Abstract
Objectives
This study investigated p-octyloxy-phenyl-phenyl iodonium hexafuoroantimonate (OPPI) as a photoinitiator, in combination with camphorquinone/amine photoinitiation systems, for use with di(meth)acrylate-based composite resins. The investigation determined if the inclusion of OPPI improved degree and rate of conversion, initial color and color stability of a representative composite resin dental material.
Methods
Camphorquinone (CQ) and OPPI were combined in various proportions with the amine co-initiator 2-dimethylaminoethyl methacrylate (DMAEMA) and used at two levels in which CQ+OPPI+DMAEMA = 1wt.% or 3wt.% to photo-initiate a BisGMA/BisEMA/TEGDMA (37.5:37.5:25 wt.%) monomer blend.
A total of eight groups (4 groups for each level of total photoinitiator, 1% and 3%) were tested according to the following proportion of components in the photoinitiator system:
| Group C: | CQ only |
| Group CO: | CQ + OPPI (1:2) |
| Group CA: | CQ + DMAEMA (1:2) |
| Group COA: | CQ + OPPI + DMAEMA (1:1:1) |
Each monomer was polymerized using a quatz-halogen curing unit (Demetron 400, Demetron Research Corp., Danbury, CT)) with an intensity of 400 mW/cm2 for 5s, 20s, 40s, 60s, 300s and their conversion levels (DC) were determined at each exposure time using a Fourier transform infrared spectrophotometer (FTIR).
To examine color stability, experimental composite resins were made by mixing 3.2% silanated barium glass (78 %wt., average filler size; 1 micron) with each monomer system, except both CQ only groups and 1% CO group, which were found to cure insufficiently to be able to prepare useful specimens. Disk shaped samples (10 mm in diameter and 1.5 mm in thickness) were made and stored under the conditions of dry or saline solution at room temperature (25 C) or 60 C water bath.
Each CIELAB scale was determined with a colorimeter (CHROMA METER CR-400) at the time of baseline (day after curing), 1 week, 2 weeks, and 4 weeks later.
Results
The high level (3%) photoinitiated groups exhibited greater DC than the low level (1%) groups. In the 3% group, the COA group showed the fastest and the highest DC, while in the 1% group the CA and COA groups showed the greatest DC.
In the color stability test, both CA groups were darker and more yellow than the CO and COA groups. Color was more stable in composite resins containing OPPI than those containing only the CQ and amine components. The least color change (greatest color stability) was found using 25C saline solution ageing, and the most change (least color stability) occurred 60C dry air aging.
Significance
This study suggests that OPPI can be used to replace the amine in a given CQ/amine photoinitiator system to accelerate cure rate, increase conversion, reduce initial color and increase color stability.
Keywords: Degree of conversion, Color stability, Photoinitiator, OPPI, Light curing
1. Introduction
Photopolymerization uses light energy to initiate photochemical and chemical reactions in organic oligomers, to form a new polymeric materials by the photo-induced increase of molecular weight by monomer to polymer conversion, as well as crosslinking of developing or preexisting macromolecules. It has been noted that improved photopolymerization is critical for the optimization of mechanical properties [1,2], biocompatibility [3], and color stability [4] of light-activated dental resins.
Free-radical polymerization can be initiated by the excitation of suitable photoinitiating systems (PISs) under lights, since direct formation of reactive species on the monomer by light absorption is not an efficient way. Most of these systems were originally sensitive to UV lights, but by now a large number of various systems allow to extend the spectral sensitivity (that corresponds to the best matching between the emission spectrum of the light source and the absorption spectrum of the formulation) to visible lights [5]. In addition, the number and reactivity of these primary radicals modulate the early stages of polymerization kinetics, the polymeric chain lengths and even the final degree of conversion [6].
The visible light photosensitizer camphoroquinone (CQ), an alpha dicarbonyl that absorbs at 468 nm, is widely used in dental resin and adhesive formulations. This can produce a pair of free radicals by proton abstraction, besides this process can be efficiently accomplished by the formation of a complex between the photoexcited sensitizer and an electron donating (reducing) agent such as a tertiary amine [7]. CQ is a solid yellow compound with an unbleachable chromophore group, so that large amounts of CQ in resin formulations lead to an undesirable yellow color, affecting the final aesthetic appearance of the cured material [8–10]. This, in turn, places practical limits on the concentration of CQ and, consequently, limits the degree of polymerization and depth of cure that can be attained [11]. Therefore, there has been substantial effort to improve this curing system by the use of alternative photosensitizers [12–17] and various amine reducing agents [18,19]. In recent years, manufacturers have also included different photoinitiators in the organic matrix to act either alone or synergistically with CQ [20]. For example, the α-diketone PPD (1-phenyl 1,2-propanedione) is used in adhesives and composite resin formulations to improve the polymerization kinetics and lessen photo-yellowing effects [11].
In this study, the onium compound p-octyloxy-phenyl-phenyl iodonium hexafluoroantimonate (OPPI) was used as a photoinitiator. It absorbs at 300-380 nm [21], which is outside the range of visible wavelengths, and is therefore colorless.
Although it was reported that almost no polymerization occurred when OPPI was used alone [22], Eick et al used this kind of OPPI as a cationic photoacid initiator in combination with a visible light sensitizer, CQ and an electron donor, ethyl 4-dimethylaminobenzoate (EDMAB) to polymerize an oxirane/polyol resin. In their study, photocuring was accomplished by the generation of a strong acid released during the generation of a strong acid released during the fragmentation of the diphenyliodonium salt photoinitiator. The acid protonates the oxirane groups and initiates cationic polymerization [23].
This study was carried out in order to determine if OPPI could be used as one component of a free-radical photoinitiator system, and can improve color stability in acrylic resins such as those used in dental (e.g., adhesives and restorations) and other biomedical applications. For this, OPPI was evaluated for its effect on the degree and rate of conversion and color stability of photoinitiated BisGMA-based resins.
2. Materials and methods
Monomer mixture (GTE) was made by mixing 37.5 wt% BISGMA (lot # 568-21-07, ESSTECH, Essington, PA), 37.5 wt% BISEMA (lot # 474-32-02, ESSCHEM Inc. Linwood, PA), and 25 wt% TEGDMA (lot # 597-23-02, ESSTECH).
Total concentrations of 1 wt% and 3 wt% of variously proportioned photo-initiators (CQ / OPPI) or/and DMAEMA were added to make the monomer mixture curable. Eight groups (four groups in each concentration) according to the weight proportion of those components were tested (Table 1). Those kinds of works were done under filtered orange light.
Table 1.
Experimental groups for degree and rate of conversion.
| Components and proportion by weight | Code | |
|---|---|---|
| 1% | 3% | |
| CQ only | 1C | 3C |
| 1 part of CQ and 2 parts of OPPI | 1CO | 3CO |
| 1 part of CQ and 2 parts of DMAEMA | 1CA | 3CA |
| Equally proportioned CQ, OPPI, and DMAEMA | 1COA | 3COA |
CQ, Camphoroquinone; OPPI, p-octyloxy-phenyl-phenyl iodonium hexafluoroantimonate; DMAEMA, ethyl 4-dimethylaminobenzoate.
