Abstract
Precise localization of proteins and mRNA in histological sections is necessary for evaluating spatial gene expression patterns. Here we report sensitive detection of the gene products in fish tissues by immunohistochemistry (IHC) and in situ hybridization (ISH) assays on sections of whole specimens and vertebra embedded in methyl methacrylate (MMA) resin. This plastic resin favors easy preparation of various specimen types and enables preparation of large sections with well-preserved cell morphology. IHC analysis of the muscle regulatory factor MyoD in transverse sections of juvenile cod revealed MyoD-positive cells in the dorsolateral parts of the adaxial muscle. ISH revealed less spatially restricted signals of the bone morphogenic protein bmp4 in muscle and brain. To assess the applicability of ISH on sections of bony tissue, col1a1 and col2a1 expression was investigated in non-decalcified vertebra sections of Atlantic salmon. The former was identified in both chondrocytes and osteoblasts, whereas the latter was mostly evident in chondrocytes. We conclude that MMA resin offers easy preparation of large and problematic tissues and the possibility of carrying out both IHC and ISH analyses using standard protocols. (J Histochem Cytochem 57:825–830, 2009)
Keywords: methyl methacrylate, whole embedding, large sections, in situ hybridization, immunohistochemistry
Localization of gene transcripts and the translated proteins by in situ hybridization (ISH) and immunohistochemistry (IHC), respectively, is commonly carried out on micrometer-thin sections of tissues. Thus, the sample must be embedded in a solid supporting medium for preservation of the finer tissue structures and cell morphology. The embedding material should minimize optimization of tissue preprocessing, and mRNAs and proteins should be available for binding to probes and antibodies, respectively. Embedding in a cryomedium offers optimal preservation and availability of biomolecules, but often results in inferior cellular resolution. Conversely, paraffin embedding improves the preservation of cellular morphology, but certain proteins may lose their antigenicity because of formalin-induced cross-linking and the high temperatures applied. Large and heterologous specimens are difficult to section and often require tedious optimization of the preprocessing steps in both methods, and bony tissue must be decalcified. Plastic resins facilitate difficult sectioning and offer improved morphological qualities, but impede accessibility for probes and antibodies. One exception is methyl methacrylate (MMA), which can be chemically removed from the tissue after sectioning (Erben 1997; Warren et al. 1998). Here we present MMA resin as a good alternative for embedding whole-specimen and bony tissues. ISH and IHC analyses of selected genes demonstrated that MMA offers easy preparation of difficult samples to evaluate the distribution of both mRNA and proteins in large tissue sections.
Materials and Methods
Tissue Preparation
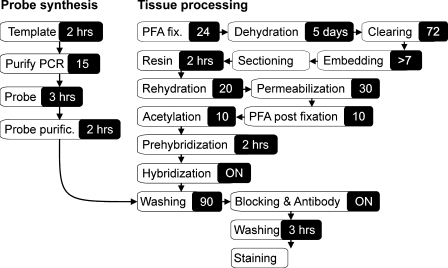
Preprocessing of whole Atlantic cod (Gadus morhua) juveniles and Atlantic salmon (Salmo salar) vertebrae dissected from 15 g salmon was initiated by fixation in 4% paraformaldehyde (PFA) for 24 hr at 4C. Successive dehydration steps were carried out in 50%, 75%, and 96% ethanol for 24 hr each, before four changes of absolute ethanol. Clearing was carried out for 3 × 24 hr in xylene and finalized with 10 min degassing. Destabilization of MMA (Technovit 9100 New; Heraeus Kulzer GmbH, Wehrheim, Germany) was accomplished by pressing 100 ml resin through a 50-ml syringe one fourth filled with aluminum oxide (90 basic; Merck, Darmstadt, Germany). Also, to prevent aluminum oxide in the resin, a Millex Nylon filter was attached to the syringe (product nr. SLHN M25 NS; Millipore, Billerica, MA). For infiltration and embedding, a total of five different resin mixtures were applied as described in Table 1. The initial three infiltration steps were carried out at 4C for 24 hr each, whereas incubation in mixture 4 was extended to 1 week or more. The specimens were then placed in polyethylene molds filled with the embedding mix and sealed to exclude oxygen, and polymerized for 7 days at −6C. Following polymerization, the resin blocks were cut and trimmed for correct orientation and then attached to microtome chucks using Technovit 3040 (Heraeus Kulzer). Sections were cut from the embedded specimens using a Microm HM 355S fitted with a D-profile tungsten carbide blade and a cutting angle of 3–4°. The cutting surface was kept wet with 30% ethanol, and the sections were mounted on precoated slides [0.01% poly-l-lysine (Sigma; St. Louis, MO) and 2% polyvinyl acetate glue (Casco; Arnheim, Germany)]. To ensure good adherence, a few drops of xylene were added before mounting and covering with a polyethylene foil and a clean slide. Several slide pairs were stacked and firmly pressed at room temperature for 1 hr and then overnight at 60C. Before use or storage, the slides were allowed to cool before careful removal of the polyethylene foil. Resin removal was carried out by successive incubations in xylene (1 hr), 2-methoxyethylacetate (1 hr), and acetone (10 min). The slides were then rehydrated in an ethanol series and rinsed for 5 min in diethylpyrocarbonate-treated dH2O before ISH and IHC analyses.
