Abstract
Prolyl oligopeptidase (POP) is a serine endopeptidase that hydrolyses proline-containing peptides shorter than 30 amino acids. POP may be associated with cognitive functions, possibly via the cleavage of neuropeptides. Recent studies have also suggested novel non-hydrolytic and non-catalytic functions for POP. Moreover, POP has also been proposed as a regulator of inositol 1,4,5-triphosphate signaling and several other functions such as cell proliferation and differentiation, as well as signal transduction in the central nervous system, and it is suspected to be involved in pathological conditions such as Parkinson's and Alzheimer's diseases and cancer. POP inhibitors have been developed to restore the depleted neuropeptide levels encountered in aging or in neurodegenerative disorders. These compounds have shown some antiamnesic effects in animal models. However, the mechanisms of these hypothesized actions are still far from clear. Moreover, the physiological role of POP has remained unknown, and a lack of basic studies, including its distribution, is obvious. The aim of this review is to gather information about POP and to propose some novel roles for this enzyme based on its distribution and its discordant spatial association with its best known substrates. (J Histochem Cytochem 57:831–848, 2009)
Keywords: brain, cell division, electron microscopy, fluorescence microscopy, neuroanatomy, neuropeptides, neurotransmitters
Prolyl oligopeptidase (POP) activity was identified first by Walter et al. (1971) in the human uterus, where it was found to cleave oxytocin. POP is an 80-kDa serine protease belonging to the family S9 of the serine carboxypeptidase clan (Rawlings and Barrett 1994). The closest phylogenetic relatives to POP are dipeptidyl peptidase IV (DPPIV; EC 3.4.14.5), acylaminoacyl peptidase (EC 3.4.19.1), and oligopeptidase B (EC 3.4.21.83) (Rawlings and Barrett 1994; Venäläinen et al. 2004). The POP family has ancient origins, and it is widely distributed in organisms ranging from bacterial and archaeal species to humans; only fungi do not seem to possess a POP enzyme (Venäläinen et al. 2004). However, homologs of the S9A peptidase family are present in some fungi species (MEROPS peptidase database; Rawlings et al. 2008).
In humans and rats, POP enzyme activities have been found in most tissues, with the highest enzyme activity generally detected in brains (Kato et al. 1980b; Daly et al. 1985; Fuse et al. 1990; Irazusta et al. 2002). Moreover, low POP-like activities can be measured in all body fluids (Goossens et al. 1996; Garcia-Horsman et al. 2007). POP is mainly a cytosolic enzyme (Dresdner et al. 1982), although Schulz et al. (2005) reported a close association between POP and microtubules in glial and neural cell lines. POP activity has also been detected in close association with cell membranes (Tenorio-Laranga et al. 2008).
In most animal species, POP is 710 amino acids long. It has a cylindrical shape with a height of 60 Å and a diameter of 50 Å (Fülop et al. 1998). The peptidase domain is formed by N and C termini (residues 1–72 and 428–710) containing the catalytic triad (Ser554, Asp641, His680). POP has a cylindrical shape, where in addition to the peptidase component, the seven-bladed β-propeller domain is radially arranged around the central tunnel and is embedded within the cylinder. Concerted movements of the peptidase and β-propeller domains are thought to be required for enzyme function in which a substrate induces an opening at the interface of the two domains when entering the active site of POP (Szeltner et al. 2004). It has been suggested that the small size of the interface opening presumably prevents peptide substrates larger than 30 mer from entering into the active site of the mammalian POP.
The primary function of POP is thought to be the hydrolysis of the -Pro-Xaa- bond, where Xaa is any amino acid other than proline (Polgar 1994; Cunningham and O'Connor 1998). Several bioactive neuropeptides, such as substance P (SP), thyrotropin-releasing hormone (TRH), arginine-vasopressin (AVP), bradykinin, and neurotensin, are known to be POP substrates in vitro (for reviews, see Polgar 1994; Garcia-Horsman et al. 2007; Männistö et al. 2007). Many of these neuropeptides are associated with memory and learning (Huston and Hasenohrl 1995; Cunningham and O'Connor 1997), and also with neurodegenerative disorders such as Parkinson's and Alzheimer's diseases (Hasenohrl et al. 2000; Harrison and Geppetti 2001; Hökfelt et al. 2003). Furthermore, changes in POP enzyme activity or expression of the POP protein in tissues have been observed during aging (Agirregoitia et al. 2003; Rossner et al. 2005) and in various diseases such as Parkinson's and Alzheimer's diseases, suggesting a role for POP in these disorders via the relevant neuropeptide cleavage (Table 1; Aoyagi et al. 1990; Mantle et al. 1996; Kato et al. 1997; Shinoda et al. 1997). These findings have served as a rationale for the development of POP inhibitors, several of which have been successfully tested in different animal models of memory and learning (Yoshimoto et al. 1987; Toide et al. 1995a; Shinoda et al. 1996; Toide et al. 1997; Morain et al. 2002; Jalkanen et al. 2007; Männistö et al. 2007). In some studies, POP inhibitors have also restored declining neuropeptide levels, e.g., SP (Toide et al. 1996; Bellemere et al. 2003), TRH, AVP (Toide et al. 1995b,1996; Bellemere et al. 2005), in the rat cerebral cortex and hippocampus. In addition to the restoring effects of POP inhibitors on brain neuropeptide levels, studies with opposite results have been published (Jalkanen et al. 2007). Moreover, when critically evaluating the results achieved by treatment with POP inhibitors, their effects on neuropeptide levels in the brain have not been convincing. It is, for example, hard to explain why repeated treatment with POP inhibitors does not elevate neuropeptide levels although acute administration does (Männistö et al. 2007).