2.1. Degree and Rate of Conversion
FT-IR, HPLC, and NMR have mainly been used for the determination of the photopolymerization efficiency of dental resins. However, the use of FT-IR absorption spectroscopy is the easiest and simplest method [16]. Therefore, degree of conversion (DC) was determined using a Fourier transform infra-red (FTIR) spectrophotometer (Nicolet 520, Nicolet Instrument Corp., Madison, WI) in this study.
A small amount of uncured resin monomer (GTE) was placed between two NaCl disks and the 4 Page 4 of 18 spectrum recorded in transmission with 40 scans at a resolution of 1 cm−1 (baseline). After the IR spectral scan, the monomer mixture was cured between the transparent NaCl disks for 5, 20, 40, 60, and 300 seconds with a Demetron 400 visible light curing unit (Demetron Research Corp., Danbury, CT). The light-intensity of the curing-unit was measured prior to the fabrication of each sample set (about 440 mW/cm2) using a Model 100 Optilux radiometer (Kerr, Dansbury, CT).
After each exposure time, the specimens were again scanned for their FTIR spectra. Remaining unconverted double bonds were calculated by comparing the ratio of aliphatic C=C absorption at 1637 or 1638 cm−1 to aromatic carbon–carbon absorption at 1608 cm−1 between cured and uncured specimens. Absorption of the aromatic carbon-carbon stretching band remains constant during polymerization and serves as an internal standard where the DC of each specimen was equal to 100% minus (%C=C) equation.
Rate of conversion at the initial curing period (first 5 seconds) was also calculated by dividing the degree of conversion into 5 seconds in order to verify which group shows the fastest polymerization reaction.
All experiments were carried out three times, and the results were analyzed by ANOVA followed by pairwise multiple comparison using Student–Newman–Keuls’ multiple range comparison test, with p = 0.05 as the level of significance.
2.2. Color Stability
Experimental 78 %wt. filler loaded composite resin was made by incorporating BisGMA/BisEMA/TEGDMA resin matrix with 3.2 % silanated barium glass fillers (mean particle size; 1 mµm).
Composites were also divided into three groups according to resin matrix system for each concentration of photoinitiators (1 % and 3 %); 1 part CQ and 2 parts of OPPI group (CO) and 1 part of CQ and 2 parts of amine group (CA), and all equally proportioned three components group (COA). Among these groups, 1 % CO group was excluded because of its poor polymerization irrespective of over 80 s light-curing, which means clinical impracticability. A total of five groups were made and used to examine color stability.
With a white background, resin composites were packed into the disk-shaped mold (Flat Washers, HO1231876, Hillman, USA; 10 mm in diameter and 1.5 mm in thickness) on a cover glass. After packing the composite, another cover glass was pressed on the top of the specimen, and specimens were light cured each for 60 s with a light-curing unit (Demetron 400, Demetron Research Corp., Danbury, CT) with an intensity setting of 440 mW/cm2. Five specimens were used for each color measurement.
Baseline color was measured after immersion in distilled water for 24 h at room temperature and blot dry. CHROMA METER CR-400 (Konica Minolta, Sensing Inc., Japan) was used to measure the CIELAB coordinates of each specimen. The CIE color system (CIELAB) was determined by the International Commission on Illumination in 1978. The three attributes of color in this system are L*, a*, b*, where L* is the lightness variable proportional to Value in the Munsell system, and a* and b* are chromatically coordinates. The a* and b* coordinates designate positions on a red/green and yellow/blue axis respectively (+a = red, -a = green; +b = yellow, -b = blue).
To evaluate their color stability, five specimens in each group were put in an empty vial (dry condition) or a vial containing 1% saline solution (wet condition). These vials were kept in 60 C water bath or dark place at room temperature up to a month. Color coordinates were re-measured 1 week, 2 weeks, and 4 weeks later. All samples were stored in the dark with no light except when measured. The color change (ΔE) was calculated using the equation:
ΔL, Δa, and Δb are the mathematical differences between baseline L*, a*, b* and re-measured L*, a*, b* values. Statistical analysis was done using Kruskal-Wallis and Mann-Whitney tests at a significance level of 0.05.
3. Results
3.1. Degree and Rate of Conversion
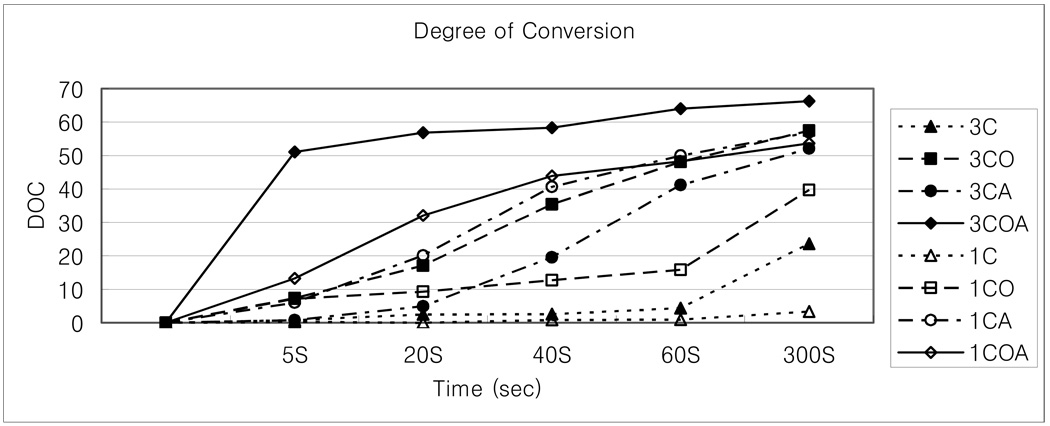
Table 2 and Figure 1 show the degree of conversion of all groups. Degree of conversion went up with the increased curing time, although, one photoinitiator component groups of 1C and 3C, in which CQ only has been used, showed a very slow and low degree of conversion as expected because that there was no reducing agent used.
Table 2.
Degree of conversion (%) of GTE with various photoinitiators.
| Curing time (sec.) | 5 s | 20 s | 40 s | 60 s | 300 s |
|---|---|---|---|---|---|
| Groups | |||||
| 1C | 0.33 | 0.00 | 0.83 | 0.96 | 3.36 |
| 1CO | 7.17 | 9.27 | 12.66 | 15.81 | 39.73 |
| 1CA | 6.02 | 20.11 | 40.65 | 49.96 | 56.93 |
| 1COA | 13.24 | 32.08 | 43.95 | 48.24 | 53.64 |
| 3C | 0.79 | 2.51 | 2.56 | 4.33 | 23.57 |
| 3CO | 7.34 | 17.04 | 35.45 | 48.10 | 57.46 |
| 3CA | 0.8 | 4.88 | 19.52 | 41.21 | 52.12 |
| 3COA | 51.11 | 56.86 | 58.28 | 63.99 | 66.24 |
Figure 1.
Degree of conversion (DC) of GTE with various photoinitiators vs. exposure time.
In general, resin monomers including 3 % photoinitiator systems converted into polymers more than those containing 1 % photoinitiator systems, except CA (CQ and amine system) groups, which showed more rapid and abundant conversion of 1 % monomer than 3 % monomer. Of the photoinitiator-component groups, 3COA showed the highest degree of conversion at all curing times. The other groups except 1CO gained similar degrees of conversion when cured more than 60 seconds, while 1COA acquired a greater degree of conversion with relatively shorter curing times (less than 20 seconds).
