Abstract
The comorbidity between attention deficit hyperactivity disorder (ADHD) and substance abuse and dependence disorders may have multiple causes and consequences. In this review, we will describe neurobehavioral, genetic and animal model studies that support the notion that a common, genetically-determined failure of response inhibition function is an endophenotype for both disorders. Through an impairment in the ability to cognitively control pre-potent behaviors, subjects can exhibit a collection of ADHD-like traits (impulsivity and hyperactivity), as well as susceptibility for the initiation of drug-taking and its ultimate progression to an inflexible, uncontrollable form. At the neural level, dysfunction within circuitry that includes the ventrolateral frontal and cingulate cortices, as well as in associated basal ganglia zones, contributes to a common pattern of behavioral impairment, explaining aspects of comorbidity. Animal models of substance abuse/dependence and ADHD that exhibit deficits in response inhibition have substantiated the role of this endophenotype in both disorders and their co-morbidity and should provide a testing ground for interventions targeting it. New directions for research that will further explore this hypothesis and begin to reveal the underlying biological mechanisms will be proposed.
Keywords: Cognitive control, norepinephrine, dopamine, Impulsivity, prefrontal cortex, animal models
1. Co-Morbidity, ADHD and Substance Abuse Disorders
As the evidence for the genetic influences on risk for major mental disorders becomes increasingly clear, the search for quantitative trait indicators of specific risk-associated alleles is underway. The sought-after traits are often conceptualized as behavioral, cognitive or biological phenotypes that are “simpler” than multi-dimensional psychiatric disorders and that are, consequently, determined by a simpler set of genetic mechanisms than is the complex disorder phenotype. As a result, these traits are potentially more fruitful candidates for gene discovery efforts (Bearden and Freimer 2006; Gottesman and Gould 2003). In addition, these “endophenotypes” would ideally be reliable indicators of the brain dysfunction that represents risk for the disorders, thereby enabling our understanding of the neural systems affected by genetic risk factors. While putative endophenotypes for a particular psychiatric disorder need not be directly related to functional outcome in affected individuals, they often are (Green 1996), making them of increased interest for treatment research.
While attempts to identify these traits have largely been spurred by their potential for gene discovery, the identification of endophenotypes has enabled our understanding of the increasingly apparent co-morbidity between disorders, as well. As will be discussed at depth in the sections that follow, certain quantitative traits may be stable indicators of neural systems-level dysfunction that represents risk for multiple, partially-overlapping disorders at once, providing a mechanistic explanation for the apparent co-morbidity. This review argues that this is true for two clearly associated psychiatric disorders: substance abuse/dependence and attention deficit/hyperactivity disorder (ADHD).
Taken individually, these two psychiatric conditions can have a profound impact on the long-term functional capacities of an individual. As both of these syndromes often appear first prior to full adulthood, their consequences can be pervasive and life-long, by altering the trajectory of an individual during the period of most significant biological, behavioral and social development. What is particularly concerning is that these two syndromes co-occur in individuals at a rate much higher than that predicted by chance alone; in other words, individuals are often “co-morbid”, in that they suffer from symptoms of both disorders at once (Gordon et al. 2004; Wilens 2004).
ADHD is an early-onset disorder that is behaviorally identified by impulsive actions (trouble waiting turns and disruptive behavior), hyperactivity (fidgety) and inattention (difficulty focusing, distractibility, poor organizational skills and forgetfulness) (American-Psychiatric-Association 1994). Other cognitive impairments (poor working memory, executive function impairments) are common in ADHD patients and may represent key indicators of genetic liability to ADHD (Aron and Poldrack 2005; Barkley 1997; Castellanos and Tannock 2002; Doyle et al. 2005; Nigg et al. 2004), although they are not diagnostic features. The consequences of the constellation of symptoms and deficits are, oftentimes, academic and/or occupational problems.
On the other hand, substance abuse involves the maladaptive, non-medical use of illicit substances that leads to functional impairments and/or to undue hazards or risks (“driving under the influence”); additional physiological criteria (namely, the presence of tolerance or withdrawal) differentiate substance dependence from abuse (American-Psychiatric-Association 1994). Conceptually, the critical factors that identify “drug addiction” include pre-occupation with drug-seeking and —taking, despite knowledge of the associated risks and despite repeated attempts to stop and the functional consequences (socially, occupationally and otherwise) of drug use.
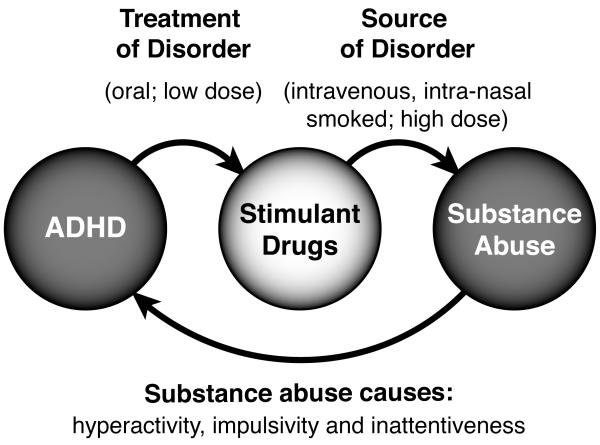
Though the phenotypic manifestations of ADHD and substance dependence seem considerably divergent (e.g., impulsive speech and scholastic impairment versus drug tolerance and compulsive drug-seeking), substance abuse/dependence disorders and ADHD are syndromes with long-recognized relationships (Figure 1). First of all, the addiction phenotype is a pervasive form of impulsive drug-seeking and —taking behavior (Jentsch and Taylor 1999; Robinson and Berridge 2003), indicating that it shares phenotypic aspects with ADHD. Second, ADHD, when left untreated, is a significant risk factor for the later development of substance abuse or dependence (ADHD→substance abuse/dependence). Third, individuals diagnosed with substance abuse/dependence often exhibit symptomatic features of ADHD, and studies from animal models (see Section 4.2) indicate that these effects could plausibly be caused by drug intake (substance abuse→ADHD-like traits). Finally, ADHD is often effectively treated with the very drugs that support addictive behavior (e.g., stimulants), albeit at doses and via routes of administration that do not typically produce the required brain levels of the drug that support reward and abuse (Volkow and Swanson 2003).
