Abstract
Although several studies implicate small declines in blood glucose levels as stimulus for spontaneous meal initiation, no mechanism is known for how these dips might initiate feeding. To assess the role of ventromedial hypothalamus (VMH) (arcuate plus ventromedial nucleus) glucosensing neurons as potential mediators of spontaneous and glucoprivic feeding, meal patterns were observed, and blood and VMH microdialysis fluid were sampled in 15 rats every 10 min for 3.5 h after dark onset and 2 h after insulin (5 U/kg, i.v.) infusion. Blood glucose levels declined by 11% beginning ∼5 min before 65% of all spontaneous meals, with no fall in VMH levels. After insulin, blood and VMH glucose reached nadirs by 30–40 min, and the same rats ate 60% faster and spent 84% more time eating during the ensuing hypoglycemia. Although 83% of first hypoglycemic meals were preceded by 5 min dips in VMH (but not blood) glucose levels, neither blood nor VMH levels declined before second meals, suggesting that low glucose, rather than changing levels, was the stimulus for glucoprivic meals. Furthermore, altering VMH glucosensing by raising or lowering glucokinase (GK) activity failed to affect spontaneous feeding, body or adipose weights, or glucose tolerance. However, chronic depletion by 26–70% of VMH GK mRNA reduced glucoprivic feeding. Thus, although VMH glucosensing does not appear to be involved in either spontaneous feeding or long-term body-weight regulation, it does participate in glucoprivic feeding, similar to its role in the counter-regulatory neurohumoral responses to glucoprivation.
Introduction
The “glucostatic hypothesis” of Mayer, which postulated that changes in glucose utilization were sensed by hypothalamic “glucoreceptor neurons” as a proximate stimulus for meal initiation (Mayer, 1953, 1955), gave rise to studies in which blood (Smith and Epstein, 1969; Rodin et al., 1985; Mitrakou et al., 1991; Smith and Campfield, 1993; Sanders et al., 2006), brain (Grossman, 1986; Gilbert et al., 2003), and/or hepatic portal (Tordoff et al., 1989) glucose levels or cellular glucose availability were manipulated to assess the effect on feeding. However, most of these manipulations are not physiologically relevant to normal spontaneous feeding. To address this issue, the relationship between spontaneous changes in blood glucose levels and meal initiation in freely feeding rodents (Strubbe et al., 1977; Louis-Sylvestre and Le Magnen, 1980; Campfield et al., 1985; Campfield and Smith, 1986) was assessed. Most, but not all (Strubbe et al., 1977), of these studies found that many spontaneous meals were preceded by spontaneous, 5–10% declines in blood glucose levels during the 5–12 min before meal onset. However, such studies raised several questions about the source of the spontaneous fluctuations in blood glucose, the site and mechanism by which they are sensed, and the overall roles of central glucose and glucosensing in spontaneous feeding.
It is known that brain glucose levels are ∼15–20% of blood levels (Fellows and Boutelle, 1993; Silver and Erecińska, 1994; McNay and Gold, 1999; Levin, 2000; de Vries et al., 2003) and that glucose levels are monitored by specialized glucosensing cells that alter their activity in response to changes in ambient glucose levels. Peripheral glucosensing elements reside in the portal vein (Adachi et al., 1984; Hevener et al., 1997), carotid body (Pardal and López-Barneo, 2002) and gastrointestinal tract (Gribble et al., 2003). Brain glucosensing neurons reside in the arcuate nucleus (ARC) and ventromedial nucleus (VMN), which together compose the ventromedial hypothalamus (VMH) (Anand et al., 1964; Oomura et al., 1964; Ashford et al., 1990; Dunn-Meynell et al., 2002; Kang et al., 2004, 2006), as well as several other brain areas (Anand et al., 1964; Adachi et al., 1984). Some glucosensing neurons also receive afferents from peripheral glucosensing elements, making them potentially important integrators of changes in blood and brain glucose levels (Adachi et al., 1984).
Despite the clear demonstration of glucosensing elements throughout the brain and periphery, there are no studies that causally link such glucosensing with alterations in blood and brain glucose levels and spontaneous feeding. In addition, although cellular glucoprivation or hypoglycemia can elicit feeding (Smith and Epstein, 1969), no studies have examined similarities and differences between glucoprivic and spontaneous feeding. The current studies were undertaken to make these comparisons and to assess whether manipulation of VMH glucosensing by altering glucokinase (GK) activity or mRNA expression can alter either spontaneous or glucoprivic feeding.
Materials and Methods
Animals.
Outbred male Sprague Dawley rats (Charles River Laboratories) were used with starting weights between 300 and 450 g. Rats were housed at 22–24°C on a 12 h light/dark cycle and were provided with ad libitum Purina lab chow (catalog #5001) and water. In some studies, lights were turned off at 10:00 P.M., and, in studies in which feeding patterns were assessed, rats were kept on a reversed light/dark schedule with lights off at 10:00 A.M.
Placement of hypothalamic cannulae and vascular catheters.
All procedures were approved by the East Orange Veterans Affairs Medical Center Institutional Animal Care and Use Committee. Rats were anesthetized with chloropent (pentobarbital, chloral hydrate, and magnesium sulfate) and given buprenorphine postoperatively. For VMH cannula placements, rats were held in a stereotaxic frame with the head level with the horizontal, and 26 gauge guide cannulae or injection cannulae were angled at 20° to the vertical aiming at the junction between the ARC and VMN (−2.9 mm bregma, ±3.7 mm midline, and −8.5 mm dura) as determined from preliminary experiments. These injection sites are referred to as the “VMH” because the 1.0 μl injection volumes used spread to both the ARC and VMN. The guide cannulae were secured with four screws and cranioplast, and an obturator was inserted. After 2–3 d, animals were again anesthetized, and vascular (jugular venous or carotid artery) catheters were implanted, exteriorized by connecting to tubing attached to the skull, and filled with heparinized polypropylene glycol according to previously described methods (Tkacs et al., 2000). Animals were allowed 6–7 d to recover their preoperative body weight. During this time, they were handled and underwent sham injections through the implanted cannulae daily to acclimate them to the procedures when applicable.