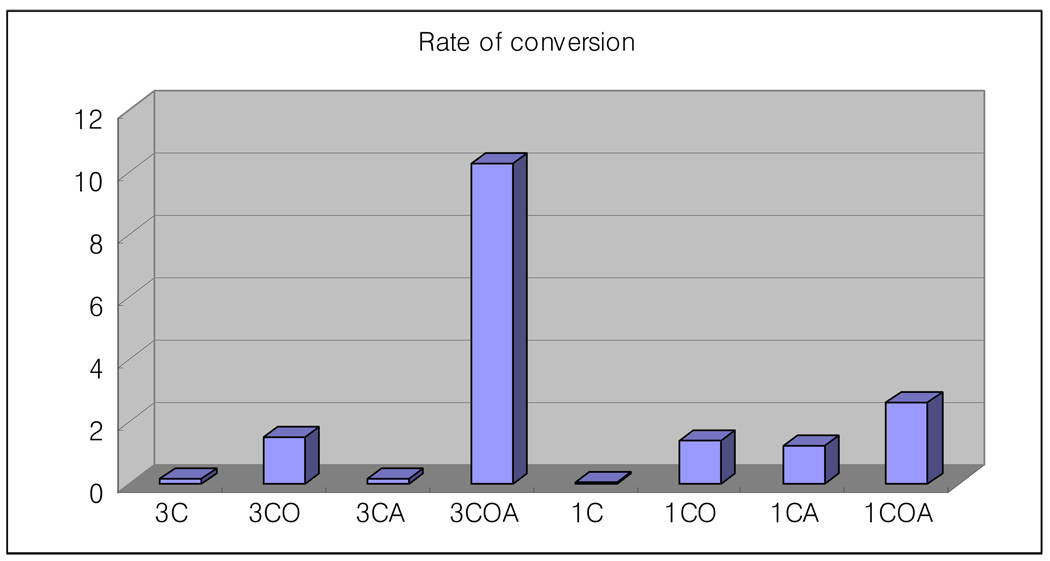
Figure 2 shows the rate of initial conversion of the groups (rate taken from the five seconds curing time interval). The 3COA group showed a faster conversion rate than any of the other groups. Furthermore, the 1COA group showed a faster cure rate than any of the 3 % groups.
Figure 2.
Rate of initial conversion (first 5 seconds) of GTE with various photoinitiators (% conversion/sec.).
In summary, CQ photosensitized OPPI in a blue light photo-cure resin and this OPPI can substitute for an amine in a CQ/amine photoinitiation system. Also, OPPI acts as a photoinitiator in the presence of CQ. Furthermore, in the presence of CQ, OPPI combined with amine (DMAEMA) increases ultimate conversion and rate of conversion more than OPPI or amine alone.
3.2. Color Stability
Table 3 reveals the change of color coordinates and Table 4 and Figure 3–6 show the color change (ΔE). At baseline, there was no difference in color coordinates among five samples in each group, that is, every specimen has a similar initial color.
Table 3.
Color coordinates.
| Groups | Storage conditions |
Baseline | Week 1 | Week 2 | Week 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | L* | a* | b* | ||
| 3CO | Room, wet | 87.29 | −3.82 | 15.96 | 87.11 | −3.87 | 16.97 | 86.75 | −3.98 | 18.61 | 86.49 | −3.82 | 19.26 |
| 60 C, wet | 87.17 | −3.85 | 16.06 | 87.05 | −4.24 | 21.59 | 87.17 | −4.17 | 20.99 | 86.93 | −4.24 | 21.34 | |
| Room, dry | 87.17 | −3.97 | 16.50 | 86.70 | −4.23 | 18.34 | 86.63 | −4.27 | 18.83 | 86.31 | −4.31 | 19.22 | |
| 60 C, dry | 87.17 | −3.94 | 16.48 | 86.19 | −4.61 | 24.21 | 86.56 | −4.64 | 23.33 | 86.68 | −4.74 | 23.51 | |
| 3CA | Room, wet | 84.30 | −1.06 | 27.86 | 82.99 | 0.78 | 32.49 | 83.75 | 0.66 | 31.81 | 84.28 | 0.20 | 32.23 |
| 60 C, wet | 84.09 | −1.01 | 27.97 | 84.39 | −1.73 | 31.72 | 84.62 | −1.75 | 30.94 | 84.66 | −1.91 | 29.92 | |
| Room, dry | 84.10 | −0.94 | 28.11 | 82.29 | 0.26 | 33.38 | 82.03 | 0.48 | 34.71 | 81.74 | 0.59 | 35.21 | |
| 60 C, dry | 84.17 | −0.99 | 27.88 | 83.61 | −0.93 | 36.37 | 84.12 | −2.14 | 34.90 | 83.79 | −2.41 | 35.55 | |
| 3COA | Room, wet | 86.89 | −3.37 | 16.60 | 86.12 | −3.41 | 19.75 | 86.09 | −3.28 | 19.79 | 86.66 | −2.91 | 18.24 |
| 60 C, wet | 86.77 | −3.96 | 18.39 | 86.96 | −4.05 | 23.99 | 86.65 | −3.64 | 23.86 | 86.31 | −3.45 | 24.62 | |
| Room, dry | 86.83 | −3.74 | 17.56 | 85.88 | −3.75 | 21.21 | 85.48 | −3.80 | 22.18 | 85.16 | −3.58 | 22.65 | |
| 60 C, dry | 86.94 | −3.95 | 18.31 | 86.88 | −4.96 | 25.44 | 86.51 | −4.6 | 25.96 | 86.40 | −4.85 | 26.37 | |
| 1CA | Room, wet | 87.03 | −1.75 | 13.67 | 86.33 | −0.22 | 15.14 | 86.52 | 0.02 | 14.80 | 86.72 | 0.02 | 14.58 |
| 60 C, wet | 87.07 | −1.75 | 13.61 | 87.33 | −1.78 | 15.75 | 86.97 | −1.68 | 15.66 | 86.91 | −1.60 | 15.45 | |
| Room, dry | 87.09 | −1.76 | 13.58 | 85.84 | −0.72 | 15.93 | 85.61 | −0.66 | 16.28 | 85.51 | −0.62 | 16.77 | |
| 60 C, dry | 87.12 | −1.71 | 13.47 | 87.08 | −2.69 | 16.61 | 87.16 | −2.66 | 16.03 | 87.23 | −2.65 | 15.60 | |
| 1COA | Room, wet | 88.11 | −1.97 | 9.81 | 87.88 | −1.53 | 10.55 | 87.84 | −1.24 | 10.41 | 87.81 | −1.00 | 10.37 |
| 60 C, wet | 88.11 | −2.13 | 10.08 | 87.77 | −1.42 | 12.59 | 87.44 | −1.37 | 12.76 | 87.42 | −1.36 | 12.92 | |
| Room, dry | 88.05 | −2.12 | 10.34 | 87.47 | −2.05 | 11.61 | 87.17 | −2.00 | 11.74 | 87.12 | −1.94 | 11.83 | |
| 60 C, dry | 88.10 | −2.13 | 10.23 | 87.77 | −1.82 | 11.97 | 87.66 | −2.03 | 12.41 | 87.68 | −2.06 | 12.44 | |
Table 4.
