Abstract
Applying Rusbult's investment model of dyadic relationships, we examined the effect of caregiver–care recipient relationship closeness (RC) on cognitive and functional decline in Alzheimer's disease. After diagnosis, 167 participants completed up to six visits, observed over an average of 20 months. Participants were 64% women, had a mean age of 86 years, and mean dementia duration of 4 years. Caregiver-rated closeness was measured using a six-item scale. In mixed models adjusted for dementia severity, dyads with higher levels of closeness (p < .05) and with spouse caregivers (p = .01) had slower cognitive decline. Effect of higher RC on functional decline was greater with spouse caregivers (p = .007). These findings of attenuated Alzheimer's dementia (AD) decline with closer relationships, particularly with spouse caregivers, are consistent with investment theory. Future interventions designed to enhance the caregiving dyadic relationship may help slow decline in AD.
Keywords: Alzheimer's disease, Caregiving, Interpersonal relations
ALZHEIMER'S dementia (AD) remains a major public health problem, with a prevalence of 11–16 million cases projected by the year 2050 in the United States alone (Alzheimer's Association, 2008). Strong efforts have been made to identify factors that might delay or prevent its onset. These factors include several potentially disease-modifying interventions intended to modify cleavage of the amyloid precursor protein or to control the hyperphosphorylation of tau, inflammation, oxidation, or excitotoxicity (Salloway, Mintzer, Weiner, & Cummings, 2008). Less thoroughly studied are nonpharmacological factors such as caregiving environment or practices that may delay the progression of symptoms in AD.
Estimates from clinic-based studies suggest that the cognitive abilities of persons with AD decline by 0.8–4.4 points per year on the Mini-Mental State Exam (MMSE), with the typical decline of about 3 points per year (Behl, Stefurak, & Black, 2005). Two population-based studies have reported decline rates of 2.3–2.9 MMSE points per year (Aguero-Torres, Fratiglioni, Guo, Viitanen, & Winblad, 1998; Slooter et al., 1999), and we recently published a mean decline of 1.9 (95% CI = 1.7–2.1) MMSE points per year in persons with newly diagnosed AD from the population of Cache County, Utah (Mielke et al., 2007). More rapid decline has been associated with higher education in some (Stern, Albert, Tang, & Tsai, 1999; Teri, McCurry, Edland, Kukull, & Larson, 1995) but not all (Bowler, Munoz, Merskey, & Hachinski, 1998; Regan et al., 2006; Small, Viitanen, Winblad, & Backman, 1997) samples. Similarly, more rapid decline has been reported in the presence of comorbid medical conditions, especially vascular risk factors (Mielke et al., 2007) and with younger onset age in some (Teri et al., 1995; Lucca, Comelli, Tettamanti, Tiraboschi, & Spagnoli, 1993) but not all (Bowler et al., 1998; Small et al., 1997; R. G. Stern et al., 1994) Studies. Finally, more rapid cognitive decline has also been associated with baseline behavioral disturbances such as agitation and psychosis (Scarmeas et al., 2005; Y. Stern et al., 1994). Thus, there is evidence that education, comorbid medical conditions—in particular, vascular health conditions—and behavioral disturbances are important moderators of decline in AD.
Although several studies have examined the effects of the clinical features of dementia on caregiver well-being (Gaugler, Davey, Pearlin, & Zarit, 2000; Ory, Hoffman, Yee, Tennstedt, & Schulz, 1999), little is known about the extent to which decline in dementia is modified by the care environment. Kitwood (1993) has argued that dementia caregiving is a “cooperative and reciprocal engagement” (pp. 64–65) that requires a caregiver to be emotionally available to the care recipient (CR), have high levels of empathy and imagination, and engage in flexible thinking. This approach posits that acceptance of the validity of CRs' experiences and accurate identification of their needs are crucial to development of a positive care environment. This hypothesis is supported by studies demonstrating that “nonadapting” (nonacceptance) strategies appear to predict worse outcomes than “supporting” strategies (adapting to the CR's level; de Vugt et al., 2004), and a longitudinal study of spousal dementia care demonstrating that positive spousal interactions, high caregiver commitment, good caregiver health, and shorter caregiving duration were all associated with delayed nursing home placement in dementia (Wright, 1994). Support for the hypothesis that the care environment influences the progression of dementia symptoms is provided by clinical trial findings that caregiver interventions intended to stimulate cognitive abilities in dementia patients may also attenuate their cognitive decline (Quayhagen & Quayhagen, 2001), improve the quality of life for both caregivers and their CRs (Quayhagen & Quayhagen, 1996), and delay nursing home placement (Mittelman, Haley, Clay, & Roth, 2006).