Table 1.
Suggested associations for POP in different disorders and physiological conditions
| Condition | Based on | Reference |
|---|---|---|
| Diseases | ||
| Alzheimer's disease | Increased enzyme activity | Aoyagi et al. 1990 |
| Decreased enzyme activity | Mantle et al. 1996 | |
| Bipolar disorder | Decreased plasma enzyme activity | Breen et al. 2004 |
| Cancer | Increased enzyme activity | Goossens et al. 1996 |
| Increased protein amount | Myöhänen et al. unpublished data | |
| Huntington's disease | Decreased enzyme activity | Mantle et al. 1996 |
| Hypertension | Increased enzyme activity | Cicilini et al. 1994; Goossens et al. 1996 |
| Inflammation | Increased enzyme activity | Kamori et al. 1991; Kakegawa et al. 2004 |
| Lewy's body dementia | Decreased enzyme activity | Mantle et al. 1996 |
| Parkinson's disease | Decreased enzyme activity | Mantle et al. 1996 |
| Physiological functions | ||
| Aging | Increased enzyme activity | Agirregoitia et al. 2003 |
| Increased protein amount | Rossner et al. 2005 | |
| Cell proliferation/differentiation/maturation | Increased enzyme activity during ontogenesis | Kato et al. 1980a; Fuse et al. 1990; Agirregoitia et al. 2007 |
| Changes in POP protein localization during maturation | Moreno-Baylach et al. 2008 | |
| Nuclear localization in peripheral tissues | Myöhänen et al. 2008c | |
| Blockade of cell differentiation and proliferation after POP inhibitor administration | Ohtsuki et al. 1994,1997b | |
| Inositol (1,4,5)-triphosphate (IP3) signalling | Increased IP3 levels after POP inhibitor dosing | Schulz et al. 2002; Williams et al. 2002 |
| Neuronal protection | POP inhibitors prevented the formation of cell stress-related factors | Puttonen et al. 2006 |
| Protein secretion | POP closely localized with microtubules | Schulz et al. 2005 |
POP, prolyl oligopeptidase.
POP is able to hydrolyze also some other peptide bonds in addition to -Pro-Xaa. Leprince et al. (2006) observed that POP can break Ala-Thr and Val-Gly peptide bonds of octadecaneuropeptide in a POP inhibitor–sensitive manner but with lower efficacy than the proline bond. POP has also been shown to hydrolyze the Cys-Xaa peptide bond in the novel neuroprotective peptide, humanin (Bär et al. 2006). Moreover, Cavasin et al. (2004) revealed that POP is responsible for the production of the antifibrotic peptide Ac-SDKP derived from its precursor, thymosin β4. However, because thymosin-β4 is 43 amino acids long, they suggested that it must be hydrolyzed into a smaller size by other peptidases, resulting in the truncation of thymosin β4 before being hydrolyzed by POP and release of Ac-SDKP from the N terminus. Similarly, Brandt et al. (2005) showed in vitro that POP cleaved fragments that belong to larger proteins, such as α-synuclein and β-hemoglobin. Additionally, POP is also able to metabolize the oncolytic drug tasidotin at the carboxyl side of tert-butylalamine (Bai et al, 2009).
Nevertheless, some roles beyond direct neuropeptide cleavage have quite early been proposed for POP. Williams and Harwood (2000) found that POP may be involved in the regulation of inositol 1,4,5-triphosphate (IP3) signaling, because Dictyostelium with a disrupted POP gene (dpoA) was resistant to lithium (Li+)-induced depletion of IP3 levels. In further support of this hypothesis, administration of a POP inhibitor restored this Li+-induced depletion of IP3 in the wild-type Dictyostelium cells and mammalian cells (Williams and Harwood 2000; Schulz et al. 2002). The mechanism of this action is still unclear, but POP may be able to regulate the synthesis of IP3 via the multiple inositol polyphosphate phosphatases (Williams and Harwood 2000; Harwood and Agam 2003) or intracellular calcium levels via the short sequence of PEP-19, a calmodulin binding polypeptide (Brandt et al. 2005). Recently, Di Daniel et al. (2009) suggested that POP is able to regulate cell phosphoinositode system via protein–protein interactions with growth-associated protein (GAP)-43.
