Abstract
Telomerase is a ribonucleoprotein (RNP) complex that synthesizes telomere repeats in tissue progenitor cells and cancer cells. Active human telomerase consists of at least three principal subunits, including the telomerase reverse transcriptase (TERT), the telomerase RNA (TERC), and dyskerin. Here, we identify a holoenzyme subunit, TCAB1 (telomerase Cajal body protein1), uniquely enriched in Cajal bodies, nuclear sites of RNP processing important for telomerase function. TCAB1 associates with active telomerase enzyme, with established telomerase components, and with small Cajal body RNAs involved in modifying splicing RNAs. Depletion of TCAB1 using RNA interference prevents TERC from associating with Cajal bodies, disrupts telomerase-telomere association and abrogates telomere synthesis by telomerase. Thus, TCAB1 controls telomerase trafficking and is required for telomere synthesis in human cancer cells.
TERT and TERC comprise the minimal catalytic core of the telomerase enzyme (1), whereas dyskerin is an RNA binding protein that recognizes the H/ACA sequence motif shared by TERC and two groups of non-coding RNAs involved in RNA modification - small Cajal body (sca) RNAs and small nucleolar (sno) RNAs (2, 3). Dyskerin functions in part to support telomerase RNP biogenesis and TERC stability (4, 5). TERT, TERC and dyskerin are all components of active telomerase (6), and mutations in any of these genes can cause the human stem cell disorder dyskeratosis congenita (7). Other potential components of active telomerase include three evolutionarily conserved dyskerin-associated proteins, NOP10, NHP2 and GAR1 (8-10), and EST1A, a homologue of the yeast telomerase protein Est1p (11, 12). However, the size of active human telomerase, estimated in the 0.65 to 2 MDa range (6, 13, 14), suggests the existence of additional components. We reasoned that other dyskerin-associated proteins may be telomerase components, and we therefore sought to purify dyskerin complexes.
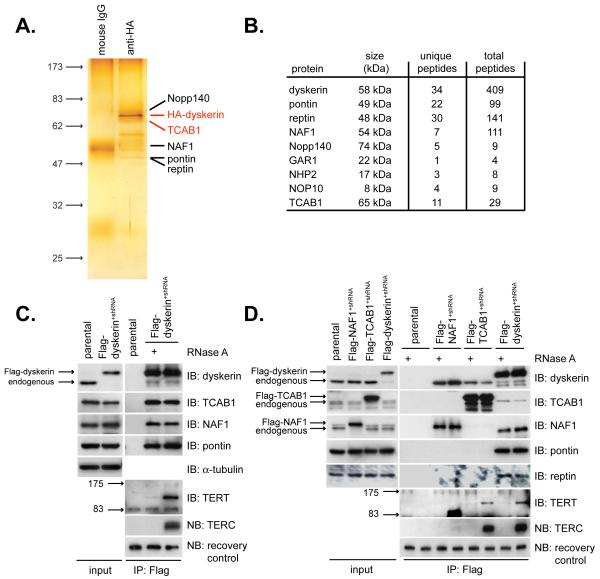
To study dyskerin, we expressed tagged dyskerin protein at endogenous levels in the absence of competing endogenous protein (Fig. S1) and isolated dyskerin complexes using a dual affinity chromatography strategy. Purified dyskerin complexes were analyzed by SDS-PAGE and nanoLC-MS/MS for identification of co-purifying proteins (Fig. 1A,B). Dense peptide coverage was obtained for dyskerin and for the dyskerin-associated ATPases pontin and reptin (14). Each of the evolutionarily conserved dyskerin-binding proteins NHP2, NOP10 and GAR1 was detected, as were the dyskerin-associated proteins Nopp140 and NAF1, a nucleoplasmic factor required for assembly of H/ACA RNPs including telomerase (15)(Fig. 1B). In addition, this approach identified the WD40 repeat protein WDR79, a protein that had not been previously implicated in dyskerin or telomerase function.
Fig. 1.
Identification of TCAB1 as a dyskerin- and telomerase-interacting protein.