Several studies have examined the association of caregiver and CR relationships and selected outcomes. Closer perceived relationships are associated with better adjustment to nursing home placement in persons with dementia (Whitlatch, Schur, Noelker, Ejaz, & Looman, 2001) and improved psychological well-being and problem-solving abilities (Burgener & Twigg, 2002). Conversely, avoidance by caregivers or insecure attachment styles in their CRs have been associated with more behavioral problems in the latter (Perren, Schmid, Herrmann, & Wettstein, 2007). Graham and Bassett's (2006) longitudinal ethnographic study of persons with Alzheimer's disease and their family caregivers presents strong evidence that caring relationships are “dynamic co-constructions built upon everyday events, interactions, environments, and disease progression” (p. 335). In their study, cooperative care relationships were built on foundations of mutual respect and sensitivity to persons with dementia, whereas lack of trust and compassion leads to unrealistic expectations and negative reciprocity. In the current study, we used interdependence theory (Kelley & Thibaut, 1978) and the investment model of commitment (Rusbult & Buunk, 1993) to guide development of hypotheses. Formulated to explain behavior in dyadic relationships, interdependence theory holds that relationship partners become interdependent over time through their interactions. As interdependence increases, so does concern for the partner's outcomes, and a transformation of motives occurs from motives of self-interest to prorelationship motives (Lewis et al., 2006). The investment model suggests that interdependence is felt as commitment, characterized by desire to maintain the relationship through good and bad times (Rusbult & Buunk, 1993). To that end, investments are made, including sacrifice for one's partner. Using these theories, and the significant association between positive spousal interactions, high caregiver commitment, and favorable outcomes in persons with dementia noted previously (Wright, 1994), we would expect relationships between AD caregivers and their CRs characterized as close to result in more favorable cognitive and functional outcomes.
Given the evidence that aspects of the care environment are associated with more favorable outcomes in dementia, we examined whether rate of progression of dementia is influenced by type of relationship (spouse vs adult child) and caregiver–CR relationship closeness (RC) in a population-based sample of persons with AD. We hypothesized that CRs with spouse caregivers, and those whose caregiver rated their relationship as closer, would experience slower rates of cognitive and functional decline.
METHODS
Participants
The Cache County Dementia Progression Study (CC-DPS) is one of few population-based studies of dementia progression in an incidence cohort, examining longitudinal cognitive, functional, and behavioral outcomes and the factors that may modify their course. Individuals were enrolled in the CC-DPS between 2002 and 2004 after first being diagnosed between 1998 and 2002 with new-onset dementia in the Cache County Memory Study (CCMS), a longitudinal population-based study of dementia that has now completed four triennial “waves” of dementia ascertainment using a multistage case detection protocol (Breitner et al., 1999; Miech et al., 2002). The CCMS has identified 357 prevalent and 473 new-onset (after baseline) cases of dementia.
Since the start of the CC-DPS in 2002, 241 individuals (87% of those still living when recruited for CC-DPS) diagnosed in the parent study with new-onset dementia, along with their principal caregivers, were enrolled. Among this panel, 183 (75.9%) had been diagnosed by a clinical review panel with possible or probable AD according to the National Institute of Neurological and communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCSA-ADRDA) criteria (McKhann et al., 1984). Of these, we excluded nine persons with dementia who had caregivers other than spouses or adult children, two missing relationship type, and five whose caregiver did not complete the closeness measure, for a final sample size of 167 persons with AD and their caregivers.
Participants with AD were 64% female, 99% Caucasian, with mean age of 86.4 (SD = 5.7) years, mean education of 13.2 (SD = 3.0) years, and mean dementia duration of 4.0 (SD = 2.0) years. At enrollment visit, 20 participants (12%) were living in a skilled nursing facility (SNF), 37 (22%) in residential or assisted living facility (ALF), and the remainder at home (or caregiver's home). Over the subsequent observations (almost 2 years, on average), 7 participants moved into an SNF and 15 moved into an ALF; however, the association between baseline closeness level and subsequent move to either SNF or ALF was nonsignificant (χ2 = 2.93, df = 4, p = .570). The final sample of 167 persons with AD comprised 63 male CRs whose caregivers were wife (n = 45), daughter or daughter-in-law (n = 12), or son or son-in-law (n = 6) and 104 female CRs whose caregivers were husband (n = 18), daughter or daughter-in-law (n = 69), or son or son-in-law (n = 17). Participants were observed for a mean of 20.3 (SD = 13.0) months and 49% coresided with their caregiver. Caregivers were 77% female with 43% spouses and 57% adult children, mean age of 65.2 (SD = 14.9) years, mean education of 14.3 (SD = 2.5) years, and mean length of caregiving of 3.8 (SD = 4.6) years.
Procedures
After enrollment, participants were examined every 6 months at their place of residence and observed for 4–51 months (M = 20.3, SD = 13.0 months). Of the 167 who completed the enrollment visit, there were 129 with at least two visits, 80 with at least three visits, 50 with at least four visits, 28 with at least five visits, and 21 with at least six visits.
The examinations included manometric measurement of blood pressure, a brief neurological exam, an inventory of functional status, and administration of a brief (∼45 min) neuropsychological test battery. At each visit, caregivers were also asked about the caregiving environment, as described subsequently. Written informed consent was obtained for each interview. All procedures were approved by the institutional review boards of Utah State University and the Johns Hopkins University.