Table 1.
Overview of the resin mixtures applied in infiltration and embedding in Technovit 9100 New
| Resin | Xylene (ml) | S-resin (ml) | DS-resin (ml) | PMMA (g) | H 1 (g) | H2 (ml) | Regulator (ml) |
|---|---|---|---|---|---|---|---|
| R1 | 20 | 20 | |||||
| R2 | 40 | 0.2 | |||||
| R3 | 40 | 0.2 | |||||
| R4a | 40a | 3.2 | 0.16 | ||||
| R5 Aa | 40a | 6.4 | 0.24 | ||||
| R5 B | 5 | 0.4 | 0.2 |
To ensure homogeneity of the two mixtures containing PMMA powder, these solutions were stirred overnight at 4C.
S-resin, stable resin directly from the bottle; DS-resin, destabilized resin after activation with aluminum oxide. The poly-methyl methacrylate (PMMA) filler, hardeners H1 and H2, and the regulator are included to ensure optimal polymerization and block quality. The indicated volumes of resin mixtures equal total volume. Embedding, 9 vol R5 A + 1 vol R5 B.
Detection of mRNA and Proteins
Both ISH and IHC were carried out according to standard protocols (Beesley 2000) with sense probes and secondary antibody as controls, respectively (Figure 1). Riboprobes specific for Atlantic cod bmp4 (FJ435089) were synthesized from a Sp6- and T7-tailed 377-bp-long PCR fragment, whereas Atlantic salmon col1a1 (FJ195608) and col2a1 (FJ195613) probes were synthesized from a 1-kb and 256-bp fragment, respectively. The riboprobes and their sense variants were digoxigenin labeled (Roche; Basel, Switzerland). After resin removal and rehydration, the sections were washed in 1 × PBS Tween-20 (PBST), postfixed in 4% PFA, acetylated (0.1 M triethanolamine with 0.25% acetic anhydride), and permeabilized for 30 min in 10 μg/ml proteinase K in 1 × PBST at room temperature. Prehybridization and hybridization were carried out at 50C in 50% formamide, 5 × SSC, 5 mM EDTA, 10% dextran sulfate, 500 μg/ml tRNA, 460 μl 1 M citric acid, and 0.05% Tween-20. Posthybridization washes were carried out twice in 50% formamide with 1 × SSC Tween-20 at 60C and then three times in 1 × PBST at room temperature. Next, the sections were blocked for 2 hr [1 × maleic acid, pH 7.4, 1% blocking reagent (Roche), and 20% lamb serum], before addition of fresh blocking solution with antidigoxygenin alkaline phosphatase–conjugated F(ab′) fragments (1:2000) (Roche). The slides were incubated overnight at 4C before five 30-min washes in 1 × maleic acid and coloration using nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indoly phosphate p-toluidine salt (NBT/BCIP) (Roche). Prior to IHC analysis, the resin was removed as described above, and epitope retrieval was achieved by boiling the slides for 3 min in 10 mM Tris-HCl, pH 10, using a microwave oven. The slides were cooled and blocked for 1 hr in 1 × TBS with 5% dry milk. A polyclonal antibody against Atlantic salmon MyoD (generous gift from Dr. I.A. Johnston), was used at a 500-fold dilution in 1 × TBS with 2% dry milk. After five 30-min washes in 1 × TBS, a secondary alkaline phosphatase–conjugated goat anti-rabbit F(ab′) fragment (Invitrogen; Life Technologies Corporation, Carlsbad, CA) was used at 1000-fold dilution in 1 × TBS with 2% dry milk for 1 hr. The sections were washed in 1 × TBS five times and developed as for the ISH sections. After NBT/BCIP coloration, both ISH and IHC slides were rinsed in water and mounted in aqueous mounting media. All images were captured with a Leica DM6000B microscope (Leica Microsystems GmbH; Wetzlar, Germany), and MosaicJ (Biomedical Imaging Group; Lausanne, Switzerland, http://bigwww.epfl.ch/thevenaz/mosaicj/) was applied to create images with an increased lateral view of the field for some sections (Thevenaz and Unser 2007). Image brightness, contrast, and color balance were adjusted in Corel Draw X3 (Corel Corporation; Ontario, Canada).