Furthermore, the effects of different mood-stabilizing drugs on both POP and IP3 levels in the cell have been studied (Williams et al. 2002; Harwood and Agam 2003; Williams 2005), and Cheng et al. (2005) even suggested that POP could be a direct target for valproic acid (VPA), because this anti-epileptic drug was shown to inhibit POP in vitro. However, subchronic administration of a POP inhibitor (JTP-4819) had no significant effect on the IP3 levels in rat cerebral cortex and hippocampus (Jalkanen et al. 2007). One suggested function for POP is as a molecular timer via the cis-trans isomerization of proline (Lu et al. 2007; Ikura et al. 2008), although the ability of POP to undertake cis-trans isomerization may not be very significant (Brandt et al. 2008). Prolyl isomerization has been considered to be involved in several biological processes, such as cell cycle, cell signaling, and gene expression (Lu et al. 2007; Ikura et al. 2008), that would have explained the mechanism of some of the POP effects.
Reported involvement of POP in proliferation and differentiation in Sarcophaga peregrina (flesh fly; Ohtsuki et al. 1994), mouse cells (Ishino et al. 1998), or cerebellar granule cells in culture (Moreno-Baylach et al. 2008) is difficult to explain in terms of the peptide hydrolytic capacity of this protein. However, POP inhibitors have been able to arrest cell cycle and differentiation in some of these systems, although this effect might occur owing to neurotoxic effects, because the concentration of the POP inhibitor in these studies was extremely high (Ohtsuki et al. 1994,1997b). Interestingly, Di Daniel et al. (2009) showed, using POP knock-out mice, that POP is involved in neuronal growth and that this action is not dependent on catalytic activity but rather on protein–protein interactions. POP inhibitors have also shown some minor neuroprotective characteristics (Puttonen et al. 2006). Moreover, Schulz et al. (2005) have proposed a role for POP in protein secretion due to the close association between POP and cell microtubules (Schulz et al. 2005). However, it is not known whether these functions result from putative peptide cleavage or from a non-hydrolytic function.
Despite intensive research, the true physiological role of POP is still practically unknown. Using tissue, cellular, and subcellular distribution data, we have tried to clarify whether the spatial distribution of the POP enzyme and its under-30-mer proline-containing peptide substrates would make hydrolysis possible. If that does not appear realistic, we also explored the possibility that a colocalization of POP and some cellular mediators would provide a rationale for alternative, perhaps non-hydrolytic, even non-catalytic functions for POP protein.
Distribution of POP mRNA, Amount of POP Protein, and Enzymatic Activity of POP in Peripheral Tissues
Distribution of POP Coding mRNA in Peripheral Tissues
In high-throughput gene expression profiling (Genomics Institute of the Novartis Research Foundation, GNF SymAtlas; Su et al. 2002), the highest levels of human POP mRNA have been found in the testis and different types of lymphocytes (Table 2). In contrast to the enzymatic activity studies, the amount of POP mRNA was approximately the same in internal organs, such as the liver, lungs, and pancreas, and the brain. In the rat, the most abundant POP mRNA expression was observed in the kidney and endothelial cells, whereas in the mouse, the expression was the highest in the thymus.
Table 2.
The distribution of immunoreactive POP protein (POP-IHC), POP coding mRNA, and POP enzyme activity in various studies
| Enzyme activity | mRNA | POP-IHC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain | ||||||||||
| References | Irazusta et al. 2002 | Kato et al. 1980b | Agirregoitia et al. 2005 | Irazusta et al. 2002 | Fuse et al. 1990 | Daly et al. 1985 | GNF SymAtlas, Su et al. 2002 | Bellemere et al. 2004 | Myöhänen et al. 2007 | Myöhänen et al. 2008b |
| Species | Human | Human | Sprague-Dawley rat | Sprague-Dawley rat | Wistar rat | Wistar rat | Human | Wistar rat | Human | Wistar rat |
| Cortex | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | +++ | +++ |
| Striatum | ++ | + | +++ | +++ | + | ++ | +++ | +++ | ||
| Cerebellum | + | + | +++ | ++ | + | +++ | +++ | +++ | ||
| Hippocampus | ++ | +++ | ++ | +++ | +++ | |||||
| Hypothalamus | ++ | ++ | ++ | ++ | +++ | ND | ||||
| Thalamus | ++ | ++ | ++ | |||||||
| Substantia nigra | + | +++ | +++ | |||||||
| Peripheral tissues | ||||||||||
| References | Goossens et al. 1996 | Kato et al. 1980b | Agirregoitia et al. 2005 | Myöhänen et al. 2008c | Fuse et al. 1990 | Daly et al. 1985 | GNF SymAtlas, Su et al. 2002 | Myöhänen et al. 2008c | ||
| Species | Human | Human | Sprague-Dawley rat | NMRI mouse | Wistar rat | Wistar rat | Human | NMRI mouse | ||
| Lung | ++ | + | ++ | + | +++ | + | ++ | |||
| Kidney | +++ | +++ | + | + | +++ | + | +++ | |||
| Spleen | ++ | + | + | |||||||
| Liver | +++ | +++ | +++ | + | + | |||||
| Urinary bladder | + | + | ++ | |||||||
| Testis | +++ | +++ | ++ | ++ | +++ | |||||
| Heart | + | ++ | + | + | + | + | ||||
| Gut | + | |||||||||
| Skeletal muscle | ++ | |||||||||
| Cells | ||||||||||
| References | Goossens et al. 1996 | Schulz et al. 2005 | Mentlein et al. 1990 | Koshiya et al. 1984 | GNF SymAtlas, Su et al. 2002 | Myöhänen et al. 2008b | ||||
| Species | Human | Human | Wistar rat | Wistar rat | Human | Wistar rat | ||||
| Neurons | +++ | +++ | +++ | +++ | ||||||
| Astrocytes | ++ | +++ | ND | |||||||
| Oligodendrocytes | + | + | ||||||||
| Lymphocytes | ++ | +++ | ||||||||
| Epithelial cells | +++ | |||||||||
+++, high expression; ++, moderate expression; +, low expression; ND, not detectable; NMRI, Navy Medical Research Institution.