Exposure Measurement: RC
Closeness of the caregiving relationship was measured using a six-item instrument developed by Noelker (1996) and Whitlach et al. (2001). This Relationship Closeness Scale (RCS) used 4-point Likert scale responses that captured caregivers’ degree of agreement with six statements about their relationship with the individual for whom they provided care. The six statements were presented twice, once soliciting responses with respect to their current relationship and once with respect to the relationship “prior to the time when you began to provide care to him/her.” Correlation between assessments of current versus prior RC was r = .628 (p < .001). Only the responses assessing their current relationship were used in this study. Total scores (range: 6–24) were calculated by summing the scores on the six individual items. Higher values on the RCS indicated closer relationships. This measure was collected at enrollment into the dementia progression study (Visit 1).
Outcomes: Cognitive and Functional Progression in AD
Symptom progression was assessed every 6 months using standard measures administered by specially trained neuropsychological technicians and research nurses. The assessment included the Consortium to Establish a Registry for Alzheimer's Disease adaptation of the MMSE (Morris et al., 1993), a 30-point cognitive screening test that includes 10 items on orientation, a brief test of immediate and delayed recall, as well as varied items assessing language, and praxis. When three or fewer points were missed owing to sensory impairments, we adjusted scores by extrapolating the proportion of items answered correctly over a total of 30 points. For individuals who had progressed to advanced dementia, the technician attempted to administer the test according to standard instructions. However, in a few instances (one testing session for 10 participants, two testing sessions for 5 participants, and three testing sessions for 1 participant), the technician discontinued test administration due to poor comprehension. In such instances, we summed across the items attempted to obtain a total score.
Progression of functional impairment was measured using the “sum of boxes” score on the Clinical Dementia Rating (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982). Individual item ratings were made by the nurse after the examination and a clinical interview with the caregiver. Individual item scores (boxes) were endorsed after comparing the participant's abilities with standard text descriptions of six different levels of severity ranging from 0 (no impairment) to 5 (total loss of function). The six individual CDR items assess memory, orientation, problem solving and community involvement, and functional abilities at home and in personal affairs (CDR range: 0–30). In assigning the individual CDR item scores, the research nurse incorporated caregiver report and his or her own clinical observations during interaction with the participant while conducting the neurological examination. The nurse additionally incorporated ongoing knowledge of the individual participant's dementia progression from having consistently visited the participant every 6 months over several years. Furthermore, in cases where the caregiver's report was clearly incongruent with the nurse's clinical observations, an additional assessment was sought by interviewing another knowledgeable informant, where available. Thus, although caregiver burden or stress or poor RC may influence the caregiver's assessment of the participant's functional status (making these measures not entirely independent), a great deal of clinical judgment by the nurse also entered into the final CDR ratings.
Covariates
Neuropsychiatric disturbance was evaluated by the nurses using the ten-item version of the Neuropsychiatric Inventory (Cummings, et al. 1994) that assesses delusions, hallucinations, dysphoria, anxiety, agitation or aggression, euphoria, disinhibition, irritability or lability, apathy, and aberrant motor activity. Symptoms not endorsed are assigned a score of 0, and each endorsed symptom is rated on frequency from 1 (occasionally) to 4 (very frequently) and on severity from 1 (mild) to 3 (marked). A composite neuropsychiatric disturbance score (range: 0–120) then sums the product of Frequency × Severity ratings across the 10 domains.
Contextual factors describing the caregiving environment assessed whether the participant with AD resided at home or coresided with the caregiver and whether anyone else assisted the caregiver with provision of care. The use of formal or informal services by the caregiver was assessed using the Service Utilization and Resource Form (Schneider et al., 2001) dichotomized into one or more services versus no services used. Caregivers reported on felt stress by rating “the degree, if any, that the participant's present condition interferes with your ability to carry on a normal life style,” coded from 1 (no problem) to 10 (can no longer cope).
Analysis
Linear mixed models (Fitzmaurice, Laird, & Ware, 2004) were computed on the trajectory of MMSE scores and CDR scores from enrollment visit forward, with key independent variables: RC and caregiver “type” (spouse or adult child of the participant). The main effect of closeness (or caregiver type) assessed whether scores on the dependent variable were significantly different, on average, between levels of the main effect. The interaction with time tested whether or not closeness (or caregiver type) was significantly associated with decline over time on the MMSE or CDR. Mixed model parameter estimates for all effects including time give information about annual rates of change. Model fitting proceeded with initial models to test the effect of closeness alone, then caregiver type alone. To determine whether observed effects of closeness and caregiver type were confounded by AD severity, a final model controlled for indicators of dementia severity at enrollment visit (dementia duration, functional status, and behavioral disturbances). The quadratic effect of time (time2) was examined to test for curvilinear effects on cognitive and functional status trajectories and to examine whether predictor variables exerted curvilinear effects on these outcomes via interaction of the time**2 term with each predictor. Effects of age, gender, and education of the CR were also examined (along with their interactions with time). All analyses were performed using SPSS version 15.0 (Chicago, IL).
