Abstract
Although the presence of a BH4 domain distinguishes the antiapoptotic protein Bcl-2 from its proapoptotic relatives, little is known about its function. BH4 deletion converts Bcl-2 into a proapoptotic protein, whereas a TAT-BH4 fusion peptide inhibits apoptosis and improves survival in models of disease due to accelerated apoptosis. Thus, the BH4 domain has antiapoptotic activity independent of full-length Bcl-2. Here we report that the BH4 domain mediates interaction of Bcl-2 with the inositol 1,4,5-trisphosphate (IP3) receptor, an IP3-gated Ca2+ channel on the endoplasmic reticulum (ER). BH4 peptide binds to the regulatory and coupling domain of the IP3 receptor and inhibits IP3-dependent channel opening, Ca2+ release from the ER, and Ca2+-mediated apoptosis. A peptide inhibitor of Bcl-2-IP3 receptor interaction prevents these BH4-mediated effects. By inhibiting proapoptotic Ca2+ signals at their point of origin, the Bcl-2 BH4 domain has the facility to block diverse pathways through which Ca2+ induces apoptosis.
Keywords: inositol 1,4,5-trisphosphate receptor; TAT-BH4; T cell receptor; WEHI7.2; Jurkat
The inositol 1,4,5-trisphosphate (IP3) receptor is an IP3-gated Ca2+ channel on the endoplasmic reticulum (ER) (1). IP3-induced Ca2+ release from the ER generates Ca2+ signals that regulate many processes including cell proliferation and survival (2). However Ca2+ signals initiated by IP3 can also promote cell death (3). Therefore, IP3 receptor channel opening is closely regulated by phosphorylation and accessory proteins that interact with the IP3 receptor (1, 4). Among the accessory proteins are the antiapoptotic proteins BcI-2 and Bcl-XL (5).
Our work has documented the interaction of Bcl-2 with IP3 receptors by co-immunoprecipitation, blue native gel electrophoresis, and FRET (6, 7). Through this interaction, Bcl-2 reversibly inhibits IP3-dependent channel opening and Ca2+ release from the ER, thus inhibiting T-cell−receptor–induced apoptosis (6, 8). Conversely, an interaction of Bcl-XL with the IP3 receptor is reported to enhance IP3-mediated Ca2+ release from the ER (9, 10). Recently, the site of Bcl-2 interaction was mapped to the IP3 receptor regulatory and coupling domain (7). This domain functions both to keep the inactivated IP3 receptor channel closed and to transfer the ligand-binding signal from the N-terminal IP3-binding domain to the C-terminal channel domain, thus causing the channel to open (1, 11). A 20-aa peptide corresponding to the Bcl-2 binding site functions as a competitive inhibitor of Bcl-2-IP3 receptor interaction (7). This peptide, referred to as peptide 2 (Pep2), reverses Bcl-2–mediated inhibition of IP3 receptor channel opening in vitro (7). Also, when delivered into cells via Chariot peptide uptake reagent or by fusion with HIV TAT cell–penetrating peptide, Pep2 reverses Bcl-2-imposed inhibition of IP3-mediated Ca2+ elevation and apoptosis (7).
Members of the Bcl-2 protein family share regions of sequence similarity, the Bcl-2 homology (BH) domains (12). Antiapoptotic family members, including Bcl-2 and Bcl-XL, have four BH domains, BH1–4, whereas proapoptotic family members lack the BH4 domain. The three-dimensional structures of Bcl-2 and Bcl-XL, determined by NMR spectroscopy, reveal that the BH1, 2 and 3 domains form a hydrophobic groove where proapoptotic proteins bind (13, 14). The interaction between Bcl-2 and its proapoptotic relatives accounts for much of the antiapoptotic activity of Bcl-2. This activity is currently being targeted therapeutically because of the important role of Bcl-2 in promoting cancer cell survival (15, 16). Molecules such as ABT-737 bind in the hydrophobic groove and displace proapoptotic proteins, thereby promoting apoptosis. However, BH1, 2, and 3 are not the only domains important for the antiapoptotic activity of Bcl-2. The BH4 domain is also important for the antiapoptotic activity of Bcl-2, as Bcl-2 lacking its BH4 domain (ΔBH4Bcl-2) promotes rather than inhibits apoptosis, even though it still heterodimerizes with proapoptotic family members (17, 18). Also, removal of the BH4 domain by caspase-mediated cleavage converts Bcl-2 to a Bax-like death effector (19, 20). Finally, the BH4 domains of Bcl-2 and Bcl-XL inhibit apoptosis when introduced into cells by fusion with the HIV TAT cell–penetrating peptide (21, 22). Thus, the BH4 domain has intrinsic antiapoptotic activity independent of BH domains 1–3, although the function(s) of the BH4 domain are not fully understood. Nevertheless, this antiapoptotic activity is currently exploited in experimental animal models for treatment of disorders associated with accelerated apoptosis, including Alzheimer's disease, ischemia reperfusion injury, spinal cord injury, and sepsis-induced lymphocyte death (23, 24). Thus, TAT-BH4 peptides have therapeutic value in these disease models by prolonging cell survival.