(A) Dual affinity-purified dyskerin complexes fractionated by SDS-PAGE and silver stained. (B) Unique and total peptides corresponding to dyskerin-associated proteins identified by nanoLC MS/MS (C) Flag-dyskerin interactions with endogenous TCAB1 and telomerase components and (D) Flag-TCAB1 and Flag-NAF1 interactions with endogenous telomerase components. Immunoprecipitation-western blot using extracts from Flag-dyskerin+shRNA cells, Flag-TCAB1+shRNA cells or Flag-NAF1+shRNA cells. IB, immunoblot. NB, northern blot. parental, HeLa cells. +, treatment of extracts with RNase A during immunoprecipitation. Recovery control, exogenous RNA spiked into samples after IP to control for differential RNA recovery.
We further characterized WDR79, hereafter referred to as TCAB1 (Fig. 1B, S2). Endogenous TCAB1 was specifically bound to Flag-dyskerin immunoprecipitated from Flag-dyskerin+shRNA HeLa cells, as were endogenous pontin, NAF1, TERT and TERC (Fig. 1C). Interactions between TERT and dyskerin were disrupted by RNase A treatment of the extract, which degraded TERC. In contrast, dyskerin binding to TCAB1, NAF1 and pontin were not RNase A-sensitive, indicating that these associations occur through protein-protein contacts (Fig. 1C). Reciprocal immunoprecipitation of Flag-TCAB1 from Flag-TCAB1+shRNA HeLa cells showed that Flag-TCAB1 not only associates with endogenous dyskerin, but also with TERT and TERC, the catalytic core of telomerase (Fig. 1D). Like the TERT-dyskerin association, TERT binding to Flag-TCAB1 was RNase A-sensitive, suggesting that the interaction of TCAB1 with telomerase is dependent on TERC (Fig. 1D). Thus, TCAB1 interacts specifically with dyskerin, TERT and TERC, all three known components of active telomerase.
Although TCAB1 associated with TERT, TERC and dyskerin, it did not interact with the assembly factors NAF1, pontin or reptin (Fig. 1D), suggesting that TCAB1 may be a component of the enzymatically active telomerase complex rather than a pre-telomerase complex. To test this hypothesis, we asked to what extent telomerase activity in cell extracts was associated with overexpressed TCAB1. Flag-tagged TCAB1, dyskerin and NAF1 were depleted from extracts by immunoprecipitation (Fig. 2A, lanes 14-21). Flag-TCAB1 and Flag-dyskerin immunoprecipitates were associated with high telomerase activity (Fig. 2A, lanes 12 and 13). Moreover, purification of either Flag-TCAB1 or Flag-dyskerin depleted these extracts of telomerase activity, indicating that both Flag-TCAB1 and Flag-dyskerin were associated with nearly all telomerase activity in the extract. In contrast, Flag-NAF1 bound only a small percentage of telomerase and did not deplete telomerase activity from cell extracts (Fig. 2A, lanes 1-8). Furthermore, immunoprecipitation of either Flag-TCAB1 or Flag-dyskerin depleted TERC but not unrelated RNAs such as U1 from cell extracts (Fig. 2A, lanes 14-21). Flag-TCAB1 and Flag-dyskerin were associated with similar amounts of endogenous TERT and TERC, further suggesting that TCAB1 and dyskerin reside together in the same active telomerase complexes (Fig 2A, lanes 24-25).
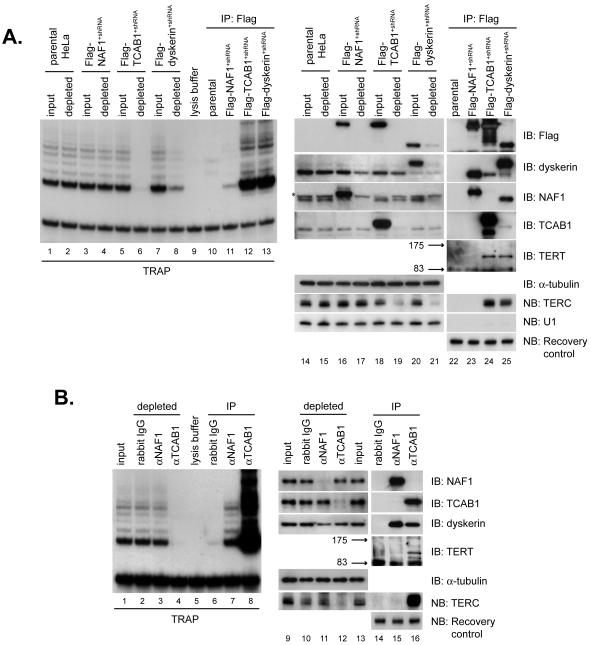
Fig. 2.