Color change (ΔE).
| Mean (S.D.) | |||||
|---|---|---|---|---|---|
| Groups | Storage conditions |
Week 1 | Week 2 | Week 4 | |
| 3CO | Room, wet | 1.03 (1.27) | 2.71 (1.17) | 3.40 (1.65) | |
| 60 C, wet | 5.54 (1.53) | 4.94 (1.67) | 5.30 (0.92) | ||
| Room, dry | 1.91 (1.42) | 2.41 (1.73) | 2.87 (1.73) | ||
| 60 C, dry | 7.82 (0.30) | 6.92 (0.67) | 7.09 (0.74) | ||
| 3CA | Room, wet | 5.16 (0.28) | 4.35 (1.05) | 4.56 (0.35) | |
| 60 C, wet | 3.83 (0.38) | 3.11 (0.49) | 2.23 (0.47) | ||
| Room, dry | 5.70 (0.95) | 7.06 (0.57) | 7.64 (0.60) | ||
| 60 C, dry | 8.51 (0.23) | 7.12 (1.20) | 7.81 (1.45) | ||
| 3COA | Room, wet | 3.25 (0.88) | 3.29 (0.67) | 1.72 (0.84) | |
| 60 C, wet | 5.61 (1.48) | 5.48 (1.44) | 6.27 (1.35) | ||
| Room, dry | 3.76 (1.71) | 4.81 (1.70) | 5.36 (0.62) | ||
| 60 C, dry | 7.20 (2.12) | 7.72 (0.87) | 8.13 (0.33) | ||
| 1CA | Room, wet | 2.23 (0.29) | 2.16 (0.24) | 2.02 (0.10) | |
| 60 C, wet | 2.15 (0.39) | 2.05 (0.51) | 1.85 (0.52) | ||
| Room, dry | 2.86 (0.13) | 3.28 (0.55) | 3.74 (0.55) | ||
| 60 C, dry | 3.29 (0.46) | 2.73 (0.38) | 2.33 (0.31) | ||
| 1COA | Room, wet | 0.89 (0.78) | 0.99 (0.53) | 1.16 (0.47) | |
| 60 C, wet | 2.63 (0.55) | 2.86 (0.53) | 3.02 (0.30) | ||
| Room, dry | 1.40 (1.13) | 1.66 (0.87) | 1.77 (0.19) | ||
| 60 C, dry | 1.80 (1.13) | 2.23 (1.21) | 2.26 (1.45) | ||
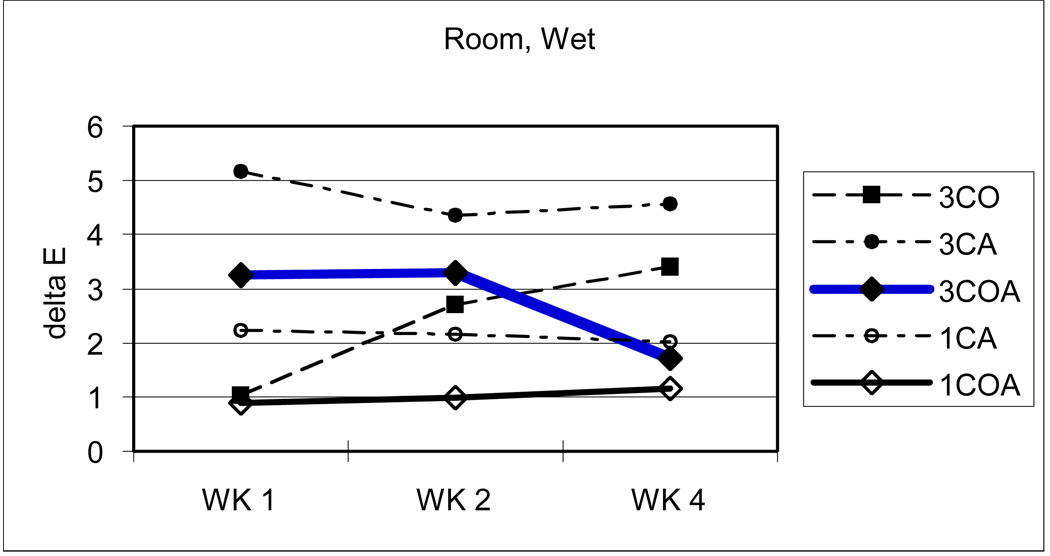
Figure 3.
Change of ΔE values with room, wet condition.
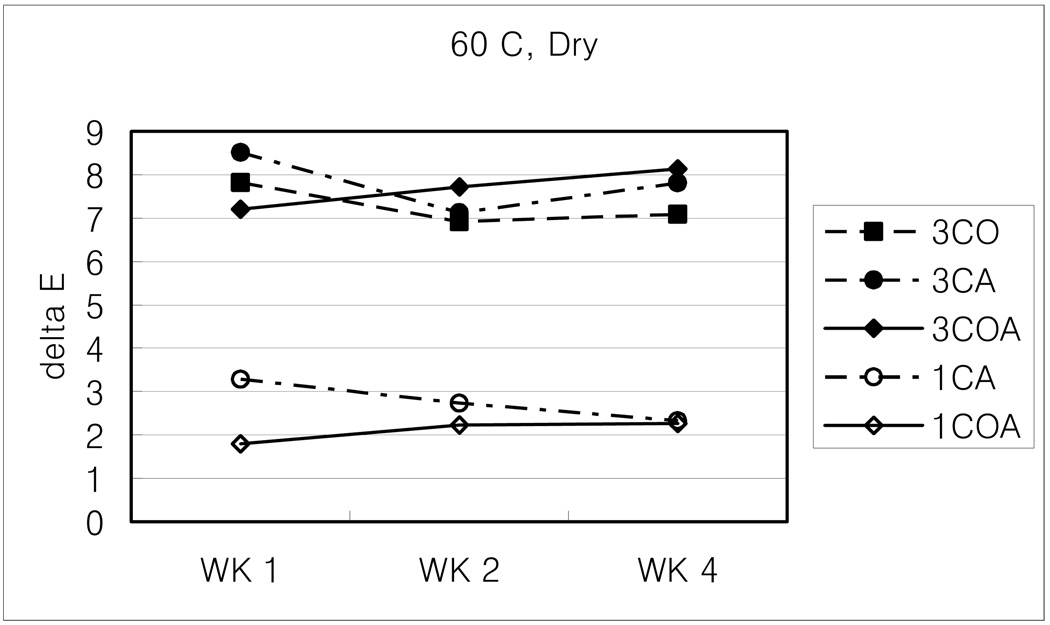
Figure 6.
Change of ΔE values with 60 C, dry condition.