For the microdialysis feeding studies, rats were anesthetized as above. Two sets of studies were performed. In the one designed to assess glucose levels in individual hypothalamic nuclei by the zero net flux method (see below), microdialysis probes with 1 mm dialysis membrane exposure (CMA) were placed into their guide cannulae, which were stereotaxically placed in three rats in the ventromedial part of the ARC with the probe tip 2.9 mm caudal to bregma, 0.2 mm lateral to midline, and 9.5 mm ventral to dura. VMN cannulae were placed in two other rats with the probe tip in the midportion of the ventromedial hypothalamic nucleus (2.9 mm caudal to bregma, 0.5 mm lateral to midline, 9.2 mm caudal to dura). In the second set of studies designed to assess blood and hypothalamic glucose levels during spontaneous and insulin-induced hypoglycemia (IIH), guide cannulae were placed unilaterally in the VMH, and jugular venous catheters were implanted as above but during the same procedure time. When applicable, these animals were acclimated to experimental conditions, which included being connected to exteriorized tubing and to the feeding device (see below), by repeated exposures before the actual testing day. On the day before testing, CMA microdialysis probes with 2 mm dialysis membrane exposure were calibrated by inserting them into a beaker of 1.0 mm glucose. When placed in their guide cannulae, these probes were located in the VMH site as described above, and microdialysis was performed as described previously (Levin, 2000).
Assessment of ARC and VMN brain glucose levels.
Food was removed 2 h before dark onset, and microdialysis probes and saline infusions were begun at 1.0 μl/min through the probes with monitoring of effluent glucose levels. Rats remained in the fasting condition throughout the procedure. At lights out, glucose was infused for 40 min at each of five concentrations between 0 and 2.0 mm with monitoring of effluent glucose concentrations at 10 min intervals over a total testing period of 200 min. Glucose levels in the ARC and VMN were calculated using the zero net flux method (Fellows et al., 1993).
Feeding responses to VMH glucoprivation.
Rats (n = 17) had bilateral VMH guide cannulae placed using the coordinates given above. After recovery of body weight to preoperative levels, they were allowed to feed overnight and then were injected in random order bilaterally with either 1 μl of saline or saline containing 120 μg of 5-thioglucose (5TG) per side with 3–4 d between injections. Food intake was monitored at 3 and 24 h after each injection.
Relationship of blood and VMH glucose to spontaneous and glucoprivic feeding.
Rats were either kept in their home cages for manual assessment of food intake (n = 8) or were housed in a BioDAQ Food Intake Monitor (BioDAQ; Research Diets) for 3–5 d before testing (n = 8). At 8:00 A.M. (2 h before lights off) on the day of testing, food was removed, microdialysis probes were inserted into their guide cannulae, and the probes and jugular catheters were attached to external tubing and saline infusions were begun through the probes. At dark onset (10:00 A.M.), food was returned, and venous blood (0.02 ml) and microdialysis effluents for glucose measurements were taken at 10 min intervals over a total of 5.5 h. To maintain plasma volume, 0.31 ml of saline was returned after each blood drawing and the rat's own washed red cells were returned at 10 min intervals over the course of 5.5 h. Spontaneous feeding was monitored for the first 3.5 h, then insulin (5 U/kg) was infused intravenously, and blood and VMH glucose and food intake were monitored for an additional 2 h. For those rats not run in the BioDAQ, preweighed food was placed in the home cages at dark onset, reweighed after rats had finished eating a given meal, and then placed back in the cage. The BioDAQ system was set so that a decrease in the amount of food in the feeding hopper was registered as being eaten. A meal was defined as intake of 0.2 g or more with a break in eating of no more than 10 min during a given bout (Zorrilla et al., 2005). There were no significant differences in food intake measures or other parameters between rats run with manually and automatically measured intake. For this reason, the data were pooled.
Effects of acute manipulation of VMH GK on spontaneous and glucoprivic feeding.
The rats used in this set of studies were part of a larger, previously published study in which they were assessed for their counter-regulatory responses to acute and chronic manipulations of VMH GK activity under baseline and IIH conditions (Levin et al., 2008). Here we report the effects of those VMH GK manipulations on food intake. Beginning after blood drawing (0.5 ml) by jugular vein catheter at baseline, 30, 60, 90, and 120 min for plasma glucose, norepinephrine, epinephrine, and glucagon after VMH drug injections and/or induction of IIH, food intake measures were performed over 4 and 24 h. These plasma values were reported previously in a study dealing with the effects of the various VMH GK manipulations used in the same rats to study food intake here (Levin et al., 2008). Plasma volume was maintained by immediate infusion of 0.3 ml of 0.9% NaCl, and red cell mass was maintained by return of washed red cells through the jugular catheters after each subsequent blood samplings (Tkacs et al., 2000)
Analyses of feeding responses after acute VMH GK manipulation were performed twice, once in the basal condition and once after IIH. The general procedure for these studies was to provide rats with three chow pellets (∼15 g) at dark onset (10:00 P.M.) so that they were semifasted at the time of testing the next day. At 2:00 P.M. on the next day (4 h after light onset), remaining food was removed, intracranial injection cannulae attached to tubing were inserted into the guide cannulae, and vascular catheters were attached to tubing leading outside the cages for injections and blood sampling, respectively. At 3:00 P.M., baseline venous blood samples were drawn over 120 min as described above. Two groups of rats (n = 10 per group) were then injected bilaterally over 5 min in the VMH with either 1 μl of alloxan (4 μg) in saline, pH 5.0, to reduce GK activity (Lenzen et al., 1987; Kang et al., 2006) or its saline vehicle. Another two groups (n = 10 per group) were injected with 0.5 nmol in 1 μl of 1% DMSO of the GK activator compound A to increase VMH GK activity (Merck Research Labs) (Kang et al., 2006; Levin et al., 2008). Blood samples were drawn as described above over 120 min, and, immediately after the last blood sample, a preweighed amount of food was returned and reweighed at 1, 3, and 24 h to assess cumulative food intake.
After an additional 2–3 d, this procedure was repeated in the same rats except that the vehicles or drugs were infused at 2:00 P.M. and the animals were injected intravenously with 5 U/kg pork insulin (Eli Lilly & Co.) to produce hypoglycemia (30 mg/dl nadir) over 120 min (Levin et al., 2008). Again, baseline and four blood samples were drawn over 120 min as described above. At the end of the 120 min blood drawing period, preweighed food was returned and reweighed at 1, 3, and 24 h for assessment of cumulative food intake.
Effects of chronic reduction in VMH GK mRNA on energy and glucose homeostasis.