TCAB1 is a component of active telomerase.
(A) Flag-TCAB1 or Flag-dyskerin immunoprecipitation (IP) quantitatively co-depletes telomerase activity and TERC from extracts. TRAP assays were performed on extracts before and after IP (lanes 1-8) and on each immunoprecipitated complex (lanes 10-13). Depletion of tagged proteins and depletion of endogenous TERC on extracts before and after IP (lanes 14-21). IP of tagged proteins and associated telomerase components (lanes 22-25). U1 splicing RNA, negative control. (B) IP of endogenous TCAB1 co-depletes telomerase activity and TERC. TRAP assays were performed on extracts before and after each IP (lanes 1-4) and on the IP (lanes 6-8). Depletion of endogenous NAF1 or endogenous TCAB1 and depletion of TERC (lanes 9-13). Association of NAF1 and TCAB1 with telomerase components (lanes 14-16). Recovery control, exogenous RNA spiked into samples after IP to control for differential RNA recovery.
To understand the composition of endogenous telomerase, immunoprecipitations were performed using anti-TCAB1 and anti-NAF1 antibodies, each of which efficiently depleted its cognate protein from cell extracts (Fig. 2B, lanes 9-13). High telomerase activity was associated with TCAB1 immunoprecipitates and anti-TCAB1 antibodies quantitatively depleted telomerase activity from cell extracts (Fig. 2B, lanes 1-8, S4). In contrast, anti-NAF1 antibodies pulled down only a small percentage of telomerase activity. Assessing the TCAB1 immunoprecipitates for telomerase components revealed that TCAB1 interacts with endogenous dyskerin, TERT and TERC (Fig. 2B, lanes 14-16). TCAB1 immunoprecipitation effectively depleted cell extracts of TERC by northern blot (Fig. 2B, lanes 9-13, S4, S5). We conclude that TCAB1, like dyskerin, associates stably with a vast majority of active telomerase and TERC, and therefore is a component of a human telomerase holoenzyme.
By immunofluorescence, stably overexpressed HA-tagged TCAB1 was distributed weakly throughout the nucleoplasm, but was strongly enriched within nuclear foci resembling Cajal bodies, sites of RNP processing shown to contain dyskerin and TERC (16-18). Immunofluorescence showed that the HA-TCAB1 foci overlapped with p80-coilin and were therefore Cajal bodies (Fig. 3A). Endogenous TCAB1 was also highly enriched in Cajal bodies, with smaller amounts distributed in the nucleoplasm (Fig. 3B). Whereas dyskerin accumulates in both Cajal bodies and nucleoli, the Cajal body-restricted localization of TCAB1 suggested that TCAB1 may function specifically with telomerase and with other non-coding RNAs found in Cajal bodies.
