancreaticogastroduodenosplenic venous inflow underwent a weight increase relative to the left lobes which were given intestinal venous blood. This hypertrophic effect was almost eliminated by alloxan-induced diabetes or by concomitant total pancreatectomy. The bars represent the means of the number, N, of experiments, while the vertical lines show ± one standard deviation.
The weight increases in the right versus the left liver lobes caused by splanchnic division were largely prevented in the four dogs of group 3 by the prior establishment of alloxan-induced diabetes which was controlled by 17.5 to 20.0 units of insulin per day. After two months, the right to left liver lobe weight ratio was 32.8:67.2 ± 8.8 (S.D.) per cent (Fig. 3). These values in the dogs with alloxan-induced diabetes represented a significant alteration from those in nondiabetic dogs with splanchnic division, p<0.05. Although the diabetic dogs still had right lobes that were slightly heavier than those in normal unaltered dogs (Fig. 3), the difference was not statistically significant.
In the six dogs which were submitted to splanchnic division plus total pancreatectomy and then managed with 15 to 20 units of insulin per day, the right to left hepatic lobar ratio two months postoperatively was 32.8:67.2 ± 3.0 (S.D.) per cent (Fig. 3). Thus, the mean right to left lobar ratio was exactly the same as in the dogs with alloxan-induced diabetes. However, the larger number of dogs and the smaller standard deviation in group 4 changed the significance figures. Compared with the nondiabetic dogs of group 2, the change in right to left liver weight ratios in group 4 was highly significant, p< 0.001. Moreover, the right lobes of group 4 dogs were now significantly heavier, p< 0.05, than those of normal unaltered dogs.
When the lobar weight ratios for all ten diabetic dogs of groups 3 and 4 were pooled and compared with those of the ten nondiabetic dogs of group 2 which also had the procedure of splanchnic division, the liver lobar ratios were significantly different at the p< 0.001 confidence level. When the pooled diabetic dogs of groups 3 and 4 were compared with normal unaltered dogs, the liver lobar ratios were not significantly different.
Pathologic studies
There were no significant differences in the sizes of either the lobules or the hepatocytes in the right and left liver lobes of the 11 normal unaltered dogs in group 1 (Fig. 4). All of these livers lacked fat, and nine contained normal amounts of glycogen and appeared normal ultra-structurally; the other two livers were deficient in glycogen. Autoradiographs of the livers of the dogs in group 1 showed similar low numbers of labeled hepatocytes in the right and left lobes (Table I).
FIG. 4.
The effect upon hepatocyte size of alloxan-induced diabetes and the diabetes of total pancreatectomy in dogs with splanchnic division and partial portacaval transpostion. All the splanchnic division procedures of groups 2, 3 and 4 diverted pancreaticogastroduodenosplenic blood to the right lobes, whereas the left lobes received intestinal venous blood. All the partial transposition procedures of groups 5, 6 and 7 diverted the total splanchnic venous blood to the right lobes, whereas the left lobes received systemic venous blood from the hindquarters and kidneys. Note that the difference in hepatocyte size caused in the nondiabetic dog by either splanchnic division or partial portacaval transposition was partly or completely eliminated by both kinds of diabetes. The generally larger cell size in the diabetic dogs was presumably, at least in part, due to a variety of abnormalities related to the diabetic state. The bars represent the means of the number, N, of experiments while the vertical lines show ± one standard deviation. The p values refer to the significance of differences in hepatocyte size between the right, R, and left, L, lobes within each experimental group.
TABLE I.
Number of Labeled Hepatocytes Per 1,000 Hepatocytes in Livers of Normal Dogs and Dogs with Splanchnic Division and Partial Portacaval Transposition
| Group | No. of experiments | Right lobes, mean S.D. | Left lobes, mean S.D. |
|---|---|---|---|
| 1 | 11 | 1.6±0.5 | 1.5±0.4 |
| 2 | 6 | 17.3±3.8 | 4.0±1.0 |
| 3 | 4 | 4.9±0.4 | 17.8±3.6 |
| 4 | 5 | 5.1±1.0 | 17.5±3.9 |
| 5 | 12 | 15.7±2.2 | 4.8±0.5 |
| 6 | 3 | 11.0±1.2 | 4.3±0.9 |
| 7 | 5 | 11.1±1.1 | 5.2±1.2 |
Two months after splanchnic division in the nondiabetic dogs of group 2, the hepatocytes in the left liver lobes, which had received the nutrient rich intestinal venous blood, had decreased in size (Fig. 4), were irregular in shape and were depleted of glycogen. The lobules were shrunken, and in some of the livers, there was centrilobular reticulin condensation as well as an increase in the number and size of the Kupffer cells, some of which contained hemosiderin. Ultrastructurally, the rough endoplasmic reticulum was greatly reduced in amount, glycogen granules were scarce, and there were increased numbers of small fat vacuoles. The right liver lobes, which had been perfused by the hormone rich pancreaticogastroduodenosplenic blood, had larger lobules and hepatocytes than were present in the normal dogs of group 1 (Fig. 4). The enlarged hepatocytes contained plenty of glycogen and were free of fat. There were also binucleate liver cells, mitoses and proliferating bile ductules. Ultrastructurally, the enlarged cells were essentially normal. Autoradiographs showed about four times as many labeled hepatocytes in the enlarged right lobes as in the atrophic left lobes (Table I).