GK mRNA was reduced chronically in the VMH using two methods. First, those rats that had been tested previously with injection of 4 μg of alloxan (n = 10) or saline (n = 10) into the VMH were injected with 24 μg of alloxan or saline bilaterally through the VMH cannulae 2 d after their last test, respectively. As published previously, these injections reduced GK mRNA by 27% at 8–12 d after the injections (Levin et al., 2008). Additional rats (n = 12 per group) were injected bilaterally in both the ARC (−9.6 mm dura) and VMN (−9.3 mm dura) at 5.9 mm anterior to the intra-aural line and 0.3 mm lateral to the midline with a total of 15 million infectious units in 1.0 μl of adenovirus expressing either GK short hairpin RNA (shRNA) or scrambled RNA (Bain et al., 2004). The GK shRNA injections produced ∼70% decreases in VMH GK mRNA from 4 to 10 d after injections (Levin et al., 2008). Rats injected with alloxan and adenovirus were tested at 6–10 d after those injections (Levin et al., 2008). Ad libitum food intake was tested in both sets of rats first at dark onset. Food intake after IIH was assessed 2 d later as described above.
At 2 d after the last bout of hypoglycemia, semifasted adenovirus-injected rats had baseline blood samples drawn at 3:00 P.M., and an oral glucose tolerance test was performed by gavaging the rats with glucose (0.5 g/kg). Blood samples (0.2 ml) were drawn at baseline and 15, 30, 60, 90, and 120 min for glucose and insulin levels. After this test, they were decapitated with weighing of carcasses and of retroperitoneal, epididymal, perirenal, mesenteric, and inguinal fat pads as an index of carcass adiposity. Brains were removed for histological verification of injection sites and micropunch of the VMH (ARC plus VMN) for real-time quantitative PCR.
Assays of blood and brain tissues.
Blood glucose levels were determined using an automated glucose analyzer (Analox). Microdialysis effluent glucose levels were determined using the Amplex red glucose/glucose oxidase assay kit (Invitrogen). In rats treated with high dose (24 μg) alloxan and with adenoviral vectors, brains were removed, and the VMH was micropunched and assayed for GK by real-time quantitative PCR according to previously described methods (Levin et al., 2004; Kang et al., 2006) and as reported previously (Levin et al., 2008).
Statistics.
Serial blood and VMH determinations were analyzed by repeated measures one-way ANOVA with post hoc correction by Bonferroni's test. Pairs of blood and VMH glucose levels and meal pattern data from the same rats were compared using paired t tests. Correlations were performed using Pearson's coefficient of correlation. For single measures, groups were compared by unpaired t test. Zero net flux calculations were performed as described previously (Fellows et al., 1993). Area under the curve was calculated for blood glucose and insulin levels with the oral glucose tolerance test using the trapezoidal rule.
Results
Assessment of ARC and VMN brain glucose levels
Using the zero net flux method for accurately determining brain glucose levels by microdialysis (Fellows et al., 1993), we found that ARC glucose levels in rats at dark onset were 1.34 ± 0.31 mm (n = 3) and VMN glucose levels were 1.85 ± 0.15 (n = 2). Although no plasma glucose levels were measured, our results below show that comparable levels at dark onset are 6.0–7.0 mm. Thus, despite its proximity to the median eminence, ARC glucose levels were comparable with those in the VMN and were ∼20% of blood levels.
Relationship of blood and VMH glucose to spontaneous and glucoprivic feeding
Tables 1 and 2 provide the data for the characteristics of the spontaneous meals that occurred during the first 3.5 h after dark onset and those that occurred over the 2 h after the induction of IIH. During the 3.5 h after lights on, rats ate 2.5 ± 0.5 meals. Of these, eight rats ate two meals, three ate three meals, one ate four meals, and one ate five meals over this period. Over the entire 3.5 h period, rats spent 10% of their time eating and consumed an average of 4 g of chow with an average latency of 50 min to eat the first meal and an intermeal interval of 59 min before the second meal. Over the 2 h after injection of insulin with subsequent induction of hypoglycemia, rats spent 81% more time eating than they did during spontaneous meals (relative to the amount of time studied), and the latency to eat the first meal was 39% shorter than that for the first spontaneous meal. However, there were no differences in the intermeal intervals preceding the second spontaneous and hypoglycemia-induced meals, neither were there significant differences in the amount eaten during either the first or second meals. For all the animals tested, blood glucose levels were 49 and 32% and VMH levels were 52 and 68% lower before the first and second hypoglycemia-induced versus spontaneous meals, respectively (Table 1). However, whereas the ratio of VMH/blood glucose levels were slightly lower preceding hypoglycemia-induced meals, there were no statistically significant differences in these ratios preceding the two types of meals.
Table 1.
Blood, VMH, and VMH/blood glucose levels in relationship to patterns of spontaneous and glucoprivic feeding
| Spontaneous | Hypoglycemia | |
|---|---|---|
| Latency to 1st meal (min) | 49.9 ± 9.2 | 30.2 ± 3.9* |
| Latency to 2nd meal (min) | 58.5 ± 8.6 | 42.3 ± 5.7 |
| Duration 1st meal (min) | 9.0 ± 1.5 | 15.0 ± 3.3 |
| Duration 2nd meal (min) | 9.6 ± 3.8 | 6.8 ± 1.0 |
| Total time spent eating (min) | 21.8 ± 4.1 | 22.7 ± 3.1 |
| Time eating as percentage of total time | 10.1 ± 1.8 | 18.6 ± 2.5* |
| Amount 1st meal (g) | 1.8 ± 0.4 | 2.1 ± 0.4 |
| Amount 2nd meal (g) | 1.5 ± 0.3 | 1.6 ± 0.3 |
| Total amount eaten (g) | 4.0 ± 0.7 | 3.8 ± 0.4 |
| Rate of eating (mg/min) | 19.2 ± 3.3 | 31.1 ± 2.5* |
| Blood glucose at dark onset/before insulin (mm) | 6.7 ± 0.3 | 6.6 ± 0.4 |
| Blood glucose before 1st meal (mm) | 6.2 ± 0.3 | 3.1 ± 0.4* |
| Blood glucose before 2nd meal (mm) | 5.7 ± 0.4 | 3.9 ± 0.5* |
| VMH glucose at dark onset/before insulin (mm) | 1.0 ± 0.2 | 0.9 ± 0.2 |
| VMH glucose before 1st meal (mm) | 1.1 ± 0.2 | 0.5 ± 0.2* |
| VMH glucose before 2nd meal (mm) | 1.0 ± 0.2 | 0.3 ± 0.1* |
| VMH/blood glucose at dark onset/before insulin | 0.16 ± 0.02 | 0.14 ± 0.02 |
| VMH/blood glucose before 1st meal (mm) | 0.18 ± 0.04 | 0.12 ± 0.03 |
| VMH/blood glucose before 2nd meal (mm) | 0.17 ± 0.04 | 0.09 ± 0.03 |
Rats (n = 12) had food removed 2 h before lights out, and their jugular venous and VMH microdialysis probes were attached. Food was returned at lights out, spontaneous feeding was monitored continuously, and blood and VMH glucose levels were monitored every 10 min for 3.5 h. Rats were then administered insulin (5 U/kg, i.v.) to induce hypoglycemia and were monitored as before. Total amount eaten and time spent eating is for all meals occurring during the first 2 h of spontaneous and postinsulin feeding. Data are mean ± SEM. *p = 0.05 or less when various parameters were compared by paired t test for the same animal between spontaneous and hypoglycemic conditions.