Distribution of Immunoreactive POP Protein in Peripheral Tissues
The distribution of POP by immunohistochemistry was not comprehensively studied before our studies, and only partial localizations from cell cultures and brains (Ishino et al. 1998; Rossner et al. 2005; Schulz et al. 2005), testis (Kimura et al. 2002), and flesh fly (Ohtsuki et al. 1997b) had been done. The specificity of our antibody in various tissues/species was tested with several methods (Myöhänen et al. 2007,2008b). It did not react with DPPIV, the closest relative of POP. However, most of our distribution studies are made by using one POP antibody because reliable commercial antibodies are not available.
In peripheral tissues, POP protein was widely but unevenly distributed, and it was present in various cells of different organs, without any cell-type specificity (Myöhänen et al. 2008c). The highest amount of POP protein was seen in the brain, but almost equally high levels of POP immunoreactivity were found in the kidney, testis, and thymus. POP was also clearly detectable in the urinary bladder, lungs, and heart. However, only a low amount of POP protein was detected in the liver.
It is important to note that in peripheral tissues, immunoreactive POP was present also in the nuclei and not only in the cytoplasm of the cells (Myöhänen et al. 2008c). Generally, POP has been considered a cytosolic enzyme, but a membrane-bound form has been described (Dresdner et al. 1982; O'Leary and O'Connor 1995; Garcia-Horsman et al. 2007; Tenorio-Laranga et al. 2008). POP protein and enzymatic activity have been detected in the nucleus of the non-neuronal cell lines (Ishino et al. 1998) and also in neuronal cell cultures, but only early in development (Table 3; Moreno-Baylach et al. 2008). Furthermore, in Sarcophaga peregrina (flesh fly), POP was widely distributed in the tissues, in both cytoplasm and nucleus (Table 3; Ohtsuki et al. 1997a,b).
Table 3.
Subcellular localization of POP in tissues and cell lines
| Tissue/cell line | Cytoplasm | Cell membranes | Microtubulus | RER | Golgi apparatus | Mitochondria | Nucleus | Method | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rat brain | +++ | + | − | + | + | − | − | IHC-EM | Myöhänen et al. 2008b |
| Rabbit brain | +++ | + | Activity | Dresdner et al. 1982 | |||||
| Mouse brain | +++ | IFL | Rossner et al. 2005 | ||||||
| Human glioma cell line (LN-405) | +++ | ++ | IFL | Schulz et al. 2005 | |||||
| Neuroblastoma cell line (SH-SY5Y) | +++ | ++ | IFL | Schulz et al. 2005 | |||||
| Mouse peripheral tissues | ++ | +++ | IHC | Myöhänen et al. 2008c | |||||
| Cerebellar neurons (primary culture) | |||||||||
| Young | ++ | +++ | FLI/WB/Activity | Moreno-Baylach et al. 2008 | |||||
| Mature | ++ | + | 0 | FLI/WB/Activity | Moreno-Baylach et al. 2008 | ||||
| Aged | +++ | + | 0 | FLI/WB/Activity | Moreno-Baylach et al. 2008 | ||||
| Swiss 3T3 cell culture | ++ | ++ | IFL | Ishino et al. 1998 | |||||
| Sarcophaga peregrina (flesh fly) embryonic cells | + | +++ | IFL | Ohtsuki et al. 1994 | |||||
The densities of POP in cell structures: −, no POP; +, low density of POP; ++, moderate density of POP; +++, high density of POP. FLI, fluorescent POP-inhibitor; IFL, immunofluorescence; IHC, immunohistochemistry; IHC-EM, immuno-electronmicroscopy; RER, rough endoplasmic reticulum; WB, Western blotting.
It is interesting that POP sequence does not contain information for nuclear transport (NetNES; la Cour et al. 2004). That may be important when defining the physiological role of POP.
Distribution and Regulation of Enzymatic Activity of POP in Peripheral Tissues
The knowledge of distribution of POP in peripheral tissues is based primarily on enzyme activity measurements. These measurements have been made using substrates with a suitable Pro-X bond, where X is a fluorescent compound such as β-naphthylamine or 4-methylcoumarin that is liberated after POP cleavage. POP activities have been found in peripheral tissues such as the rat skeletal muscle (Daly et al. 1985; Fuse et al. 1990), testis, liver, kidney, lung, renal cortex, heart, and gut (Kato et al. 1980a,b; Fuse et al. 1990; Goossens et al. 1996; Agirregoitia et al. 2005). The results of these studies are somewhat inconsistent (Table 2), possibly owing to different assay conditions, especially regarding the nature of the substrate and its concentration. Generally, in the rat, the highest POP activities have been found in the brain (Kato et al. 1980b; Irazusta et al. 2002; Agirregoitia et al. 2005), but Fuse et al. (1990) found the highest activities in the kidney. In humans, the highest enzyme activities were reported from cancerous tissues and in healthy samples from the epithelial cells, kidney, and testis (Goossens et al. 1996). In the mouse, the highest enzyme activity was detected in the liver, followed by testis (Myöhänen et al. 2008c). Surprisingly, the activity in the brain was only moderate, and even lower activities were measured from the urinary bladder, adrenal gland, and kidney (Myöhänen et al. 2008c).
