Abstract
Caspase (Casp) family proteases regulate not only lymphocyte apoptosis but also lymphocyte activation and development. In this study, we show that Casp6 regulates B cell activation and differentiation into plasma cells by modifying cell cycle entry. B cells from Casp6 knockout (Casp6 KO) mice examined ex vivo have more cells in G1 than wild-type B cells, and mitogen-induced G1 entry of Casp6 KO B cells is much faster than that of wild-type B cells. Even so, S phase entry and proliferation are not increased in Casp6 KO B cells. Rather than proliferating, activated Casp6 KO B cells preferentially differentiate into syndecan-1+ plasma cells and produce Abs. In Casp6 KO mice compared with WT mice, serum levels of IgG1, IgG2a, and IgG2b are increased and Ag-specific Ab responses are also enhanced along with increased percentages of syndecan-1+ plasma cells. Casp6 may regulate both B cell activation and differentiation by modifying requirements for G0 B cells to enter G1.
The caspase (Casp)4 family of the cysteine aspartate-specific proteases is best known for its involvement in apoptosis through cleavage of specific substrates. However, the 14 Casps identified in mammals are involved not only in cell death, including apoptosis and autophagy, but also in the maturation of proinflammatory cytokines and lymphocyte development (1-3). Casp family members can be divided into three groups: activators of cytokines, initiator Casps, and effector Casps. The cytokine activators (Casp1, 4, 5, 11, and 12) trigger the release of inflammatory mediators through the cleavage of precursors of the inflammatory cytokines IL-1β and IL-18 (4). During apoptosis, effector Casps including Casp3, 6, and 7 are usually cleaved and activated by initiator Casps such as Casp2, 8, 9, and 10.
As the executioners in apoptosis, activated effector Casps cleave crucial specific cellular substrates (5, 6). Casp6, an effector Casp, cleaves substrates involved in cell cycle, survival, or development such as SATB1, p27Kip1, Notch1, AP-2α, lamin A, Akt, and 5-lipoxygenase (7-14). Casp6 also may cleave other Casps including initiator Casps (15-17). Recently, mutations in the Casp6 gene (Casp6) were found in human gastric and colorectal carcinomas, suggesting that dysregulation of Casp6 may contribute to the pathogenesis of gastrointestinal cancers (18). In addition, invasive melanomas and metastatic cancers express activated Casp6 and Casp3 and down-regulate the Casp substrate AP-2α, a transcriptional factor required for cell proliferation that suppresses genes controlling terminal differentiation and apoptosis (19, 20). Thus, Casp6 may not only be involved in the development of tumors as a proapoptotic executor, but may also contribute to tumor progression in melanomas.
Some Casps also regulate nonapoptotic functions, including cell proliferation and differentiation (2, 21). Terminal differentiation is characterized by prolonged cell cycle arrest in the G1 phase, where cells become refractory to signals for proliferation (22). Some Casps are involved in terminal differentiation of cells such as keratinocytes and erythroblasts (23, 24). In the immune system, Casp3 activity is detectable in immature dendritic cells and can inhibit dendritic cell maturation, while macrophage differentiation requires both Casp3 and other Casps, including Casp8 (25-27). During the initial stages of human Th 2 cell differentiation, Casp3 is inhibited by IL-4 (28). In terminally differentiated plasma cells, Casp6 expression is also strongly repressed with other apoptosis-associated genes (29).
Casps regulate cell proliferation; Casp8, in particular, may play a critical role in the activation of NF-κB by TLRs or Ag receptors (30-32). In B cells, Casp8 may be required for B cell activation by the TLR agonists, dsRNA, and LPS (33, 34). The effector caspase Casp3 is also involved in B cell activation and cleaves its substrate cyclin-dependent kinase (CDK) inhibitor p21Cip1 during B cell proliferation (35). We previously demonstrated that Casp6 influences B cell activation and that both Casp6 and its substrate SATB1 are cleaved after CD40-induced stimulation of resting human B cells (36). Benzyloxycarbonyl-(Cbz)-Val-Glu-Ile-Asp(Ome)-fluoromethylketone (VEID), a Casp6 selective inhibitor, blocked human B cell proliferation and expression of proteins, which promote cell cycle progression including cyclin D2, cyclin A, and CDK4. Recently, Werz et al. (13) showed that Casp6 cleaves human 5-lipoxygenase, which initiates the synthesis of bioactive leukotrienes from arachidonic acid. This cleavage correlated with the proliferation of the Burkitt lymphoma line BL41, again suggesting Casp6 may have an important role in regulating B cell activation.
Casp6 knockout (KO) mice are grossly normal, breed with Mendelian ratio, and are only slightly protected from anti-Fas/CD95-induced cell death (37). To further analyze the role of Casp6 in B cell biology, we examined B cell activation and differentiation in Casp6 KO mice. We found that G1 cell cycle entry from a quiescent state is accelerated in Casp6 KO B cells. Surprisingly, despite this accelerated G1 entry, neither the S phase entry nor cell death is increased in Casp6 KO B cells. Instead, more Casp6 KO B cells differentiate into syndecan-1+ plasma cells compared with wild-type (WT) B cells. Thus, Casp6 may play a role in balancing B cell proliferation and differentiation at the point of cell cycle entry.
Materials and Methods
Mice
Casp6 KO mice were generated as described previously (37). Mice were backcrossed for >15 generations to C57BL/6 and housed under specific pathogen-free conditions at the University of Washington. All experiments were performed in compliance with the University of Washington Institutional Animal Care and Use Committee.
B cell isolation
After RBC depletion by Gey’s solution, splenic B cells were purified by magnetic depletion using anti-Thy 1.2 and anti-CD11b MACS beads (BD Biosciences) or, after anti-Thy 1.2 bead depletion, the negative fraction was subjected to plastic adherence for 1 h at 37°C to remove monocytes/macrophages. Splenic resting B cells were isolated by negative selection using the EasySep mouse B cell enrichment kit (StemCell Technologies), which includes CD4, CD8, CD11b, CD43, CD49b, Gr-1, TER119 Abs, or by using the 66/70% interface cells from a Percoll gradient after negative selection using Thy1.2 beads (38). The purity of the resting B cell preparations was >90%.
Western blotting
Resting B cells were treated with CpG oligodeoxynucleotide 1826 (1 μg/ml; Coley Pharmaceutical Group), LPS (5 μg/ml; Sigma-Aldrich), or staurosporine (50 nM; Sigma-Aldrich) at the indicated times at 37°C. Samples were harvested and washed with PBS, then lysed with Nonidet P-40 lysis buffer (10 mM Na2HPO4, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, pH 7.2) containing 1 mM Na3VO4, 50 mM NaF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin, and 1 mM PMSF. Cell lysates were subjected to brief sonication on ice. Proteins were quantified using the Bio-Rad protein assay and then 20 μg of cell lysate was applied per lane.