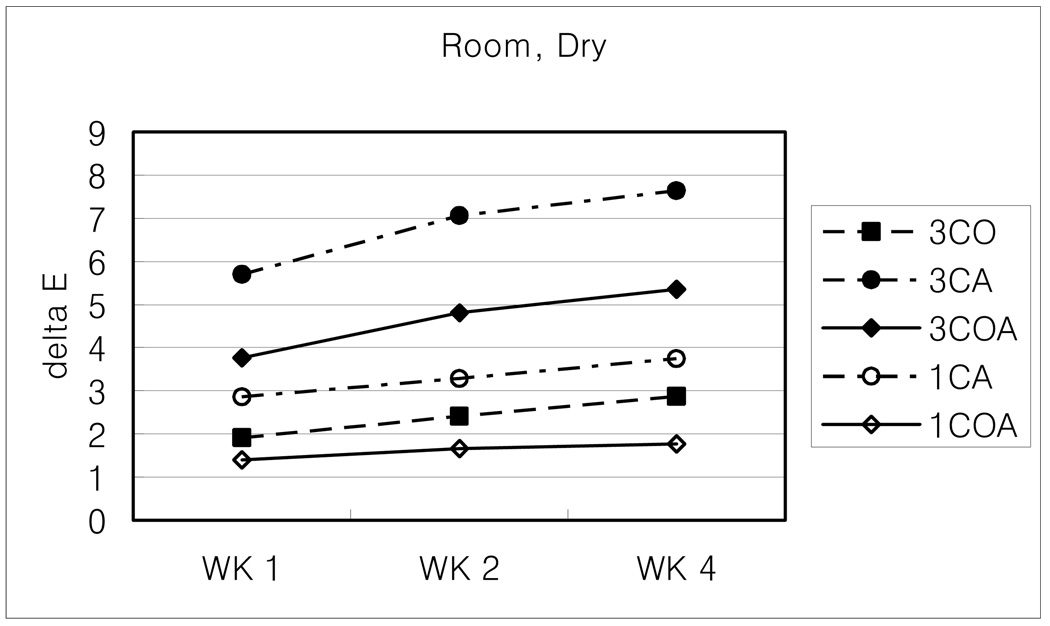
When the color coordinates were compared between different concentrations but the same proportions, such as 3CO and 1CO, higher sensitizer and initiator concentrations generally showed darker and/or more yellow colors, and changed more during aging than did lower photoinitiator concentrations.
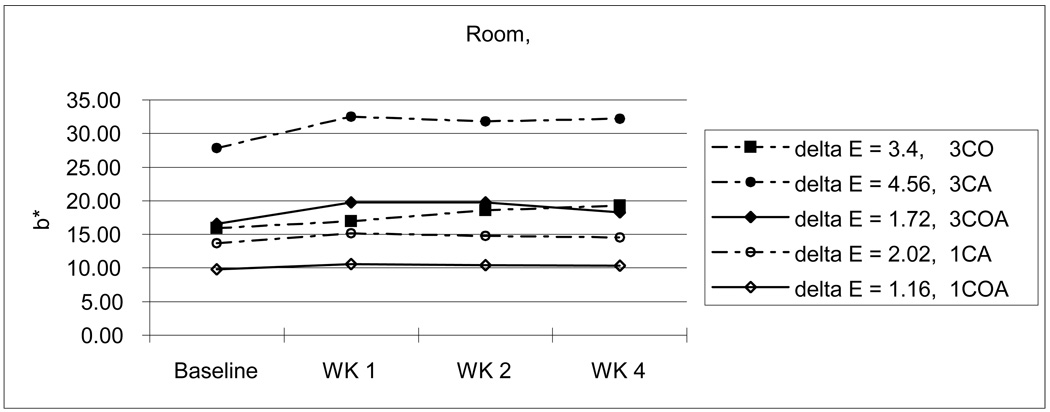
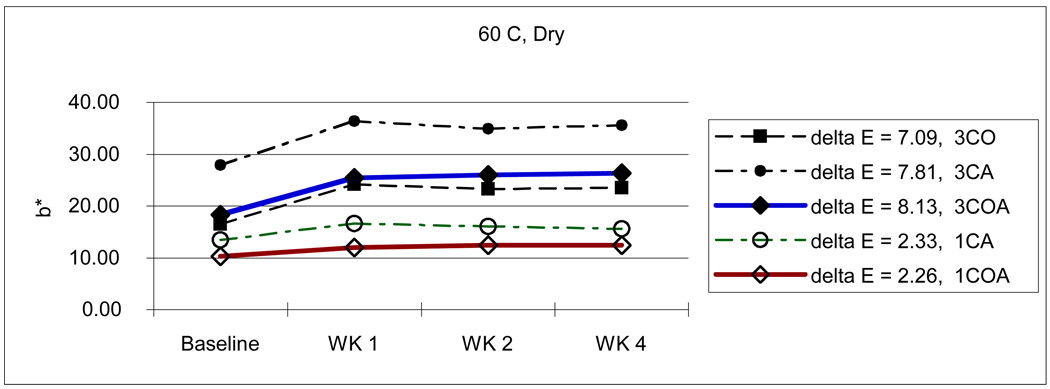
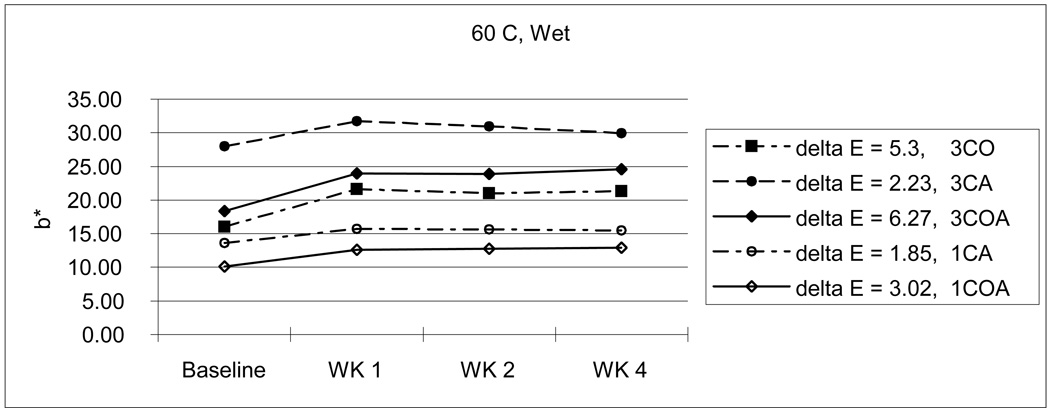
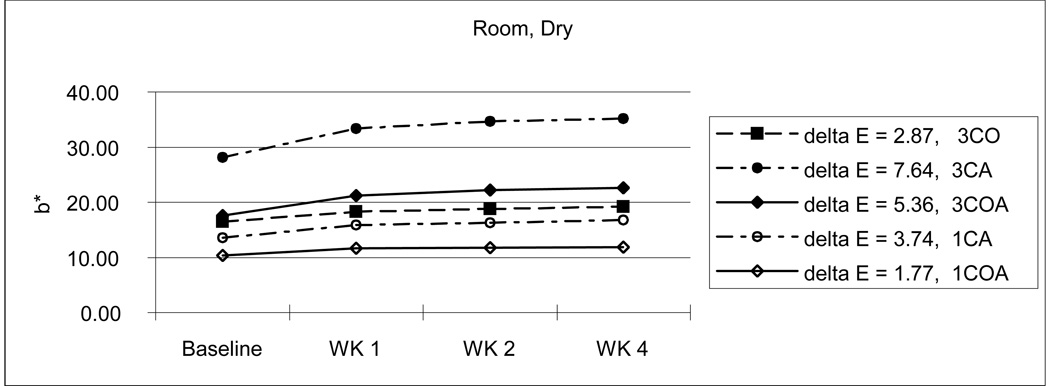
From Table 3, we can also see the initial color reduction, especially in b* parameter (3CA-27.95; 3CO-16.25; 3COA-17.71 / 1CA-13.58; 1COA-10.11), when OPPI has partially or totally replaced the amine. For example, 3CA was more dark and yellow than 3CO and 3COA, and 1CA was more yellow than 1COA. This tendency continues throughout the experiment. Even after 4 weeks storage in various conditions, the amine-containing 3CA group showed the highest yellow color value although the 3COA group changed a lot more than the 3CA group at the higher temperature with wet and dry conditions. (Fig. 7–10)
Figure 7.