RESULTS
Cognitive status, as measured by the MMSE, varied across time from Visit 1 (M = 19.4, SD = 7.2) to Visit 6 (M = 11.5, SD = 10.2), whereas functional status, based on the SumCDR, varied across time from Visit 1 (M = 7.7, SD = 5.3) to Visit 6 (M = 14.6, SD = 7.9). Individual RC statements describing the current relationship between caregiver and “CR” ranged from 1 (strongly disagree) to 4 (strongly agree) and included the following: CR always understands what I value in life (M = 2.88, SD = 0.92), My relationship with CR is close (M = 3.35, SD = 0.76), My relative always makes me feel that whatever I do for him/her, it is not enough (reverse coded; M = 3.42, SD = 0.77), CR makes me feel like a special person (M = 3.27, SD = 0.71), CR is often critical of me (reverse coded; M = 3.29, SD = 0.75), and CR and I can always discuss things together (M = 2.80, SD = 0.90). The composite closeness score had a baseline mean of 18.0 (SD = 4.2). Cronbach's alpha reliability for the scale was .88. Caregiver stress responses had a mean of 3.64 (SD = 1.74) for adult child and a mean of 3.93 (SD = 1.88) for spouse caregivers (p = .321), whereas coresidency was more common for spouse (92%) than for adult child (22%) caregivers.
We examined attrition effects by comparing persons with AD who remained in the study through all six semi-annual visits with those who dropped out (or died) at some point after the initial visit. These two groups did not differ on enrollment visit age (p = .390), education (p = .128), gender (p = .234), whether coresiding with caregiver (p = .455), whether institutionalized (p = .260), or caregiver gender (p = .109).
Cognitive Trajectory
In the initial MMSE model, there was a significant Closeness × Time interaction (p = .009): Higher levels of closeness were associated with a significantly slower rate of decline in MMSE scores (Table 1). In a separate model comparing caregiver type, there was also a significant Type × Time interaction (p = .003); those with spouse caregivers experienced a slower rate of decline than those with adult child caregivers. A quadratic effect for time was consistently nonsignificant in all models (p = .27–.40) as was the interaction of the quadratic time effect and each predictor (p = .26–.97); thus, these terms were removed.
Table 1.
Mixed Models of Repeated MMSE Scores as a Function of Caregiving Relationship and Caregiver Type, Controlling Also for Additional Explanatory Factors (including age, sex, and education)
| Model 1: closeness | Model 2: type | Model 3: full model | |
| Closeness | 0.15 (p = .287) | −0.06 (p = .557) | |
| Type | 0.06 (p = .962) | −1.34 (p = .113) | |
| Time | −5.20 (p = .000) | −3.40 (p = .000) | −5.18 (p = .000) |
| Closeness × Time | 0.16 (p = .009) | 0.12 (p = .048) | |
| Type × Time | 1.44 (p = .003) | 1.16 (p = .014) | |
| Dementia duration | −0.39 (p = .035) | ||
| Baseline functional status | −1.04 (p = .000) | ||
| Behavioral disturbance | −0.07 (p = .093) |
Notes: Parameters are estimates of the average difference in MMSE score, adjusted for all terms in the model; Type: 1 = spouse caregiver, 2 = adult child caregiver (adult child is reference category); Closeness: range from 6 to 24; Time2 not significant (p = .529), so was removed; Closeness × Type × Time not significant (p = .625), so removed from Model 3 and later models; age (p = .718), gender (p = .091), and education (p = .275) not significant (nor interactions with time), so were removed; Dementia duration × Time (p = .084), Functional status × Time (p = .650), and Behavioral disturbance × Time (p = .198) were not significant, so were removed; a simple model (data not shown) with only the effect for time yields a parameter estimate indicating a 2.52-point average annual decline, consistent with other studies cited herein with a typical 3-point average annual decline on the MMSE. MMSE = Mini-Mental State Exam
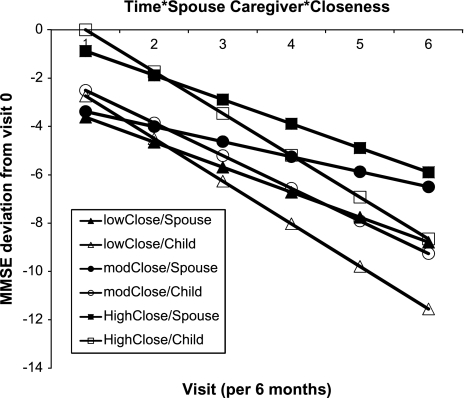
In the final model, longer dementia duration (p = .035) and poorer baseline functional status (p < .001) were associated with lower cognitive status (but not rate of progression), whereas higher closeness (p = .048) and spouse caregiver type (p = .014) were associated with slower progression. In this model, an average 1-unit increase per closeness item (i.e., a 6-unit increase across the closeness composite score) was associated with 0.72 points per year slower MMSE decline. Participants with spouse caregivers showed an average 1.16 points per year slower MMSE decline than those with adult child caregivers. At each level of closeness, participants with spouse caregivers had higher MMSE scores and slower decline than those with adult child caregivers (Figure 1). Furthermore, within each caregiver type, increasing closeness was associated with higher MMSE scores. In intermediate models (data not shown), the effects of age (p = .718), gender (p = .091), and education (p = .275) of the person with AD were nonsignificant, so they were removed from the final model.