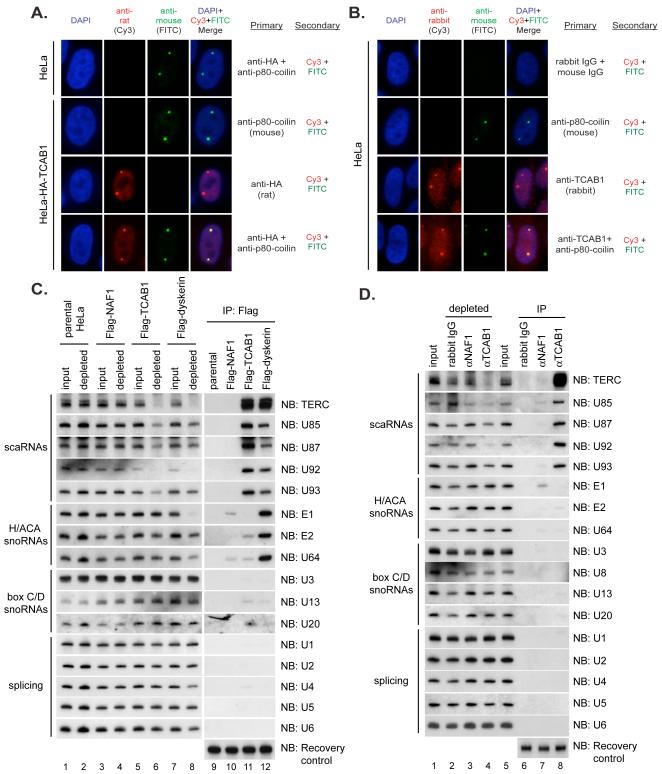
Fig. 3.
TCAB1 is enriched in Cajal bodies and associates specifically with small Cajal body RNAs (scaRNAs).
(A) HA-TCAB1 colocalizes with the Cajal body marker p80-coilin. Immunofluorescence (IF) using anti-HA (rat) and anti-p80-coilin (mouse) antibodies on fixed HeLa cells stably overexpressing HA-TCAB1. (B) Co-localization of endogenous TCAB1 with p80-coilin. IF using anti-TCAB1 (rabbit) and anti-p80-coilin antibodies (mouse) on fixed HeLa cells. (C) Flag-TCAB1 associates specifically with scaRNAs by immunoprecipitation-northern blot. Cell extract from parental HeLa cells, Flag-NAF1+shRNA cells, Flag-TCAB1+shRNA cells or Flag-dyskerin+shRNA cells was incubated with Flag antibody beads. RNA was isolated from extracts before (input) and after (depleted) antibody treatment. Two μg total RNA was fractionated by urea-PAGE (lanes 1-8), representing 2% of the input. RNAs from IPs, lanes 9-12 (D) Endogenous TCAB1 associates specifically with scaRNAs. NAF1 antibodies, TCAB1 antibodies or IgG control were used in assays at the same scale as in (C). RNAs from input and depleted extracts, lanes 1-5. RNAs from IPs, lanes 6-8. Recovery control, exogenous RNA spiked into samples after IP to control for differential RNA recovery.
Immunoprecipitates of Flag-TCAB1 (Fig. 3C) and endogenous TCAB1 (Fig. 3D) were assayed for scaRNAs and representatives of other classes of nuclear non-coding RNAs by northern blot. Both overexpressed TCAB1 and endogenous TCAB1 specifically bound H/ACA scaRNAs, but associated with neither snoRNAs nor splicing RNAs. In contrast, Flag-dyskerin specifically bound both scaRNAs and H/ACA snoRNAs, while NAF1 did not associate stably with any non-coding RNAs studied. For each H/ACA scaRNA tested, a significant proportion was depleted from the extract by immunoprecipitation of TCAB1 (Fig. 3C, lanes 5-6; 3D, lanes 1-5). Thus, TCAB1 specifically binds scaRNAs, which share a CAB box sequence that controls Cajal body localization, and provides a potential mechanism to explain how scaRNAs, including TERC, are retained in Cajal bodies.
TCAB1 depletion using retroviral encoded shRNAs reduced neither telomerase activity nor TERC levels, in contrast to dyskerin depletion, which markedly diminished TERC and telomerase activity (Fig. 4A). Thus, TCAB1 may be required in vivo for a step after assembly of a catalytically competent telomerase complex. To determine the role of TCAB1 in Cajal body localization of telomerase, TERC localization was measured by RNA fluorescence in situ hybridization (FISH) in TCAB1-depleted HeLa cells. FISH revealed that TCAB1 knockdown significantly reduced the percentage of cells in which TERC was found in Cajal bodies (Fig. 4B,C) without affecting overall TERC RNA levels (Fig. 4A). Cajal bodies have been directly implicated in the delivery of TERC to telomeres during S phase (19-21). Therefore, we also examined the effect of TCAB1 knockdown on the localization of TERC to telomeres, using FISH for TERC and TRF2 immunofluorescence. Depletion of TCAB1 significantly reduced the presence of TERC at telomeres during S phase (Fig. 4B,C). Thus, TCAB1 is required for TERC localization in Cajal bodies and for delivery of TERC to telomeres during S phase.