Change of b* values with room temperature and wet condition.
Figure 10.
Change of b* values with high temperature and dry condition.
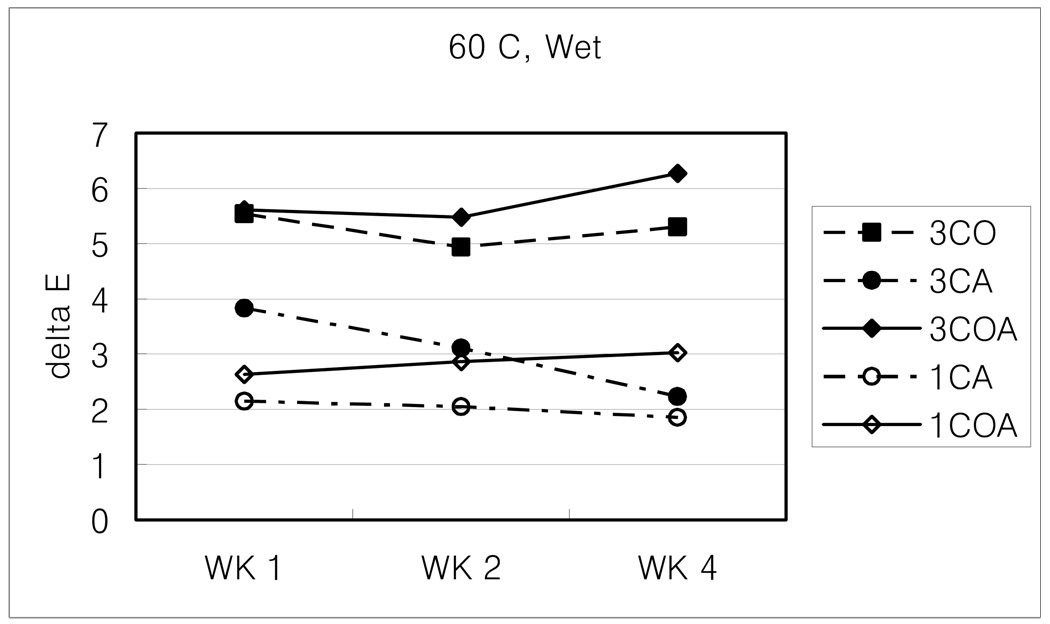
Considering the average ΔE for all storage conditions, amine-containing CA groups (3CA-5.56; 1CA-2.49) showed a little bit more color instability than OPPI-containing groups (3CO-4.67; 3COA-5.37; 1COA-2.05).
Generally, high temperature (60 C) or dry condition brought about more color change than room temperature or wet conditions. Among them, 60 C and dry combined condition is the harshest, under which composites could not keep their color stability and developed the greatest changes. Room temperature and wet condition is just the opposite.
In summary, OPPI in place of amine reduces initial color, mainly in the yellow (b*) parameter. In addition, OPPI in place of all or part of the amine has better color stability than the CQ/amine system and resists yellowing, maintaining a value 2/3 to half of that of the CQ/amine system (at the baseline: 3CA-27.86; 3CO-15.96; 3COA-16.60; 1CA-13.67; 1COA-9.81 / after 4 weeks: 3CA-32.23; 3CO-19.26; 3COA-18.24; 1CA-14.58; 1COA-10.37).
From the results of this study on degree and rate of conversion and color stability, the following results could be obtained ultimately. Substitution of OPPI for the amine provides a faster cure rate, higher conversion, lower initial color and better color stability. Moreover, substitution of OPPI for some or all of both CQ and amine provides very low initial color and exceptional color stability.
4. Discussions
The organic formulations of dental composite resin include photoinitiation systems that absorb light and take the molecules to excited states. From there, free radicals or other initiating species form and initiate conversion of oligomer blends to a polymeric crosslinked network [7,24]
Proper combinations of light sources, exposure time and photo-sensitizer/co-initiator systems are regarded as major factors to optimize light curing of dental composites [25], because improved conversion is critical for the optimization of mechanical properties [26], clinical performance, longevity, and biocompatibility.
CQ has been used as a photoinitiator the most commonly in dental composites because it has a light absorption maximum at 468 nm which matches well with the conventional dental curing lamps. However, it has some drawbacks such as low polymerization efficiency and toxicity [27]. The photolysis of a diketone leads to the homolytic cleavage of the C-C bond between the two carbonyl groups, resulting in two carbonyl radicals. This radical pair can undergo cage escape to form photodecomposed products. However, the two carbonyl radicals in CQ are structurally connected to each other and the probability of their recombination in CQ is great [16]. The consequent low polymerization efficiency of CQ results in relatively low mechanical properties without relatively high CQ concentrations and/or relatively long exposure times, as well as possible toxic effects from unreacted residual monomers [27]. In this study, experimental groups “C”, which used CQ alone as both photosensitizer and photoinitiator, showed very low degrees of conversion even after 300 seconds exposure (1C-3.36%; 3C- 23.57%).
High degrees of conversion could not be obtained until CQ was combined with an amine (1CA-56.93%; 3CA-52.12% at 300 seconds). The irradiation of the diketone and amine mixture resulted in the formation of an exciplex (excited state complex) via an electron transfer from amine to excited ketone [28]. A hydrogen transfer from hydrogen of amine to the diketone in the exciplex resulted in the production of an aminyl radical and a hydryl radical. The aminyl radical is responsible for the initiation of the polymerization reaction, whereas the CQ hydryl radical is almost inactive. The photo-decomposition rate of CQ increases with the addition of amine [16].
Meanwhile, it was also reported that high concentrations of CQ in resin formulations results in undesirable photoyellowing effects on the final aesthetic appearance of the cured material [8,9,29]. Therefore, there have been numerous tries to enhance the photo-initiator efficiency at lower concentrations by adding different co-initiators [12–14,18,19,26,30]. In addition, the claim of synergistic effects when two or more initiators are used together has also been reported. Park et al. [11] observed that equal amounts of CQ and PPD produced a higher degree of conversion than the sum of the effects of CQ and PPD used separately. This increase in the effectiveness was attributed to the wider absorption profile when both photoinitiators were used together, and the combination of two mechanisms of free radical production [20].
In this study, OPPI was used as an additional or alternative photoinitiator to enhance the degree and rate of conversion and, furthermore, to overcome the color problems associated with CQ. It was noted that the optimal photosensitizer/co-initiator concentration depends on many factors such as solubility of these compounds in the monomer mixture, absorption characteristics of the sensitizer, photo-reactivity (ability to form free radicals when the photosensitizer and coinitiator react), the effects of these compounds on color, the overlap between the wavelengths of light-source emission and photosensitizer absorption, and the biocompatibility of the components in the photoinitiatior system [31].