The difference in size of the hepatocytes in the left versus the right lobes, brought about by splanchnic division, did not occur in the ten insulin-treated diabetic dogs of groups 3 and 4. After two months, the hepatocytes in the right and left lobes were almost equal in size and larger than those of normal, unaltered dogs (Fig. 4). The enlarged hepatocytes on both sides contained lots of fat droplets, many of which were obvious by light microscopy. The amount of fat seen in frozen sections was greatest in the left lobes of the dogs in group 4 which had been submitted to splanchnic division plus total pancreatectomy. The quantity of stainable glycogen was reduced in the hepatocytes in the right lobes of the alloxan-induced diabetic dogs and was normal in the right lobes of the dogs after pancreatectomy and in the left lobes of all the dogs. In these ten diabetic dogs, binucleate liver cells, mitoses and proliferating bile ductules were more common in the left lobes than in the right. Ultrastructurally, the cells of the left lobes contained little rough endoplasmic reticulum, and there were increased numbers of large lipid droplets, and lysosomes were prominent; glycogen granules were normal in number. The hepatocytes in the right lobes contained few lipid droplets, the amount of rough endoplasmic reticulum was normal, but there were fewer glycogen granules in the insulin treated alloxan-induced diabetic dogs of group 3 than normal. The number of glycogen granules was normal in both sides of the dogs of group 4 after pancreatectomy. Autoradiographs showed more labeled hepatocytes in the left lobes than in the right lobes in dogs with both kinds of diabetes (Table I).
In vivo deoxyribonucleic acid synthesis
There were only minor differences in the deoxyribonucleic acid concentrations of the right and left liver lobes of normal unaltered dogs. In nondiabetic dogs with splanchnic division, the atrophic left lobes had higher deoxyribonucleic acid concentrations, presumably because of the larger number of cells per unit weight of tissue analyzed. The right lobe concentrations were 2.3 ± 0.07 (S.D.), whereas the left lobes were 3.2 ± 1.0 (S.D.) micrograms per milligrams of liver. The difference between the right and left sides was statistically significant, p< 0.02 The dogs with splanchnic division and either kind of diabetes did not have significant differences between the right and left lobes in deoxyribonucleic acid concentrations.
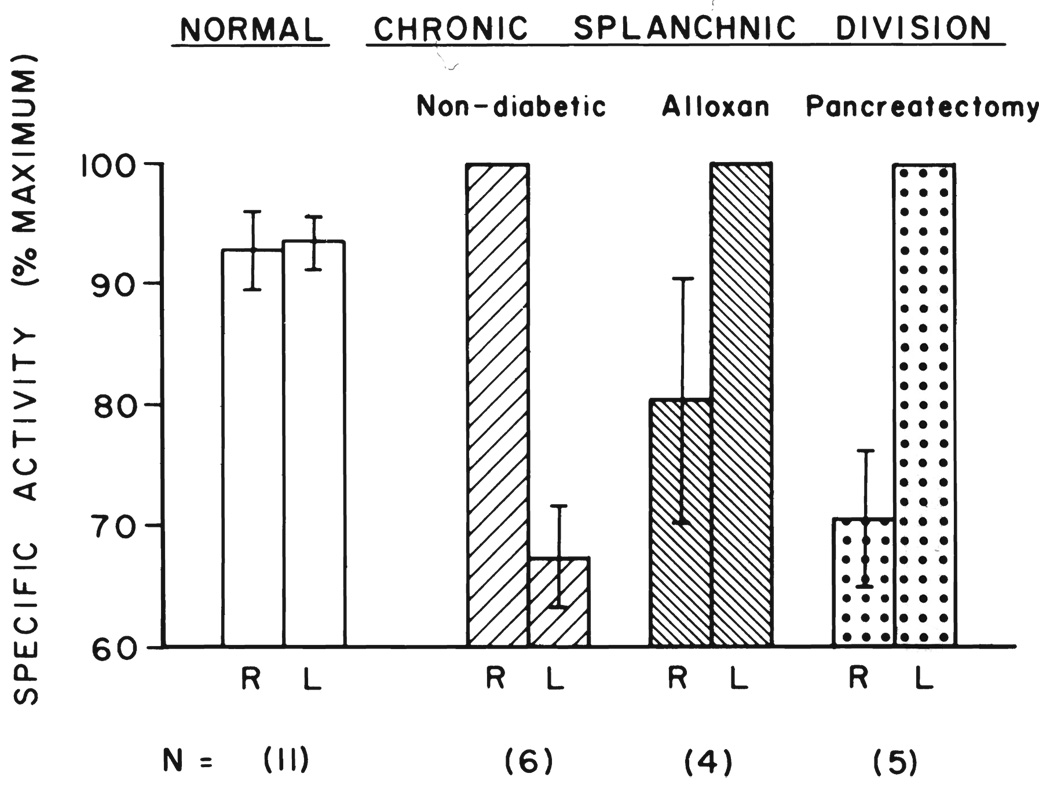
The incorporation of (CH33-3H) thymidine into deoxyribonucleic acid as a reflection of deoxyribonucleic acid synthesis was bilaterally equal in normal unaltered dogs of group 1 (Table II). In nondiabetic dogs with chronic splanchnic division, the right lobes always had the higher specific activity. These findings were significantly influenced by the presence of diabetes. In the dogs of group 3 with alloxan-induced diabetes, the dominance two months after chronic splanchnic division was transferred in each of four experiments to the left side (Table II). The same shift in nucleic acid synthesis occurred in the dogs of group 4 studied two months after being submitted to total pancreatectomy and splanchnic division (Table II). These results are summarized graphically in Figure 5, using an analytic technique in which the side with the greater specific activity is assigned a value of 100 per cent, and a proportionally smaller percentage is calculated for the side with lesser specific activity.