Figure 1.
The relationships between ADHD and stimulant abuse are multi-fold. First, impulsive traits and behaviors in ADHD are often treated with low, orally-delivered doses of stimulant drugs. At higher doses, or after alternative routes of administration (e.g., intra-nasal administration of crushed tablets), euphoria and reward can develop. Stimulant drugs, under these circumstances, can support substance abuse behavior, which ultimately leads to further molecular adaptations in the brain that exacerbate the deficits of response inhibition that the stimulant drugs were prescribed to treat, in the first place.
The many aspects of the relationship between ADHD and substance abuse have led to various neural and psychological explanations that attempt to explain their apparent co-morbidity; many proposals have focused on a common pattern of dysfunction within circuitry associated with motivational and cognitive processes. For example, several investigators have proposed that ADHD can be characterized as a state of aberrant motivational and reinforcement processes, enhanced sensitivity to delay, inability to properly allocate and sustain attention, poor motor planning and impaired executive functions, each resulting from hypofunctional dopamine system (Sagvolden et al. 2005). Correspondingly, some descriptive theories describe substance abuse/dependence disorders as resulting from drug-induced dysregulation of systems involved in reward and motivation (Koob and Le Moal 1997; Berridge and Robinson 2000) and executive control over reward-related behavior (Jentsch and Taylor 1999). Therefore, while there are clearly components of ADHD not shared with substance abuse problems (and vice versa), there is considerable empirical support for a partially overlapping set of problems with reward sensitivity, motivation and cognitive control.
The sources of the co-morbidity between ADHD and substance abuse/dependence are presently unknown. The thesis of this article is that a common pattern of neural systems-level dysfunction, stemming from partially-overlapping genetic and neurochemical determinants, leads to a characteristic impairment of cognitive control over behavior; this failure of control leads to the signs and symptoms of ADHD and represents a vulnerability factor for the progression to substance abuse. In the next section, the presence of common impulsivity-related phenotypes in these two disorders will be discussed, and the systems-level dysfunction that relates to these phenotypes in the two conditions will be reviewed.
2. Cognitive Control Deficits in ADHD and Addiction
Cognitive control is a rubric that incorporates multiple cognitive mechanisms that organisms use to effectively enable adaptive behavior (e.g., planning, updating representations of goals, of attentional biases or of action; inhibitory control of pre-potent responses; (Miller and Cohen 2001)); individually, multiple components of cognitive control are impaired in a variety of psychiatric disorders, including ADHD and substance abuse/dependence, and these effects will be described in more detail here.
A lack of cognitive control over behavior is likely to directly underpin the impulsive behavior that is a cardinal feature of ADHD and substance abuse/dependence. In naturalistic settings, children with ADHD exhibit difficulty suppressing situationally-inappropriate behavior, and it is the consequences of these control failures that lead to the disruptive behaviors that characterize the disorder and contribute to the scholastic deficits. Furthermore, addictive disorders critically include a failure of effective, voluntary control over reward-directed behavior (Jentsch and Taylor 1999).
Because multiple dimensions of psychological dysfunction are found in these disorders, it does not immediately follow that the impulsive behavioral patterns in ADHD and substance abuse stem from an empirically-measurable lack of cognitive control; direct investigation of this possibility, using laboratory measures, is necessary. A variety of tests have been used to evaluate the ability to stop or change responses in these clinical populations, and together, these different experimental tasks have provided convergent evidence for a substantial deficit in response inhibition in ADHD and substance abuse. One dimension of cognitive control that has been the focus of considerable study is response inhibition. Response inhibition encompasses the ability to adaptively suppress behavior when environmental contingencies demand it. Laboratory measures of response inhibition normally involve the establishment of a response that becomes the default (“pre-potent”) response. Each of the empirically-validated tasks (see Table 1) incorporates situational requirements for inhibiting, stopping, delaying or modifying the pre-potent response. The nature of control exerted over the pre-potent response can vary widely (e.g. stopping an ongoing behavior, inhibiting responding when reward is no longer delivered, eliminating inappropriate or excessive responding, or inhibiting responses to a previously rewarded stimulus); additionally, the cognitive processes response control may vary, as well (e.g. motor inhibition, ability to bridge a delay, ability to shift responding to new stimuli, ability to weigh magnitude of reward effectively). Importantly, the measures are not suggested to index a singular, invariant construct, but they do all appear to be procedures that allow one to quantify aspects of neural systems dysfunction that occurs in ADHD and substance abuse/dependence.
Table 1.