Table 2.
Change (Δ) in blood and VMH glucose levels in relationship to patterns of spontaneous and glucoprivic feeding for the 12 rats described in Table 1 and Figures 2 and 3
| Spontaneous | Hypoglycemia | |
|---|---|---|
| Blood glucose Δ before 1st meal (#/total) | 8/12 down none up | None down 8/12 up |
| Blood glucose Δ before 2nd meal (#/total) | 6/10 down none up | 4/12 down 8/12 up |
| VMH glucose Δ before 1st meal (#/total) | None down 9/12 up | 10/12 down 2/12 up |
| VMH glucose Δ before 2nd meal (#/total) | None down 4/11 up | None down 9/12 up |
Blood and VMH glucose Δ before first and second meals is the number of rats of the total for which complete data were available in which glucose levels went up or down significantly (p = 0.05 or less) within the 3.6–5 min before each meal as specified in Results. Data are mean ± SEM.
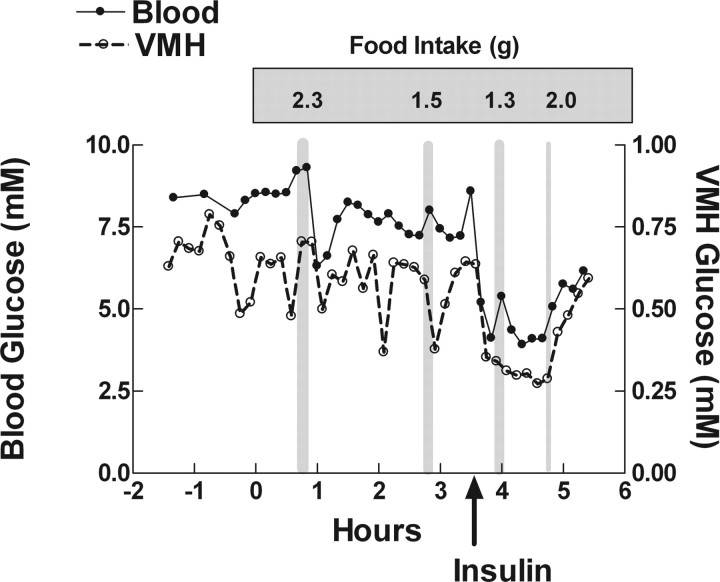
Figure 1 is an example of the interrelationship among blood and VMH glucose and both spontaneous and hypoglycemia-induced meal interval, duration, and amount in a single animal. The general lack of correlation between either a rise or fall in blood or VMH glucose levels and meal onset, amount, or duration in this rat is representative of the overall findings in 12 rats from which full data were available for either blood or VMH glucose levels. Figure 2 provides a graphic representation of the collective changes in blood and VMH glucose levels over the 25 min preceding and after the first meal. These data are grouped according to whether there was a fall or rise in levels over the ∼5 min period before each meal. Because blood samples were obtained every 10 min and VMH microdialysis eluates were collected over the same 10 min bins, regardless of the time at which meal onset occurred, the relationship to a given meal was estimated within this framework. However, these were clearly two very different types of measures, one taken at single time points and the other integrated across a 10 min interval of collection. Thus, the results from these tests must be interpreted within that framework.
Figure 1.
Example of a rat in which food was removed 2 h before lights out and lines were attached VMH microdialysis probes and jugular venous catheters. Food was returned at dark onset (0 h on the graph). Spontaneous intake was monitored over the next 3.5 h, and then insulin (5 U/kg, i.v.) was given and intake was monitored. The duration of each meal is presented by the width of vertical gray bars, and the amount of food eaten during that meal (grams) is given above each bar. Blood and VMH glucose levels are represented by filled and open circles, respectively.
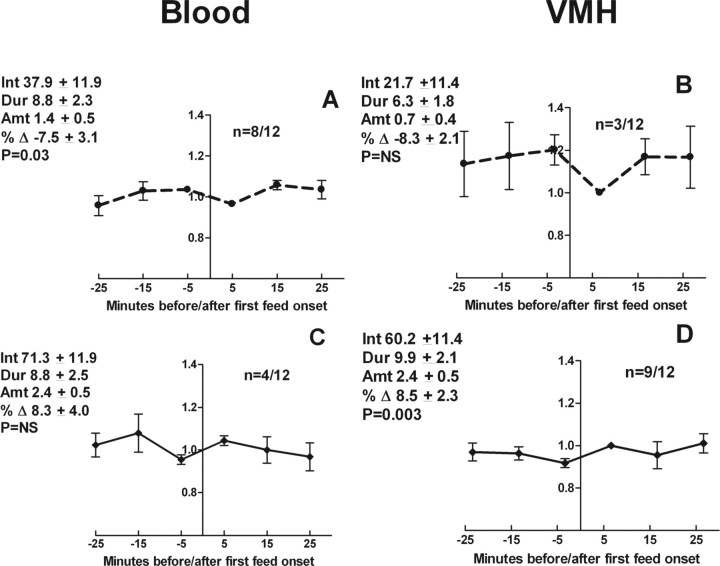
Figure 2.
Blood and VMH glucose levels surrounding the first spontaneous meals. The number, duration, and amount eaten during meals were monitored along with monitoring of blood and microdialysis probe effluent glucose levels in 10 min bins. Data were grouped by either a fall (A, B) or rise (C, D) in blood or VMH glucose levels beginning before the meal. Blood samples were taken at 10 min intervals, and VMH samples were collected continuously over the same 10 min interval. The y-axis is percentage change and is set to represent the beginning of the first meal as time 0. Data are provided for each grouping for the mean ± SEM interval from lights out (Int; minutes), duration (Dur; minutes), and amount eaten (Amt; grams) during the first meal, as well as the percentage change (% Δ) in glucose levels during the premeal period.