OPPI is a readily soluble white onium salt [21] and can be used as a somewhat hybrid photoinitiator, designed to be able to induce not only cationic polymerization radical but polymerization as well [32]. The onium salt immediately decomposes forming phenyl radicals, which may initiate cure or, abstract a hydrogen from the amine or monomer forming initiating radicals [21], therefore, it has sufficient energy to initiate the free-radical polymerization [32]. OPPI alone has an absorption maximum around 300 nm with a tail out to 380 nm, which is not in accord with the irradiated wavelengths from the conventional dental curing lamps. However, It was informed that the use of photosensitizers is one of the approaches to extend its absorption to longer wavelength ranges by forming bimolecular photosensitization systems with onium salts although the efficiency of bimolecular systems are usually limited by diffusion, especially in highly viscous polymeric systems [33]. For instance, combined, the borate/OPPI complex exhibits a substantial shift of 50 nm towards the visible light, which extends the tail of the absorbance to about 440 nm [21].
It is not clear whether there was a shift like this or not in this study. If it is true, increase in the degree and rate of conversion of the monomer would be anticipated, however, there may be another concern about the use of LED curing unit, because of its narrow range of irradiated visible light.
Of all the experimental groups, 3COA showed the highest degree and the fastest rate of conversion during all the curing time. While, 1COA acquired similar degree of conversion to that of conventional 1CA group [(1COA): 43.95 %, 48.24 %, 53.64 %; (1CA): 40.65 %, 49.96 %, 56.93 % at the times of 40, 60, 300 seconds], however, more degree of conversion was obtained within a relatively short time [(1COA): 13.24 %, 32.08 %; (1CA): 6.02 %, 20.11 % at the times of 5, 20 seconds]. These results meant that OPPI combined with amine increases ultimate conversion and rate of conversion more than OPPI or amine alone in the presence of CQ.
Sun and Chae also claimed that the photopolymerization efficiency of the BisGMA resins increased appreciably with the photosensitizer concentration up to 0.3 mol%, but not significantly over 3 mol% when 1,2-diketones, 1-phenyl-1,2-propanedione (PD), 2,3-butanedione (BD) and CQ were compared as a photosensitizer [16].
In this study, resin monomers with the higher concentration of photoinitiator systems (3 %) showed greater conversion than those containing the lower concentration (1 %). This result implies that improved converted composite resins with optimized mechanical properties could be obtained by using higher concentrations of the photoinitiator system if color problems and accompanying possible polymerization shrinkage could be mitigated.
Ideally, a composite resin should have the same color, translucency, and transparency as tooth structure in order to achieve a perfect color match between tooth and restoration. This is currently one of the more demanding factors in restorative dentistry. In addition, one of the most common reasons for replacing restorations is esthetic failure [34], therefore, color stability is another important factor.
As mentioned above earlier, composite resin with large amounts of CQ will create undesirable photo-yellowing effects [8,9,29]. Similarly, high concentrations of photoinitiator (3 %) showed darker and/or more yellow color and changed more during aging than do resins with lower photoinitiator concentrations (1 % in this study). However, when compared with monomers containing CQ and amine, the initial color reduction, especially in b* parameter (3CA-27.95; 3CO-16.25; 3COA-17.71 / 1CA-13.58; 1COA-10.11) was gained if OPPI was present. That is, if OPPI has either partially or totally replaced the amine initiator. For example, 3CA [L*,a*,b*: 84.16, −1.00, 27.95] was darker and more yellow than 3CO [L*,a*,b*: 87.20, −3.90, 16.25] and 3COA [L*,a*,b*: 86.86, −3.75, 17.71] and 1CA [L*,a*,b*: 87.08, −1.74, 13.58] was more yellow than 1COA [L*,a*,b*: 88.09, −2.09, 10.11]. Even after 4 weeks storage in various conditions, amine-containing 3CA (33.23) showed the highest yellow color value (3CO-20.83; 3COA-22.97) although 3COA changed a lot more than 3CA at the higher temperature in a wet environment [ΔE: 3COA-6.27; 3CA-2.23] and under dry conditions [ΔE: 3COA-8.13; 3CA-7.81]. (Fig. 7–10) Furthermore, the OPPI containing groups (ΔE: 3CO-4.67; 3COA-5.37; 1COA-2.05) showed somewhat more color stability than the amine-containing CA groups (ΔE: 3CA-5.56; 1CA-2.49), when considering average ΔE of all storage conditions.
As expected, high temperature or dry conditions brought about more color change than room temperature or wet conditions. Among them, combined high temperature and dry condition has the greatest effect (ΔE: 5.52), in which the composite has the least color stability (greatest increase in color). The combination of room temperature and wet conditions has just the opposite effect (ΔE: 2.57, the least color change).
Even though very promising results, such as increased rate and extent of monomer conversion, and increased color stability, can be obtained by using OPPI as a photoinitiator, other factors, such as biocompatibility, proper proportion, etc., must also be considered.
Generally, aqueous extracts of the cured resins are found to be less cytotoxic than aqueous extracts of the individual components. This means that the individual components may have been well polymerized and incorporated into the resin formulation such that elution of leachables does not reach toxic levels for the individual components [35]. However, the widely used BisGMA oligomer has been found to be somewhat cytotoxic in many cell culture systems [3,36] and there is a report that the photoinitiator Sarcat™ CD 1012, a diaryliodonium salt with a structure similar to OPPI, has greater cytotoxicity [TC50 = 14 mM] than CQ [TC50 = 779 mM] [23].
It has also been reported that a decreased degree of conversion can result if insufficient amine is used, and in addition, that resin color instability is aggravated and residual color problems develop at higher onium levels [21].
Only two values of total concentration (1%, 3%) and four proportions of the CQ/OPPI photoinitiator systems were investigated in this study. Therefore, the biocompatibility of composite resins containing OPPI, optimum concentrations and proper proportion of the three components (CQ/OPPI/amine), and other, additional, photosensitizers, photoinitiators, and coinitiator amines should be the subject of further studies.
5. Conclusions
Within the limits of this study, the following may be concluded:
OPPI can be used to replace the amine in a given CQ/amine photoinitiator system to accelerate cure rate, increase conversion, reduce initial color and increase color stability.
OPPI can be included with CQ and amine to allow reduction in CQ and amine concentration while maintaining or improving rate and degree of conversion and producing a very low color (e.g., b* value: 10) with improved color stability.
Including OPPI as a co-initiator with CQ and amine provides an increased range of trade-offs and flexibility of choice among curing and esthetic characteristics.
Figure 4.
Change of ΔE values with 60 C, wet condition.
Figure 5.
Change of ΔE values with room, dry condition.
Figure 8.
Change of b* values with high temperature and wet condition.
Figure 9.