Figure 1.
Model-based adjusted MMSE trajectory by caregiver type (spouse vs adult child) and relationship closeness (grouped into tertiles); MMSE range: 0–30. MMSE = Mini-Mental State Exam.
Functional Trajectory
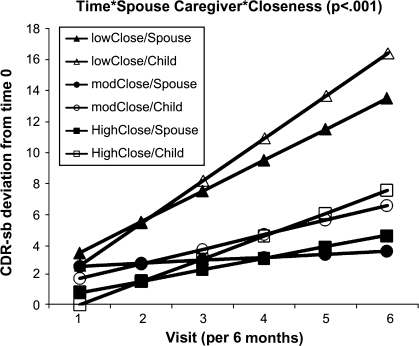
Results with assessment of functional capacities were similar. In the initial mixed model of CDR, there was a significant Closeness × Time interaction (p = .020); higher levels of closeness were associated with a significantly slower functional decline (Table 2). In a separate model, there was a significant Type × Time interaction (p = .041), indicating that participants with spouse caregivers experienced a slower rate of decline than did those with adult child caregivers (nonsignificant quadratic term). In the final model, longer dementia duration and greater behavioral disturbance were significantly associated with poorer functional status (both p < .001) but not rate of progression. The significant Closeness × Type × Time interaction (p = .007, graphically depicted in Figure 2) revealed that for each 1-unit increase per closeness item (i.e., a 6-unit increase across the closeness composite score), participants with spouse caregivers showed 1.7 CDR points per year slower functional decline than those with adult child caregivers.
Table 2.
Mixed Models of Repeated Functional Status (CDR) Scores as a Function of Caregiving Relationship and Caregiver Type, Controlling Also for Additional Explanatory Factors (including age, sex, and education)
| Model 1: closeness | Model 2: type | Model 3: full model | |
| Closeness | −0.12 (p = .278) | −0.01 (p = .941) | |
| Type | −1.10 (p = .291) | 1.27 (p = .189) | |
| Time | 4.40 (p = .000) | 2.80 (p = .000) | 1.24 (p = .002) |
| Closeness × Time | −0.12 (p = .020) | 0.08 (p = .433) | |
| Type × Time | −0.90 (p = .041) | 3.82 (p = .026) | |
| Closeness × Type × Time | −0.28 (p = .007) | ||
| Female gender | −1.71 (p = .058) | ||
| Dementia duration | 1.11 (p = .000) | ||
| Behavioral disturbances | 0.18 (p = .000) |
Notes: Type: 1 = spouse caregiver, 2 = adult child caregiver (adult child is reference category); Closeness: range from 6 to 24; Time2 not significant (p = .057), so was removed; age (p = .361) and education (p = .844) were not significant (nor interactions with time), so were removed; Dementia duration × Time (p = .886) and Behavioral disturbance × Time (p = .100) were not significant, so were removed. CDR = Clinical Dementia Rating.
Figure 2.
Model-based adjusted functional trajectory (CDR) by caregiver type (spouse vs adult child) and relationship closeness (grouped into tertiles); CDR range: 0–30. CDR = Clinical Dementia Rating.
Other Covariate Effects
Caregiver stress and use of support services did not differ by caregiver type. However, participants with spouse caregivers were more likely to be living at home and to coreside with the caregiver than those whose caregiver was an adult child. Therefore, we constructed other models that adjusted for coresidence. On average, MMSE scores were higher among those who coresided with their caregiver (p = .032), but coresidence was not associated with rate of MMSE decline (p = .491; results not shown). Adjustment for coresidence had negligible change on the apparent effects of other factors in the model.
DISCUSSION
This is the first study to directly examine the association of RC between the CR and the care provider, as reported by caregivers, with subsequent rate of cognitive and functional decline in CRs with AD. With closer relationships, participants declined more slowly in cognition and functional capacity, even after adjustment for several potential confounders. These findings were stronger when caregivers were the spouses of the participants with AD. Furthermore, effects observed in the present study were on the same order of magnitude as reported in a recent meta-analysis of nine clinical trials using acetylcholinesterase inhibitors (AChEIs; Birks, 2006). Averaging across the nine studies, mean difference in MMSE score between those treated with AChEIs versus placebo was 1.37 MMSE points annual change (95% CI = 1.13–1.61) measured over an interval ranging from 6 to 12 months. Our findings of 0.72- and 1.16-point differences in annual rate of change of MMSE (for effect of closer relationships and spouse caregivers, respectively) are promising. They suggest that interventions enhancing the caregiving dyadic relationship, including those already developed to improve care management strategies (Logsdon, McCurry, Moore, & Teri, 1997), would slow the progression of dementia.