For the 12 rats, the average time from the blood sampling to the onset of the first meal was 3.6 ± 1.0 min so that the second samples were taken ∼6.4 min into the meal. A similar time period applies to the VMH samples. Defined in this way, beginning at ∼3.6 min before the first meal, statistically significant changes in blood glucose levels occurred in blood and VMH but in opposite directions. Blood levels fell in 8 of 12 rats by 7.5%, whereas VMH levels rose significantly in 9 of 12 rats by 8.5% (Table 2, Fig. 2). When the time period of observation was extended to include the 10 min before the 3.6 min time point before the first meal, there were no significant increases or decreases in blood or VMH glucose levels beginning ∼13.6 min before and continuing ∼6.4 min into the first meal.
Blood sampling and VMH glucose determinations occurred 4.7 ± 0.9 min preceding the second spontaneous meal. Data for the second spontaneous meals are summarized in Tables 1 and 2. When the data for the times 3.6 and 4.7 min before the first and second meals were taken together, 14 of 22 blood glucose levels fell by 11%, although none rose significantly before these meals. In the VMH, there was no significant fall in glucose levels during this period, whereas levels rose before 13 of 23 meals by 7% for which full VMH data were available. Thus, there was no significant pattern by which blood or VMH glucose levels fell or rose beginning 4–5 min before and continuing 5–6 min into the first and second spontaneous meals. Finally, the ratio of VMH to blood glucose was essentially the same at dark onset and preceding both the first and second spontaneous meals (Table 1).
After IIH, the first and second meals began 4.6 ± 0.8 and 4.8 ± 1.1 min after initiation of blood and VMH glucose sampling, respectively. After insulin infusion, blood levels fell to 3.1 mm and VMH levels to 0.5 mm over 30 min before the first meal (Table 1). Despite these low levels, beginning 5 min before and ending 5 min into the first meal, blood levels did not fall significantly in any of the animals and actually rose significantly in 8 of 12 rats by 13% (Table 2, Fig. 3). However, VMH glucose levels fell by 44% (p = 0.014) over this same period in 10 of 12 rats but rose by 6% in 2 of 12 rats (Table 2, Fig. 3). Although blood levels did not fall significantly in the 5 min period just before the first meal during hypoglycemia, blood levels did fall significantly in 12 of 12 rats and VMH levels in 10 of 12 rats over the entire 25 min before and 5 min into the first meal (Fig. 3). The second meal during hypoglycemia occurred ∼42 min later when blood glucose levels had risen slightly from 3.1 to 3.9 mm, whereas VMH levels fell from 0.5 to 0.3 mm (Table 1). In this case, blood glucose levels did fall by 64% in 4 of 12 rats but rose by 15% in 8 of 12 rats beginning 5 min before and ending 5 min into the second meal. There was no significant fall in VMH glucose levels in any rat over this same period (or over the 25 min before the second meal; data not shown), whereas levels rose by 24% in 9 of 12 rats over the 5 min before and during the second meal (Table 2). Thus, during hypoglycemia, there was a steady decline in blood and VMH glucose levels for 25–30 min before the first meal, although blood levels actually rose from 5 min before to 5 min into the first meal in 67% of rats. During this same period, VMH glucose levels did fall in 83% of the rats. In the 5 min before the second meal during hypoglycemia, only 33% of rats had a fall in blood glucose and none had a fall in VMH glucose levels. Finally, although they were slightly lower, the ratios of VMH to blood glucose levels preceding the first and second meals during hypoglycemia were not statistically different from each other or from those seen preceding the first two spontaneous meals (Table 1).
Figure 3.
Blood and VMH glucose levels surrounding the first hypoglycemia-induced meals. Data are presented in the same manner as in Figure 2.
In summary, although falling blood glucose did immediately precede the first spontaneous meal in a majority of rats, overall there was no consistent pattern of rising or falling blood or glucose levels preceding the majority of spontaneous meals to suggest a causal relationship between short-term changes in blood or brain glucose levels and initiation of the next meal. However, absolute levels of low blood and VMH glucose and falling VMH glucose levels were the major association with initiation of first meals during hypoglycemia. Second meals during hypoglycemia were, if anything, associated with even lower absolute but rising VMH glucose levels in the majority of rats.
Feeding response to VMH glucoprivation
Because IIH induces feeding and we chose to measure VMH glucose levels during spontaneous and IIH-induced feeding, we next investigated the possibility that bilateral VMH glucoprivation produced by injections of 5TG could produce feeding. Compared with intake after saline injection bilaterally into the VMH (1.5 ± 0.2 g), injections of 5TG increased food intake by 55% at 3 h after injection (3.8 ± 0.3 g; p = 0.001). Despite this initial stimulation of feeding by 5TG, rats reduced their intake so that, over 24 h after the injections, there was no difference in cumulative intake between saline (22.2 ± 0.4 g) and 5TG VMH injections in the same animal (20.7 ± 0.8 g).
Effects of acute manipulation of VMH GK on spontaneous and glucoprivic feeding
Spontaneous food intake in these studies was assessed in semifasted rats that had been given ∼15 g of chow at dark onset, injected bilaterally with drug or vehicle in the VMH at light onset, and then provided with food 2 h later. Delayed glucoprivic feeding (Ritter et al., 1978) was assessed in the same rats in the semifasted state that were injected in the VMH with drug or vehicle and insulin intravenously and then given food 2 h after IIH. We previously demonstrated that low-dose alloxan acts as a pharmacologic inhibitor of GK activity in VMN glucosensing neurons, reduces VMH GK activity in vitro (Dunn-Meynell et al., 2002; Kang et al., 2006), and increases the sympathoadrenal response to IIH when injected into the VMH in vivo (Levin et al., 2008). Here, acute, low-dose (4 μg) injections of alloxan into the VMH had no significant effect on spontaneous food intake over the entire 24 h postinjection period (Table 3). Also, acute alloxan injections had no effect on delayed glucoprivic feeding at any time over the 24 h after a 2 h bout of IIH (Table 3).
Table 3.