Figure 1.
Flow diagram of methyl methacrylate embedding, resin removal, and in situ hybridization (ISH) analysis of a large specimen. The total hands-on time applied in embedding is brief and does not depend upon sample size. Immunohistochemistry (IHC) can be carried out after the resin removal step.
Results
Tissue Embedding and Sectioning
Here we have investigated the embedding and sectioning of large and difficult tissues in MMA resin and the subsequent detection of mRNA and proteins of selected genes. We applied the commercially available Technovit 9100 New, although it is possible to prepare cold curable MMA from stock chemicals (Erben 1997). Fine and fragile structures of both hard and soft tissues were well preserved in MMA resin, and few sections exhibited holes and tears, as can be experienced with paraffin embedding. Both transverse and longitudinal sections were prepared from the whole juvenile cod and from the adult salmon vertebra, resulting in up to 2–3-cm-long sections. Sizable sections were easily prepared at 6 μm, whereas smaller specimens could be cut thinner, down to 3 μm.
IHC and ISH Analysis
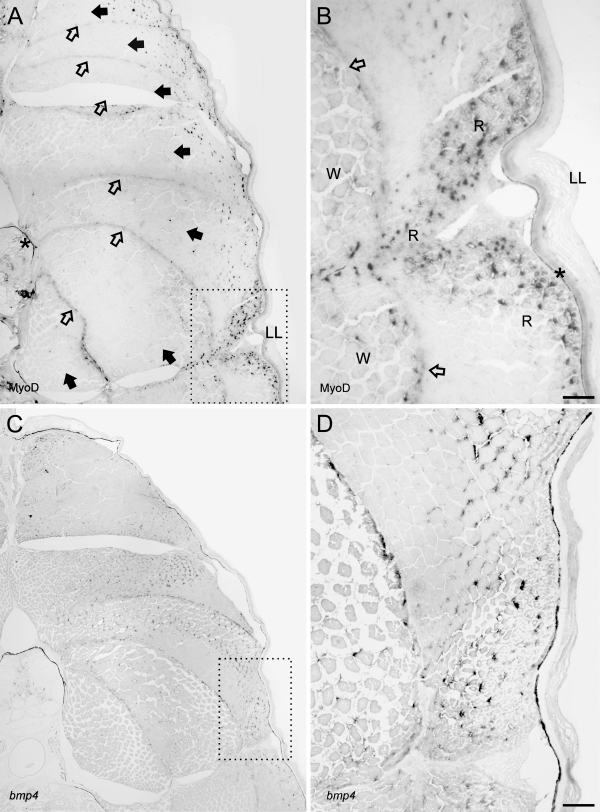
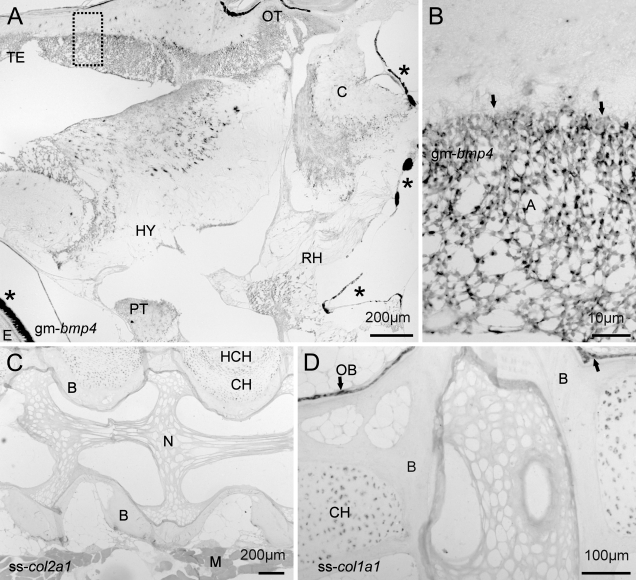
The detection of gene activity in MMA sections was performed using standard IHC and ISH protocols without any need for optimizations. Localization of MyoD by IHC in transverse sections of the juvenile cod revealed that positive cells were mainly located to the dorsolateral parts of the adaxial muscle, although some MyoD activity was also evident along the myoseptums. (Figures 2A and 2B). Large numbers of MyoD-positive satellite cells were also observed in the slow muscle layer underneath the skin and along the lateral line. Analysis of bmp4 expression was investigated in both cross and sagital sections of a whole juvenile cod. In cross sections, the bmp4 signal was abundant in most cells of the axial muscle, with the exception of some larger myocytes deep inside the myotome (Figures 2C and 2D). The sagital sections revealed spatially restricted expression of bmp4 in the brain, with strong expression in astrocytes, but also other cells (Figures 3A and 3B). Microscopy of the salmon vertebra revealed that both collagen genes were expressed in a cell-specific manner, with col1a1 abundantly expressed in chondrocytes and osteoblasts, whereas col2a1 was more prominent in chondrocytes (Figures 3C and 3D).