The slower rates of cognitive and functional decline in those with AD who have spouse caregivers suggest a particular importance of marital relationships in dementia care. Persons with dementia and their spouses are noted to be “living in relationship” (Davies & Gregory, 2007) with shared meanings continuously cocreated by the couple (Graham & Bassett, 2006). Older married couples in our sample are probably typical of such couples elsewhere in their shared long-term commitment to mutual assistance through adversity (the duration of their marriages averaged 51.3 years, SD = 16.6). Interdependence theory and the investment model support the notion that such commitment makes spouses more willing to accept a caregiving role and sacrifice self-interest than adult children, who must balance parent care responsibilities with responsibilities to other family members, including spouses and children (Piercy, 1998). The need for such a balance may explain why adult child and spouse caregivers reported similarly moderate stress levels despite the fact that coresidency was more common for spouse than adult child caregivers.
Although caregiver–CR relationships have developed over the lifetime of the marriage (for spouse caregivers) or child (for adult child caregivers), work done by Kitwood (1990, 1993) and Graham and Bassett (2006) affirm the dynamic nature of dyadic relationships, thus suggesting their potential for change through intervention, even in the late stages of a relationship.
Because commitment to and investment in the relationship with the CR are likely to be important to the success of dyadic interventions in dementia caregiving, interventions that focus on collaborative aspects of care dyads have shown promise in improving these relationships. Quayhagen and Quayhagen's (1996) 4-month cognitive remediation intervention improved spouse relationships as dyads rediscovered life quality. More recently, a counselor-guided dyadic intervention for family caregivers and persons with early-stage dementia designed to help care partners (including non–spouse caregivers) plan for future care needs showed success in attaining concrete plans (Whitlatch, Judge, Zarit, & Femia, 2006). Critical to the success of this intervention was a sense that persons with dementia felt listened to and understood by their caregivers.
One way in which closer relationships might predict improved outcomes is their tendency toward more successful and adaptive care management strategies. For example, engagement of persons with AD in cognitively and socially stimulating activities (Graham & Bassett, 2006) may in turn slow the rate of cognitive decline (Quayhagen & Quayhagen, 1996; Quayhagen & Quayhagen, 2001). We also note, however, that caregivers in our sample who reported higher levels of closeness also described greater use of services such as respite care, meal delivery, housekeeping, and so forth (data not shown)—any or all of which might lessen the daily demands of caregiving and allow more time for the pair to share meaningful activities. Respected service providers may also teach caregivers to become more skillful at care provision (Piercy & Dunkley, 2004).
Among this study's strengths is its use of a population-based sample, often more representative than clinic-based samples with higher occupational and educational status and younger onset of AD (Kokmen, Ozsarfati, Beard, O'Brien, & Rocca, 1996). Other strengths include a detailed and standardized diagnostic workup of the participants with new-onset AD, a high enrollment rate, and a longitudinal design with semi-annual visits.
An important limitation of this work is the brevity and simplicity of the RC instrument, which was included among many other measures used in the CC-DPS. The six-item instrument was originally designed for studying adjustment to nursing home placement (Whitlatch et al., 2001). Notwithstanding its apparent face validity, this scale has not specifically been validated for measurement of such important variables as communication quality, mutual respect, empathy, and affection. Furthermore, given the nature of the sample, we cannot assess the extent to which the participants' perceptions of RC might be predictive of clinical course. Seventy-five percent of caregivers were female (45 wives, 81 daughters/daughters-in-law), as is typically the case with dementia caregiving. We therefore did not have sufficient numbers to be able to stratify analyses by all combinations of spouse versus adult child caregiver type, gender of caregiver, and gender of CR. Thus, the study of differential effects of these eight combinations awaits further data collection, with results presented here somewhat more generalizable to dyads with female caregivers. Finally, our findings may be relatively specific to mild-to-moderate AD. Additional years of follow-up will be needed to learn whether the effects reported here appear only in the mild and moderate stages of AD or whether they will remain important as symptoms progress.
In this population-based study of AD progression, a closer caregiving relationship was associated with slower progression of cognitive and functional symptoms, particularly for persons with spouse caregivers. Although caution is warranted because the direction of effect may be one in which slower cognitive or functional decline promotes closer relationships, findings were robust after control for dementia severity, raising questions about caregiver strategies that may promote better functioning in dementia. Furthermore, although our measure of functional status was not entirely independent of the caregiver (and therefore the caregiver's RC report), the consistency of our results across both the functional and the objectively measured cognitive domains lends further support to them.
It is conceivable that RC may reflect caregiver strategies that flexibly adapt to the needs of the person with AD, encouraging, when appropriate, participation in cognitively and socially stimulating activities, which help promote sustained functioning. Conversely, less close caregivers may experience more negative stress from the burden of care provision to a person with whom they feel more distant, especially if the caregiver has little understanding of what to expect as dementia progresses. Furthermore, persons cared for by more stressed caregivers may be at greater risk of behavioral neuropsychiatric symptoms; this may result in increased use of psychotropic medications or interfere with treatment of comorbid medical conditions and thereby accelerate decline. Additional studies can help to clarify if RC is a marker for caregiver personality, stress, burden, interdependence with CR, care management strategies, or other factors that affect clinical course of dementia. Such factors could be targets of future interventions designed to enhance the caregiving dyadic relationship in an effort to slow decline in AD.