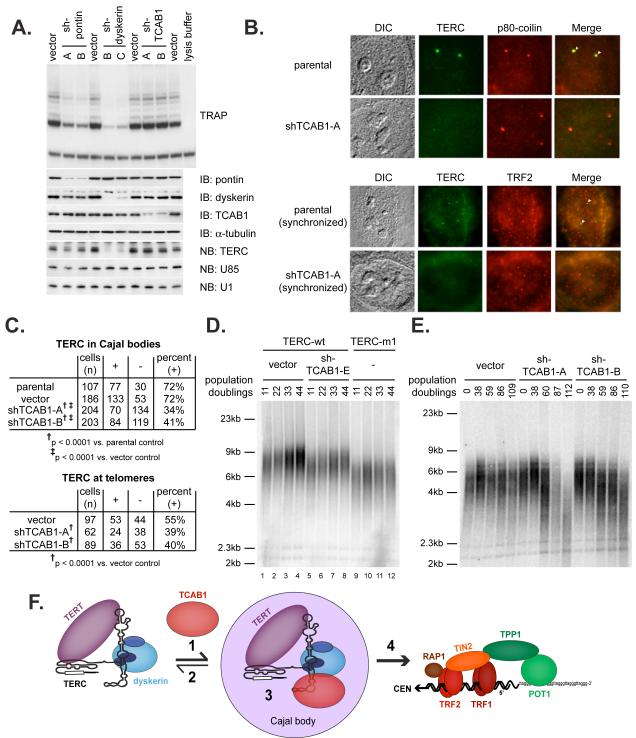
Fig. 4.
TCAB1 is essential for TERC localization to Cajal bodies and for telomere synthesis by telomerase.
(A) HeLa cells were transduced with retroviruses expressing independent shRNA sequences targeting the indicated proteins or with empty vector control. Telomerase activity was measured by TRAP assay. (B) TERC colocalization with p80-coilin was determined by RNA FISH for TERC (green) and immunofluorescence for p80-coilin (red)(top). Cells synchronized in S phase were assayed for TERC by RNA FISH (green) and for telomeres with TRF2 antibodies (red) to assess trafficking of TERC to telomeres (bottom). (C) Quantification of data in (B). Top: cells in which TERC colocalized with p80-coilin (+) versus cells in which TERC was not detected in Cajal bodies (-), p<0.0001 by Fisher’s exact test. Bottom: cells in which TERC colocalized with telomeres (+) versus cells in which TERC was not detected at telomeres (-), p<0.0001 by Fisher’s exact test. (D) Telomere lengths were measured by TRF Southern blot in HTC75 cells overexpressing wild-type TERC (lanes 1-8) or mutant TERC-m1 (lanes 9-12). Cells overexpressing wild-type TERC were transduced with shRNA retroviruses targeting TCAB1 or with empty vector. (E) Effect of TCAB1 depletion on endogenous telomerase was assessed by TRF Southern blot in parental HTC75 cells transduced with empty vector or retroviruses expressing independent TCAB1 shRNAs. Cells were counted at each passage and population doublings are indicated (F) Model for TCAB1 function in the telomere synthesis pathway.
To determine whether TCAB1 is required for telomere addition by telomerase, we first induced telomere elongation through TERC overexpression in HTC75 fibrosarcoma cells. Overexpression of wildtype TERC lengthened telomeres with cell passage, but telomere lengthening was inhibited in cells overexpressing a CAB box-mutant TERC that fails to accumulate in Cajal bodies, as previously shown (19). Depletion of TCAB1 in cells overexpressing wildtype TERC significantly inhibited telomere elongation, mimicking the effect of the CAB box mutation (Fig. 4D, S6). To determine whether TCAB1 is required for telomere synthesis by endogenous telomerase, we assayed telomere lengths with serial passage in HTC75 cells transduced with TCAB1 shRNAs or with empty vector. Both shRNAs targeting TCAB1 led to progressive telomere shortening compared to the empty vector control (Fig. 4E, S7), indicating that TCAB1 is required for telomere synthesis in human cancer cells.