Change of b* values with room temperature and dry condition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dong-Hoon Shin, Dankook Univ. School of Dentistry, South Korea
H. Ralph Rawls, Dept. of Restorative Dentistry, Biomaterials Div., UTHSCSA Dental School, San Antonio, TX.
References
- 1.Ferracane JL, Greener EH. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res. 1986;20:121–131. doi: 10.1002/jbm.820200111. [DOI] [PubMed] [Google Scholar]
- 2.Ferracane JL, Mitchem JC, Condon JR, Todd R. Wear and marginal breakdown of composites with various degrees of cure. J Dent Res. 1997;76:1508–1516. doi: 10.1177/00220345970760081401. [DOI] [PubMed] [Google Scholar]
- 3.Wataha JC, Hanks CT, Strawn S, Fat GC. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehab. 1994;21:453–462. doi: 10.1111/j.1365-2842.1994.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 4.Imazato S, Tarumi H, Kobayashi K, Hiraguri H, Oda K, Tsuchitani Y. Relationship between the degree of conversion and internal discoloration of light-activated composite. Dent Mater J. 1995;14:23–30. doi: 10.4012/dmj.14.23. [DOI] [PubMed] [Google Scholar]
- 5.Fouassier JP, Allonas X, Burget D. Photopolymerization reactions under visible lights: principle, mechanisms and examples of applications. Progress in Organic Coatings. 2003;47:16–36. [Google Scholar]
- 6.Watts DC. Reaction kinetics and mechanics in photopolymeised networks. Dent Mater. 2005;21:27–35. doi: 10.1016/j.dental.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent. 2000;12:300–308. doi: 10.1111/j.1708-8240.2000.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 8.Rueggeberg FA, Ergle JW, Lockwood PE. Effect of photoinitiator level on properties of a light-cured and post-cure heated model resin system. Dent Mater. 1997;13:360–364. doi: 10.1016/s0109-5641(97)80107-8. [DOI] [PubMed] [Google Scholar]
- 9.Suh BI. Controlling and understanding the polymerization shrinkage-induced stresses in light-cured composites. Compendium. 1999;20 suppl. 25:34–41. [PubMed] [Google Scholar]
- 10.Brackett MG, Brackett WW, Browning WD, Rueggeberg FA. The effect of light curing source on the residual yellowing of resin composites. Oper Dent. 2007;32(5):443–450. doi: 10.2341/06-129. [DOI] [PubMed] [Google Scholar]
- 11.Park YJ, Chae KH, Rawls HR. Development of a new photoinitiation system for dental light-cure composite resins. Dent Mater. 1999;15:120–127. doi: 10.1016/s0109-5641(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 12.Peutzfeldt A. Quantity of remaining double bonds of diacetyl containing resins. J Dent Res. 1994;73:511–515. doi: 10.1177/00220345940730020501. [DOI] [PubMed] [Google Scholar]
- 13.Peutzfeldt A. Quantity of remaining double bonds of propanal containing resins. J Dent Res. 1994;73:1657–1662. doi: 10.1177/00220345940730101101. [DOI] [PubMed] [Google Scholar]
- 14.Peutzfeldt A, Asmussen E. Effect of propanal and diacetyl on quantity of remaining double bonds of chemically cured BisGMA/TEGDMA resins. Eur J Oral Sci. 1996;104:309–312. doi: 10.1111/j.1600-0722.1996.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Burtscher P, Rheinberger V. Efficiency of various light initiators after curing with different light-curing units. J Dent Res. 2003;81 [Abstr. No. 42] [Google Scholar]
- 16.Sun GJ, Chae KH. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer. 2000;41:6205–6212. [Google Scholar]
- 17.Ogunyinka A, Palin WM, Shortall AC, Marquis PM. Photoinitiation chemistry affects light transmission and degree of conversion of curing experimental dental resin composites. Dent Mater. 2007;23(7):807–813. doi: 10.1016/j.dental.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SG, Chao HM. Photoreduction of aromatic ketones by amine. Studies of quantum yields and mechanism. J Am Chem Soc. 1968;90:165–173. [Google Scholar]
- 19.Antonucci JM, Venz S. Tertiary amine salts and complexes as chemical and photochemical accelerators. J Dent Res. 1987;66:128. [Abstr. No. 170] [Google Scholar]
- 20.Neumann MG, Schmitt CC, Ferreira GC, Corrêa IC. The initiating radical yields and the efficiency of polymerization for various dental photoinitiators excited by different light curing units. Dent Mater. 2006;22:576–584. doi: 10.1016/j.dental.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.De Raaff AM, Marino TL, Neckers DC. Optimized cure efficiency using a fluorone visible light photoinitiator and a novel charge transfer: Complex initiating system. RAI/TECH conference; May.1996. [Google Scholar]
- 22.Feng K, Zang H, Martin D, Marino TL, Neckers DC. Synthesis and study of iodonium borate salts as photoinitiators. J Polym Chem. 1998;36:1667–1677. [Google Scholar]
- 23.Eick JD, Kostoryz EL, Rozzi SM, Jacobs DW, Oxman JD, Chappelow CC, Glaros AG, Yourtee DM. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dent Mater. 2002;18:413–421. doi: 10.1016/s0109-5641(01)00071-9. [DOI] [PubMed] [Google Scholar]
- 24.Cook WD. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer. 1992;33:600–609. [Google Scholar]
- 25.Emami N, Soderholm KJ. Influence of light-curing procedures and photo-initiator/ co-initiator composition on the degree of conversion of light-curing resins. J Mater Sci Mater Med. 2005;16(1):47–52. doi: 10.1007/s10856-005-6445-1. [DOI] [PubMed] [Google Scholar]
- 26.Peutzfeldt A, Asmussen E. In vitro wear, hardness, and conversion of diacetyl-containing and propanal-containing resin materials. Dent Mater. 1996;12:103–108. doi: 10.1016/S0109-5641(96)80076-5. [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa S, Kadoma Y, Masuhara E. Effects of photoinitiators for the visible-light resin system on hemolysis of dog erythrocytes and lipid peroxidation of their components. J Dent Res. 1986;65:1186–1190. doi: 10.1177/00220345860650091401. [DOI] [PubMed] [Google Scholar]
- 28.Rabek JF. Mechanisms of photophysical processes and photochemical reactions in polymers. Wiley: Chichester; 1987. p. 269. [Google Scholar]
- 29.Studer K, Koniger R. Initial photoyellowing of photocrosslinked coatings. Eur Coat J. 2001;1:26–37. [Google Scholar]
- 30.Peutzfeldt A, Asmussen E. Influence of ketones on selected mechanical properties of resin composites. J Dent Res. 1992;71:1847–1850. doi: 10.1177/00220345920710111601. [DOI] [PubMed] [Google Scholar]
- 31.Jun N. PhD thesis. Division of Biomaterials Science, Karolinska institutet; 1998. ISBN 91-628-3231-X. [Google Scholar]
- 32.He JH, Mendoza VS. Synthesis and Study of a Novel Hybrid UV Photoinitiator:p-Benzoyldiphenyliodonium Hexafluorophosphate (PhCOPhl+PhPF;) J Polym Sci Part A Polym Chem. 1996;34:2809–2816. [Google Scholar]
- 33.Pappas SP, Jilek JH. Photogr. Sci. Eng. 1979;23:140. [Google Scholar]
- 34.Wilson NH, Burke FJ, Mjor IA. Reasons for placement and replacement of restorations of direct restorative materials by a selected group of practitioners in the United Kingdom. Quintessence Int. 1997;28(4):245–248. [PubMed] [Google Scholar]
- 35.Kostoryz EL, Tong PY, Chappelow CC, Eick JD, Glaros AG, Yourtee DM. In vitro cytotoxicity of solid epoxy-based dental resins and their components. Dent Mater. 1999;15:363–373. doi: 10.1016/s0109-5641(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 36.Hanks CT, Anderson M, Craig RG. Cytotoxic effects of dental cements on two cell culture systems. J Oral Pathol. 1981;10:101–112. doi: 10.1111/j.1600-0714.1981.tb01255.x. [DOI] [PubMed] [Google Scholar]