Effects of acutely manipulating VMH GK activity on spontaneous and glucoprivic feeding
| Saline | Alloxan | DMSO | Compound A | |
|---|---|---|---|---|
| 4 μg of alloxan alone | ||||
| 1 h intake (g) | 5.2 ± 1.4 | 4.7 ± 0.9 | ||
| 3 h intake (g) | 9.4 ± 0.8 | 8.4 ± 0.5 | ||
| 24 h intake (g) | 40.9 ± 1.0 | 37.6 ± 1.2 | ||
| 4 μg of alloxan + IIH | ||||
| 1 h intake (g) | 5.1 ± 1.2 | 5.3 ± 1.4 | ||
| 3 h intake (g) | 9.4 ± 1.1 | 9.3 ± 1.2 | ||
| 24 h intake (g) | 40.2 ± 1.6 | 36.8 ± 1.4 | ||
| Compound A alone | ||||
| 1 h IIH intake (g) | 5.5 ± 0.3 | 5.4 ± 0.1 | ||
| 3 h IIH intake (g) | 8.7 ± 0.3 | 8.4 ± 0.1 | ||
| 24 h IIH intake (g) | 41.3 ± 2.1 | 41.0 ± 3.4 | ||
| Compound A + IIH | ||||
| 1 h IIH intake (g) | 4.9 ± 0.7 | 4.9 ± 0.8 | ||
| 3 h IIH intake (g) | 9.1 ± 1.0 | 8.5 ± 1.1 | ||
| 24 h IIH intake (g) | 36.6 ± 2.1 | 34.6 ± 1.6 |
Semifasted rats (6–10 per group) were injected in the VMH with alloxan (4 μg), compound A (0.5 nmol), or their saline and 1% DMSO vehicles, respectively, and food intake was measured over the next 24 h. The same rats were retested 3–7 d later for food intake beginning 2 h after the induction of insulin-induced (5 U/kg, i.v.; 30 mg/dl nadir) hypoglycemia. Data are mean ± SEM. There were no significant differences in intake between the respective saline and alloxan groups.
We demonstrated previously that compound A increases VMH GK activity in vitro, increases the sensitivity of VMN neurons to glucose (Kang et al., 2006), and markedly attenuates the counter-regulatory response to IIH in vivo (Levin et al., 2008). Despite its prominent effects on counter-regulatory responses, injection of compound A into the VMH to increase GK activity had no significant effect on either spontaneous or hypoglycemia-induced feeding for >24 h period after its injection (Table 3).
Effects of chronic reduction in VMH GK mRNA on energy and glucose homeostasis
As we reported previously (Levin et al., 2008), GK mRNA was reduced by 26% after high-dose (24 μg) alloxan and by ∼70% of controls with VMH injections of an adenovirus expressing GK shRNA in the same rats used for the current studies. In those original studies, both of these reductions in VMH GK mRNA caused an increase in the counter-regulatory response to IIH (Levin et al., 2008). Despite this effect on the counter-regulatory responses, high-dose (24 μg) alloxan injections had no effect on spontaneous, dark-onset food intake over 24 h in these same rats in the current studies (Table 4). However, there was an overall 21% decrease and a positive correlation (r = 0.55; p = 0.05) between food intake over the 24 h after IIH and VMH GK mRNA expression. Similar to the 26% reduction produced by alloxan, 70% reduction in VMH GK mRNA had no effect on dark-onset feeding. However, unlike alloxan, the threefold greater reduction in VMH GK with GK shRNA led to a 43% reduction in delayed glucoprivic feeding over the first 3 h but had no effect on the 24 h intake (Table 4). In this case, there was a positive correlation (r = 0.68; p = 0.03) between VMH GK mRNA expression and 3 h posthypoglycemic intake.
Table 4.
Effects of chronic reduction of VMH GK mRNA on dark onset and glucoprivic feeding
| Saline | Alloxan | Scrambled RNA | GK shRNA | |
|---|---|---|---|---|
| Dark-onset intake after 24 μg of alloxan | ||||
| 3 h intake (g) | 4.0 ± 0.6 | 3.8 ± 1.1 | ||
| 24 h intake (g) | 39.2 ± 2.5 | 36.8 ± 2.1 | ||
| IIH-induced intake after 24 μg of alloxan | ||||
| 3 h intake (g) | 11.0 ± 1.6 | 9.8 ± 1.5 | ||
| 24 h intake (g) | 44.3 ± 1.3 | 35.1 ± 2.3* | ||
| Dark-onset intake after GK shRNA | ||||
| 3 h intake (g) | 4.2 ± 0.4 | 3.8 ± 0.5 | ||
| 24 h intake (g) | 39.0 ± 0.8 | 41.1 ± 0.4 | ||
| IIH-induced intake after GK shRNA | ||||
| IIH 3 h intake (g) | 9.2 ± 0.3 | 5.2 ± 1.1* | ||
| IIH 24 h intake (g) | 32.1 ± 0.4 | 29.8 ± 0.8 |
Rats (n = 10–12 per group) were injected in the VMH with 24 μg of alloxan or saline or with adenovirus expressing GK shRNA or scrambled RNA. They were assessed for feeding beginning at dark onset and at 2 h after IIH (30 mg/dl nadir). Data are mean ± SEM. *p = 0.05 when alloxan- or GK shRNA-treated rats were compared with saline controls.
In keeping with the lack of effect of reducing VMH GK mRNA with either high-dose alloxan or GK shRNA on dark-onset feeding, there were no effects on terminal body, fat pad, or liver weights, or fasting glucose or insulin levels compared with controls over a 8–9 and 10–14 d periods, respectively (Table 4), neither did reducing VMH GK mRNA with GK shRNA affect the excursions of either glucose or insulin after an oral glucose tolerance test (Fig. 4).
Figure 4.
Rats were given bilateral injections of adenovirus-expressing GK shRNA and, in the semifasted state, were gavaged with glucose (0.5 kg) 8–12 d later to assess blood glucose and insulin responses over the next 2 h.
Discussion
The current studies comparing the relationship between VMH glucosensing and spontaneous and hypoglycemia-induced meal initiation suggest that the two types of meals are mediated by different mechanisms. Although we partly confirmed studies showing that many spontaneous meals are preceded by small declines in blood glucose levels (Louis-Sylvestre and Le Magnen, 1980; Campfield et al., 1985, 1996; Campfield and Smith, 1986), we also found that there was no consistent relationship between previous changes in VMH glucose levels and spontaneous meal initiation, neither did altering VMH neuronal glucosensing by manipulating GK mRNA or activity affect spontaneous feeding, body or adipose weights, or glucose tolerance. However, absolute levels of low glucose, rather than dynamically changing blood and/or VMH glucose levels, was most consistently associated with meal initiation during hypoglycemia. Finally, a role in VMH neuronal glucosensing in glucoprivic feeding was supported by demonstrating that acute VMH glucoprivation stimulated feeding, whereas chronic lowering of VMH GK mRNA was associated with a reduction in hypoglycemia-induced feeding.