Distribution of POP mRNA, Amount of POP Protein, and Enzymatic Activity in the Central Nervous System (CNS)
POP has been studied most intensively in the brain. In enzyme activity measurements, the highest activities have been generally found in the brain, especially in the cerebral cortex (Kato et al. 1980b; Daly et al. 1985; Irazusta et al. 2002; Agirregoitia et al. 2005). Moreover, a rationale for POP inhibitor development was to prevent memory and learning disorders in neurodegenerative disease by inhibiting the POP-mediated degradation of neuropeptides to elevate neuropeptide levels in the brain (Kato et al. 1980a; Cunningham and O'Connor 1997). High POP activity in the brain and effects of POP inhibitors on memory and learning models gave rise to the presumption that POP would have an important physiological function in the brain.
Distribution of POP Coding mRNA in the CNS
Bellemere et al. (2004) studied the distribution of the POP mRNA in the rat brain and pituitary by quantitative RT-PCR analysis and in situ hybridization. The highest amounts of POP mRNA were found in the cerebellum and hypothalamus. Interestingly, the amounts of mRNA in the cerebral cortex were only approximately half those found in the cerebellum (Bellemere et al. 2004), even though in terms of enzyme activity measurements, the situation was reversed (Table 2). Minor amounts of POP mRNA were observed in the substantia nigra, medulla oblongata, and spinal cord. In the high-throughput gene expression profiling, the POP mRNA levels have been generally rather similar throughout the different brain areas. In the human brain, the highest POP mRNA levels have been found in the hypothalamus and prefrontal cortex, with the lowest levels present in the cerebellum (Table 2, GNF SymAtlas; Su et al. 2002). In the mouse brain, substantial amounts of POP mRNA were found in the cerebellum, whereas in the rat, the highest levels were detected in the hippocampus and dorsal striatum. In these species, there were no major differences in the levels of the expression of POP mRNA in different brain regions (GNF SymAtlas; Su et al. 2002).
Distribution of Immunoreactive POP Protein in the CNS
We recently studied the distribution of POP protein at the microscopic level in human and rat brains (Myöhänen et al. 2007) and at the cellular and subcellular level in the rat brain (Myöhänen et al. 2008b). A summary of these studies is presented in Tables 2–4.
Table 4.
The distribution of POP immunoreactivity in the rat brain and its colocalizations with γ-aminobutyric acid (GABA)ergic (GAD 65/67), cholinergic (ChAT), inositol 1,4,5-triphosphate type 1 receptor (IP3R1), and substance P (SP) markers
| Structure | Expression of POPa | Colocalization of POP with GAD 65/67a (%) | Colocalization of POP with ChATa (%) | Colocalization of POP with IP3R1b (%) | Colocalization of POP with SPb (%) |
|---|---|---|---|---|---|
| Forebrain and cerebral cortex | |||||
| Agranular insular cortex | ++ | 58 ± 2.6 | 42 ± 3.1 | 58 ± 4.7 | 20 ± 3.5 |
| Auditory cortex, primary | +++ | 37 ± 4.4 | 41 ± 2.9 | 46 ± 5.7 | 39 ± 2.9 |
| Cingulated cortex | + | 23 ± 1.7 | 31 ± 3.9 | 42 ± 3.8 | 31 ± 5.6 |
| Granular insular cortex | +++ | 34 ± 2.2 | 36 ± 2.0 | 47 ± 2.8 | 33 ± 3.5 |