Common tasks used for the assessment of cognitive control of behavior, including response inhibition
| Task | Pre-Potent Response | Adaptive Response | Consequence of Failure |
|---|---|---|---|
| Reversal Learning | Rapid responding to a conditioned stimulus, despite a change in conditional rules |
Inhibiting previously trained response in the favor of new conditional rules |
Loss of reward |
| Delay Discounting | Emission of responses that produce immediate feedback (reward) |
Maximizing reward by tolerating delay period | Smaller reward magnitude |
| Choice Reaction Time | Anticipatory responses, made during inter-trial intervals |
Withholding responses until contextually appropriate |
Delay in reward availability |
| Extinction | Continuing to emit conditional responses despite outcome omission |
Cessation of responding | Excessive, inappropriate behavior |
| Stop Signal Reaction Time | Rapid instrumental responding | Inhibition of an initiated sequence when infrequent stop cues presented |
Loss of reward |
| Go-No Go | Rapid instrumental responding | Withholding an response sequence in response to infrequent no-go trials |
Loss of reward |
Using laboratory tests of response inhibition and cognitive control, patients with ADHD have been shown to exhibit difficulties with withholding, stopping or changing an established response (Aron et al. 2003; Casey et al. 1997; Chamberlain et al. 2007; Clark et al. 2007; Itami and Uno 2002; Kuntsi et al. 2005; Schachar et al. 1995), and these effects are generally associated with physiological dysfunction within a network that includes inferior frontal cortical regions (Aron and Poldrack 2005; Casey et al. 1997; Clark et al. 2007; Itami and Uno 2002). With respect to addiction, deficits in response inhibition have been found in patients diagnosed with drug abuse and dependence, particularly those that abuse stimulants (Ersche et al. 2008; Fillmore and Rush 2002; 2006; Monterosso et al. 2005). As in ADHD, anatomical and physiological dysfunction within ventrolateral frontal cortex is associated with these deficits (London et al. 2004; Thompson et al. 2004), suggesting that a common neural adaptation within this part of the brain likely mediates the deficiencies in inhibitory control function in both disorders.
Neuroimaging studies have revealed a common pattern of brain dysfunction that extends beyond anatomical and functional abnormalities in ventrolateral prefrontal cortex. Molecular imaging studies have demonstrated that both ADHD and substance-dependent individuals have altered dopaminergic function and production, particularly in striatal regions (Ernst et al. 1998; Heinz et al. 2005; Ludolph et al. 2008; Martinez et al. 2007). Beyond these dopaminergic alterations, both disorders show a consistent pattern of lower gray matter density in prefrontal regions (Matochik et al. 2003; Semrud-Clikeman et al. 2006) and striatal areas (Castellanos et al. 1994; Jacobsen et al. 2001). Functional imaging has also demonstrated hypoactivation of the anterior cingulate when performing a response inhibition task in both ADHD and substance dependent individuals (Hester and Garavan 2004; Leland et al. 2008). Together, these data indicate a shared neural dysfunction and dysregulation that may contribute to the shared behavioral deficits, indicative of a parallel neuronal pathway. Although the relationship between these functional, anatomical and biochemical alterations is not well understood, the fact that similarities exist beyond the behavioral output substantiates the claim that a shared neural pathway exists between ADHD and substance abuse.
Amongst neuropsychiatric disorders, ADHD is somewhat unique in that the available pharmacological treatments, while not without side effects, are remarkably effective at controlling symptomatology (Arnsten 2006b; Biederman et al. 2006). Additionally, methylphenidate and atomoxetine lessen deficits of inhibitory control in ADHD when given at therapeutically effective doses (Aron et al. 2003; Chamberlain et al. 2007; Scheres et al. 2003; Tannock et al. 1989). Pre-clinical studies have provided clues as to the neurotransmitter systems that mediate its effects on response inhibition measures. Methylphenidate and amphetamine (both of which non-selectively increase monoamine output in brain) have mixed effects on response inhibition tasks in rats that vary depending upon dose, route of administration and procedure (Cardinal et al. 2000; Cole and Robbins 1987; Eagle et al. 2007; Richards et al. 1999). On the other hand, atomoxetine, a selective norepinephrine reuptake inhibitor, appears to consistently improve response inhibition in a variety of pre-clinical measures (Robinson et al. 2007; Seu et al. 2008). Selective norepinephrine transporter inhibitors differ from traditional stimulant treatments in that stimulants increase extracellular dopamine levels in the striatum, while atomoxetine does not (Bymaster et al. 2002). Notably, however, both stimulants and atomoxetine both increase dopamine and norepinephrine in prefrontal regions (Berridge et al. 2006; Bymaster et al. 2002). These similar prefrontal monoaminergic effects of different drug classes suggest a critical role of prefrontal dopamine and norepinephrine in regulating and recruiting the neural systems that are believed to be critical for inhibiting behavior.
What is less clear, however, is the relationship between early, effective treatment of symptoms of ADHD and later risk for substance abuse disorders. Pre-clinical studies have suggested that developmentally-early treatment with stimulant medications used to treat ADHD reduce sensitivity to addictive drugs in adulthood (Andersen et al. 2002; Mague et al. 2005), but see also (Brandon et al. 2003). These results are seemingly congruent with recent prospective studies indicating that early methylphenidate treatment in ADHD does not increase, and may actually decrease, risk for substance use disorders (Biederman et al. 2008; Mannuzza et al. 2008). Because of the potential reductions in substance abuse risk associated with effective treatment of ADHD, it is of empirical interest to further determine whether clinical improvement in treated patients tracks along with effective modulation of the deficits in response inhibition (Nigg et al. 2006); if that were the case, it would strengthen support for the idea that the relationship between ADHD and substance abuse depends upon an aberrant response inhibition mechanism.