Table II.
In Vivo Deoxyribonucleic Acid Synthesis in Normal Dogs and in Dogs Having Chronic Splanchnic Division, with or without Pancreatectomy or Alloxan-Induced Diabetes
| Thymidine uptake, specific activity in counts per min./10 mcgm. DNA, mean±S.D. | |||||
|---|---|---|---|---|---|
| Experiment | Group | No. of experiments | Right lobes | Left lobes | p* |
| Normal dogs | 1 | 11 | 22.4±6.3 | 22.5±5.8 | N.S. |
| Chronic splanchnic division | |||||
| Nondiabetic | 2 | 6 | 31.0±10.5 | 21.0±7.4 | <0.01 |
| Alloxan-induced diabetic | 3 | 4 | 25.0±12.4 | 31.0±13.7 | N.S. |
| Total pancreatectomy | 4 | 5 | 24.8±8.3 | 35.8±12.9 | <0.05 |
The p values given are for comparisons of the right to left lobes in each group.
DNA, Deoxyribonucleic acid.
N.S., Not statistically significant.
FIG. 5.
Deoxyribonucleic acid synthesis in the right, R, and left, L, liver lobes of normal dogs and of nondiabetic or diabetic dogs approximately two months after splanchnic division. In nondiabetic dogs, the right lobes which received pancreaticogastroduodenosplenic blood had the dominant deoxyribonucleic acid synthesis, but in the diabetic dogs, this dominance was transferred to the left liver lobes. The actual values for [CH33-3H] thymidine uptake, which are given in Table II, were converted to percentages in which the side with the greater thymidine uptake was accorded 100 per cent. The bars represent the means of the number, N, of experiments while the vertical lines show ± one standard deviation.
Biochemical studies
There was no significant difference in the glycogen contents of the right and left lobes of nine normal unaltered dogs of group 1 (Fig. 6). The right liver lobes in nondiabetic dogs with splanchnic division had significantly more glycogen after 60 days than did the left liver lobes which were receiving intestinal venous blood, p< 0.001 (Fig. 6). The differential glycogenation was reversed by alloxan-induced diabetes, so that the glycogen storage was now significantly greater on the left side, p<0.01 (Fig. 6). After total pancreatectomy, both sides had a high glycogen content, but the difference between the right and left sides was not statistically significant, as shown in Figure 6.
FIG. 6.
Liver glycogen concentrations in the normal dogs of group 1 and in the nondiabetic and diabetic dogs which had splanchnic flow division or partial portacaval transpostition. In the splanchnic division experiments of groups 2, 3 and 4, the right lobes were given pancreaticogastroduodenosplenic blood, while the left lobes received intestinal venous blood. In the partial portacaval transposition experiments of groups 5, 6 and 7, the right lobes received the total splanchnic venous return, while the left lobes were given the inferior vena caval blood from the hindquarters and kidneys. The bars represent the means of the number, N, of experiments, while the vertical lines show ± one standard deviation. The p values refer to the significance of the differences of the right, R, and left, L, lobar concentrations of glycogen within each experimental group.
The protein and lipid concentrations in the right and left liver lobes were not different to a statistically significant degree in any of the foregoing experiments of groups 1 to 4. However, the lipid content of both sides seemed to be increased by alloxan-induced diabetes (Fig. 7).
FIG. 7.
Lipid contents in the right, R, and left, L, liver lobes of the dogs in all the experimental groups. For comparison, the histopathologic estimates of fat in the hepatocytes are noted at the bottom. The latter values were obtained from light and electron microscopic observations by grading the amount of fat on a zero to 5 plus scale, zero indicating no detectable fat and 5 plus being maximum. The index presented for each group represents the mean of the gradings from individual experiments.
Partial Portacaval Transposition
Gross findings
In nine of the 12 nondiabetic dogs of group 5, the right and left liver lobes were weighed at the time of sacrifice, the mean follow-up period for these dogs being 61.3 ± 0.5 (S.D.) days. The right to left liver lobar ratio was 57.2:42.8 ± 3.8 (S.D.) per cent, a proportion drastically different from the approximately 30:70 ratio in normal unaltered dogs, p< 0.001 (Fig. 8).
FIG. 8.
The effect of partial portacaval transposition, PPT, upon the weights of the right and left liver lobes which normally have a 30:70 ratio, as shown. Note that the right lobes given the total splanchnic venous inflow underwent hypertrophy in nondiabetic dogs relative to the left lobes which were given vena caval blood. This hypertrophic effect was only slightly less evident in the dogs with alloxan-induced diabetes but was reduced by about one-half in dogs with the diabetes of total pancreatectomy. The bars represent the means of the number, N, of experiments while the vertical lines show ± one standard deviation.