Relationship of blood and VMH glucose levels to spontaneous and glucoprivic meals
In partial support of previous studies that found small dips in blood glucose levels over 5–12 min before virtually all spontaneous meals in rats (Campfield et al., 1985; Campfield and Smith, 1986), we found similar dips in blood glucose in the ∼5–10 min period preceding spontaneous meals but only in ∼65% of rats. These differences may be partly attributable to our less frequent sampling. However, there were also major differences in the number of spontaneous meals taken by our rats. In the Campfield studies, in 14 of 31 experiments, rats ate no meals over 2.25 h (Campfield et al., 1985; Campfield and Smith, 1986). In contrast, 12 of our 15 rats ate at least one spontaneous meal during the first 2 h after dark onset. Our results compare favorably with those of Zorrilla et al. (2005) in spontaneously feeding, undisturbed rats, suggesting that our results reflect relatively physiological conditions. Thus, although our data support the contention that many spontaneous meals in rats are preceded by small dips in blood glucose, we found no evidence that such meals were preceded by any predictable fall or rise in VMH glucose levels.
VMH glucosensing and spontaneous meal initiation
It remains unclear what mechanism underlies the spontaneous dips in blood glucose that occur in freely feeding rats. Neither preventing access to nor the absence of food affects the time course of these declines in blood glucose or the latency to food-seeking behavior (Campfield and Smith, 1986). In fact, 67% of our rats had spontaneous dips in blood glucose before their first meals despite the absence of food for up to 3 h, suggesting that they do not reflect ongoing metabolic events associated with feeding. Although the dips might originate in the brain, it is unlikely that any relationship between them and spontaneous feeding is attributable to VMH glucosensing because there was no consistent relationship between fluctuations in VMH glucose levels and meal initiation. Also, altering VMH glucosensing both acutely and chronically by manipulating GK activity affected neither spontaneous nor chronic food intake. Although we might have missed small transient dips in VMH glucose because of the 10 min intervals, this method was sufficient to detect 8–9% increases in VMH glucose levels preceding meals preceding 56% of spontaneous meals.
Although glucose levels might differ from VMH levels in one of the several other brain areas containing glucosensing neurons, differences among these areas are relatively small (Fellows and Boutelle, 1993; Silver and Erecińska, 1994, 1998; McNay and Gold, 1999; Levin, 2000; de Vries et al., 2003). Alternatively, dips in blood glucose levels might be sensed by peripheral glucosensors and relayed to central sites as is seen for nucleus tractus solitarius neurons that both sense glucose directly and receive vagal afferents from peripheral glucosensors in the portal vein (Adachi et al., 1984). Another explanation for the lack of effect on feeding by altering VMH GK expression and activity on spontaneous feeding is the fact that some glucosensing neurons do not use GK as a gatekeeper for neuronal glucosensing (Gribble et al., 2003; Burdakov et al., 2006; Kang et al., 2006; Gonzàlez et al., 2009).
Finally, it was also possible that ARC glucosensing neurons are exposed to systemic glucose levels because of their proximity to the fenestrated capillaries of the median eminence. However, our data demonstrate that glucose levels in the ARC are essentially the same as those in the VMN. In fact, ARC neurons and their dendrites are effectively segregated from the median eminence by processes from tanycytes at the base of the third ventricle (van den Pol and Cassidy, 1982; Peruzzo et al., 2000). Similarly, there appears to be a diffusion barrier present between the nucleus tractus solitarius and the area postrema that would isolate neurons there from blood glucose levels (Wang et al., 2008). Given all these possibilities, it seems most likely that, if the spontaneous dips in blood glucose serve as a signal for spontaneous meal initiation, these dips neither originate in VMH glucosensing neurons nor are monitored directly by them.
Blood and VMH glucose, glucosensing, and glucoprivic feeding
The situation for meals initiated during IIH differed markedly from that seen with spontaneous meals. During the total 2 h of hypoglycemia, rats ate comparable amounts of food and spent more total time eating than they did during the preceding 3.5 h of spontaneous intake. Despite the steady decline in blood and VMH glucose levels over 30–40 min preceding the first hypoglycemic meal, there were no significant declines in blood glucose levels, whereas VMH levels did fall significantly over the 5 min before that meal in 83% of rats. This is just the opposite of what occurred before spontaneous meals in the same rats. However, the second meal during hypoglycemia was preceded by a fall in neither VMH nor blood glucose levels; VMH levels actually rose in 75% of rats before that meal. This suggests that absolute, low levels of both blood and brain glucose levels were the major cause of meal initiation during hypoglycemia.
Also, in contrast to spontaneous feeding, chronic lowering of VMH GK mRNA expression reduced glucoprivic feeding. Although it is unclear why the greater reduction in GK expression with GK shRNA than with high-dose alloxan resulted in differential effects on 3 vs 24 h intake, these results are similar to our previous studies in which impaired VMH glucosensing significantly reduced glucoprivic feeding (Sanders et al., 2004). The robust feeding response seen in our rats with VMH 5TG injections further supports a role for VMH glucosensing in glucoprivic feeding. Together with previous studies demonstrating the importance of the VMH (Borg et al., 1994, 1995, 1997), and particularly VMH GK activity and glucosensing in producing the counter-regulatory neuroendocrine responses to glucopenia and hypoglycemia (Tkacs et al., 2000; Dunn-Meynell et al., 2002; Kang et al., 2008; Levin et al., 2008), the current studies support VMH glucosensing as a mediator of both the behavioral and neuroendocrine responses to systemic glucoprivation. Among the VMH glucosensing neurons, the orexigenic ARC neuropeptide Y neurons are a likely candidate as an effector of this feeding because they express GK mRNA (Dunn-Meynell et al., 2002) and are activated by hypoglycemia (Muroya et al., 1999; Fioramonti et al., 2007), and NPY-deficient mice have defective glucoprivic feeding (Sindelar et al., 2004).
Summary and conclusions
We have shown that, although declines in blood glucose levels do precede many spontaneous meals, it is unlikely that these dips are mediated by central processes involving VMH glucosensing or comparable changes in brain glucose levels. To the contrary, feeding in response to acute hypoglycemia clearly involves VMH glucosensing as mediated by GK. As opposed to spontaneous meal initiation, glucoprivic feeding appears to occur primarily in response to low, rather than dynamically changing, blood and/or VMH glucose levels. Although hypoglycemia of the degree required to evoke glucoprivic feeding rarely occurs in nature, both central and peripheral mechanisms by which both feeding and neurohumoral counter-regulatory responses can be activated clearly exist as can be seen in the clinical situation of IIH. Thus, there may well be a point of overlap between spontaneous and glucoprivic feeding, for example, during short-term fasting, when lowered blood and brain glucose levels (de Vries et al., 2003) might stimulate feeding by engaging these counter-regulatory glucosensing sites, even at glucose levels above those required to produce feeding to rapid induction of hypoglycemia. Finally, our data suggest that VMH glucosensing, in general, probably has little to do with long-term body-weight regulation.