Virtually nothing is known about the pharmacological regulation of response inhibition deficits in substance abuse. Theoretically, effective treatments for ADHD may be expected to accomplish this effect; however, the abuse liability of methylphenidate and amphetamine make them practically problematic in the treatment of substance abuse. On the other hand, atomoxetine lacks abuse liability (Michelson et al. 2003), but its effects on response inhibition have not yet been evaluated and/or reported in substance abuse. If effective at modulating these deficits in substance-dependent persons, atomoxetine would represent an important tool in determining whether agents that lessen response inhibition deficits could be expected to enable voluntary cessation of drug intake, as is hypothesized by earlier models (Jentsch and Taylor 1999).
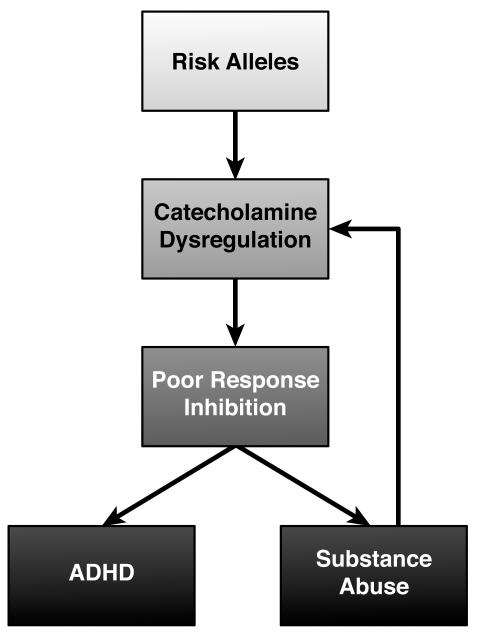
What are the implications of this common pattern of neurophysiological and response inhibition impairments in the two disorders? Some of the potential relationships are exhibited in Figure 2. First, and most simplistically, the co-morbidity of the two disorders may explain the concordance of neural and behavioral phenotypes. Second, ADHD, and its corresponding neural and behavioral traits, is a risk factor for addiction, leading to an over-representation of ADHD-like phenotypes in stimulant-dependent subjects. Third, chronic intake of psychostimulants may directly change the function of the orbitofrontal cortex in a manner that mimics neural and behavioral aspects of ADHD. Studies in animal models are particularly helpful in disambiguating the directionality of these sorts of associations present in clinical populations.
Figure 2.
This hypothetical model graphically represents the concept that poor response inhibition, mediated by genetically-determined alterations in forebrain catecholamine transmission, is a risk factor for both ADHD and substance abuse. The abuse of illicit substances is also an environmental risk factor that produces further adaptations in catecholamine systems, leading to increasing impairments in response inhibition.
3. Genetic Mechanisms and Comorbidity
The proposal that genetically-determined variation in a set of endophenotypes can represent a risk factor for multiple co-morbid psychiatric disorders is best supported by the identification of a common set of candidate risk genes that 1) are in association with the presumed endophenotype and 2) are, consequently, in association with both disorders. Of relevance to this review, there are genetic mechanisms that satisfy these criteria for ADHD and substance abuse/dependence, supporting the general view that both are determined by a common endophenotype of poor response inhibition.
An important, albeit limited effect-size, gene that has received attention for its relationship to ADHD and substance abuse is DAT1 (Faraone et al. 2005), the gene that encodes the dopamine transporter. A variable number tandem repeat polymorphism in the 3′-untranslated region of the gene has been repeatedly associated with ADHD (Cornish et al. 2005; Gill et al. 1997; Lee et al. 2007b; Roman et al. 2001; Swanson et al. 2000) and, more recently, with cocaine abuse and inflexible smoking behavior (Guindalini et al. 2006; Stapleton et al. 2007). Notably, because a significant association between the DAT1 risk genotype and response inhibition remains after controlling for degree of ADHD symptoms (Cornish et al. 2005), the proximal effect of the risk genotype may lie at the level of response inhibition, not disorder severity. It therefore appears to be the case that certain genes influencing risk for both ADHD and substance abuse behavior may do so by mediating a common endophenotype of poor response inhibition, consistent with the assertion that this behavioral mechanism lies at the heart of the comorbidity.
While further studies of this type are needed, it remains possible that other candidate genes (e.g., DRD4; COMT) influencing these disorders may exert their effects through a common set of cognitive control-related constructs. The completion of largescale phenomics studies in genotyped individuals (Bilder 2008) may further expose the critical relationships required to support this hypothesis. Additionally, animal models that allow a more direct analysis of neurochemical and behavioral mechanisms critical to these disorders can functionally define these associations and explain their mechanistic foundation. The following section deals with the current state of knowledge regarding animal models relevant to ADHD and addiction that illuminate the potential role for response inhibition in both disorder phenotypes.