Our data identify TCAB1 as a Cajal body-enriched protein that associates with TERC and other scaRNAs, and explains how TERC, and perhaps other scaRNAs, localize in Cajal bodies. TCAB1 functions as a telomerase holoenzyme component in the telomere synthesis pathway at a step after assembly of a minimal telomerase complex containing TERT, TERC and dyskerin. NAF1 and the ATPases pontin and reptin are required for assembly of the catalytically competent complex (14, 15). In contrast, TCAB1 stably associates with active telomerase enzyme and directs it through Cajal bodies to telomeres. In this manner, TCAB1 may act as a Cajal body-targeting or retention factor, may facilitate additional assembly steps of the enzyme in Cajal bodies, and/or may facilitate translocation of telomerase to telomeres (Fig. 4F). Once at telomeres, the activity of the telomerase holoenzyme may be enhanced by interaction with the telomere binding proteins TPP1 and POT1 (22, 23), as well as other factors that remain to be discovered.
Supplementary Material
Acknowledgments
Supported by Medical Scientist Training Program Grant GM07365 (A.S.V), NCI CA104676 (M.P.T., R.M.T) with a Supplement to Promote Diversity in Health-Related Research (E.B.A.). This project has been funded in part with federal funds from the NCI under Contract NO1-CO-12400 (T.D.V). This work was supported by NCI CA111691 and CA125453 (S.E.A.) and by a Leukemia and Lymphoma Society SCOR grant (S.E.A).
REFERENCES
- 1.Collins K. Nat Rev Mol Cell Biol. 2006;7:484. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier UT. Chromosoma. 2005;114:1. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matera AG, Terns RM, Terns MP. Nat Rev Mol Cell Biol. 2007;8:209. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JR, Wood E, Collins K. Nature. 1999;402:551. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10756. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SB, et al. Science. 2007;315:1850. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 7.Kirwan M, Dokal I. Clin. Genet. 2008;73:103. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 8.Fu D, Collins K. Mol. Cell. 2007;28:773. doi: 10.1016/j.molcel.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pogacic V, Dragon F, Filipowicz W. Mol. Cell. Biol. 2000;20:9028. doi: 10.1128/mcb.20.23.9028-9040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dragon F, Pogacic V, Filipowicz W. Mol. Cell. Biol. 2000;20:3037. doi: 10.1128/mcb.20.9.3037-3048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snow BE, et al. Curr. Biol. 2003;13:698. doi: 10.1016/s0960-9822(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 12.Reichenbach P, et al. Curr. Biol. 2003;13:568. doi: 10.1016/s0960-9822(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 13.Schnapp G, Rodi HP, Rettig WJ, Schnapp A, Damm K. Nucleic Acids Res. 1998;26:3311. doi: 10.1093/nar/26.13.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Cell. 2008;132:945. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoareau-Aveilla C, Bonoli M, Caizergues-Ferrer M, Henry Y. RNA. 2006;12:832. doi: 10.1261/rna.2344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier UT, Blobel G. J. Cell Biol. 1994;127:1505. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jady BE, Bertrand E, Kiss T. J. Cell Biol. 2004;164:647. doi: 10.1083/jcb.200310138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Tomlinson RL, Lukowiak AA, Terns RM, Terns MP. Mol. Biol. Cell. 2004;15:81. doi: 10.1091/mbc.E03-07-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristofari G, et al. Mol. Cell. 2007;27:882. doi: 10.1016/j.molcel.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Jady BE, Richard P, Bertrand E, Kiss T. Mol. Biol. Cell. 2006;17:944. doi: 10.1091/mbc.E05-09-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Mol. Biol. Cell. 2006;17:955. doi: 10.1091/mbc.E05-09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, et al. Nature. 2007;445:506. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 23.Xin H, et al. Nature. 2007;445:559. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.