Footnotes
This work was funded by the Research Service of the Veterans Administration, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-53181, and Merck Research Labs. Research Diets provided the BioDAQ Food Intake Monitor. We thank Sunny Park, Charlie Salter, and Antoinette Moralishvilli for their expert technical assistance.
References
- Adachi A, Shimizu N, Oomura Y, Kobáshi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Bain JR, Schisler JC, Takeuchi K, Newgard CB, Becker TC. An adenovirus vector for efficient RNAi-mediated suppression of target genes in insulinoma cells and pancreatic islets of Langerhans. Diabetes. 2004;53:2190–2194. doi: 10.2337/diabetes.53.9.2190. [DOI] [PubMed] [Google Scholar]
- Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamic glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ. Functional coupling between transient declines in blood glucose and feeding behavior: temporal relationships. Brain Res Bull. 1986;17:427–433. doi: 10.1016/0361-9230(86)90250-9. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Brandon P, Smith FJ. On-line continuous measurement of blood glucose and meal pattern in free feeding rats: the role of glucose in meal initiation. Brain Res Bull. 1985;14:605–616. doi: 10.1016/0361-9230(85)90110-8. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Rosenbaum M, Hirsch J. Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev. 1996;20:133–137. doi: 10.1016/0149-7634(95)00043-e. [DOI] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose excited and glucose inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG. Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res. 1993;604:225–231. doi: 10.1016/0006-8993(93)90373-u. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem. 1993;60:1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: Integration in NPY and POMC networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Magnan C, Turban S, André J, Guerre-Millo M. Leptin receptor-deficient obese Zucker rats reduce their food intake in response to a systemic supply of calories from glucose. Diabetes. 2003;52:277–282. doi: 10.2337/diabetes.52.2.277. [DOI] [PubMed] [Google Scholar]
- Gonzàlez JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar-sensing neurons. J Physiol. 2009;587:41–48. doi: 10.1113/jphysiol.2008.163410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev. 1986;10:295–315. doi: 10.1016/0149-7634(86)90015-1. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- Kang L, Sanders NM, Dunn-Meynell AA, Gaspers LD, Routh VH, Thomas AP, Levin BE. Prior hypoglycemia enhances glucose responsiveness in some ventromedial hypothalamic glucosensing neurons. Am J Physiol Regul Integ Comp. 2008;294:R784–R792. doi: 10.1152/ajpregu.00645.2007. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Tiedge M, Panten U. Glucokinase in pancreatic B-cells and its inhibition by alloxan. Acta Endocrinol. 1987;115:21–29. doi: 10.1530/acta.0.1150021. [DOI] [PubMed] [Google Scholar]
- Levin BE. Glucose-regulated dopamine release from substantia nigra neurons. Brain Res. 2000;874:158–164. doi: 10.1016/s0006-8993(00)02573-7. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling prior to obesity onset. Am J Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- Levin BE, Becker TC, Eiki J, Zhang BB, Dunn-Meynell AA. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2008;57:1371–1379. doi: 10.2337/db07-1755. [DOI] [PubMed] [Google Scholar]
- Louis-Sylvestre J, Le Magnen J. Fall in blood glucose level precedes meal onset in free-feeding rats. Neurosci Biobehav Rev. 1980;4:13–15. doi: 10.1016/0149-7634(80)90041-x. [DOI] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and lipostatic hypothesis. Ann N Y Acad Sci. 1955;63:15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. J Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett. 1999;264:113–116. doi: 10.1016/s0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi N. Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Roelke M, Neville M. Glucoprivic feeding behavior in absence of other signs of glucoprivation. Am J Physiol. 1978;234:E617–E621. doi: 10.1152/ajpendo.1978.234.6.E617. [DOI] [PubMed] [Google Scholar]
- Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34:826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes. 2004;53:1230–1236. doi: 10.2337/diabetes.53.5.1230. [DOI] [PubMed] [Google Scholar]
- Sanders NM, Figlewicz DP, Taborsky GJ, Jr, Wilkinson CW, Daumen W, Levin BE. Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol Behav. 2006;87:700–706. doi: 10.1016/j.physbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecińska M. Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecińska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Ste Marie L, Miura GI, Palmiter RD, McMinn JE, Morton GJ, Schwartz MW. Neuropeptide Y is required for hyperphagic feeding in response to neuroglucopenia. Endocrinology. 2004;145:3363–3368. doi: 10.1210/en.2003-1727. [DOI] [PubMed] [Google Scholar]
- Smith FJ, Campfield LA. Meal initiation occurs after experimental induction of transient declines in blood glucose. Am J Physiol. 1993;265:R1423–R1429. doi: 10.1152/ajpregu.1993.265.6.R1423. [DOI] [PubMed] [Google Scholar]
- Smith GP, Epstein AN. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol. 1969;217:1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Steffens AB, De Ruiter L. Plasma insulin and the time pattern of feeding in the rat. Physiol Behav. 1977;18:81–86. doi: 10.1016/0031-9384(77)90097-x. [DOI] [PubMed] [Google Scholar]
- Tkacs NC, Dunn-Meynell AA, Levin BE. Presumed apoptosis and reduced arcuate nucleus neuropeptide Y and pro-opiomelanocortin mRNA in non-coma hypoglycemia. Diabetes. 2000;49:820–826. doi: 10.2337/diabetes.49.5.820. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Tluczek JP, Friedman MI. Effect of hepatic portal glucose concentration on food intake and metabolism. Am J Physiol. 1989;257:R1474–R1480. doi: 10.1152/ajpregu.1989.257.6.R1474. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Cassidy JR. The hypothalamic arcuate nucleus of rat: a quantitative Golgi analysis. J Comp Neurol. 1982;204:65–98. doi: 10.1002/cne.902040108. [DOI] [PubMed] [Google Scholar]
- Wang QP, Guan JL, Pan W, Kastin AJ, Shioda S. A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem Res. 2008;33:2035–2043. doi: 10.1007/s11064-008-9676-y. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]