| Infralimbic cortex | + | 50 ± 4.7 | 38 ± 3.3 | 53 ± 2.4 | 27 ± 2.5 |
| Lateral entorhinal cortex | ++ | 45 ± 4.0 | 36 ± 3.3 | 57 ± 1.0 | 35 ± 2.7 |
| Motor cortex, primary | |||||
| Layer 1 | 0 | − | − | − | − |
| Layer 2 | ++ | 54 ± 4.9 | 21 ± 2.8 | 56 ± 1.5 | 29 ± 4.8 |
| Layer 3 | +++ | 50 ± 2.4 | 32 ± 2.5 | 31 ± 2.9 | 35 ± 3.8 |
| Layer 4 | ++ | 43 ± 1.4 | 30 ± 7.0 | 35 ± 1.5 | 34 ± 0.5 |
| Layer 5 | +++ | 35 ± 4.2 | 37 ± 3.3 | 40 ± 2.5 | 35 ± 2.3 |
| Layer 6 | +++ | 45 ± 5.3 | 40 ± 2.7 | 37 ± 2.5 | 22 ± 2.9 |
| Piriform cortex | +++ | 43 ± 3.6 | 53 ± 3.4 | 63 ± 9.8 | 25 ± 1.2 |
| Prelimbic cortex | + | 34 ± 4.7 | 38 ± 3.4 | 52 ± 2.1 | 35 ± 4.8 |
| Retrosplenial cortex | ++ | 46 ± 2.7 | 57 ± 4.6 | 38 ± 4.2 | 25 ± 5.6 |
| Somatosensory cortex, primary | |||||
| Layer 1 | 0 | − | − | − | − |
| Layer 2 | +++ | 37 ± 1.5 | 33 ± 5.6 | 40 ± 7.1 | 24 ± 1.9 |
| Layer 3 | +++ | 43 ± 6.7 | 39 ± 4.2 | 32 ± 4.3 | 28 ± 1.1 |
| Layer 4 | ++ | 37 ± 4.4 | 38 ± 1.7 | 53 ± 6.4 | 29 ± 3.6 |
| Layer 5 | +++ | 42 ± 2.3 | 36 ± 3.8 | 54 ± 7.0 | 38 ± 3.0 |
| Layer 6 | +++ | 44 ± 5.6 | 39 ± 2.8 | 57 ± 2.8 | 39 ± 6.4 |
| Visual cortex, primary | ++ | 35 ± 3.4 | 28 ± 3.6 | 31 ± 2.3 | 33 ± 1.9 |
| Claustrum | +++ | 43 ± 3.3 | 44 ± 6.4 | 51 ± 4.8 | 26 ± 3.4 |
| Lateral globus pallidus | ++ | 44 ± 2.3 | 26 ± 1.8 | 38 ± 2.7 | 30 ± 4.5 |
| Striatum | +++ | 61 ± 2.9 | 29 ± 3.2 | 36 ± 5.2 | 26 ± 2.2 |
| Ventral pallidum | +++ | 45 ± 2.9 | 28 ± 3.6 | 59 ± 7.9 | 29 ± 6.1 |
| Nucleus accumbens | |||||
| Core | ++ | 37 ± 3.2 | 42 ± 4.9 | 38 ± 3.0 | 31 ± 2.8 |
| Shell | + | 32 ± 3.1 | 45 ± 3.3 | 42 ± 2.3 | 35 ± 1.5 |
| Hippocampus | |||||
| CA1 | +++ | 27 ± 2.1 | 31 ± 3.7 | 87 ± 7.6 | 27 ± 2.9 |
| CA2 | ++ | 25 ± 2.2 | 29 ± 4.3 | 83 ± 3.3 | 28 ± 4.3 |
| CA3 | ++ | 23 ± 3.4 | 26 ± 3.6 | 87 ± 4.6 | 26 ± 3.2 |
| Dentate gyrus | + | ||||
| Stratum laconosum | +++ | ||||
| Indusium griseum | ++ | 55 ± 4.1 | 26 ± 2.5 | 34 ± 4.5 | 21 ± 4.7 |
| Lateral septal area | +++ | 36 ± 7.0 | − | ||
| Midbrain | |||||
| Substantia nigra | ++ | 58 ± 3.5 | 27 ± 4.4 | 25 ± 4.1 | 23 ± 2.9 |
| Ventral tegmental area | 0 | ||||
| Red nucleus | 0 | ||||
| Superior colliculus | 0 | ||||
| Inferior colliculus | 0 | ||||
| Thalamus and hypothalamus | |||||
| Ventroposterior nuclei | ++ | 43 ± 3.9 | 34 ± 5.2 | 78 ± 3.9 | 32 ± 3.5 |
| Posterior thalamic nuclear group | ++ | 46 ± 2.4 | 43 ± 2.5 | 86 ± 4.3 | 40 ± 3.8 |
| Laterodorsal thalamic nucleus | ++ | 42 ± 2.4 | 34 ± 3.2 | 85 ± 3.5 | 32 ± 3.7 |
| Mediodorsal thalamic nucleus | ++ | 45 ± 6.4 | 35 ± 2.6 | 83 ± 1.6 | 35 ± 3.6 |
| Ventromedial thalamic nucleus | ++ | 42 ± 4.9 | 34 ± 1.5 | 66 ± 7.2 | 38 ± 6.6 |
| Medial geniculate nucleus | ++ | 38 ± 5.2 | 34 ± 1.9 | 71 ± 6.6 | 25 ± 4.6 |
| Lateral geniculate nucleus | ++ | 49 ± 5.5 | 35 ± 3.3 | 44 ± 7.4 | 30 ± 2.2 |
| Medial mammillary nucleus | + | ||||
| Cerebellum and pons | |||||
| Cerebellum | |||||
| Purkinje cells and molecular layer | +++ | 90 ± 3.7 | − | 77 ± 5.8 | 45 ± 6.1 |
| Granular layer | 0 | − | − | ||
| Medulla | 0 | ||||
Data from Myöhänen et al. (2008b).
Data from Myöhänen et al. (2008a).
The densities of POP immunoreactive cells in brain structures: 0, no POP immunoreactivity; +, low density of POP; ++, moderate density of POP; +++, high density of POP; −, not detectable. Data are presented as mean ± SEM.