In the work reported here, the BH4 domain of Bcl-2 is found to be both necessary and sufficient for interaction with the IP3 receptor. These findings identify a novel function of the BH4 domain that contributes to the overall antiapoptotic activity of the Bcl-2 protein and that may be of value as a potential therapeutic target.
Results
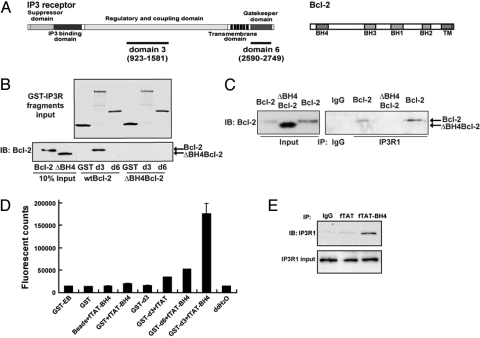
A diagram depicting the location of the BH domains within Bcl-2 is included in Fig. 1A. A series of GST-IP3 receptor fragments that correspond to natural domains of type 1 IP3 receptor (also shown in Fig. 1A) were previously used to map the Bcl-2 binding region on the IP3 receptor (7). Bcl-2 interacts with IP3 receptor domain 3 but not with domain 6. Therefore, GST fragments corresponding to domains 3 and 6 were used in GST-pulldown experiments with Bcl-2 and ΔBH4Bcl-2, a Bcl-2 mutant lacking the BH4 domain, to determine whether the BH4 domain is necessary for the interaction of Bcl-2 with the IP3 receptor (Fig. 1B). Full-length Bcl-2 was pulled down by GST-domain 3 but not by GST-domain 6, whereas ΔBH4Bcl-2 was not pulled down by either of the IP3 receptor fragments. Thus, BH4 domain deletion abrogates the interaction of Bcl-2 with the IP3 receptor. This conclusion is substantiated by evidence that Bcl-2, but not ΔBH4Bcl-2, co-immunoprecipitates with the IP3 receptor (Fig. 1C). The amount of ΔBH4Bcl-2 was intentionally higher than the amount of Bcl-2 in co-immunoprecipitations to detect a potential weak interaction of ΔBH4Bcl-2 with the IP3 receptor, but no interaction was detected.
Fig. 1.
BH4 domain is necessary for Bcl-2-IP3 receptor interaction. (A) (Upper) Diagram depicting IP3 receptor type 1 and its functional domains. (Lower) Diagram depicting Bcl-2, its four BH domains and the C-terminal hydrophobic domain (TM). Diagrams are not drawn to scale. (B) GST pull down showing that BH4 deletion inhibits interaction of Bcl-2 with IP3 receptor domain 3 (d3). (Upper) Coomassie blue–stained gel showing GST-IP3 receptor fragment input levels. (Lower) Immunoblot showing input levels of Bcl-2 and ΔBH4Bcl-2 and Bcl-2 pulled down by IP3 receptor domain 3 (d3). (C) Co-immunoprecipitation of Bcl-2, but not ΔBH4Bcl-2, with the IP3 receptor. Type 1 IP3 receptor was immunoprecipitated from WEHI7.2 cells expressing either Bcl-2 or ΔBH4Bcl-2. (D) Binding of FITC-labeled TAT-BH4 (fTAT-BH4) to IP3 receptor domain 3. fTAT-BH4 (40 μM) was incubated with GST-IP3 receptor fragments corresponding to IP3 receptor domains 3 (GST-d3) and 6 (GST-d6) and the amount of fTAT-BH4 bound was quantified by fluorescence measurements. Negative controls included GST-EB (elution buffer), GST alone (GST), glutathione beads alone, GST-d3 alone, and FITC-TAT (fTAT). Symbols represent mean ± SEM of three experiments. (E) Co-immunoprecipitation of fTAT-BH4 peptide with IP3 receptor 1 in WEHI7.2 cell extracts. The WEHI7.2 cell extracts were incubated with 40 μM fTAT or fTAT-BH4 for 2 h before pull down by anti-fluorescein antibody. Immunoblot analysis was performed with anti-IP3 receptor 1 antibody.