4. Inhibitory Control Deficits in Animal Models of ADHD or Substance Abuse
4.1. Animal models of ADHD
Traditionally, animal models of ADHD have focused on hyperactive phenotypes, but as the dimensionality of the clinical syndrome has become more apparent, animals models have become, correspondingly, more sophisticated. This section deals with the evidence that deficits of response inhibition are a common feature of animal models of ADHD, including those with etiological validity, suggesting that poor cognitive control over behavior is a key mechanism associated with the biological determinants of the disorder.
Amongst the variety of commonly-used animal models for ADHD, several exhibit phenotypes of relevance to response inhibition which stem, in some cases, from determinants thought to influence risk for ADHD in humans. Importantly, many of these models, along with exhibiting classic ADHD-like symptoms such as inattentiveness and hyperactivity, show deficits in response inhibition. These models also help to specify the neurochemical mechanisms directly underlying the phenotypes, and as will be discussed below, many directly point towards disturbances in catecholamine transmitter systems in the expression of impulsive behavior.
Spontaneously hypertensive rats (SHR) are a selectively-bred line originating from normotensive Wistar-Kyoto (WKY) rats and display many characteristics that resemble ADHD symptoms, such as hyperactivity and inattention (Sagvolden 2000). Though the specific neurochemical abnormalities mediating behavioral phenotypes in the SHR model are unknown, these animals do show an increase in norepinephrine release in the prefrontal cortex that depends upon autoreceptor sub-sensitivity (Russell et al. 2000; Russell and Wiggins 2000); alterations in prefrontal cortical noradrenergic tone have been implicated in the cognitive control deficits in ADHD (Arnsten 2006a). Moreover, genetic variation in the gene encoding the dopamine transporter may play a role in the hyperactive and impulsive behavior of SHR rats (Mill et al. 2005), an effect notable due to the fact that variation in the dopamine transporter gene associates with ADHD in humans (Faraone et al. 2005).
Evidence for a deficit in response inhibition in SHR rats is derived from multiple sources. For example, as compared with WKY rats, SHRs exhibit persistence of responding on an extinction (reward omission) schedule, suggesting that they have difficulty inhibiting a conditioned response when task rules change (Johansen and Sagvolden 2004). In delayed reward tasks (that measure the ability of subjects to choose larger, delayed rewards rather than immediate, suboptimal rewards), SHR rats also exhibit abnormal preferences for small, immediate rewards, as well as more burst responding and a steeper delay-discounting curve (Johansen et al. 2005). Delay gradients that are characterized by a tendency to trade larger, delayed rewards for smaller, immediate rewards, are consistent with poor response inhibition capability.
Dopamine transporter knock-out and knock-down (DAT KO and DAT KD) mice have also been investigated as a putative animal model for ADHD. DAT KOs exhibit hyperactivity, especially in response to a novel environment, and this hyperactivity is reduced by systemic administration of stimulant drugs (Gainetdinov et al. 1999), paralleling their effects in ADHD patients. Deficits in response inhibition in DAT KOs are evident in the persistent responding observed during extinction of food-reinforced operant responses (Hironaka et al. 2004), as well as in increased perseverative responding to previously-visited arms in a win-shift task (Gainetdinov et al. 1999). DAT KD mice, which have 10% of wild-type DAT levels and lack several of the developmental problems characteristic of DAT KOs (Zhuang et al. 2001), exhibit normal instrumental conditioning but poorly extinguish a previously established instrumental response (Yin et al. 2006). These results indicate that animals with impaired dopamine clearance exhibit deficits in adaptive inhibition of previously established responses, independent of their associative learning capacity.
Coloboma mice carry a semidominant deletion mutation within the SNAP-25 gene which results in a 50% reduction of SNAP-25 expression, a protein critical for calcium-triggered exocytosis (Hess et al. 1996). Poor response inhibition in the coloboma mouse is indicated by delay discounting tasks: coloboma mice exhibit reduced tolerance for delay compared to controls, indicating an impulsive tendency to trade quality of reward for immediacy (Bruno et al. 2007). This model, and its associated phenotypes, are potentially of substantial relevance for ADHD because variation in the gene encoding SNAP-25 associates with ADHD in humans (Faraone et al. 2005). Moreover, this model links clearly with the SHR and DAT genetic models because it similarly exhibits dysregulation of basal ganglia catecholamine transmission (Jones et al. 2001), a neurochemical abnormality that likely contribute directly to its behavioral phenotypes.
Irrespective of the genetic determinants of each of these models, they each support impairments of response inhibition as being a key aspect to ADHD that likely relates directly to abnormal catecholamine function in the prefrontal cortex and/or basal ganglia. Ultimately, these models may be useful in further disentangling the specific nature of the relationship between genetic and neurochemical mechanisms that influence this phenotypic dimension of ADHD.
While the above models have focused on putative models of the disease-associated pathophysiology, the actions of effective treatments for ADHD in otherwise “normal” animals also supports the relevance of behavioral measures of response inhibition. Using a number of tasks, it is now clear that effective ADHD treatments, including methylphenidate, amphetamine and atomoxetine, reduce impulsive behavior, probably by enhancing response inhibition, in rodents (Blondeau and Dellu-Hagedorn 2007; Eagle et al. 2007; Navarra et al. 2007; Rivalan et al. 2007; Robinson et al. 2007; Seu et al. 2008). Therefore, beyond an animal model of ADHD pathophysiology, these important behavioral features of the disorder can be used to generate phenotype models that nevertheless are helpful in understanding the neuropharmacology of effective treatment. Further exploitation of this approach is of critical importance.