In the brain, POP was expressed rather evenly and widely in all cortical brain areas and more specifically in some nuclei (Myöhänen et al. 2007). Generally, POP was present in the gray matter, whereas axons were devoid of POP immunoreactivity. In both human and rat brains, POP was extensively present in the nigrostriatal pathway, i.e., substantia nigra and striatum (Tables 2 and 4; Myöhänen et al. 2007,2008b). Generally, in the rat brain cortex, the staining was particularly intense in the large and medium-sized pyramidal cells and apical dendrites of layers II-VI (Table 4; Myöhänen et al. 2008b).
High levels of POP protein were seen in both the rat and human hippocampi (Myöhänen et al. 2007), especially in the pyramidal neurons and apical dendrites of the CA1 field (Myöhänen et al. 2008b). Furthermore, in the areas with close connections to the hippocampus, such as the indusium griseum and lateral septal area, POP immunoreactivity was particularly intense. (Myöhänen et al. 2008b). In other limbic system areas, such as nucleus accumbens and frontal cortex, POP protein was present from a low to a moderate degree (Table 4; Myöhänen et al. 2008b).
In the cerebellum, the expression of POP protein was high in the cortex and Purkinje cells. Both the somas and the dendrites of the molecular cell layer were intensively immunostained. In contrast, the granular cell layer virtually lacked POP-immunoreactive cells (Myöhänen et al. 2007,2008b).
Moderate POP staining was found in different anterior and medial thalamic nuclei, and in lateral and medial geniculate nuclei, the expression of POP protein was high (Table 4; Myöhänen et al. 2007,2008b). In the hypothalamus, practically no immunoreactive POP has been detected, with the exception of the medial mamillary nucleus (Table 4; Myöhänen et al. 2007,2008b).
Even though POP activities have been detected in astroglial cell lines (Table 2; Mentlein et al. 1990; Schulz et al. 2005), no immunoreactive POP protein was seen in the brain astrocytes (Rossner et al. 2005; Myöhänen et al. 2008b).
Enzymatic Activity of POP in the CNS
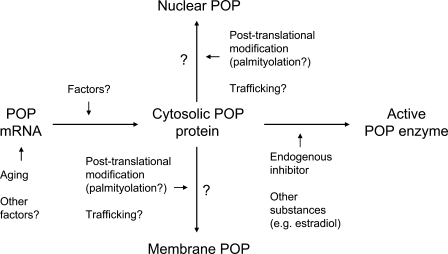
POP activities have been found in various brain areas. Kato et al. (1980b) studied the distribution of POP activity in the human brain and found the highest activity in the cerebral cortex, whereas other areas exhibited much lower activities. Similar results were obtained in the rat brain by Daly et al. (1985), who also found a rather high POP activity in the cerebellum and slightly lower activity in the brain stem. Also, in the other distribution studies conducted in the rat, the highest POP activities were found in the cortex (Table 2; Fuse et al. 1990; Irazusta et al. 2002; Agirregoitia et al. 2005). In the rat brain, lower POP activities have been analyzed in the hypothalamus (Fuse et al. 1990; Irazusta et al. 2002), hippocampus, cerebellum, and amygdala (Irazusta et al. 2002). Moreover, Agirregoitia et al. (2005) measured similar levels of POP activities in the rat cortex, striatum, and cerebellum. However, it was difficult to make a valid comparison of various studies because different substrates and conditions have been used (Table 2). Furthermore, the presence of a putative endogenous POP inhibitor (Yoshimoto et al. 1982; Salers 1994; Yamakawa et al. 1994) and other possible regulators of POP activity may have influenced the results (Figure 1).
Figure 1.
The relationship between prolyl oligopeptidase (POP) mRNA, protein, and active enzyme is not straightforward. Posttranslational modification or trafficking of POP protein might explain the differences between POP mRNA and mature protein. An endogenous POP inhibitor or other compounds may be able to modulate the activity of POP. However, it is not known how these compounds are regulated.
The distribution of POP activity between different neuronal cell types has been analyzed in a few studies (Table 2). Mentlein et al. (1990) measured the highest enzyme activity in rat neurons, followed closely by astrocytes, and concluded that POP was expressed in both the neurons and glial cells. The POP activity was low in the oligodendrocytes. Schulz et al. (2005) measured POP activities in rat neuron-, astrocyte-, oligodendrocyte-, and microglial-rich primary cultures, with POP activity significantly higher in neurons than in glial cells, where moderate POP activity was seen only in the astrocytes.
Subcellular Localization of POP in the CNS
In addition to cytosol (Dresdner et al. 1982; Schulz et al. 2005), POP activity has been found bound to the membranes of a variety of cell lines (Chappell et al. 1990) and in the synaptosomal fractions of the bovine brain (O'Leary and O'Connor 1995). This form of POP is able to hydrolyze in vitro the same substrates as the soluble, cytosolic form (O'Leary et al. 1996), but its activity or amounts are lower than those of the cytosolic POP (Irazusta et al. 2002; Agirregoitia et al. 2005).