Also, pulldown and co-immunoprecipitation experiments were performed to determine whether the Bcl-2 BH4 domain alone is sufficient to interact with the IP3 receptor. These experiments used a fusion peptide, TAT-BH4, in which the cell-penetrating peptide of human immunodeficiency virus (HIV) TAT (25) is fused to a synthetic peptide corresponding to the BH4 domain. FITC-labeled TAT-BH4 (fTAT-BH4) was pulled down by GST-IP3 receptor-domain 3 but not by GST-IP3 receptor-domain 6, GST alone or by glutathione beads alone (Fig. 1D). Also, the interaction of fTAT-BH4 with full-length IP3 receptor was detected by co-immunoprecipitation (Fig. 1E). Collectively, the findings in Fig. 1 indicate that the BH4 domain of Bcl-2 is both necessary and sufficient to interact with the IP3 receptor.
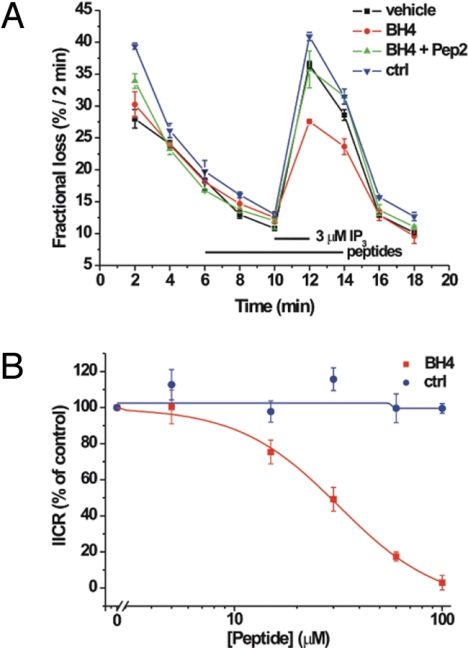
The effect of BH4 peptide (without TAT) on IP3R-mediated Ca2+ release was assessed in unidirectional Ca2+-flux assays (Fig. 2A). In these experiments, the nonmitochondrial Ca2+ stores of saponin-permeabilized wild-type MEF cells were loaded to steady state with 45Ca2+. After incubation with 4 μM thapsigargin, the efflux of 45Ca2+ was followed in the presence of 1 mM EGTA. This approach allows a direct and very accurate quantification of Ca2+ release through IP3 receptors. The addition of IP3 (3 μM) in the efflux medium leads to Ca2+ release, which is observed as an increase in the rate of 45Ca2+ efflux. Preincubation with the BH4 peptide (40 μM) but not with the control peptide (60 μM) caused a marked inhibition of the IP3-induced Ca2+ release. In addition, Pep2 (see introductory section) alleviated this inhibition of IP3-induced Ca2+ release by the BH4 peptide. Finally, we also performed a dose–response analysis, showing that the BH4 peptide inhibits IP3 receptor-mediated Ca2+ release with an IC50 of approximately 32 μM and provokes a nearly complete inhibition at 100 μM (Fig. 2B). Consistent with these findings, planar lipid bilayer analysis indicated that the BH4 domain is sufficient to inhibit IP3 receptor channel opening in vitro (SI Text and Fig. S1).
Fig. 2.
BH4 peptide inhibits IP3 receptor channel activity. (A) BH4 peptide (without TAT) inhibits IP3-induced Ca2+ release from the ER. A typical unidirectional 45Ca2+-efflux experiment showing the Ca2+ release induced by 3 μM IP3 from permeabilized 45Ca2+-loaded wild-type MEF cells in the presence of vehicle (filled squares), 40 μM BH4 peptide (filled circles), 40 μM BH4 peptide, and 40 μM Pep2 (filled triangles) or 60 μM ctrl peptide (inverted filled triangles). All peptides were incubated from 4 min before the addition of IP3 to 2 min after its addition (bars). Data points of a representative experiment, plotted as fractional loss (%/2 min) as a function of time, were obtained in duplicate and represent mean ± SD. Findings are representative of three independent experiments performed in duplicate. (B) Dose–response curve summarizing the effect of different concentrations of BH4 peptide and ctrl peptide on the IP3-induced Ca2+ release from permeabilized wild-type MEF cells. Data points represent mean ± SEM, obtained from at least three independent experiments performed in duplicate and normalized to the amount of Ca2+ release provoked by IP3 under control conditions (vehicle). Logistic curve fitting indicates an IC50 of about 32 μM for the BH4 peptide.