For detection of the cleavage of Casp6, we used a combination of two anti-Casp6 sera (Cell Signaling Ab 9762 and Ab 9761). The Ab 9761 antiserum was made against a region of Casp6 (aa 153-162), which is very close to the 162 aspartic acid cleavage site in the large subunit of cleaved Casp6. The Ab 9762 antiserum was generated against aa 171-190, which is in the propeptide and small subunit of Casp6. Neither the Ab 9761 antiserum nor the 9762 antiserum cross-reacts with other Casps. Using Ab 9762, our laboratory and another found that WT mouse spleen, liver, and brain express Casp6, Casp6+/- tissues express less, and Casp6-/- tissues have no detectable Casp6 (17). The Casp6 KO mice were constructed by replacing the first exon of Casp6, including the atg start codon into the Neo gene (37). Using primers derived from intron 1 or intron 2 for genotyping, we confirmed that exon 1 was completely deleted in the Casp6 KO mice.
An Ab to Casp8 (1G12; Alexis Biochemicals) was made by immunization with the recombinant mouse Casp8 large, catalytic domain p20. The Casp8 Ab recognizes full-length Casp8 (∼55-58 kDa), the apoptosis-induced cleavage fragment of Casp8 (∼20 kDa), and truncated forms of Casp8 (35-51 kDa) (39).
Abs specific for Casp3 (Cell Signaling Abs 9661 and 9662), p38 MAPK (C-20; Santa Cruz Biotechnology), c-myc (Ab 9402; Cell Signaling), p27kip1 (Ab 2552; Cell Signaling), CDK4 (Ab 2906; Cell Signaling), cyclin D2 (M-20; Santa Cruz Biotechnology), cyclin E (M-20; Santa Cruz Biotechnology), CDK2 (M2; Santa Cruz Biotechnology), phosphorylated Rb (pRb) Ser780 (Cell Signaling), pRb Ser807/811 (Cell Signaling), and retinoblastoma protein (Rb) (IF8; Santa Cruz Biotechnology) were purchased.
Flow cytometry
Single-cell suspensions were prepared from mouse spleens. Peritoneal cavity cells were collected by flushing the peritoneal cavity with PBS containing 0.5% FCS. One million cells were stained with the following mAbs: anti-CD16/CD32 (2.4G2), anti-IgD (JA12.5), anti-CD43 (S7), anti-CD8 (Ly-2), anti-B220 (RA3-6B2), anti-CD5 (53-7.3), anti-CD11b (M1/70), anti-IgM (R6-60.2), anti-CD21/35 (CR2/CR1), anti-CD19 (1D3), and syndecan-1 (CD138, 281-2) conjugated with or without FITC, PE, PerCP, allophycocyanin, or biotin (BD Biosciences). Streptavidin-FITC, -PE, and -allophycocyanin were used as second-step reagents for biotinylated Abs. Stained cells were analyzed using an LSR 1 (BD Biosciences), and the data analysis was performed using FlowJo software (Tree Star).
Cell cycle analysis
For analysis of the cell cycle using BrdU, 7-aminoactinomycin D (7AAD) and pyronin Y (PY), purified B cells were stimulated with or without LPS, then BrdU was added for the last 6 h of culture. After harvesting, BrdU-labeled cells were frozen in FBS containing 10% DMSO at -80 °C over-night. For the detection of BrdU and definition of each cell cycle stage, the manufacturer’s protocol for the FITC BrdU flow kit (BD Biosciences) was followed. Briefly, after dissolving frozen cells, cells were fixed again with BD Biosciences Cytofix/Cytoperm buffer, then treated with DNase for 1 h at 37 °C. Cells were stained with FITC-conjugated anti-BrdU Ab for 20 min at room temperature, followed by 7AAD for 10 min, and lastly incubated with PY. Stained cells were analyzed using an LSR 1 with FlowJo software.
Proliferation assays
Resting B cells (1 × 105 cells/well) purified by EasySep were cultured in RPMI 1640 medium (HyClone) supplemented with 10% FCS (Life Technologies), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 2-ME (5 × 10-5 M) in the presence or the absence of 10 μg/ml anti-CD40 mAb 1C10 (40), 1 μg/ml CpG oligodeoxynucleotide 1826, 5 μg/ml anti-CD180 (Rp105) mAb (RP/14; eBioscience), or LPS. The peptide Casp6 inhibitor was purchased from Kamiya Biomedical. After 2 days, cells were pulsed with 0.5 μCi of [3H]thymidine for 8 h. Cells were then harvested onto glass fiber filters with a cell harvester, and radioactivity was measured in a liquid scintillation counter. In some experiments, cells were pretreated for 3 h with VEID at 80 μM or DMSO as a vehicle control.
For CFSE assays, purified B cells (2 × 107cells/ml) were incubated with 5 μM CFSE (Molecular Probes) for 10 min at 37°C, then quenched with RPMI 1640 medium containing 10% FCS and 50 μM 2-ME. CFSE-labeled B cells were washed twice in RPMI 1640 medium containing FCS as above, then seeded into 6-well plates with either medium only, LPS with or without IL-4 (10 ng/ml). After culture for the indicated times, stimulated CFSE-labeled cells were analyzed by FACScan (BD Biosciences) using FlowJo software.
Cell death assay
For measurement of cell viability, cultured cells with various stimuli were stained with MitoTracker ROS red dyes (Molecular Probes). Stained cells were then analyzed by FACScan (BD Biosciences) with CellQuest software (BD Biosciences).
Ig production and plasma cell formation
Purified resting B cells were cultured in 48-well plates (5 × 105 cells/well) for 5 days at 37°C with the indicated stimuli to detect plasma cells (syndecan-1+B220-low), and for 7 days with the indicated stimuli for measurement of Ig production. IL-4 was purchased from R&D Systems. To induce Ab responses to T cell-dependent (TD) Ag in vivo, 10-wk-old mice were immunized by i.p. injection with the indicated dose of alum-precipitated OVA (alum, Pierce and OVA, Sigma-Aldrich) and bled 7 or 14 days after immunization.
Ig levels of harvested supernatants or mouse sera were determined by ELISA. Briefly, ELISA plates were coated with donkey anti-mouse IgM or IgG (The Jackson Laboratory) or OVA (Sigma-Aldrich) and blocked with 1% of BSA. Diluted samples were detected with goat anti-mouse isotype antisera (IgM, IgG1, IgG2b, and IgG3 from The Jackson Laboratory; IgG2a from GeneTex) and donkey anti-goat IgG HRP (The Jackson Laboratory). To control for plate-to-plate variation, serial dilutions of isotype standards (Zymed) were used as relative standards on each plate.
Quantitative real-time PCR
Total RNA was extracted from B cells by RNeasy (Qiagen) and cDNA was prepared by a Cloned AMV First-Strand cDNA synthesis kit (Invitrogen). Quantitative real-time PCR was performed on an Applied Biosystems 7300 machine with a cycle of 50°C, 2 min and 95°C, 10 min for 1 cycle and 95°C, 15 s and 60°C, 1 min for 40 cycles, recording data at 60°C at 1 min and using primers for Blimp-1 (5′-ATGAGTTGAATGGGAGC-3′ and 5′-CAATGCTTGTCTAGTGTC-3′) and Gapdh (5′-TGCACCACCAACTGCTTAG-3′ and 5′-GGATGCAGGGATGATGTTC-3′). Relative quantitation of samples was calculated using the comparative cycle threshold (Ct) method, relative ratio = 2˄(Ct (Gapdh) - Ct(Blimp-1)).