The relative advantage of the right lobes after partial portacaval transposition was partly lost by the superimposition of alloxan-induced diabetes or total pancreatectomy (Fig. 8), but comparisons between the nondiabetic dogs of group 5 and the diabetic dogs of groups 6 and 7 were statistically significant only for the dogs of group 7 after pancreatectomy, p< 0.001.
In the livers of dogs with total pancreatectomy, there was usually a striking color difference between the right and left lobes. The right lobes which were receiving splanchnic blood appeared normal, whereas, the left lobes which were being perfused with vena caval blood were yellowish and waxy.
Pathologic studies
After partial portacaval transposition, the hepatocytes in the left liver lobes, which had received systemic blood, decreased in size (Fig. 4) and became depleted of glycogen. The lobules were smaller than normal, and the Kupffer cells were increased in size and number and contained hemosiderin. Ultrastructurally, there was loss of rough endoplasmic reticulum, glycogen granules were scarce and there were increased numbers of small lipid droplets. The right liver lobes, which had received splanchnic venous blood, had larger lobules and hepatocytes than were present in the normal dogs of group 1 (Fig. 4). The enlarged hepatocytes contained normal quantities of glycogen and no fat droplets. Binucleate liver cells, mitoses and proliferating bile ductules were present. Ultrastructurally, the enlarged cells were essentially normal. Autoradiographs showed more labeled hepatocytes in the enlarged right lobes than in the atrophic left lobes (Table I).
The presence of insulin-treated diabetes changed a number of these findings. The atrophy of the hepatocytes in the left lobes induced by portacaval transposition either failed to occur or was greatly reduced in amount. This effect was most obvious in the dogs of group 7 made diabetic by pancreatectomy when, after two months, the hepatocytes in the right and left lobes were approximately the same size and slightly larger than those of the controls (Fig. 4). The enlarged hepatocytes of the dogs after pancreatectomy contained many large lipid droplets obvious by light microscopy; this accumulation of fat was greatest in the left lobes. The hepatocytes of the alloxan-induced diabetic dogs of group 6 contained much less fat and more in the right lobes rather than the left. The amount of stainable glycogen was reduced in the hepatocytes in the right lobes of the alloxan-induced diabetic dogs but was normal in the left lobes and in the lobes on both sides in the dogs of group 7 after pancreatectomy. Binucleate liver cells and mitoses were more common in the right lobes than in the left.
Ultrastructurally, in the diabetic dogs, the left lobe hepatocytes showed little rough endoplasmic reticulum and normal numbers of glycogen granules (Fig. 9). Lipid droplets were slightly increased in number in the alloxan-induced diabetic dogs; they were large and frequent in most of the dogs after pancreatectomy. The hepatocytes in the right lobes also contained lipid droplets, but in the dogs after pancreatectomy these were smaller and less frequent than in the alloxan-induced diabetic dogs. The amount of rough endoplasmic reticulum in the right lobes was normal; the number of glycogen granules was reduced (Fig. 10) in the insulin-treated alloxan-induced diabetic dogs of group 6 but not in the dogs of group 7 after pancreatectomy. Autoradiographs of the livers of the diabetic dogs of groups 6 and 7 showed more labeled hepatocytes in the right lobes than in the left lobes (Table I), although the right lobar dominance was less than in the nondiabetic partial portacaval transpositions of group 5.
FIG. 9.
Part of an hepatocyte from the left liver lobes of an insulin-treated, alloxan-induced diabetic dog with partial portacaval transposition from group 6. There is little rough endoplasmic reticulum, rer, but normal amounts of glycogen. ×10,250.
FIG. 10.
Part of an hepatocyte from the right liver lobes of the same dog as in Figure 9. There is abundant rough endoplasmic reticulum, rer, but less glycogen than normal. ×10,250.
In vivo deoxyribonucleic acid synthesis
In the 12 non-diabetic dogs of group 5, which were submitted to partial portacaval transposition, there was not a statistically significant difference in deoxyribonucleic acid synthesis between the right lobes which were receiving splanchnic venous blood and the left lobes which were being perfused with systemic venous blood. The prior presence of alloxan-induced diabetes or concomitant pancreatectomy did not affect these results.
Biochemical studies
Biochemical determinations were made in nine of the 12 nondiabetic dogs of group 5. The glycogen was significantly greater in the splanchnic fed right lobes, p< 0.01 (Fig. 6). With alloxan-induced diabetes, the predominant glycogen storage was in the left side, although the left lobar dominance was not statistically significant. After total pancreatectomy, the glycogen contents were nearly equal on the two sides (Fig. 6). In the nondiabetic dogs and the dogs with alloxan-induced diabetes, the protein concentrations in the left lobes were higher than on the right side, p< 0.02; the converse was true after total pancreatectomy, although not to a level of statistical significance.