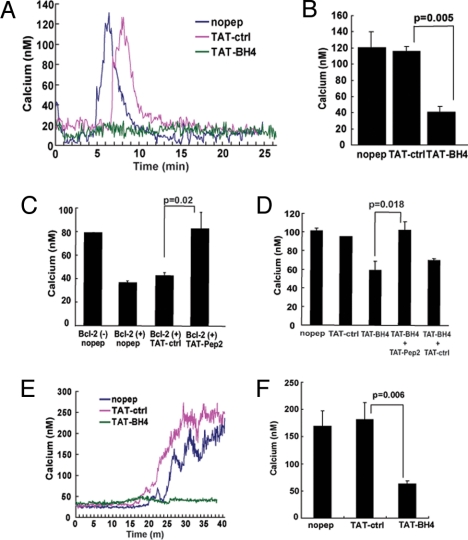
The WEHI7.2 murine T cell line has virtually no detectable Bcl-2, and consequently T-cell receptor activation by anti-CD3 antibody induces a robust, IP3-dependent elevation of cytoplasmic Ca2+ that is inhibited by enforced expression of Bcl-2 (6, 8). To determine whether the BH4 domain is sufficient by itself to inhibit anti-CD3–induced Ca2+ elevation, it was introduced into the WEHI7.2 cells as a TAT-BH4 fusion peptide. A 1-h preincubation with 2 μM TAT-BH4 inhibited anti-CD3–induced Ca2+ elevation in wild-type (Bcl-2–negative) WEHI7.2 cells, whereas the control peptide, TAT-ctrl, did not inhibit anti-CD3–induced Ca2+ elevation (Fig. 3A, B). Moreover, Pep2 prevented the inhibition of anti-CD3-induced Ca2+ elevation not only by full-length Bcl-2 (Fig. 3C) but also by TAT-BH4 (Fig. 3D). Notably, TAT-Pep2 interferes with the BH4 domain-mediated interaction of Bcl-2 with the IP3R but does not interfere with the BH4 domain-mediated interaction of Bcl-2 with VDAC, an interaction originally described by Tsujimoto et al. (21, 26) (SI Text and Fig. S2).
Fig. 3.
Inhibition of IP3-induced Ca2+ elevation by TAT-BH4 and reversal by TAT-Pep2. (A) Representative Ca2+ traces recording the Ca2+ elevation induced by 20 μg/ml anti-CD3 antibody in wild-type WEHI7.2 cells after pretreatment with 2 μM TAT-control (TAT-ctrl) or TAT-BH4 peptides for 1 h. Anti-CD3 was added 1–2 min after recording was started. (B) Histograms summarizing the average peak anti-CD3 induced Ca2+ elevation in WEHI7.2 cells treated with various peptides (mean ± SEM of seven individual experiments, >50 cells per sample per experiment). (C) Peak Ca2+ elevation induced by 20 μg/ml anti-CD3 antibody in wild-type Bcl-2(-) WEHI7.2 cells without any peptide addition, or in Bcl-2(+)WEHI7.2 cells in the absence of peptide and in the presence of either 10 μM TAT-ctrl or TAT-Pep2. Data are from three separate experiments (>50 cells per sample per experiment). Symbols represent mean ± SEM. (D) Peak Ca2+ elevation induced by 20 μg/ml anti-CD3 antibody in WEHI7.2 cells, either untreated or pretreated with 10 μM TAT-ctrl, 2 μM TAT-BH4, 2 μM TAT-BH4 + 10 μM TAT-Pep2, 2 μM TAT-BH4 + 10 μM TAT-ctrl (mean ± SEM of four separate experiments, >50 cells per sample per experiment). (E) Representative Ca2+ traces recording the Ca2+ elevation induced by 40 μM cell-permeable IP3 ester in wild-type Bcl-2 (-) WEHI7.2 cells after pretreatment with 1 μM TAT-ctrl or TAT-BH4 peptide for 1 h. IP3 ester was added 1–2 min after the recording was started. (F) Summary of the peak Ca2+ elevation induced by 50 μM IP3 ester in TAT-ctrl-and TAT-BH4-pretreated cells (mean ± SEM in three separate experiments, >50 cells per sample per experiment).
The effect of TAT-BH4 on calcium elevation induced by a cell-permeable IP3 ester (D-myo InsP3 hexakisbutyryloxymethyl ester) was also investigated to ensure that inhibition of anti-CD3–induced Ca2+ elevation by TAT-BH4 was due to an action of TAT-BH4 on the IP3 receptor rather than an unexpected effect on upstream signaling pathways triggered by anti-CD3–mediated TCR activation. TAT-BH4 inhibited Ca2+ elevation induced by IP3 ester, which bypasses the TCR signaling pathway by directly inducing IP3 receptor channel opening and Ca2+ release from the ER (Fig. 3E,F).