Results
Casp6 is cleaved after B cell activation
To investigate the in vivo effects of Casp6 on B cell function, we obtained Casp6 KO mice. Adult Casp6 KO mice showed no significant differences in the numbers of bone marrow B cell subsets (pre, pro, immature B cells), peritoneal B-1a B cells (CD11b+ B220+CD5+) and B-1b B cells (CD11b+B220+CD5-), or splenic B cell subsets including transitional 1 (IgMhighIgDlowCD21low), transitional 2-follicular precursor (IgMhighIgDhighCD21low), transitional 2-marginal zone precursor (IgMhighIgDhighCD21high), follicular (IgMlowIgDhighCD21low) B cells, and marginal zone (IgMhighIgDlowCD21high) B cell subsets (data not shown). Splenic T cell levels (CD4+ T cells, CD8+ T cells) were also normal in Casp6 KO mice (data not shown).
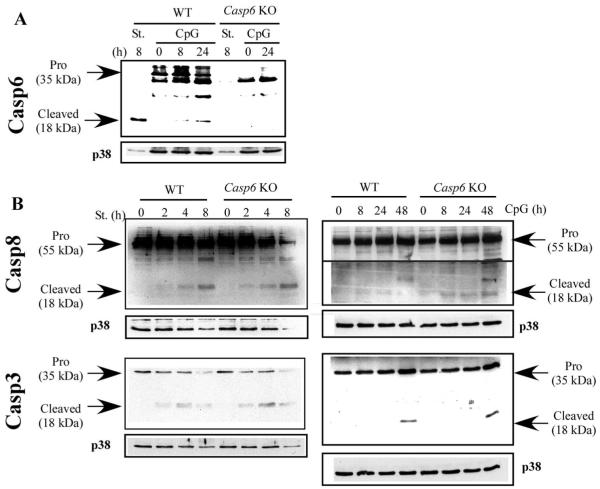
We next tested whether Casp6 is activated in murine B cells in vitro as it is in human B cells (36). Since Casp6 is an effector Casp in apoptosis pathways, we measured Casp6 expression and cleavage in both apoptotic and activated B cells from both WT and Casp6 KO mice. Using appropriate Casp6-specific antisera (see Materials and Methods), we detected the 18-kDa cleaved form of Casp6 in WT B cells treated with staurosporine, an apoptotic stimulus (Fig. 1A). As expected, these bands were not detectable in B cells from Casp6 KO mice. Furthermore, after stimulating B cells with CpG, which leads to strong B cell activation through TLR9, Casp6 cleavage again was detectable in WT B cells but not in Casp6 KO B cells. We also detected Casp6 cleavage in anti-CD40-stimulated WT B cells (data not shown).
FIGURE 1.
Casp6 is cleaved in CpG-stimulated WT B cells. A and B, Small splenic resting B cells from WT or Casp6 KO mice were treated with staurosporine (St.; 50 nM) or CpG (1 μg/ml) for the indicated times. Each cell lysate was subjected to Western blotting with antisera specific for the indicated proteins. Membranes were blotted with Abs to p38 MAPK (p38) as a loading control. The data are representative of three independent experiments.
Both Casp3 and Casp8 are involved in B cell activation and B cell death (35, 36, 41) and both were cleaved after treating B cells with either staurosporine or CpG in both WT and Casp6 KO B cells (Fig. 1B). The kinetics and intensities of cleavage were the same between WT and Casp6 KO B cells, and the kinetics of Casp3 and Casp8 cleavage induced by CpG was slower (detectable at 48 h) than that of Casp6 cleavage (detectable at 8 h). Thus, these Casps may be cleaved in proliferating or dying B cells via a Casp6-independent pathway.
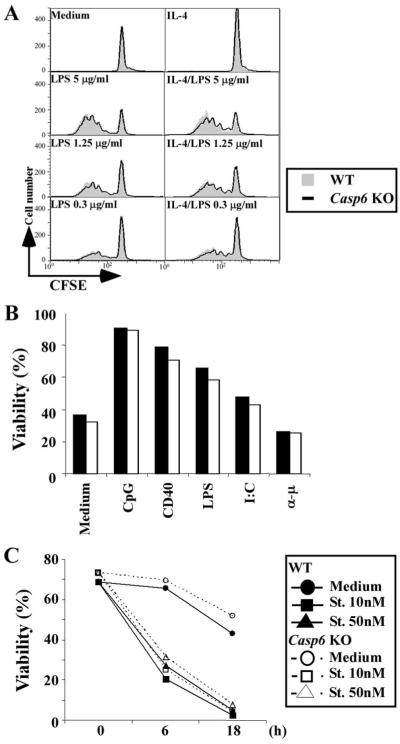
We also compared proliferation induced by various stimuli between WT and Casp6 KO murine B cells, because VEID, a Casp6-selective inhibitor peptide, blocked human B cell proliferation strongly in our previous study (36). To our surprise, Casp6 KO B cells did not show any impaired proliferation induced by graded doses of various stimuli, including anti-CD40, anti-CD180 (Rp105), LPS, or CpG (data not shown). Pretreatment with VEID not only blocked the proliferation of WT B cells but also the proliferation of Casp6 KO B cells (Fig. 2). These data demonstrate that VEID affects something other than Casp6 in mouse B cells and thus require that our previous results based solely on the use of VEID be reevaluated (see Discussion).
FIGURE 2.
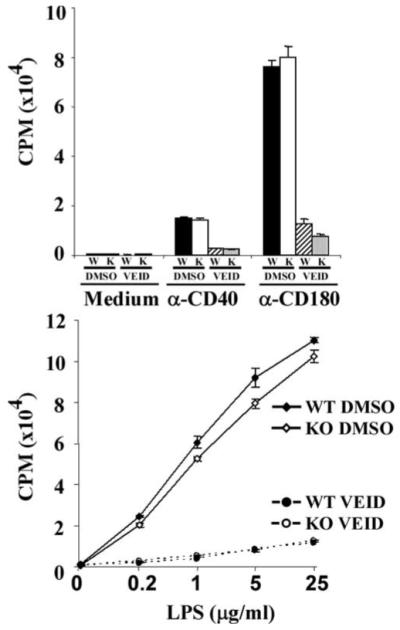
Casp6 inhibitor blocked Casp6 KO B cell proliferation. Splenic B cells were purified by anti-Thy1.2 and anti-CD11b magnetic bead negative selection, then stimulated with anti-CD40 mAb (1 μg/ml), anti-CD180 mAb (5 μg/ml), or LPS (indicated doses) for 48 h. [3H]Thymidine was added for the last 8 h of culture. ■ or ◆, WT DMSO; □ or ◇, Casp6 KO DMSO;  or ●, WT VEID;
or ●, WT VEID;  or ○; KO VEID. The data are representative of five independent experiments.
or ○; KO VEID. The data are representative of five independent experiments.
G1 entry into cell cycle is accelerated in Casp6 KO B cells
To assess the role of Casp6 in B cell activation, we next looked at the cell cycle status of Casp6 KO B cells. To examine each stage of the cell cycle, we used PY, 7AAD, and BrdU. PY is a dye which specifically binds to RNA in the presence of a DNA dye, such as 7AAD or 4′,6-diamidino-2-phenylindole, and can be used as a marker of cells in G1 within a mixed G0-G1 population (BrdU-7AAD-) (42). Using this method, we gated on BrdU- 7AAD- cells (Fig. 3A, top) and measured binding to PY (Fig. 3A, bottom). We found that the number of G1 (BrdU- 7AAD- PY high) splenic B cells from Casp6 KO mice was significantly greater than those in WT mice when examined ex situ (Fig. 3A). In the spleens of adult WT mice, ∼80% of all B cells were in the quiescent G0 stage. In contrast, only ∼60% of splenic B cells in Casp6 KO mice were in G0 and 30% were already in G1without stimulation. Both the percentage and absolute cell number of G1 CD19+ B cells in spleens of Casp6 KO mice were significantly increased compared with WT B cells (Fig. 3B).