FUNDING
National Institutes of Health grants AG021136, AG011380, AG031272, AG018712, and AG027841 including study design, data collection, data management and analysis, interpretation of the data and preparation, review, and approval of the manuscript.
Acknowledgments
The authors wish to thank the study participants and their families for their generosity and willingness to participate. Other Cache County Study investigators involved in the project include (in alphabetic order): James Anthony, PhD; Erin Bigler, PhD; Ron Brookmeyer, PhD; James Burke, MD; Michelle Carlson, PhD; Eric Christopher, MD; Jane Gagliardi, MD; Andrea Hart, PhD; Kate Hayden, PhD; Christine Hulette, MD; Liz Klein, MPH; Carol Leslie, MA; Richard A. Miech, PhD; John Morris, MD; Ronald Munger, PhD; Chiadi Onyike, MD; Ron Petersen, MD; Roxane Pfister, MS; Carl Pieper, PhD; Brenda Plassman, PhD; Pritham Raj, MD; Russell Ray, MS; Linda Sanders, MPH; Ingmar Skoog, MD; David C. Steffens, MD; Martin Steinberg, MD; Marty Toohill, PhD; Leslie Toone, MS; Jeannette J. Townsend, MD; Heidi Wengreen, PhD; Nancy West, PhD; Michael Williams, MD; Bonita W. Wyse, PhD; and Peter P. Zandi, PhD. Portions of this paper were presented at the annual conference of the American Association for Geriatric Psychiatry, Orlando, Florida, in March, 2008.
References
- Alzheimer's Association. (2008). Alzheimer's disease facts and figures. Alzheimer's & Dementia. 4(2):110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Prognostic factors in very old demented adults: A seven-year follow-up from a population-based survey in Stockholm. Journal of the American Geriatric Society. 1998;46:444–452. doi: 10.1111/j.1532-5415.1998.tb02464.x. [DOI] [PubMed] [Google Scholar]
- Behl P, Stefurak TL, Black SE. Progress in clinical neurosciences: Cognitive markers of progression in Alzheimer's disease. Canadian Journal of Neurological Sciences. 2005;32(2):140–151. doi: 10.1017/s0317167100003917. [DOI] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD005593. CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler JV, Munoz DG, Merskey H, Hachinski V. Factors affecting the age of onset and rate of progression of Alzheimer's disease. Journal of Neurology, Neurosurgery, & Psychiatry. 1998;65:184–190. doi: 10.1136/jnnp.65.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JCS, Wyse BW, Anthony JCA, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, et al. APOE4 count predicts age when prevalence of AD increases, then declines, the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Burgener S, Twigg P. Relationships among caregiver factors and quality of life in care recipients with irreversible dementia. Alzheimer Disease and Associated Disorders. 2002;16:88–102. doi: 10.1097/00002093-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davies J, Gregory D. Entering the dialogue: Marriage biographies and dementia care. Dementia. 2007;6:481–488. [Google Scholar]
- de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Winkens I, Jolles J, Verhey FR. Do caregiver management strategies influence patient behaviour in dementia? International Journal of Geriatric Psychiatry. 2004;19:85–92. doi: 10.1002/gps.1044. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New York: Wiley-Interscience; 2004. [Google Scholar]
- Gaugler JE, Davey A, Pearlin LI, Zarit SH. Modeling caregiver adaptation over time: The longitudinal impact of behavior problems. Psychology and Aging. 2000;15:437–450. doi: 10.1037//0882-7974.15.3.437. [DOI] [PubMed] [Google Scholar]
- Graham JE, Bassett R. Reciprocal relations: The recognition and co-construction of caring with Alzheimer's disease. Journal of Aging Studies. 2006;20:335–349. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Kelley HH, Thibaut JW. Interpersonal relations: A theory of interdependence. New York: Wiley; 1978. [Google Scholar]
- Kitwood T. The dialectics of dementia: With particular reference to Alzheimer's disease. Ageing and Society. 1990;10:177–196. [Google Scholar]
- Kitwood T. Towards a theory of dementia care: The interpersonal process. Ageing and Society. 1993;13:51–67. doi: 10.1017/s0144686x0000502x. [DOI] [PubMed] [Google Scholar]
- Kokmen E, Ozsarfati Y, Beard CM, O'Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer's disease. Journal of Clinical Epidemiology. 1996;49:79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: An interdependence and communal coping approach. Social Science & Medicine. 2006;62:1369–1380. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, McCurry SM, Moore AL, Teri L. Family and caregiver issues in the treatment of patients with Alzheimer's disease. Seminars in Clinical Neuropsychiatry. 1997;2(2):138–151. doi: 10.1053/SCNP00200138. [DOI] [PubMed] [Google Scholar]