The hepatic lipid contents were elevated in all the partial portacaval transposition experiments and to an extraordinary degree in the dogs also submitted to total pancreatectomy (Fig. 7). The major increases were in the triglyceride fractions, which in turn were reflected in the less quantitative collateral histopathologic assessments of lipid (Fig. 7). Both the right and left lobes showed fat accumulation.
DISCUSSION
Our earlier suggestion that the principal portal hepatotrophic factors are interreacting hormones was based on circumstantial evidence that imputed a special importance to insulin (33,34). This hypothesis was directly tested in the canine investigations herein reported by inducing diabetes mellitus with alloxan or by total pancreatectomy and by seeing how the diabetic state affected the results after splanchnic division and partial portacaval transposition. These latter procedures divide the liver into two fragments with qualitatively different portal venous inflows. The most important variable had previously been shown to be the presence or absence of pancreatic venous blood (33, 34).
Because the dogs with both kinds of diabetes were treated with exogenous insulin for the two month period of postoperative study, the consequences to the two liver fragments in any given experiment were not those of an absolute insulinoprival state. Instead, exogenous insulin introduced by subcutaneous injection was distributed to both fragments after absorption; dilution; passage through the cardiac mixing chamber; and in the case of the venous inflow, after transmission through a capillary bed. In comparison with nondiabetic dogs submitted to splanchnic division or partial transposition, what was lost to one fragment was the transportal supply of endogenous insulin normally delivered in the pancreatic effluent to the liver in high concentrations and in a physiologic ratio to endogenous glucagon.
The effectiveness of replacement therapy by subcutaneously administered insulin was not uniform in the different groups of diabetic dogs, and this apparently was the explanation for the highly variable hepatic lipid concentrations. Banting and Best and their associates (1), Hersey (15) and many subsequent investigators have emphasized the importance of insulin management if fatty infiltration, such as that found bilaterally in the livers of the dogs of groups 4 and 7, is to be avoided. When it was excessive, the fat undoubtedly distorted the hepatocyte size data, and it may have created other artifacts.
The dogs with splanchnic division provided the most consistent and easily interprétable observations. In nondiabetic dogs, earlier experiments were confirmed (33). Liver tissues of the right lobes which were perfused with the hormone rich venous return from the pancreaticogastroduodenosplenic area underwent hypertrophy, hyperplasia and glycogen storage during the two month duration of study compared with the liver tissues of the left lobes which were supplied by the nutrient rich venous effluent returning from the intestine.
The superimposition of diabetes mellitus which was produced either with alloxan or by total pancreatectomy altered these findings. The hypertrophy of the right lobes and their hepatocytes combined with relative atrophy on the left side was no longer a prominent feature. Instead, the hepatocytes on both sides were slightly bigger than in normal dog livers. The greatest cell division as reflected morphologically, by autoradiography and by deoxyribonucleic acid synthesis, was transferred from the right to the left. Histopathologically, it was of interest that neither side was completely normal in the diabetic state as the right lobes had been in the nondiabetic dogs subjected to splanchnic division. The most uniform structural abnormality in the diabetic dog livers was increased lipid vacuolization which was not necessarily accurately reflected in the biochemical measures of tissue lipid concentration.
The effects caused by alloxan-induced diabetes compared with those caused by total pancreatectomy in the dogs with splanchnic division were not particularly different, except that the degree of fat infiltration tended to be less on both sides of the liver. In addition, there was a lower glycogen concentration in the right liver lobes of the dogs with alloxan-induced diabetes, as would be expected from the continuing action of glucagon on this hepatic tissue. With the elimination of glucagon by total pancreatectomy, the hepatic glycogen concentrations became bilaterally equal. The fact that no other demonstrable hepatotrophic effects of pancreatectomy were identified compared with those of alloxan-induced diabetes suggested that the most important single experimental variable in both circumstances had been the elimination of endogenous insulin. As a corollary, glucagon and other substances from the pancreas, stomach, duodenum and spleen singly or together did not have a hepatotrophic role approaching in importance that of insulin. Finally, the similarity of results indicated that the diminution in the volume of venous flow to the right lobes following pancreatectomy was not sufficiently great to introduce a major artifact into the comparisons between pancreatectomy and alloxan-induced diabetes.
Although insulin thus appeared to be the most important hepatotrophic substance in portal venous blood, there was no reason to believe that it was the only one. This was particularly well shown by the partial transposition experiments in which the right lobes were given blood returning from all the splanchnic organs, thus providing both hormone and nutrient enrichment. The left liver lobes were perfused with systemic venous blood from the hindquarters, kidneys and adrenal glands. In nondiabetic dogs, the resulting advantages to the right lobes and the disadvantages to the left lobes were similar to but greater than after the experiments with splanchnic division. The disparity between the sides in terms of hypertrophy, hyperplasia and other findings was lessened by alloxan-induced diabetes and especially by total pancreatectomy, but the right lobar dominance was by no means eliminated. Translating these findings into more practical terms, the most favorable condition for portal perfusion was with splanchnic venous blood which contained normal amounts of endogenous insulin. The least favorable condition was perfusion with systemic venous blood. Intermediate in quality was splanchnic venous blood that was deficient in endogenous insulin but which was rich in other elements.