FIGURE 3.
G1 entry is accelerated in Casp6 KO B cells. A, Splenic B cells were purified by anti-Thy1.2 and anti-CD11b magnetic bead negative selection. BrdU was added for 6 h. After harvesting the B cells with incorporated BrdU, samples were stained with anti-BrdU, 7AAD, and PY. From the BrdU-7AADlow gate (G0-G1 cells), the population of PYhigh group was determined to be cells in G1. The BrdU+ population was designated as S phase cells and the BrdU-7AADhigh population as G2-M phase cells. B, Percentages or cell numbers are shown for G1 WT B cells (■) and Casp6 KO B cells (□) of CD19+ gated splenic cells without negative selection. C, Small resting B cells were prepared by Percoll gradient centrifugation after negative selection. Large activated cells were collected from the 50/60% interface of the gradient and small resting cells were from the 66/70% interface of the gradient. Small resting B cells were stimulated with LPS and BrdU was added for the last 6 h of culture. Harvested cells were stained with BrdU, PY, and 7AAD. Only live and single cells were gated for analysis. Closed bars (WT B cells) and open bars (Casp6 KO B cells) show percentages of G1 cell cycle stage after LPS stimulation for the indicated times. The data are representative of more than three independent experiments.
Based on this result, we next tested whether G1 entry was accelerated in Casp6 KO B cells. We examined cell cycle status by using resting small B cells obtained from Percoll density gradients. High-density small resting B cells isolated from Percoll density gradients had 99% of G0 cells in both WT and Casp6 KO mice (data not shown). However, 24 h after LPS stimulation (10 μg/ml) of G0 B cells, about twice as many Casp6 KO B cells had entered G1 compared with WT B cells (Fig. 3C, left). Over time, more LPS-stimulated Casp6 KO B cells moved into G1 (Fig. 3C). The differences between WT and Casp6 KO B cells were statistically significant in separate independent experiments (e.g., p < 0.05 at 48 h after 10 μg/ml LPS, WT = 3.1 ± 0.04%, Casp6 KO = 27.3 ± 10.4%). Interestingly, more Casp6 KO B cells than WT B cells were in G1, yet the number of Casp6 KO B cells proliferating, if anything, was less than the number of proliferating WT B cells (Fig. 2). Similar results were also obtained when we used anti-CD40 or CpG for stimulation (data not shown). These results indicate that entry into the G1 stage of the cell cycle was accelerated in Casp6 KO B cells, but that the S phase entry was not similarly affected.
Proliferation and cell death are not enhanced in Casp6 KO B cells
Casp6 KO mice have more G1 B cells ex situ and have B cells that rapidly enter G1 after activation. However, B cell subset numbers in bone marrow and spleen are normal in adult Casp6 KO mice and, after activation, the number of S phase Casp6 KO B cells is not increased compared with WT B cells. To further assess the basis of these phenotypes, we next examined more closely the effect of Casp6 on B cell division. CFSE analysis revealed that cell division of Casp6 KO B cells after LPS with or without IL-4 stimulation was almost the same compared with that of WT B cells (Fig. 4A). Similar results were obtained when B cells were stimulated with graded doses of anti-CD40, anti-IgM, or CpG (data not shown). After activation with various stimuli, there were no differences in cell viability between Casp6 KO and WT B cells as measured using MitoTracker (Fig. 4B), propidium iodide, annexin V, or TUNEL (data not shown). We also looked at B cell death to see whether activated B cells were dying faster in Casp6 KO mice than in WT mice. After treatment with staurosporine, cell viability as measured by MitoTracker was not significantly elevated in Casp6 KO B cells (Fig. 4C). Taken together, these results indicate that the absence of Casp6 leads to an acceleration of B cell entry into the G1 stage of the cell cycle following either LPS, CD40, or CpG stimulation, but does not result in increased cell proliferation or increased susceptibility to cell death.
FIGURE 4.
B cell proliferation and cell death are not increased in Casp6 KO mice. A, Resting B cells were stained with CFSE, then stimulated with or without LPS (1 μg/ml) or LPS plus IL-4 (10 ng/ml) for 48 h. Cell division was measured on FL-1 using a FACScan. Gray areas show WT responses and thick lines show Casp6 KO responses. B and C, Purified resting B cells were treated with medium, CpG (1 μg/ml), CD40 (10 μg/ml), LPS (1 μg/ml), poly(I:C) (100 μg/ml), or anti-μ (10 μg/ml) for 48 h (B) or with staurosporine (St.; 10-50 nM) for 6 or 18 h (C). Cells were stained with MitoTracker ROS Red and analyzed with FACScan and CellQuest software. Percent viability is indicated for WT mice (closed bars or closed symbols) and Casp6 KO mice (open bars and open symbols). The data are representative of three independent experiments.
Activated Casp6 KO B cells do not reduce expression of Rb
To further investigate the underlying mechanisms of accelerated G1 entry in Casp6 KO B cells, we examined the expression of several cell cycle-related proteins in activated WT and Casp6 KO B cells, including the retinoblastoma-susceptibility protein Rb. In the mammalian cell cycle, Rb negatively regulates cell proliferation through a restriction point in late G1. After mitogenic stimulation, CDKs phosphorylate Rb, leading to the release of E2F family transcription factors from Rb, thereby allowing subsequent cell cycle progression (43). Rb is known to block apoptosis and is degraded by Casps in response to apoptotic stimuli (44-45), and thus was a good candidate for being directly or indirectly affected by Casp6.
Using Western blotting analysis, we compared the expression of cell cycle-related proteins in WT vs Casp6 KO B cells after activation with LPS (Fig. 5). Rb is phosphorylated on Ser780 by cyclin D-CDK4 in early G1 and on Ser807/811 in late G1 (46). The phosphorylation of Rb on Ser780 and Ser807/811 was significantly up-regulated in LPS-activated Casp6 KO B cells (Fig. 5A). Hyperphosphorylation of Rb in activated Casp6 KO B cells was also seen with different stimuli, including LPS plus IL-4, CD40, or CpG (data not shown). In contrast, the expression levels of total Rb were not very different in activated WT and Casp6 KO B cells. Changes in c-myc, CDK inhibitor p27kip1, cyclin D2, cyclin D3, cyclin E, CDK2, and CDK4 (up until 48 h) were also not different in activated WT and Casp6 KO B cells (data not shown and Fig. 5B). Thus, although changes in pRb Ser780 and pRb Ser807/811 correlated with enhanced cell cycle entry in Casp6 KO B cells, the levels of Rb degradation and other G1 indicators were normal. Although activated Casp6 KO B cells enter the cell cycle rapidly, their progression through S phase may not be accelerated due to the lack of down-regulation of Rb.
FIGURE 5.