4.2. Animal models of substance abuse
Animal models of substance abuse/dependence demonstrate that deficits of response inhibition can be a direct consequence of long-term exposure to illicit substances of abuse and are beginning to help us understand the mechanistic basis of these deficits. Conversely, response inhibition tasks have also been used to predict future self-administration behavior in animals, suggesting that naturally-occurring poor response inhibition can alter the susceptibility to drug-taking. The following section will explore these relationships in more detail.
As discussed above, reversal learning tasks measure the ability to adaptively change a conditioned response when task circumstances change, and this procedure has been used to detect response inhibition deficits in models of drug dependence. Mice treated chronically with an escalating dose exposure to ethanol exhibit no difficulty with learning a simple spatial discrimination task but require more trials to reach criterion when spatial contingencies are serially reversed (Borde and Beracochea 1999). Similarly, studies in rats have revealed that chronic exposure to cocaine or phencyclidine persistently impairs serial reversal learning performance (Abdul-Monim et al. 2006; Calu et al. 2007; Jentsch and Taylor 2001; Schoenbaum et al. 2004). Of clearest relevance to humans, non-human primates trained on a 3-choice object discrimination task and then chronically exposed to cocaine have no problems with acquiring an object discrimination, but when stimulus-reward contingencies are reversed, they exhibit a selective increase in perseverative errors, an effect that persists for at least one month after the last exposure to cocaine (Jentsch et al. 2002).
Similar studies utilizing delayed reward tasks are in agreement with the above results. The degree of response inhibition deficit is measured by calculating an indifference point, which is defined as the delay at which animals choose either option with equal frequency. As compared to saline-treated controls, rats chronically exposed to cocaine exhibit poor delay discounting (Paine et al. 2003; Simon et al. 2007). Because treatment groups were counterbalanced for locomotor activity, this finding suggests a drug-induced deficit in response inhibition that cannot be explained by non-specific differences in activity. Similarly, rats exposed to cocaine prior to an odor discrimination task show elevated discounting and sensitivity to the independent modulation of reward delay and magnitude (Roesch et al. 2007). Steeper delay discounting gradients have also been found after chronic treatment with methamphetamine (Richards et al. 1999), and nicotine increases preference for immediate, small rewards in a dose-independent manner when chronically administered (Dallery and Locey 2005). Collectively, these studies indicate that chronic exposure (voluntary or otherwise) in rats produces deficits in reversal learning and performance in a delayed reward task in a manner that suggests that the abuse of drugs leads directly to response inhibition deficits. Although this helps to clarify one facet of the relationship between addiction and response inhibition deficits, it does not immediately preclude the possibility that naturally-occurring impairments in this domain of function (including those attributable to ADHD) are risk factors for the development of impulsive drug taking.
In fact, a variety of studies have found that several dimensions of behavior related to response inhibition are predictive of future patterns of drug self-administration behavior in rodents. This was initially investigated by assessing locomotor response to a novel environment and dividing rats into groups the exhibited greater “impulsive” exploratory behavior (high responders) versus those that exhibited little (low responders). High-responding rats more readily acquire amphetamine self-administration (Piazza et al. 1989), though, interestingly, this self-administration behavior is not predictive of response inhibition abilities as measured by a fixed consecutive number task (Bardo et al. 2006). On the other hand, self-administration behavior can also be predicted based upon performance in delay discounting tasks. Animals exhibiting the steepest delay discounting effects are more susceptible to behavioral sensitization to repeated ethanol administration (Mitchell et al. 2006) and self-administer more ethanol and cocaine than their low impulsivity counterparts (Perry et al. 2005; Poulos et al. 1995). Furthermore, impulsive delay discounting performance predicts resistance to extinction and susceptibility to conditioned-cue reinstatement in rats self-administering nicotine (Diergaarde et al. 2008). Finally, anticipatory responding in a choice reaction-time task, which also measures a simple form of inhibitory control, predicts overall amounts of cocaine taking in rats (Dalley et al. 2007), as well as acquisition of nicotine self-administration behavior (Diergaarde et al. 2008).
4.3. Comorbidity in Animal Models
Together, these studies clearly support the conclusion that impulsive patterns of responding, probably related to poor cognitive control over behavior, is predictive of drug-taking liability. In that sense, animal models have provided explicit support for the plausibility of two theories relating ADHD-like behavior to addiction (impulsivity causes addiction; addiction causes impulsivity). Animal models can illuminate aspects of addiction and ADHD, as well as the processes that may contribute to their comorbidity. As described above, examination of results garnered from animal models of addiction and ADHD reveals similarity in several phenotypic domains related to response inhibition, including low delay discounting indifference points, resistance to extinction, and perseverative responding.
Additionally, these models can further exhibit direct evidence for “comorbidity”, although this issue has not been examined as extensively. For example, the SHR rat model of ADHD has been shown consume more ethanol than both their normotensive counterparts and ethanol-preferring Lewis rats (Da Silva et al. 2004; Da Silva et al. 2005; Khanna et al. 1990). It is possible that the common endophenotype of poor response inhibition is a major contributor to these overlapping phenotypic characteristics, providing an explanation for high rates of comorbidity in humans. Nevertheless, more work directly investigating whether animal models exhibiting response inhibition deficits display addiction-like and ADHD-like behaviors that are mutually predictive will assist in determining how response inhibition plays a role in comorbidity, as well as expose the shared neural mechanisms that underlie it.
