Abstract
A rapid inoculum standardization system for antimicrobial susceptibility testing without incubation or the conventional turbidity adjustment has been developed. The rapid inoculum standardization system consists of a plastic rod with cross-hatched grooves on one end and a specific nutrient medium in a vial. The crosshatched grooves are designed to pick up and release a known number of viable microorganisms. In use, the end of the rod is touched to five colonies 1 to 2 mm in diameter from a primary agar plate, thus filling the grooves with bacteria. The rod is placed into the vial, and the bacteria are suspended in the medium by agitation with a Vortex Genie Mixer. The resulting suspension contains 5 X 10(7) to 5 X 10(8) CFU/ml for most gram-negative bacilli and gram-positive cocci. Microorganisms such as streptococci that have colonies less than 1 mm in diameter require as many as 10 colonies for an adequate inoculum suspension. Ninety-five commonly encountered bacterial isolates were tested in triplicate by agar plate counts. The resulting overall geometric mean of the agar plate counts was 1.52 X 10(8) CFU/ml for the species tested. We have found that the rapid inoculum standardization system provides a consistent and reproducible method for the standardization of inoculum for antimicrobial susceptibility testing without the incubation period and turbidity adjustment.
Full text
PDF
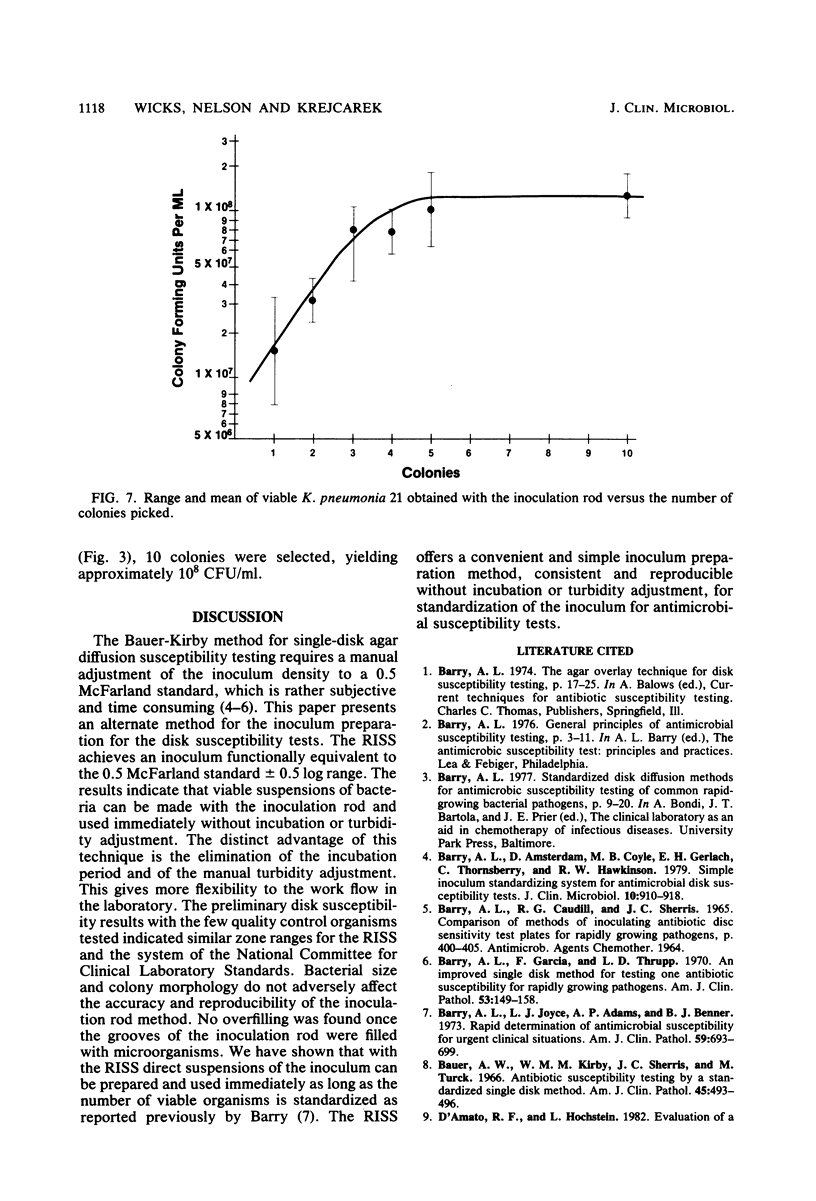

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Amsterdam D., Coyle M. B., Gerlach E. H., Thornsberry C., Hawkinson R. W. Simple inoculum standardizing system for antimicrobial disk susceptibility tests. J Clin Microbiol. 1979 Dec;10(6):910–918. doi: 10.1128/jcm.10.6.910-918.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Garcia F., Thrupp L. D. An improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens. Am J Clin Pathol. 1970 Feb;53(2):149–158. doi: 10.1093/ajcp/53.2.149. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Joyce L. J., Adams A. P., Benner E. J. Rapid determination of antimicrobial susceptibility for urgent clinical situations. Am J Clin Pathol. 1973 May;59(5):693–699. doi: 10.1093/ajcp/59.5.693. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- D'Amato R. F., Hochstein L. Evaluation of a rapid inoculum preparation method for agar disk diffusion susceptibility testing. J Clin Microbiol. 1982 Feb;15(2):282–285. doi: 10.1128/jcm.15.2.282-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]