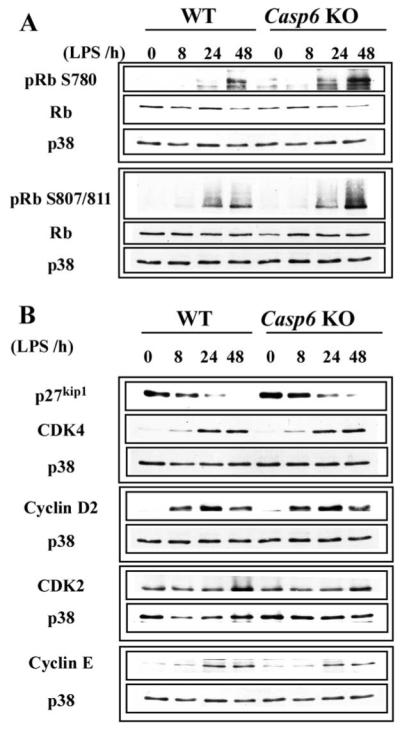
Phosphorylation of Rb is up-regulated in activated Casp6 KO B cells. A and B, Resting B cells were stimulated with LPS (5 μg/ml) for the specified times and harvested. Each lysate was subjected to Western blotting with antisera specific for the indicated proteins. p38 MAPK (p38) served as a loading control for each blot. The data are representative of three independent experiments.
B cell differentiation into plasma cells is accelerated in Casp6 KO mice
Next, we sought to determine why accelerated G1 entry in Casp6 KO B cells did not affect proliferation or cell death. During T cell-dependent humoral immune responses, Ag-specific B cells divide and form germinal centers (GCs). B cells later exit from GCs and terminally differentiate into plasma cells in extrafollicular foci. In many cell types, decisions are made during G1 to either return to quiescence or terminally differentiate (47). Furthermore, as B cells terminally differentiate into plasma cells, they arrest in G1 and secrete Igs (48). Thus, we tested whether the accelerated G1 entry of Casp6 KO B cells, rather than translating into increased S phase entry, shifted B cells toward differentiating into plasma cells. Indeed, syndecan-1+ plasma cell formation compared with WT B cells was clearly increased in Casp6 KO B cells cultured with either graded doses of LPS, LPS plus IL-4, or with low doses of anti-CD40 mAb (Fig. 6A and data not shown). Higher doses of LPS induce more B cell G1 entry and proliferation than lower doses (e.g., Fig. 3C); the increased proliferation with high doses of LPS, however, is associated with fewer plasma cells being induced than at lower doses of LPS (Fig. 6A). In fact, as little as 0.1 μg/ml LPS stimulated less B cell proliferation but more plasma cell production than 0.3 μg/ml LPS (data not shown). Overall, these findings indicate that plasma cell formation is increased in Casp6 KO B cells.
FIGURE 6.
In vitro plasma cell formation and Ig production are enhanced in Casp6 KO mice. A, Purified resting B cells were stimulated with graded doses of various stimuli with or without IL-4 (10 ng/ml). Stimulated cells were harvested on day 5 and stained with PE-syndecan-1 and PerCP-B220. Plasma cells were identified as syndecan-1+B220 -low and percentages were calculated on live gated cells. B, Resting B cells were stimulated with LPS (1.25 μg/ml) with or without IL-4 (10 ng/ml). After 7 days of culture, supernatants were harvested and the levels of IgM and IgG1 were determined by ELISA. The WT mice are shown as closed bars and Casp6 KO mice as open bars. C, Quantitative real-time PCR performed on cDNA prepared from 4-day stimulation of resting B cells with LPS. Relative quantitation of Blimp-1 expression to GAPDH was calculated using the comparative Ct method, relative ratio = 2˄(Ct Gapdh - Ct sample). The data are representative of three independent experiments using three mice per genotype per experiment. Statistical analysis was defined as *, p < 0.05 and **, p < 0.01 by a paired t test.
We also compared in vitro Ig production between WT and Casp6 KO B cells. LPS-stimulated B cells mainly produced IgM, but IgG1 was made when IL-4 was added (Fig. 6B). The levels of IgM were significantly higher in Casp6 KO B cell cultures compared with WT B cells after stimulation with either LPS or LPS plus IL-4. IgG1 production was also higher in Casp6 KO B cells stimulated with LPS plus IL-4 (Fig. 6B). Furthermore, the expression levels of Blimp-1 (B lymphocyte-induced maturation protein 1), which is considered to be an essential regulator of B cell differentiation into plasma cells (49), were also up-regulated in Casp6 KO B cells activated at a low dose of LPS compared with that of WT B cells (Fig. 6C). These results suggest that in vitro B cell differentiation into plasma cells was accelerated in Casp6 KO B cells.
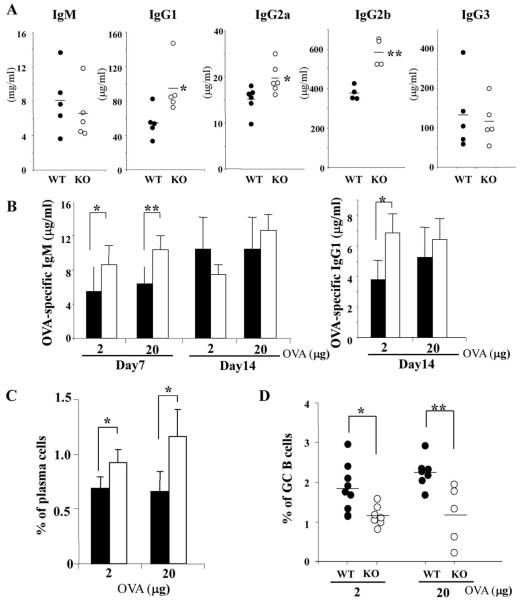
We next investigated the role of Casp6 in B cell differentiation in vivo. We first measured serum levels of Igs in WT and Casp6 KO mice. IgG1, IgG2a, and IgG2b were significantly increased in Casp6 KO mice compared with age-matched WT mice (Fig. 7A); however, serum IgM and IgG3 levels were normal. This suggested to us that TD Ab responses may be dysregulated in Casp6 KO mice. Indeed, after immunization with the TD Ag, OVA, the levels of Ag-specific IgM were elevated in sera of Casp6 KO mice at day 7 compared with those of WT mice (Fig. 7B, left). The levels of Ag-specific IgG1 were higher in the sera of Casp6 KO mice bled 14 days after immunization with a low dose (2 μg) of OVA but not in mice immunized with 20 μg of OVA (Fig. 7B, right). In addition, syndecan-1+ plasma cells were significantly higher in immunized Casp6 KO mice (Fig. 7C). In contrast, GC B cells that are PNA+B220+ were decreased in immunized Casp6 KO mice compared with those of WT mice (Fig. 7D). Together with the in vitro B cell differentiation experiments (Fig. 6), these in vivo experiments show that serum levels of some Igs are already increased ex situ in Casp6 KO mice and that both humoral immune responses to specific Ag and plasma cell differentiation are elevated in Casp6 KO mice.
FIGURE 7.