5. Future Directions for Research
Additional research is required in order to further define the relationships between ADHD and substance abuse, to explore the role for response inhibition deficits as a factor moderating this relationship and to reveal the neural mechanisms of direct relevance to the phenotypic association between these phenomena. Statistical genetic methods can be used to precisely map the inter-twin correlations in ADHD, substance abuse and response inhibition phenotypes. If our hypothesis is correct, ADHD-like, substance abuse-related and response inhibition phenotypes should correlate across twins (i.e., that poor response inhibition in one twin is correlated with ADHD or substance abuse in the other twin); such a result would directly support a shared genetic determination of the multiple phenotypic dimensions.
Neuroimaging studies are needed in order to specify the molecular mechanisms that co-vary with individual variation in response inhibition and to determine whether these biological phenotypes will ultimately be useful as quantitative estimates of genetic liability for ADHD and/or substance abuse or dependence. For example, it is known that drug-dependent individuals show lower D2 availability (Volkow et al. 2001), and cocaine self-administration in non-human primates decreases D2 receptor availability (Nader et al. 2006). For example, a recent study in rats has suggested that low ventral striatal D2/D3 receptor availability is a predictor of disinhibited responding and susceptibility for drug taking (Dalley et al. 2007); remarkably, low nucleus accumbens D2/D3 receptor availability predicts poor ability to cease an on-going response, as measured by the stop signal reaction time task, in humans (London et al. 2007). Notably, recent pharmacological studies further directly tie low D2/D3 receptor function to response inhibition, as measured by reversal learning, in monkeys (Lee et al. 2007a). Therefore, it appears that alterations in D2 receptor levels may be linked to response inhibition in a manner that confers risk to substance abuse/dependence, while chronic exposure to drugs of abuse simultaneously encourages disinhibited responding via similar D2 mechanisms. Further investigation of modifications in monoamine signaling in brain in human and animal subjects may expose additional mechanisms of relevance to the association between response inhibition and disorder phenotypes.
Finally, investigating the extent to which naturally-occurring variation in impulsivity is a risk factor for ADHD- and addiction-like traits, more work should focus on exploring the consequences of this natural variation in animal models, rather than focusing on altering the physiology of subjects to make them useful for research purposes. Recent studies in both rats, monkeys and humans have shown that natural variation in impulsive responding is predictive of a range of cognitive control-related mechanisms, including working memory maintenance and updating (Cools et al. 2007; Dellu-Hagedorn 2006; James et al. 2007). Animal models may be particularly useful in understanding the genetic and neurochemical determinants of poor response inhibition and its consequences; for instance, variation in the DRD4 gene associates with impulsive behavior and associated cognitive deficits (James et al. 2007), precisely as it may do in humans (Lynn et al. 2005). The identification of animal models with both genetic and phenotypic variation nearly identical to that in humans suggests that there are new tools for understanding the genomic determination of complex behavioral phenotypes in humans.
Finally, a new frontier in biological research on drug abuse and dependence must include concepts of susceptibility and risk for the disorder. As the behavioral and genetic factors that influence liability to impulsive drug-taking are identified, these factors can be incorporated into prevention strategies aimed at prolonging the onset and lessening the impact of drug use on young people. Our understanding of response inhibition-related phenotypes will likely be crucial in this endeavor.
Acknowledgements
Preparation of this review was supported, in part, by USPHS grants P50-MH077248, P20-DA022539 and RL1-MH083270.
References Cited
- Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169:263–73. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association . Diagnostic and statistical manual of mental disorders. 4th edn American Psychiatric Association, American Psychiatric Association; 1994. [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–4. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006a;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006b;31:2376–83. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Cain ME, Bylica KE. Effect of amphetamine on response inhibition in rats showing high or low response to novelty. Pharmacol Biochem Behav. 2006;85:98–104. doi: 10.1016/j.pbb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet. 2006;22:306–13. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Arnsten AF, Faraone SV, Doyle AE, Spencer TJ, Wilens TE, Weiss MD, Safren SA, Culpepper L. New developments in the treatment of ADHD. J Clin Psychiatry. 2006;67:148–59. doi: 10.4088/jcp.v67n0121. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens T, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: A naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Phenomics: Building scaffolds for biological hypotheses in the post-genomic era. Biol Psychiatry. 2008;63:439–40. doi: 10.1016/j.biopsych.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry. 2007;61:1340–50. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Borde N, Beracochea DJ. Effects of diazepam or chronic alcohol treatment on spatial reversal learning in mice. Pharmacol Biochem Behav. 1999;62:719–25. doi: 10.1016/s0091-3057(98)00211-1. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–44. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Bruno KJ, Freet CS, Twining RC, Egami K, Grigson PS, Hess EJ. Abnormal latent inhibition and impulsivity in coloboma mice, a model of ADHD. Neurobiol Dis. 2007;25:206–16. doi: 10.1016/j.nbd.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–8. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Vaituzis AC, Kaysen D, Hamburger SD, Rapoport JL. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:1791–6. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine Improved Response Inhibition in Adults with Attention Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, Sahakian BJ. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61:1395–401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–66. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–14. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–98. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Da Silva GE, Ramos A, Takahashi RN. Comparison of voluntary ethanol intake by two pairs of rat lines used as genetic models of anxiety. Braz J Med Biol Res. 2004;37:1511–7. doi: 10.1590/s0100-879x2004001000010. [DOI] [PubMed] [Google Scholar]