Although it is reasonable and probable as we (32, 33, 34), Popper (25) and others have suggested that the relationships of a number of hormones to each other and to nutritional substrate constitute the essence of the hepatotrophic effect, only insulin has been shown unequivocally to be hepatotrophic in intact dogs. Experimental preparations, such as we have been using, are probably too crude to demonstrate the lesser or more subtle effects of other individual hormones or nutrients which may have a substantial cumulative influence. The same limitation apparently applies to the techniques of Ozawa and his associates (24) which have focused upon the beneficial effects of insulin upon hepatocyte mitochondrial metabolism and to the historically important studies of Younger and his coworkers (39). Further progress may be expected with exploitation of the kind of monolayer hepatocyte culture methods described for separated rat liver cells by Bissell (2) and Chapman and their colleagues (9) and by Leffert (17, 18). With these techniques, the ingredients of the culture media can be controlled, and their effects upon the structure and function of hepatocytes can be determined under conditions of changing concentrations.
By exploiting the hepatocyte monolayer method, Leffert (17, 18) has been particularly successful during the last year in developing evidence of widespread endocrine control of hepatocellular growth. In this work, which he has recently summarized, Leffert (17, 18) has identified insulin, hydroxycortisone, tri-iodothyronine and somatotrophin as variable initiators or potentiators of deoxyribonucleic acid synthesis, with the powerful insulin effect antagonized by glucagon. Other serum factors which are not hormones and which probably include lipid moieties and specific amino acids were cited by Leffert (17, 18) as conditioners or inhibitors of hepatocellular growth. The potential complexity of these control mechanisms can be imagined from the demonstration of Cornell and his colleagues (10) that some of the amino acids, such as lysine, may simulate the action of hormones.
In all of our earlier studies of hepatotrophic factors and in those using the monolayer techniques, the assumption has been explicit or tacit that such portal factors were involved in the control of liver regeneration after partial hepatectomy. The main reason to believe this from our work with chronic double liver fragment preparations (20, 33) was that cell division in these experiments, as judged by morphologic criteria, was greater in liver tissue perfused for about two months by the full splanchnic venous return or the pancreaticogastroduodenosplenic portion of it than in liver tissue which was deprived of this kind of blood for the same period of time. By autoradiography and measurement of deoxyribonucleic acid synthesis, the same conclusion was reached in the experiments herein reported with the important added observation that the relatively higher cell replication on the enriched side in the splanchnic division experiments was markedly lower after total pancreatectomy or the production of alloxan-induced diabetes.
However, none of these studies actually involved partial hepatectomy. In recent years, several workers interested primarily in regeneration, beginning with Sigel (31), have devised experiments that exploit the properties of the double liver fragment model to test directly if the hepatotrophic substances are involved in the regeneration that occurs in a few hours or days after hepatic resection. Lee (16), Chandler (8) and Fisher (11) and their associates have produced convincing evidence that portal hepatotrophic factors are essential for normal liver regeneration. By employing ingenious composite grafts of liver and pancreas in isogeneic rats that also underwent partial hepatectomy, Broelsch and his colleagues (5) localized the main control of hepatic hyperplasia to the pancreas. It was of interest that Ranson and his associates (27) used an almost identical composite graft operation without hepatectomy in dogs that included an immunologically tolerant beagle recipient. They concluded by criteria other than hyperplasia that the pancreas was the source of the principal hepatotrophic factors.
Investigations of liver regeneration after the extirpation of various splanchnic organs have provided less consistent results. Sgro and his associates (30) found in rats that pancreatectomy markedly inhibited the regenerative response to partial hepatectomy but that the removal of other splanchnic organs had no such effect. In contrast, Fisher (12) claimed, after carrying out similar studies, that the principal hyperplasia-inducing factors emanate from the intestine. Price (26), Max (23) and Bucher (6) and their associates have excised all of the nonhepatic splanchnic organs in rats or dogs. The animals were kept alive with intravenous infusions that contained insulin. After both minor and major partial hepatectomy, regeneration occurred in such animals, but Bucher and Swaffield (6) demonstrated this to be later and less vigorous than normal. Bucher and Swaffield concluded that portal hepatotrophic factors were not a prerequisite for hepatic regeneration but that regeneration was conditioned and permitted to express its full potential only in the presence of these substances. This view, as well as our own, is consistent with that of Weinbren and his colleagues (38) who have envisioned regeneration as the product of several complimentary processes rather than the result of any single determinant. In spite of this basis for broad agreement, Bucher (6) and Weinbren (38) and their associates must be classified to date as skeptics about the primacy in regeneration of portal blood in general and of insulin in particular.
The multifactorial concept could help explain certain features of regeneration, including the fact that hepatocyte hypertrophy and hyperplasia during regeneration are not necessarily in parallel, as has been emphasized by Sigel (31) and by Price (26) and Weinbren (38) and their associates. In earlier publications (33, 34), we have alluded to a number of other important implications of the hepatotrophic concept in understanding the physiology of the liver in several clinical circumstances. Two more promising fields of investigation need to be added here. First, the effect of known hepatotrophic substances and especially insulin upon acute liver injury should be tested singly and in combination. It would be surprising if the course of recovery after hepatic injury could not be favorably influenced. Second, hepatotrophic factors may be of value for the prevention or treatment of the hepatic encephalopathy that occurs with distressing frequency in animals and less commonly in human beings after complete portal venous diversion.