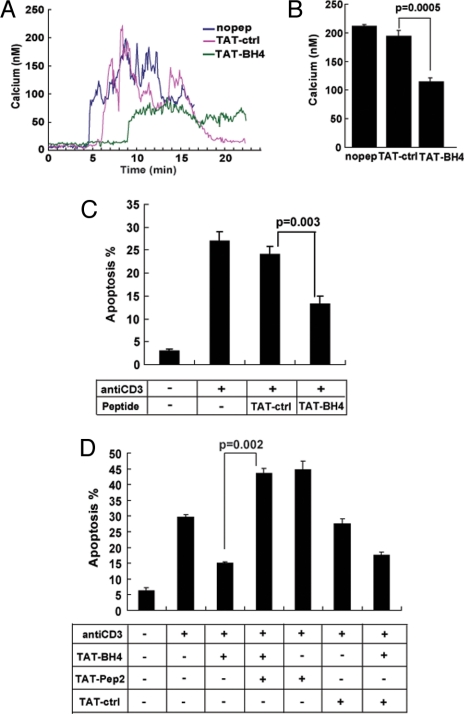
TAT-BH4 also inhibits anti-CD3–induced Ca2+ elevation in the Jurkat human T-cell leukemia line, a convenient model for studying the linkage between IP3-induced Ca2+ elevation and apoptosis (Fig. 4A, B). To determine whether TAT-BH4 has an antiapoptotic action similar to full-length Bcl-2, Jurkat cells were preincubated with TAT-BH4 for 1 h and then treated with anti-CD3 antibody in the continued presence of TAT-BH4 for 24 h. Cells were then stained with Hoechst dye and apoptosis was quantified according to the percentage of cells displaying apoptotic nuclear morphology. The results indicate that TAT-BH4 inhibited anti-CD3-induced apoptosis (Fig. 4C) and also that co-incubation with TAT-Pep2 prevented the inhibition of anti-CD3–induced apoptosis by TAT-BH4 (Fig. 4D). Note that anti-CD3 alone induced approximately 30% apoptosis, whereas the combination of anti-CD3 and TAT-Pep2 induced approximately 45% apoptosis (Fig. 4D). This is consistent with our previous findings in Jurkat cells, in which endogenous Bcl-2 repressed anti-CD3–induced apoptosis and Pep2 reversed the inhibitory action of endogenous Bcl-2 (7). Treating Jurkat cells with TAT-BH4 further inhibited apoptosis (beyond the inhibition attributed to endogenous Bcl-2), and TAT-Pep2 reversed the TAT-BH4–mediated apoptosis inhibition. The ability of TAT-Pep2 to prevent the inhibition of anti-CD3–induced apoptosis by TAT-BH4 indicates that the antiapoptotic function of TAT-BH4 is mediated through its interaction with the IP3 receptor.
Fig. 4.
Inhibition of anti-CD3-induced apoptosis by TAT-BH4 and reversal by TAT-Pep2. (A) Representative Ca2+ traces recording the Ca2+ elevation induced by 5 μg/ml anti-CD3 antibody in Jurkat cells in the presence of 2 μM TAT-ctrl or TAT-BH4 peptides. Anti-CD3 was added 1–2 min after recording was started. (B) Histograms summarize the average peak anti-CD3 induced Ca2+ elevation in Jurkat cells treated with various peptides (mean ± SEM of four- individual experiments, >50 cells per sample per experiment). (C) TAT-BH4 or TAT-ctrl peptides were added at 1-μM final concentration 1 h before and 15 h after adding 5 μg/ml anti-CD3 antibody. Symbols represent the percentage of Hoechst 33342–stained cells displaying morphology typical of apoptosis 24 h after anti-CD3 addition (mean ± SEM; >200 cells counted per coverslip). (D) TAT-Pep2 reverses TAT-BH4-mediated inhibition of anti-CD3–induced apoptosis. Jurkat cells were incubated with or without TAT-BH4, and apoptosis was induced by anti-CD3 antibody as described in (C). Also, some of the cell suspensions were co-treated with 10 μM TAT-Pep2 or TAT-ctrl, added 1 h before anti-CD3 antibody, as outlined in the accompanying diagram. Symbols represent the percentage of cells (mean ± SEM) with apoptotic nuclei in four experiments (>200 cells counted per coverslip).