Basal level of serum Igs and Ag-specific Ig production are elevated in Casp6 KO mice. A, Sera were collected from mice and Ig isotype levels were determined by ELISA. B-D, Mice were immunized i.p. with alum-precipitated OVA at indicated doses and bled at day 7 or 14. Ag-specific Ig levels of collected sera were determined by ELISA. At day 7 postimmunization, spleens were harvested and plasma cells (syndecan-1+B220-low) and GC B cells (PNA+B220+) were examined. The data (A-D) are representative of three independent experiments using four to eight mice per genotype per experiment. Statistical analysis was defined as *, p < 0.05 and **, p < 0.01 by a paired t test.
Discussion
Our data demonstrate that Casp6 regulates the balance between proliferation and differentiation in B cells by affecting both entry into G1 and the Rb-dependent restriction point. Two striking phenotypes in Casp6 KO mice were a dysregulated entry into the G1 phase of the cell cycle (Fig. 3) and an accelerated B cell differentiation into plasma cells (Figs. 6 and 7). This dual phenotype of dysregulated cell cycle entry and B cell differentiation suggests a common function for Casp6 to control the equilibrium between cell proliferation and differentiation by cleaving substrates involved in maintaining B cell quiescence.
Previously, we reported that Casp6 is cleaved early after human B cells are stimulated to proliferate (36). Similarly, CpG induces Casp6 cleavage within 8-24 h after activation of mouse B cells (Fig. 1A), suggesting Casp6 may play a role in both mouse and human activated B cells. However, our results using human tonsillar B cells and the Casp6-selective inhibitor VEID (36) differ from those that we obtained using Casp6 KO B cells: although VEID strongly blocked human B cell proliferation (36), the proliferation of Casp6 KO B cells was normal. Furthermore, VEID treatment of activated human B cells strongly reduced the levels of cell cycle-related proteins (cyclin D1/2/3, CDK4, and pRb). In contrast, the expression levels of cell cycle-related proteins were not different in activated Casp6 KO B cells compared with WT B cells except that Rb phosphorylation was markedly increased (Fig. 5).
One possibility is that these disparate results reflect some difference between mouse splenic B cells and human tonsillar B cells. Although we cannot completely rule this out, we think this is unlikely since VEID has the same effect on mouse and human B cells: it inhibits the proliferation of both (Ref. 36 and Fig. 2). A more likely explanation is that VEID has a different and broader effect on B cells than Casp6 deficiency has. VEID inhibits proliferation of both WT and Casp6 KO B cells (Fig. 2) and in a number of experiments using a number of different stimuli, we detected no differences in the responses between VEID-treated WT and Casp6 KO B cells (data not shown). Thus, VEID at least in B cells is not specific for Casp6. It is unclear how VEID blocks B cell proliferation, but the data in Fig. 2 demonstrate that Casp6 is not required for the VEID-mediated inhibition. Although our first study showed that Casp6 is activated soon after B cells are stimulated (36), our model that Casp6 is required for B cells to enter the cell cycle turned out to be incorrect; we did not take into consideration that VEID could be affecting B cell entry into the cell cycle via a Casp6-independent mechanism.
Casp6 is activated within 12 h after activation of either human (36) or mouse B cells (Fig. 1A). This occurs before activation-induced cell death. Thus, Casp6 activity appears to function early after B cell activation. G1 B cells are increased in both ex vivo B cells from Casp6 KO mice (Fig. 3) and in resting human tonsillar B cells treated with Casp6- selective inhibitor VEID (data not shown). G1 entry from G0 is also accelerated in activated Casp6 KO B cells (Fig. 3C), These observations at first suggested that Casp6 may have an inhibitory role on cell cycle entry for maintaining the B cell pool in a homeostatic balance. However, to our surprise, the increased numbers of G1 B cells in Casp6 KO mice did not translate into dysregulation of overall B cell numbers in adult Casp6 KO mice, but rather into an elevation of serum Ig levels (Fig. 7A). Consistent with this in vivo phenotype, G1 cell cycle entry and syndecan-1+ plasma cell formation in vitro were accelerated in Casp6 KO B cells, but S phase entry and cell death were normal (Figs. 2-4 and 6A).
These findings suggest that Casp6 plays a role during G1 progression in determining whether B cells decide to proliferate or differentiate. Stimuli that normally drive B cell proliferation and thereby reduce differentiation (e.g., high doses of LPS) in the absence of Casp6 were less effective at preventing differentiation (Fig. 6A). Similarly, Casp6 KO mice after immunization had fewer GC B cells and more syndecan-1+ plasma cells (Fig. 7, C and D). Thus, Casp6 may regulate the threshold favoring proliferation rather than differentiation into syndecan-1+ plasma cells. Terminal differentiation is usually coupled with cell cycle exit and maintenance of the nonproliferative state in certain cell types. Many plasma cells are arrested in G1 (48) and factors promoting cell cycle progression and proliferation, including Casp6, are down-regulated as cells terminally differentiate (29). Interestingly, microarray analyses comparing murine plasma cells to surface IgM+ naive B cells showed that Casp6 is dramatically down-regulated (435-fold) (29). It may be necessary for Casp6 to be down-regulated in order for B cells to become plasma cells.
Very few Casp substrates of the hundreds of possible candidates have been verified, since rigorous criteria must be met before it is certain a protein is a substrate (6). One possible direct or indirect target for Casp6 in B cells is Rb (44, 45). As cells go into S phase, Rb normally is inactivated by phosphorylation and then cleaved to release E2F-DP1 complexes. Rb phosphorylation was increased much more after activation of Casp6 KO B cells than WT B cells. However, the total amount of Rb, which reflects the level of Rb degradation, was not notably different between WT and Casp6 KO B cells (Fig. 5). Thus, if Rb is a substrate for Casp6 in B cells, it may be cleaved after it is phosphorylated.
The hyperphosphorylation of Rb in activated Casp6 KO B cells could have simply reflected that more Casp6 KO B cells had entered G1; however, the expression levels of other early G1 indicators, CDKs and cyclins, were similar in activated Casp6 KO B cells and activated WT B cells. Another possibility is that Rb phosphorylation is more strongly elevated in Casp6 KO B cells because activated Casp6 KO B cells do not progress readily into S phase and are predisposed to differentiate. Rb has been implicated both in promoting terminal differentiation in several cell types (50) and in inhibiting repressors of differentiation (47).
A dynamic balance between transcriptional repressors including Blimp-1, Bcl-6, and Pax-5 determine whether GC B cells continue to divide, become memory B cells, or differentiate to become plasma cells (51). Blimp-1 is a well-defined transcriptional repressor, which is induced upon B cell differentiation into plasma cells (49). The expression level of Blimp-1 was up-regulated in LPS-activated Casp6 KO B cells where plasma cell formation was increased (Fig. 6C), suggesting that Casp6 may influence the balance of transcriptional repressors. A number of molecules regulate Blimp-1 expression including NF-κB, IRF4, STAT3, STAT5, p53, and AP-1 (49, 52-56). NF-κB p50, p65, STAT3, and p53 have been reported to be cleaved by Casps (57-60). Therefore, it is possible that Blimp-1 expression is influenced by Casp6 indirectly by cleavage of Blimp-1 activators. A remaining question, as to whether G1 entry/arrest and B cell differentiation are affected directly or indirectly through Casp6 substrates needs to be further investigated.