SUMMARY
Ten nondiabetic dogs were submitted to a procedure called splanchnic division which directed the nutrient rich venous return from the intestines into the left lobes of the liver and the hormone rich pancreaticogastroduodenosplenic venous return into the right lobes. Two months later, the right lobes had undergone the expected gross and microscopic hypertrophy. Compared with the abnormal shrunken and glycogen-depleted hepatocytes of the left lobes, the large and otherwise normal hepatocytes of the right lobes had a higher rate of cell division as judged by microscopic examination, measurements of deoxyribonucleic acid synthesis and the results of autoradiography. Both sides had greater cell replication than in the livers of normal unaltered dogs.
The dominance of the right lobes following splanchnic division was almost completely eliminated by the prior creation of alloxan-induced diabetes in four dogs and by the performance of total pancreatectomy at the same time as splanchnic division in six dogs. In these ten diabetic dogs, which were treated with subcutaneously administered insulin for the two month period of the postoperative study, hepatic lobar and cell size were nearly equal on both sides. By light and electron microscopy, the hepatocytes on both sides had abnormalities, somewhat less pronounced on the right. However, the most active cell division was now transferred to the left lobes. The results with alloxan-induced diabetes were similar to those after total pancreatectomy, except that lipid deposits were less on both liver sides in the alloxan experiments, and the glycogen was selectively reduced in the right lobes. The latter finding presumably was due to the continued action of glucagon in dogs made diabetic with alloxan.
Twelve nondiabetic dogs had a procedure called partial portacaval transposition which directed systemic venous blood from the hindquarters, kidneys and adrenal glands into the left lobes of the liver and the total splanchnic venous return into the right lobes. Two months later, the degree of relative hypertrophy and hyperplasia of the glycogen rich right lobes was even greater than after splanchnic division, as was the morphologic damage to the left lobar hepatocytes.
The degree of right lobar hypertrophy following partial portacaval transposition was reduced but not eliminated by pre-existing alloxan-induced diabetes in four dogs and by concomitant total pancreatectomy in six more dogs. The dogs were subcutaneously treated with insulin. Structurally, the hepatocytes on the right side after two months were in better condition than were those on the left, although both were abnormal. The dominance of cell division on the right side was reduced, as judged by standard microscopy and by autoradiography, but there was not a shifting of sides. The biochemical analyses reflected the presence or absence of glucagon.
These findings are consistent with our earlier multifactorial hypothesis which holds that portal hepatotrophic factors are mainly interreacting hormones generated by splanchnic organs and delivered straight to the liver and that the hormone interrelationships might have augmented significance because of the high concentration of nutritional substrate in the same venous blood. The observations also substantiate by direct testing the suggestion that insulin is the most important hepatotrophic factor and that it profoundly affects many aspects of liver cell structure, division and function.
Acknowledgments
The work was supported by research grants from the Veterans Administration; by grant Nos. AI-AM-08898 and AM-07772 from the National Institutes of Health; and by grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, the National Institutes of Health.
Contributor Information
T. E. Starzl, Departments of Surgery, The University of Colorado Medical Center and The Veterans Administration Hospital, Denver, Colorado
K. A. Porter, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
N. Kashiwagi, Departments of Surgery, The University of Colorado Medical Center and The Veterans Administration Hospital, Denver, Colorado
I. Y. Lee, Departments of Surgery, The University of Colorado Medical Center and The Veterans Administration Hospital, Denver, Colorado
W. J. I. Russell, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
C. W. Putnam, Departments of Surgery, The University of Colorado Medical Center and The Veterans Administration Hospital, Denver, Colorado
REFERENCES
- 1.Banting FG, Best CH, Collip JB, et al. The effect produced in diabetes by extracts of pancreas. Tr. Assoc. Am. Physicians. 1922;37:337. [Google Scholar]
- 2.Bissell DM, Hammaker LE, Meyer UA. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. J. Cell Biol. 1973;59:722. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Bloom WL, Lewis GT, Schumpert MZ, Shen T-M. Glycogen fractions of liver and muscle. J. Biol. Chem. 1951;188:631. [PubMed] [Google Scholar]
- 5.Broelsch CE, Lee S, Charters AC, III, et al. Regeneration of liver isografts transplanted in continuity with splanchnic organs. Surg. Forum. 1974;25:394. [PubMed] [Google Scholar]
- 6.Bucher NLR, Swaffield MN. Regeneration of liver in rats in the absence of portal splanchnic organs and a portal blood supply. Cancer Res. 1973;33:3189. [PubMed] [Google Scholar]
- 7.Burton K. A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956;62:315. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler JG, Lee S, Krubel R, et al. The inter-liver competition and portal blood in regeneration of auxiliary liver transplants. Surg. Forum. 1971;22:341. [PubMed] [Google Scholar]