Discussion
Here we report that the BH4 domain of Bcl-2 mediates the interaction of Bcl-2 with the regulatory and coupling domain of the IP3 receptor. Moreover, the BH4 domain by itself is sufficient to bind the IP3 receptor and to inhibit IP3-dependent channel opening and Ca2+ release from the ER. When introduced into cells as a TAT-BH4 fusion peptide, the BH4 domain of Bcl-2 inhibits apoptosis caused by IP3-mediated Ca2+ elevation after T-cell–receptor activation. Significantly, a peptide inhibitor of Bcl-2-IP3R interaction prevented TAT-BH4–mediated suppression of T-cell receptor–induced Ca2+ elevation and apoptosis. Thus, an intrinsic antiapoptotic activity of the Bcl-2 BH4 domain is due to its ability to bind the IP3 receptor and inhibit Ca2+ release from the ER.
The major function of Bcl-2 and its antiapoptotic relatives is to preserve mitochondrial integrity, thus maintaining ATP synthesis and inhibiting release of proapoptotic factors, such as cytochrome C, that activate the caspase cascade, ultimately leading to apoptosis (12, 27). Mitochondrial membrane integrity is disrupted during apoptosis by at least two mechanisms (27, 28). One mechanism involves direct permeabilization of the outer mitochondrial membrane by the proapoptotic Bcl-2 family members Bax and Bak after their activation by BH3-only proteins Bid and Bim. The other mechanism involves induction of the mitochondrial permeability transition, mainly in response to Ca2+ release from the ER and mitochondrial Ca2+ overload. Bcl-2 is strategically located on both the outer mitochondrial membrane and the ER membrane (29, 30), positioning it to preserve mitochondrial integrity by inhibiting both of these mechanisms. The ability of Bcl-2 on the outer mitochondrial membrane to heterodimerize with proapoptotic family members inhibits direct permeabilization of the outer mitochondrial membrane (12), whereas the ability of Bcl-2 to inhibit Ca2+ release from the ER inhibits the transfer of Ca2+ to mitochondria, thus preventing mitochondrial Ca2+ overload and mitochondrial permeability transition (31).
The findings presented here indicate that separate structural features of the Bcl-2 protein endow these antiapoptotic functions. BH domains 1–3 are responsible for forming a hydrophobic groove that binds proapoptotic relatives (12). On the other hand, the BH4 domain of Bcl-2 is responsible for binding the IP3 receptor, thus inhibiting Ca2+ release from the ER. Ca2+ can induce apoptosis via mitochondrial Ca2+ overload (32, 33), by up-regulating the BH3-only protein Bim (34) and the death receptor ligand Fas (35, 36), and by Ca2+/calcineurin-mediated dephosphorylation and hence activation of the BH3-only protein Bad (37, 38). Therefore, by inhibiting proapoptotic Ca2+ signals at their point of origin, Bcl-2 blocks diverse routes through which Ca2+ can induce apoptosis.
Ca2+-mediated crosstalk between the ER and mitochondria, facilitated by the close proximity of these organelles, plays a critical role in determining the balance between cell survival and cell death (39, 33). The close proximity of ER and mitochondria places the IP3 receptor in contact with the voltage-dependent anion channel (VDAC) located in the outer mitochondrial membrane, thus facilitating transfer of Ca2+ from the ER lumen to the mitochondrial matrix (40–42). The switch that governs whether mitochondrial Ca2+ elevation favors survival or promotes cell death is mediated in part by the VDAC (42, 43) and involves targeting the permeability transition pore by Ca2+-mediated dephosphorylation of the BH3-only protein Bad (44). In earlier studies, Tsujimoto et al. identified an interaction of the BH4 domains of Bcl-2 and Bcl-XL with VDAC and thus attributed the antiapoptotic activity of the BH4 domains to this interaction (21, 26). Recent evidence that VDAC isoforms are dispensable for the induction of mitochondrial permeability transition suggests that these channels may not be required for apoptosis induction (45, 46[see discussion in the latter]). Nevertheless, the preceding evidence that the close proximity of VDACs on the outer mitochondrial membrane and IP3 receptors on the ER facilitates transfer of Ca2+ between these organelles and hence ER-mitochondrial crosstalk is strong. Thus, by positioning its BH4 domain on both the ER and mitochondria, Bcl-2 may mount a double defense against mitochondrial Ca2+ overload and apoptosis, with one barrier at the ER to limit IP3 receptor channel opening and the other at mitochondria to regulate the VDAC channel. In the present report, Pep2, a specific inhibitor of Bcl-2-IP3 receptor interaction, prevented the inhibition of anti-CD3-induced apoptosis in Jurkat cells by TAT-BH4. The ability of Pep2 to prevent the inhibition of anti-CD3–induced apoptosis by TAT-BH4 indicates that, at least in this situation, inhibition of IP3-induced Ca2+ release from the ER is the predominant antiapoptotic mechanism of the BH4 domain.