The function of Casp6 in B cells is distinct from two other Casps which regulate B cell activation and proliferation, Casp8 and Casp3. B cells from patients with Casp8 deficiency and from mice with Casp8-deleted B cells (bCasp8 KO mice) have impaired nuclear translocation of NF-κB p65 (30, 34). Adult bCasp8 KO mice, like adult Casp6 KO mice have normal numbers of B cells (33, 34). However, unlike Casp6 KO B cells, Casp8 KO B cells are more susceptible to activation-induced cell death and have defective proliferative responses to TLR3 and TLR4 agonists (33, 34). Thus, Casp8 appears to regulate B cell survival and proliferation in response to certain TLR agonists, while Casp6, in contrast, provides a brake on B cell entry into the cell cycle.
Woo et al. (35) showed that Casp3, like Casp6, is essential for cell cycle control of mature B cells. Unlike Casp6 KO mice, Casp3 KO mice have dramatically increased numbers of B cells in vivo and enhanced splenic B cell proliferation (35). Casp6 KO B cells, unlike Casp3 KO B cells (35), do not have abnormal increased expression of p21Cip1 and indeed, if anything, express less p21Cip1 (data not shown); this suggests that unlike Casp3, Casp6 does not cleave p21Cip1 in mitogen-activated B cells. After mitogenic stimulation of resting B cells, both Casp6 and Casp3 are cleaved and are presumably activated but with different kinetics; Casp6 cleavage was consistently detectable earlier after stimulation compared with Casp3 cleavage in our experiments (Fig. 1) and others (35). One possibility is that Casp6 is active and functions at an early stage of the cell cycle, while Casp3 is activated and operates at a later point in the cell cycle. However, the target of Casp6 during early cell cycle entry remains to be identified.
It is noteworthy that certain inhibitors of apoptosis (IAPs) like cIAP1 and cIAP2 (61), which are activated in B cells by LPS, CD40 ligation, and other mitogenic signals, can inhibit Casp8 and Casp3, but not Casp6 (21). B cells, in particular, appear to be regulated by a team of Casps (Casp3, Casp8, and now Casp6) during B cell activation. Further understanding as to how Casps work together to regulate steps leading to B cell proliferation may lead to new insights into how to eliminate dysregulated B cells in autoimmune disorders and cancers. This is the first report to show a role of Casp6 in B cells in vivo and our data demonstrate that Casp6 is not only an apoptotic factor, but also functions to maintain B cells in a G0 or quiescent state and can modify terminal differentiation. These findings on Casp6 KO B cells provide more information to the growing area of nonapoptotic functions of Casps, including the regulation of cell cycle and cell differentiation in B cells. Further studies are required to clarify the roles of various Casp6 substrates in these processes.
Acknowledgments
We thank Kevin Draves for taking care of the mice used in these studies and helping with flow cytometric analyses. We thank Dr. Jonathan Graves for helpful discussions early in the development of this project and for critical review of this manuscript.
Footnotes
This work was supported mainly by National Institutes of Health Grant GM37905 (to E.A.C.) and also by National Institutes of Health Grants DE16381 (E.A.C.) and RR00166 and by the Research Fellowship of Japan Society for the Promotion of Science (to C.W.).
- Casp
- caspase
- KO
- knockout
- WT
- wild type
- PY
- pyronin Y
- CDK
- cyclin-dependent kinase
- Rb
- retinoblastoma protein
- TD
- T cell dependent
- VEID
- benzyloxycarbonyl(Cbz)-Val-Glu-Ile-Asp(Ome)-fluoromethylketone
- Ct
- cycle threshold
- 7AAD
- 7-aminactinomycin D
- GC
- germinal center
- IAP
- inhibitor of apoptosis
- pRb
- phosphorylated Rb
Disclosures
The authors have no financial conflict of interest.
References
- 1.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat. Rev. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 3.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 4.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 5.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 6.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2006;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 7.Galande S, Dickinson LA, Mian IS, Sikorska M, Kohwi-Shigematsu T. SATB1 cleavage by caspase 6 disrupts PDZ domain-mediated dimerization, causing detachment from chromatin early in T-cell apoptosis. Mol. Cell. Biol. 2001;21:5591–5604. doi: 10.1128/MCB.21.16.5591-5604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eymin B, Sordet O, Droin N, Munsch B, Haugg M, Van de Craen M, Vandenabeele P, Solary E. Caspase-induced proteolysis of the cyclin-dependent kinase inhibitor p27Kip1 mediates its anti-apoptotic activity. Oncogene. 1999;18:4839–4847. doi: 10.1038/sj.onc.1202860. [DOI] [PubMed] [Google Scholar]
- 9.Cohen LY, Bourbonniere M, Sabbagh L, Bouchard A, Chew T, Jeannequin P, Lazure C, Sekaly RP. Notch1 antiapoptotic activity is abrogated by caspase cleavage in dying T lymphocytes. Cell Death Differ. 2005;12:243–254. doi: 10.1038/sj.cdd.4401568. [DOI] [PubMed] [Google Scholar]
- 10.Nyormoi O, Wang Z, Doan D, Ruiz M, McConkey D, Bar-Eli M. Transcription factor AP-2α is preferentially cleaved by caspase 6 and degraded by proteasome during tumor necrosis factor α-induced apoptosis in breast cancer cells. Mol. Cell. Biol. 2001;21:4856–4867. doi: 10.1128/MCB.21.15.4856-4867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC. Cleavage of lamin A by Mch2 α but not CPP32: multiple interleukin 1 β-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl. Acad. Sci. USA. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rokudai S, Fujita N, Hashimoto Y, Tsuruo T. Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. J. Cell. Physiol. 2000;182:290–296. doi: 10.1002/(SICI)1097-4652(200002)182:2<290::AID-JCP18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Werz O, Tretiakova I, Michel A, Ulke-Lemee A, Hornig M, Franke L, Schneider G, Samuelsson B, Radmark O, Steinhilber D. Caspase-mediated degradation of human 5-lipoxygenase in B lymphocytic cells. Proc. Natl. Acad. Sci. USA. 2005;102:13164–13169. doi: 10.1073/pnas.0505991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allsopp TE, McLuckie J, Kerr LE, Macleod M, Sharkey J, Kelly JS. Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ. 2000;7:984–993. doi: 10.1038/sj.cdd.4400733. [DOI] [PubMed] [Google Scholar]
- 16.Cowling V, Downward J. Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ. 2002;9:1046–1056. doi: 10.1038/sj.cdd.4401065. [DOI] [PubMed] [Google Scholar]
- 17.Graham RK, Deng Y, Slow EJ, Haigh B, Bissada N, Lu G, Pearson J, Shehadeh J, Bertram L, Murphy Z, et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Kim MR, Soung YH, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. Mutational analysis of the CASP6 gene in colorectal and gastric carcinomas. APMIS. 2006;114:646–650. doi: 10.1111/j.1600-0463.2006.apm_417.x. [DOI] [PubMed] [Google Scholar]
- 19.Woenckhaus C, Giebel J, Failing K, Fenic I, Dittberner T, Poetsch M. Expression of AP-2α, c-kit, and cleaved caspase-6 and -3 in naevi and malignant melanomas of the skin: a possible role for caspases in melanoma progression? J. Pathol. 2003;201:278–287. doi: 10.1002/path.1424. [DOI] [PubMed] [Google Scholar]
- 20.Pfisterer P, Ehlermann J, Hegen M, Schorle H. A subtractive gene expression screen suggests a role of transcription factor AP-2 α in control of proliferation and differentiation. J. Biol. Chem. 2002;277:6637–6644. doi: 10.1074/jbc.M108578200. [DOI] [PubMed] [Google Scholar]