- Da Silva GE, Vendruscolo LF, Takahashi RN. Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci. 2005;77:693–706. doi: 10.1016/j.lfs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2:10. doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, Pennington BF, Peart J, Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46:774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–7. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Tulak F, Troncale J. Prevalence and characteristics of adolescents patients with co-occurring ADHD and substance dependence. J Addict Dis. 2004;23:31–40. doi: 10.1300/J069v23n04_03. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Guindalini C, Howard M, Haddley K, Laranjeira R, Collier D, Ammar N, Craig I, O’Gara C, Bubb VJ, Greenwood T, Kelsoe J, Asherson P, Murray RM, Castelo A, Quinn JP, Vallada H, Breen G. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc Natl Acad Sci U S A. 2006;103:4552–7. doi: 10.1073/pnas.0504789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–20. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Collins KA, Wilson MA. Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation. J Neurosci. 1996;16:3104–11. doi: 10.1523/JNEUROSCI.16-09-03104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka N, Ikeda K, Sora I, Uhl GR, Niki H. Food-reinforced operant behavior in dopamine transporter knockout mice: enhanced resistance to extinction. Ann N Y Acad Sci. 2004;1025:140–5. doi: 10.1196/annals.1316.018. [DOI] [PubMed] [Google Scholar]
- Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–7. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–9. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27:14358–64. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T. Response disinhibition may be explained as an extinction deficit in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2004;149:183–96. doi: 10.1016/s0166-4328(03)00229-8. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T, Kvande G. Effects of delayed reinforcers on the behavior of an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2005;162:47–61. doi: 10.1016/j.bbr.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Jones MD, Williams ME, Hess EJ. Abnormal presynaptic catecholamine regulation in a hyperactive SNAP-25-deficient mouse mutant. Pharmacol Biochem Behav. 2001;68:669–76. doi: 10.1016/s0091-3057(01)00481-6. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Chau AK, Sharma H. Initial sensitivity, acute tolerance and alcohol consumption in four inbred strains of rats. Psychopharmacology (Berl) 1990;101:390–5. doi: 10.1007/BF02244059. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Borger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007a;32:2125–34. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Waldman I, Van Hulle CA, Rathouz P, Pelham WE, Loney J, Cook EH. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. Am J Med Genet B Neuropsychiatr Genet. 2007b;144:310–7. doi: 10.1002/ajmg.b.30447. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Miller DA, Paulus MP. Anterior cingulate cortex and benefit of predictive cueing on response inhibition in stimulant dependent individuals. Biol Psychiatry. 2008;63:184–90. doi: 10.1016/j.biopsych.2007.04.031. [DOI] [PubMed] [Google Scholar]
- London ED, Lee B, Tabibnia G, Monterosso J, Farahi J, Aron AR, Poldrack RA, Mandelkern MA, Dahlborn M, Bokarius A, Brody AL, Bilder RM. Association of ventral striatal dopamine D2/D3 receptors with stop-signal reaction time. Soc. Neurosci. Abstr.: 910.13. 2007 [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, Reske SN, Fegert JM, Mottaghy FM. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–27. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Lynn DE, Lubke G, Yang M, McCracken JT, McGough JJ, Ishii J, Loo SK, Nelson SF, Smalley SL. Temperament and character profiles and the dopamine D4 receptor gene in ADHD. Am J Psychiatry. 2005;162:906–13. doi: 10.1176/appi.ajp.162.5.906. [DOI] [PubMed] [Google Scholar]
- Mague SD, Andersen SL, Carlezon WA. Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–5. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–9. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17:539–55. doi: 10.1016/j.nic.2007.07.004. x. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, Kelsey D, Wernicke J, Dietrich A, Milton D. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–20. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1 in SHR and WKY rat strains. Behav Brain Funct. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–37. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–6. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2007 doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113:614–25. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, FItzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–9. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Gregoire S, Dellu-Hagedorn F. Reduction of impulsivity with amphetamine in an appetitive fixed consecutive number schedule with cue for optimal performance in rats. Psychopharmacology (Berl) 2007;192:171–82. doi: 10.1007/s00213-007-0702-6. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman T, Schmitz M, Polanczyk G, Eizirik M, Rohde LA, Hutz MH. Attention-deficit hyperactivity disorder: a study of association with both the dopamine transporter gene and the dopamine D4 receptor gene. Am J Med Genet. 2001;105:471–8. doi: 10.1002/ajmg.1408. [DOI] [PubMed] [Google Scholar]
- Russell V, Allie S, Wiggins T. Increased noradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2000;117:69–74. doi: 10.1016/s0166-4328(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Russell VA, Wiggins TM. Increased glutamate-stimulated norepinephrine release from prefrontal cortex slices of spontaneously hypertensive rats. Metab Brain Dis. 2000;15:297–304. doi: 10.1023/a:1011175225512. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1995;23:411–37. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–20. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Pliszka SR, Lancaster J, Liotti M. Volumetric MRI differences in treatment-naive vs chronically treated children with ADHD. Neurology. 2006;67:1023–7. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1250-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Sutherland G, O’Gara C. Association between dopamine transporter genotypes and smoking cessation: A meta-analysis. Addict Biol. 2007;12:221–6. doi: 10.1111/j.1369-1600.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, Posner M. Dopamine genes and ADHD. Neurosci Biobehav Rev. 2000;24:21–5. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–91. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85:283–8. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]