- Lucca U, Comelli M, Tettamanti M, Tiraboschi P, Spagnoli A. Rate of progression and prognostic factors in Alzheimer's disease: A prospective study. Journal of the American Geriatrics Society. 1993;41:45–49. doi: 10.1111/j.1532-5415.1993.tb05947.x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County Study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- Morris JC, Edland S, Clark C, Galasko D, Koss E, Mohs R, van Belle G, Fillenbaum G, Heyman A. The consortium to establish a registry for Alzheimer's disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer's disease. Neurology. 1993;43:2457–2465. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- Noelker LS. Promoting positive relationships between nursing assistants and the families of cognitively impaired nursing home residents. Final report to the Cleveland Foundation. Cleveland, OH: A Benjamin Rose Institute; 1996. [Google Scholar]
- Ory MG, Hoffman RR, 3rd, Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. The Gerontologist. 1999;39:177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- Perren S, Schmid R, Herrmann S, Wettstein A. The impact of attachment on dementia-related problem behavior and spousal caregivers' well-being. Attachment & Human Development. 2007;9:163–178. doi: 10.1080/14616730701349630. [DOI] [PubMed] [Google Scholar]
- Piercy KW. Theorizing about family caregiving: The role of responsibility. Journal of Marriage and the Family. 1998;60:109–118. [Google Scholar]
- Piercy KW, Dunkley GJ. What quality paid home care means to family caregivers. Journal of Applied Gerontology. 2004;23:175–192. [Google Scholar]
- Quayhagen MP, Quayhagen M. Discovering life quality in coping with dementia. Western Journal of Nursing Research. 1996;18:120–135. doi: 10.1177/019394599601800202. [DOI] [PubMed] [Google Scholar]
- Quayhagen MP, Quayhagen M. Testing of a cognitive stimulation intervention for dementia caregiving dyads. Neuropsychological Rehabilitation. 2001;11:319–332. [Google Scholar]
- Regan C, Katona C, Walker Z, Hooper J, Donovan J, Livingston G. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67:1357–1362. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- Rusbult CE, Buunk BP. Commitment processes in close relationships: An interdependence analysis. Journal of Social and Personal Relationships. 1993;10:175–204. [Google Scholar]
- Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer's disease. Alzheimer's & Dementia. 2008;4(2):65–79. doi: 10.1016/j.jalz.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Devanand D, Honig L, Marder K, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Archives of Neurology. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, Hsiao JK, Jeste DV, Katz IR, Olin JT, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. American Journal of Geriatric Psychiatry. 2001;9:346–360. [PubMed] [Google Scholar]
- Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, Cruts M, van Broeckhoven C, Breteler MM, Hofman A, Stijnen T, van Duijn CM. Apolipoprotein E genotype and progression of Alzheimer's disease: The Rotterdam Study. Journal of Neurology. 1999;246:304–308. doi: 10.1007/s004150050351. [DOI] [PubMed] [Google Scholar]
- Small BJ, Viitanen M, Winblad B, Backman L. Cognitive changes in very old persons with dementia: The influence of demographic, psychometric, and biological variables. Journal of Clinical and Experimental Neuropsychology. 1997;19:245–260. doi: 10.1080/01688639708403855. [DOI] [PubMed] [Google Scholar]
- Stern RG, Mohs RC, Davidson M, Schmeidler J, Silverman J, Kramer-Ginsberg E, Searcey T, Bierer L, Davis , K. L. A longitudinal study of Alzheimer's disease: Measurement, rate, and predictors of cognitive deterioration. American Journal of Psychiatry. 1994;151:390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, Bell K, Sano M, Devanand DP, Bylsma F, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer's disease: Prospective analyses from the Predictors Study. Neurology. 1994;44:2300–2307. doi: 10.1212/wnl.44.12.2300. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Teri L, McCurry SM, Edland SD, Kukull WA, Larson EB. Cognitive decline in Alzheimer's disease: A longitudinal investigation of risk factors for accelerated decline. Journal of Gerontology: Biological Sciences and Medical Sciences. 1995;50A:M49–M55. doi: 10.1093/gerona/50a.1.m49. [DOI] [PubMed] [Google Scholar]
- Whitlatch CJ, Judge K, Zarit SH, Femia E. Dyadic intervention for family caregivers and care receivers in early-stage dementia. The Gerontologist. 2006;46:688–694. doi: 10.1093/geront/46.5.688. [DOI] [PubMed] [Google Scholar]
- Whitlatch CJ, Schur D, Noelker LS, Ejaz FK, Looman WJ. The stress process of family caregiving in institutional settings. The Gerontologist. 2001;41:462–473. doi: 10.1093/geront/41.4.462. [DOI] [PubMed] [Google Scholar]
- Wright LK. Alzheimer's disease afflicted spouses who remain at home: Can human dialectics explain the findings? Social Science & Medicine. 1994;38:1037–1046. doi: 10.1016/0277-9536(94)90220-8. [DOI] [PubMed] [Google Scholar]