- 9.Chapman GS, Jones AL, Meyer UA, Bissell DM. Parenchymal cells from adult rat liver in nonproliferating monolayer culture—II, ultrastructural studies. J. Cell Biol. 1973;59:735. doi: 10.1083/jcb.59.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornell NW, Lund P, Krebs HA. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem. J. 1974;142:327. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Szuch P, Fisher ER. Evaluation of a humoral factor in liver regeneration utilizing liver transplants. Cancer Res. 1971;31:322. [PubMed] [Google Scholar]
- 12.Fisher B, Szuch P, Levine M, et al. The intestine as a source of a portal blood factor responsible for liver regeneration. Surg. Gynecol. Obstet. 1973;137:210. [PubMed] [Google Scholar]
- 13.Fletcher MJ. A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta. 1968;22:393. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- 14.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751. [PubMed] [Google Scholar]
- 15.Hersey JM. Substitution of lecithin for raw pancreas in the diet of the depancreatized dog. Am. J. Physiol. 1930;93:657. [Google Scholar]
- 16.Lee S, Keiter JE, Rosen H, et al. Influence of blood supply on regeneration of liver transplants. Surg. Forum. 1969;20:369. [PubMed] [Google Scholar]
- 17.Leffert HL. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture—VII, hormonal control of DNA synthesis and its possible significance to the problem of liver regeneration. J. Cell. Biol. 1974;62:792. doi: 10.1083/jcb.62.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idem. Hepatocellular growth control. In: Newberne P, Butler W, editors. Workshop on Rat Liver Pathology. New York: American Lesevier Publishing Co.; in press. [Google Scholar]
- 19.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J. Cell. Biol. 1968;37:27. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchioro TL, Porter KA, Brown BI, et al. The effect of partial portacaval transposition on the canine liver. Surgery. 1967;61:723. [PMC free article] [PubMed] [Google Scholar]
- 21.Marchioro TL, Porter KA, Dickinson TC, et al. Physiologic requirements for auxiliary liver homotransplantation. Surg. Gynecol. Obstet. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 22.Marchioro TL, Porter KA, Illingworth BI, et al. The specific influence of non-hepatic splanchnic venous blood flow upon the liver. Surg. Forum. 1965;16:280. [PMC free article] [PubMed] [Google Scholar]
- 23.Max MH, Price JB, Jr, Takeshige K, Voorhees AB., Jr The role of factors of portal origin in modifying hepatic regeneration. J. Surg. Res. 1972;12:120. doi: 10.1016/0022-4804(72)90131-x. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa K, Yamada T, Honjo I. Role of insulin as a portal factor in maintaining the viability of liver. Ann. Surg. 1974;180:716. doi: 10.1097/00000658-197411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popper H. Implications of portal hepatotrophic factors in hepatology. Gastroenterology. 1973;66:1227. [PubMed] [Google Scholar]
- 26.Price JB, Jr, Takeshige K, Max MH, Voorhees AB., Jr Glucagon as the portal factor modifying hepatic regeneration. Surgery. 1972;72:74. [PubMed] [Google Scholar]
- 27.Ranson JHC, Eng K, Becker FF, et al. Auxiliary transplantation of liver, duodenum, and pancreas. Surg. Forum. 1974;25:389. [PubMed] [Google Scholar]
- 28.Schneider WC, Greco AE. Incorporation of pyrimidine deoxyribonucleosides into liver lipids and other components. Biochem. Biophys. Acta. 1971;228:610. doi: 10.1016/0005-2787(71)90725-8. [DOI] [PubMed] [Google Scholar]
- 29.Seifter S, Seymour D, Novic B, Muntwyler E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950;25:191. [PubMed] [Google Scholar]
- 30.Sgro J-C, Charters AC, Chandler JB, et al. Site of origin of the hepatotrophic portal blood factor involved in liver regeneration. Surg. Forum. 1973;24:377. [PubMed] [Google Scholar]
- 31.Sigel B. The extracellular regulation of liver regeneration. J. Surg. Res. 1969;9:387. doi: 10.1016/0022-4804(69)90084-5. [DOI] [PubMed] [Google Scholar]
- 32.Starzl TE. Judd lecture—portal hepatotrophic factors; a century of controversy. In: Najarian JS, Delaney JP, editors. Surgery of the Liver, Biliary Tract and Pancreas. New York: Intercontinental Medical Book Corp.; 1974. pp. 495–524. [Google Scholar]
- 33.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg. Gynecol. Obstet. 1973;137:179. [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl TE, Lee IY, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg. Gynecol. Obstet. 1975;140:381. [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Marchioro TL, Rowlands DT, Jr, et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann. Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Putnam CW. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Co.; 1969. pp. 475–491. [Google Scholar]
- 37.Sunderman FW, Sunderman FW., Jr . A method for lipid phosphorus in serum. In: Sunderman FW, Sunderman FW Jr, editors. Lipids and Steroid Hormones in Clinical Medicine. Philadelphia: J. B. Lippincott Co.; 1960. p. 28. [Google Scholar]
- 38.Weinbren K, Washington S, Dowling F. The proliferative response after partial hepatectomy in hypophysectomized rats bearing portacaval anastomoses. Br. J. Exp. Pathol. 1973;54:429. [PMC free article] [PubMed] [Google Scholar]
- 39.Younger LR, King J, Steiner DF. Hepatic proliferative response to insulin in severe alloxan diabetes. Cancer Res. 1966;26:1408. [PubMed] [Google Scholar]
- 40.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953;41:486. [PubMed] [Google Scholar]