- 21.Graves JD, Craxton A, Clark EA. Modulation and function of caspase pathways in B lymphocytes. Immunol. Rev. 2004;197:129–146. doi: 10.1111/j.0105-2896.2004.0110.x. [DOI] [PubMed] [Google Scholar]
- 22.Buttitta LA, Edgar BA. Mechanisms controlling cell cycle exit upon terminal differentiation. Curr. Opin. Cell Biol. 2007;19:697–704. doi: 10.1016/j.ceb.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J. Cell Biol. 2008;180:451–458. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J. Exp. Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santambrogio L, Potolicchio I, Fessler SP, Wong SH, Raposo G, Strominger JL. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat. Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- 26.Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E, Dubrez-Daloz L. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- 27.Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, L’Ollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, et al. Caspase-8 prevents sustained activation of NF-κB in monocytes undergoing macrophagic differentiation. Blood. 2007;109:1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rautajoki KJ, Marttila EM, Nyman TA, Lahesmaa R. Interleukin-4 inhibits caspase-3 by regulating several proteins in the Fas pathway during initial stages of human T helper 2 cell differentiation. Mol. Cell. Proteomics. 2007;6:238–251. doi: 10.1074/mcp.M600290-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Underhill GH, George D, Bremer EG, Kansas GS. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood. 2003;101:4013–4021. doi: 10.1182/blood-2002-08-2673. [DOI] [PubMed] [Google Scholar]
- 30.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 31.Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-κB activation. Curr. Biol. 2006;16:1666–1671. doi: 10.1016/j.cub.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 33.Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 34.Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, Hakem A. Essential role for caspase-8 in Toll-like receptors and NFκB signaling. J. Biol. Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 35.Woo M, Hakem R, Furlonger C, Hakem A, Duncan GS, Sasaki T, Bouchard D, Lu L, Wu GE, Paige CJ, Mak TW. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat. Immunol. 2003;4:1016–1022. doi: 10.1038/ni976. [DOI] [PubMed] [Google Scholar]
- 36.Olson NE, Graves JD, Shu GL, Ryan EJ, Clark EA. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J. Immunol. 2003;170:6065–6072. doi: 10.4049/jimmunol.170.12.6065. [DOI] [PubMed] [Google Scholar]
- 37.Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW, Lazebnik Y, Flavell RA. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat. Med. 2000;6:1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]
- 38.Mond JJ, Brunswick M. Assay for B cell function in vitro antibody production. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Wiley; Hoboken, NJ: 1991. pp. 3.8.1–3.8.16. [Google Scholar]
- 39.O’ Reilly LA, Divisekera U, Newton K, Scalzo K, Kataoka T, Puthalakath H, Ito M, Huang DC, Strasser A. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724–736. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- 40.Heath AW, Wu WW, Howard MC. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur. J. Immunol. 1994;24:1828–1834. doi: 10.1002/eji.1830240816. [DOI] [PubMed] [Google Scholar]
- 41.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 42.Schmid I, Cole SW, Korin YD, Zack JA, Giorgi JV. Detection of cell cycle subcompartments by flow cytometric estimation of DNA-RNA content in combination with dual-color immunofluorescence. Cytometry. 2000;39:108–116. doi: 10.1002/(sici)1097-0320(20000201)39:2<108::aid-cyto3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat. Rev. Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- 44.Lemaire C, Godefroy N, Costina-Parvu I, Rincheval V, Renaud F, Trotot P, Bouleau S, Mignotte B, Vayssiere JL. Caspase-9 can antagonize p53-induced apoptosis by generating a p76Rb truncated form of Rb. Oncogene. 2005;24:3297–3308. doi: 10.1038/sj.onc.1208493. [DOI] [PubMed] [Google Scholar]
- 45.Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat. Cell Biol. 2002;4:757–765. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- 46.Guo J, Sheng G, Warner BW. Epidermal growth factor-induced rapid retinoblastoma phosphorylation at Ser780 and Ser795 is mediated by ERK1/2 in small intestine epithelial cells. J. Biol. Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- 47.Blomen VA, Boonstra J. Cell fate determination during G1 phase progression. Cell Mol. Life Sci. 2007;64:3084–3104. doi: 10.1007/s00018-007-7271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen-Kiang S. Cell-cycle control of plasma cell differentiation and tumorigenesis. Immunol. Rev. 2003;194:39–47. doi: 10.1034/j.1600-065x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 49.Calame K. Activation-dependent induction of Blimp-1. Curr. Opin. Immunol. 2008;20:259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Skapek SX, Pan YR, Lee EY. Regulation of cell lineage specification by the retinoblastoma tumor suppressor. Oncogene. 2006;25:5268–5276. doi: 10.1038/sj.onc.1209710. [DOI] [PubMed] [Google Scholar]
- 51.Johnson K, Shapiro-Shelef M, Tunyaplin C, Calame K. Regulatory events in early and late B-cell differentiation. Mol. Immunol. 2005;42:749–761. doi: 10.1016/j.molimm.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 52.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudth LM, Shingh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription-3 dependent B lymphocyte terminal differentiation by BCL-6. J. Exp. Med. 2000;192:1841–1848. doi: 10.1084/jem.192.12.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horikawa K, Takatsu K. Interleukin-5 regulates genes involved in B-cell terminal maturation. Immunology. 2006;118:497–508. doi: 10.1111/j.1365-2567.2006.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan J, Jiang J, Lim CA, Wu Q, Ng NH, Chin KC. BLIMP1 regulates cell growth through repression of p53 transcription. Proc. Natl. Acad. Sci. USA. 2007;104:1841–1846. doi: 10.1073/pnas.0605562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohkubo Y, Arima M, Argumi E, Okada S, Yamashita K, Asari S, Obata S, Sakamoto A, Hatano M, O-Wang J, et al. A role for c-fos/activator protein 1 in B lymphocyte terminal differentiation. J. Immunol. 2005;174:7703–7710. doi: 10.4049/jimmunol.174.12.7703. [DOI] [PubMed] [Google Scholar]
- 57.Ravi R, Bedi A, Fuchs EJ, Bedi A. CD95 (Fas)-induced caspase-mediated proteolysis of NF-κB. Cancer Res. 1998;58:882–886. [PubMed] [Google Scholar]
- 58.Levkau B, Scatena M, Giachelli CM, Ross R, Raines EW. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-κB loop. Nat. Cell Biol. 1999;1:227–233. doi: 10.1038/12050. [DOI] [PubMed] [Google Scholar]
- 59.Darnowski JW, Goulette FA, Guan YJ, Chatterjee D, Yang ZF, Cousens LP, Chin YE. Stat3 cleavage by caspases: impact on full-length Stat3 expression, fragment formation, and transcriptional activity. J. Biol. Chem. 2006;281:17707–17717. doi: 10.1074/jbc.M600088200. [DOI] [PubMed] [Google Scholar]
- 60.Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM. p53 is cleaved by caspases generating fragments localizing to mitochondria. J. Biol. Chem. 2006;281:13566–13573. doi: 10.1074/jbc.M512467200. [DOI] [PubMed] [Google Scholar]
- 61.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]