Understanding the multiple mechanisms by which Bcl-2 inhibits apoptosis is of both fundamental and clinical importance because of important role that Bcl-2 plays in promoting cancer. Up to this point, efforts to target Bcl-2 for therapeutic purposes have focused mainly on the interaction of Bcl-2 with proapoptotic proteins. With increased understanding of how the BH4 domain contributes to the antiapoptotic activity of Bcl-2, this domain may also become a valuable target for cancer therapy intended to overcome Bcl-2–mediated resistance to cell death. Conversely, improved understanding of the intrinsic antiapoptotic function of the BH4 domain may be exploited to develop antiapoptotic therapeutics of value in managing disease processes associated with accelerated apoptosis, including Alzheimer's disease and cardiac reperfusion injury.
Materials and Methods
Reagents and Cell Culture.
Sources of reagents and GST-IP3 receptor fragments, as well as tissue culture methods, were recently described in detail (7).
Peptide Synthesis and Delivery.
The purity of synthesized peptides was >95%, verified by mass spectrometry and high-performance liquid chromatography (HPLC) (GeneScript). Peptide sequences are as follows: TAT-BH4 Bcl-2 peptide, NH2-GRKKRRQRRRGGRTGYDNREIVMKYIHYKLSQRGYEW-COOH; TAT-ctrl, NH2-RKKRRQRRRGGLKNDDICLRVYTPVSILVNE-COOH; TAT-PEP2, NH2-RKKRRQRRRGGNVYTEIKCNSLLPLDDIVRV-COOH. The BH4 peptide without TAT was synthesized by Thermo Fisher(Germany). For some experiments, TAT-BH4 peptide and TAT-ctrl were labeled at the N terminus with FITC (EZBiolab). Peptides were incubated with 106 cells in OPTI-MEM (Invitrogen) for 60 min at 25 °C before Ca2+ measurements.
GST Pull-Down with FITC-Labeled Peptides.
GST-IP3 receptor fragments were expressed, purified, and used in GST pulldown experiments as described previously in detail (7).
Ca2+ Imaging.
Cells were loaded with Fura 2-AM in buffer containing 1.3 mM Ca2+ and adhered to polyL-lysine–coated coverslips as previously described in detail (8). Cytoplasmic Ca2+ concentration was measured by digital imaging during and after addition of anti-mouse CD3 (20 μg/ml) or IP3 ester (40 μM) to WEHI7.2 cells or anti-human CD3 antibodies (5 μg/ml) to Jurkat cells. Methods of Ca2+ imaging were described in detail elsewhere (8).
Immunoprecipitation and Western Blotting.
Co-immunoprecipitation methods were described previously (6). The following antibodies were used: anti-human Bcl-2 (BD Biosciences, 15131A), anti-mouse Bcl-2 (Santa Cruz Biotechnology, sc7382), anti-IP3 receptor type 1 (Calbiochem, 407144), anti-Fluorescein (Abcam, ab19492), anti-VDAC (Calbiochem, 529532), anti-calcineurin A (BD Biosciences, 610259), and anti-GST (Amersham Biosciences).
Unidirectional 45Ca2+-Flux Assay.
45Ca2+ fluxes were performed in 12-well clusters on confluent monolayers of MEF cells, obtained 5 days after plating the cells at 2 × 104 cm−2, as described (47). In short, after permeabilization of the cells with saponin (20 μg/ml), the nonmitochondrial Ca2+ stores were loaded with 150 nM free 45Ca2+ (28 μCi/ml) for 45 min at 30 °C in the presence of 10 mM NaN3. After washing the cells with efflux medium (120 mM KCl, 30 mM imidazole hydrochloride, pH 6.8, 1 mM EGTA) containing 4 μM thapsigargin, 45Ca2+ efflux was followed for 18 min by adding and replacing the efflux medium every 2 min. After 10 min, 3 μM IP3 (Sigma, I7012) was added. Peptides were added from 4 min before the addition of IP3 to 2 min after the addition of IP3. At the end of the experiments, the 45Ca2+ remaining in the stores was released by adding 2% sodium dodecyl sulfate (SDS) for 30 min. Ca2+ release is plotted as fractional loss, obtained by measuring the amount of Ca2+ released in 2 min divided by the total store Ca2+ content at that time. The IP3-sensitive Ca2+ release was quantified as the difference in fractional loss after and before the addition of IP3. Origin 7.0 software (OriginLab Corporation, Northampton, MA) was used to analyze, plot, and fit the data points. Dose–response curves using different concentrations of peptide were obtained by normalizing all data points to the IP3-induced Ca2+-release values obtained in control conditions (vehicle), which was set at 100%.