Figure 2.
MyoD activity as revealed by IHC and bmp4 by ISH on cross sections (5 μm) of whole juvenile cod embedded in Technovit 9100 New. (A) MyoD activity is restricted to the dorsal and lateral extremes of the myotomes and a few cells deep inside the axial muscle along the myoseptum. (B) Magnification of stippled box in A visualizes MyoD activity in myosatellite cells of the white and red muscles. (C) Expression of bmp4 in white and red muscles is widespread and abundant in myocytes. (D) Magnification of stippled box reveals high bmp4 expression in both slow and fast fibers. No coloration was visible on the sections with a sense bmp4 probe control. And for IHC, no signals were present on control sections incubated with the secondary antibody only. The asterisk indicates pigmented melanocytes in the dermis and notochord and is not unspecific background. Closed arrowhead, myotome; open arrowhead, myoseptum; LL, lateral line; W, white muscle; R, red muscle. Bar = 40 μm.
Figure 3.
ISH analysis on sagital sections (6 μm) of fish bony tissues. (A) Mosaic image of a cod brain with bmp4-positive cells in various brain structures (dorsal upwards). A small part of the eye (E) is visible in the lower left corner. The asterisks indicate pigmented melanocytes in the eye and hindbrain and are not unspecific background. (B) Magnification of the stippled rectangle showing abundant bmp4 expression in astrocytes in the molecular layer (arrows). (C) Expression of col2a1 in chondrocytes at the dorsal side of the vertebra. (D) Expression of col1a1 in chondrocytes and the bone-forming osteoblasts adjacent to the ossified bone. No coloration was visible on control sections with the corresponding sense probes. Arrows indicate osteoblasts (OB). TE, telencephalon; C, cerebellum; OB, osteoblasts; OT, optic tectum; RH, rhombencephalon; PT, pituitary; E, eye; HY, hypothalamus; A, astrocytes; B, ossified bone; N, notochord; CH, chondrocytes; HCH, hypertrophic chondrocytes; M, muscle.
Discussion
Preliminary experiments with paraffin embedding of Atlantic cod juveniles resulted in disintegrating paraffin sections despite optimization of dehydration, clearing, and paraffin-embedding regimes. In contrast, MMA resin embedding and sectioning was immediately successful without any optimization. Compared with paraffin and cryogenic embedding, the orientation of MMA-embedded specimens is easily inspected, because the blocks are clear and an optimal cutting angle can be obtained after tooling. The possibility for preparation of non-decalcified sections is beneficial for staining of calcified structures as well as for obtaining the best possible morphology (Blythe et al. 1997; Yang et al. 2003). The low-exothermic curing may also improve antigenicity, although the main cause of deteriorated antibody function is the cross-linking of proteins caused by formalin fixation (D'Amico et al. 2009). One drawback compared with paraffin is the slower cutting speed and the absence of cutting ribbons; thus, the main application of this protocol is in research and not in high-throughput routine applications.
Resin sections are prone to falling off the slides during IHC and ISH and, when compared with mounting in 70% ethanol, the reported use of xylene significantly improves the adherence during long protocols. Compared with other resin types, MMA improves detection sensitivity because it is removable (Church et al. 1997; Warren et al. 1998). Here we report strong coloration of both IHC and ISH signals, which implies good accessibility of both proteins and mRNA in MMA sections of whole fish and non-decalcified tissues. The detection of MyoD-positive myosatellite cells relatively close to the skin suggests that fiber recruitment in the adaxial muscle is spatially restricted. Further, the appearance of new cells explains the mosaic appearance of postlarval fish muscle and visualizes proliferative regions (Rowlerson and Veggetti 2001). The spatial expression of bmp4 in juvenile fish tissues is unknown, but postembryonal bmp4 expression has been investigated in the brain of rodents (Mikawa et al. 2006; Lein et al. 2007) and points toward a role for Bmp4 in astrocyte maturation (Gross et al. 1996; Gomes et al. 2003). Our findings with strong staining in cod astrocytes may suggest a similar role in teleosts, but more studies are needed. In the cod transverse sections, bmp4 was present in most cells of the adaxial muscle. The function remains to be elucidated, but bmp4 is a known inhibitor of MyoD during embryonal myogenesis (Hirsinger et al. 1997; Marcelle et al. 1997). The ISH analysis of Atlantic salmon col1a1 and col2a1 expression shows that both collagen genes are expressed in both osteoblasts and chondrocytes, although the former is much more abundant in osteoblasts. From mammals, it is known that these genes display cell specificity with col1a in differentiating osteoblasts and possibly in transdifferentiating chondrocytes (Roach et al. 1995; Gelse et al. 2003), whereas col2a1 is active in chondrocytes and the control cellular phenotype (Zhao et al. 1997).
In summary, the results from the ISH and IHC analyses show that MMA resin is a good alternative to paraffin and cryoembedding. The possibility of whole embedding of sizeable specimens enables analysis of gene activity in juvenile fish larvae and even adults of species like zebrafish. MMA sections offer good section adherence and quality, and the possibility of detecting mRNA and proteins in the same embedded tissue, including calcified structures. Further experiments in our laboratory have confirmed these findings for additional antibodies and probes.
Acknowledgments
This project was funded by Norwegian Research Council Grants 159672 (2005–2007), 164695 (2005–2007), and 172483/S40 (2006–2009).
We thank Dr. I.A. Johnston for the polyclonal MyoD antibody.
References
- Beesley JE, ed (2000) Immunocytochemistry and In Situ Hybridization in the Biomedical Sciences. Boston, Birkhäuser
- Blythe D, Hand NM, Jackson P, Barrans SL, Bradbury RD, Jack AS (1997) Use of methyl methacrylate resin for embedding bone marrow trephine biopsy specimens. J Clin Pathol 50:45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RJ, Hand NM, Rex M, Scotting PJ (1997) Non-isotopic in situ hybridization to detect chick Sox gene mRNA in plastic-embedded tissue. Histochem J 29:625–629 [DOI] [PubMed] [Google Scholar]
- D'Amico F, Skarmoutsou E, Stivala F (2009) State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods 341:1–18 [DOI] [PubMed] [Google Scholar]
- Erben RG (1997) Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem 45:307–313 [DOI] [PubMed] [Google Scholar]
- Gelse K, Soder S, Eger W, Diemtar T, Aigner T (2003) Osteophyte development: molecular characterization of differentiation stages. Osteoarthritis Cartilage 11:141–148 [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA (2003) Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol 255:164–177 [DOI] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA (1996) Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17:595–606 [DOI] [PubMed] [Google Scholar]
- Hirsinger E, Duprez D, Jouve C, Malapert P, Cooke J, Pourquie O (1997) Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development 124:4605–4614 [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176 [DOI] [PubMed] [Google Scholar]
- Marcelle C, Stark MR, Bronner-Fraser M (1997) Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development 124:3955–3963 [DOI] [PubMed] [Google Scholar]
- Mikawa S, Wang C, Sato K (2006) Bone morphogenetic protein-4 expression in the adult rat brain. J Comp Neurol 499:613–625 [DOI] [PubMed] [Google Scholar]
- Roach HI, Erenpreisa J, Aigner T (1995) Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol 131:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlerson A, Veggetti A (2001) Cellular mechanisms of post-embryonic muscle growth in aquaculture species. In Johnston IA, ed. Muscle Development and Growth. San Diego, Academic Press, 103–140
- Thevenaz P, Unser M (2007) User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc Res Tech 70:135–146 [DOI] [PubMed] [Google Scholar]
- Warren KC, Coyne KJ, Waite JH, Cary SC (1998) Use of methacrylate de-embedding protocols for in situ hybridization on semithin plastic sections with multiple detection strategies. J Histochem Cytochem 46:149–155 [DOI] [PubMed] [Google Scholar]
- Yang R, Davies CM, Archer CW, Richards RG (2003) Immunohistochemistry of matrix markers in Technovit 9100 New-embedded undecalcified bone sections. Eur Cell Mater 6:57–71 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B (1997) Parallel expression of Sox9 and Col21 in cells undergoing chondrogenesis. Dev Dyn 209:377–386 [DOI] [PubMed] [Google Scholar]