Anti-CD3-Induced Apoptosis.
Jurkat cells and WEHI7.2 cells were plated into 96-well plates at 4 × 105/ml and 2 × 105/ml respectively. After 24 h treatment with 5 μg/ml anti-human CD3 for Jurkat cells and 20 μg/ml hamster anti-mouse CD3 plus anti-hamster IgG for WEHI7.2 cells, the cells were stained with Hoechst 33342 (final concentration 10 μg/ml) for 10 min, and typical apoptotic nuclear morphology was detected by epifluorescence microscope with a 40× objective (Carl Zeiss MicroImaging, Inc.) as previously described (8).
Statistical Analysis.
Data were summarized as the mean ± SEM, and comparisons were made using the two-tailed t test for repeated measures. Differences between means were accepted as statistically significant at the 95% level (P < 0.05).
Supplementary Material
Acknowledgments.
The authors thank Stuart J. Conway for IP3 ester synthesis and Shigemi Matsuyama for helpful suggestions. This work was supported by National Institutes of Health Grants RO1 CA085804 (to C.W.D.) and HL80101 (to G.A.M.), by Research Program G.0604.07 of the Research Foundation–Flanders (FWO), and by Grant GOA/09/012 of the Concerted Actions Program of the K.U. Leuven (to J.B.P. and H.D.S.). H.L.R. is a Royal Society University Research Fellow. The authors thank Tomas Luyten for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907555106/DCSupplemental.
References
- 1.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 4.Vanderheyden V, et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca(2+) release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rong YP, Distelhorst CW. Bcl-2 protein family: Versatile regulators of calcium signaling in cell survival and apoptosis. Ann Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rong Y, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong F, Davis MC, McColl KS, Distelhorst CW. Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J Cell Biol. 2006;172:127–137. doi: 10.1083/jcb.200506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White C, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-Xl modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, et al. Apoptosis regulation by Bcl-XL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci USA. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor CW, da Fonseca PC, Morris EP. IP(3) receptors: The search for structure. Trends Biochem Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A. The Bcl-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 13.Muchmore SW, et al. X-ray and NMR structure of human Bcl-XL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 14.Petros AM, et al. Solution structure of the antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Neill J, Manion M, Schwartz P, Hockenberry DM. Promises and challenges of targeting Bcl-2 anti-apoptotic proteins for cancer therapy. Biochim Biophys Acta. 2004;1705:43–51. doi: 10.1016/j.bbcan.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 17.Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- 18.Huang DC, Adams JM, Cory S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 1998;17:1029–1039. doi: 10.1093/emboj/17.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng EH, et al. Conversion of Bcl-2 to Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 20.Kirsch DG, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugioka R, et al. BH4-domain peptide from Bcl-XL exerts anti-apoptotic activity in vivo. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- 23.Cantara S, et al. Exogenous BH4/Bcl-2 peptide reverts coronary endothelial cell apoptosis induced by oxidative stress. J Vasc Res. 2004;41:202–207. doi: 10.1159/000077408. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, et al. TAT-BH4 and TAT-Bcl-XL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 25.Gump JM, Dowdy SF. TAT transduction: The molecular mechanism and therapeutic prospects. Trends Mol Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–412. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 27.Kuwana T, et al. BH3 domain of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann T, et al. Characterization of the signal that directs Bcl-s(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annis MG, Yethon JA, Leber B, Andrews DW. There is more to life and death than mitochondria: Bcl-2 proteins at the endoplasmic reticulum. Biochim Biophys Acta. 2004;1644:115–123. doi: 10.1016/j.bbamcr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Pinton P, et al. Calcium and apoptosis: ER-mitochondrial Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabadkai G, Simoni AM, Rizzuto R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem. 2003;278:15153–15161. doi: 10.1074/jbc.M300180200. [DOI] [PubMed] [Google Scholar]
- 33.Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- 34.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of Bim of cell death. J Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 35.Hildeman DA, et al. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 36.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang HG, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 38.Saito S, et al. β-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem. 2000;275:34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- 39.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: Structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Hajnoczky G, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 42.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapizzi E, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy SS, et al. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baines CP, et al. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galluzzi L, Kroemer G. Mitochondrial apoptosis without VDAC. Nat Cell Biol. 2007;9:487–489. doi: 10.1038/ncb0507-487. [DOI] [PubMed] [Google Scholar]
- 47.Kasri NN, et al. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.