Generally, POP activity has been found in cytosol (Table 3; Dresdner et al. 1982; Rossner et al. 2005; Schulz et al. 2005; Myöhänen et al. 2008b), although in the study by Dresdner et al. (1982), POP activity was found in the mitochondrial fraction. Moreover, Schulz et al. (2005) used immunofluorescence techniques to reveal that POP was attached to the main component of the microtubulin cytoskeleton, tubulin, in human glioma cell lines. POP appeared to be mostly localized in the perinuclear space of the cell in human glioma and neuroblastoma cell lines, but not in the nucleus.
In postembedding immunoelectron microscopy of the rat brain, POP immunoreactivity was generally and most abundantly found free in the cytoplasm (Myöhänen et al. 2008b). However, some POP protein was also attached to cell membranes, especially to the intracellular membranes of the rough endoplasmic reticulum (RER) and Golgi apparatus. However, we failed to detect whether POP is in the extracellular or intracellular part of the cell membrane. Some POP was also found inside the axons and within their myelin sheaths (Table 3; Myöhänen et al. 2008b).
Interestingly, POP was seen in the nucleus of cerebellar granular primary culture early in development (Moreno-Baylach et al. 2008). However, as the culture matured, POP seemed to depart from the nucleus to the cytosol. The relevance of nuclear POP is discussed in more detail in a later section.
As a summary, there seems to be a discrepancy between the distributions of enzymatic activity of POP, POP mRNA, and POP protein (Tables 2 and 3). For example, a large amount of the POP coding mRNA (Bellemere et al. 2004) and POP protein (Tables 2 and 4) was detected in cerebellum, whereas enzyme activity fluctuated from low to high between the studies (Table 2; Irazusta et al. 2002; Agirregoitia et al. 2005). Moreover, we did not observe any POP protein in the hypothalamus (Tables 2 and 4), whereas in the mRNA study of Bellemere et al. (2004), POP coding mRNA expression was high in the hypothalamus, and some POP activities were also found in the hypothalamus (Table 2; Fuse et al. 1990; Irazusta et al. 2002). The regulation of POP activity and expression is discussed in more detail in the next section.
All of these measurements show, however, that POP is widely expressed in both the CNS and in the peripheral tissues. Generally, the highest levels of POP are measured in the cerebral cortex in the CNS and in the testis in peripheral tissues. The wide expression of POP indicates that it must have a role in physiological functions. Interestingly, there also seem to be differences between POP expression in the tissues and in cell lines. For example, POP activity has been detected in the neural cells and POP protein in a glial cell line, whereas in the tissues, this was not the case (Table 2). Moreover, in the peripheral tissues, POP was also expressed in the nucleus, in contrast to the CNS. This disparity might be explained as owing to the faster proliferation in the cell lines and peripheral tissues when compared with the CNS, and to possible effects of POP in the cell proliferation. However, the studies dealing with the expression of POP in vivo might further clarify its physiological effects.
Possible Regulators of the Enzymatic Activity and Expression of POP in the CNS
As shown above, major differences are obvious when the distribution of POP is judged based on the results of enzymatic activity, mRNA expression (high-throughput gene expression profiling or in situ hybridization), and immunohistochemistry (Figure 1; Table 2).
Several substances are able to modify the POP activity in the brain. Polyamines (e.g., spermine, spermidine) activate POP, and they are able to reverse the effect of an endogenous POP inhibitor in vitro (Soeda et al. 1986). Moreover, treatment with a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist, MK-801, was shown to increase POP activity in the rat hippocampus and cerebral cortex, whereas a γ-aminobutyric acid (GABA)A receptor blocker, pentylenetetrazol, and an atypical antipsychotic drug, clozapine, decreased POP activity in the same brain areas (Ahmed et al. 2005; Arif et al. 2007). Moreover, oxidizing agents may inhibit POP activity, at least in cell cultures (Tsukahara et al. 1990). Even changes in plasma volume and/or osmotic pressure have been reported to affect the brain POP activity (Irazusta et al. 2001).
In a microarray analysis conducted in tissue from mouse hypothalamus and cortex (Jiang et al. 2001), the POP gene expression in 22-month-old mice was 11-fold in hypothalamus, and in cerebral cortex it was 2.7-fold higher than the values in 2-month-old mice. In a supporting study, Rossner et al. (2005) used immunohistochemistry to demonstrate increased POP expression in the hippocampus of aged mouse. Furthermore, POP gene expression was downregulated by 2.6–2.7-fold after 3- to 6-hr exposure to an enriched environment (Rampon et al. 2000), pointing to the age- and learning-dependent regulation of POP expression.
An endogenous POP inhibitor has been described in several studies (Yoshimoto et al. 1982; Salers 1994; Yamakawa et al. 1994). It was originally found and purified from the rat pancreas (Yoshimoto et al. 1982) and a pancreatic cell line (Salers 1994) and subsequently identified also in regenerating rat liver (Yamakawa et al. 1994). This cytosolic substance is a 6.5-kDa POP-specific inhibitor (Ki value 2.6 μM) (Soeda et al. 1985; Salers 1994). However, this compound has been biologically rather poorly characterized, and its regulation and functions are still obscure. In addition, estradiol-17β, progesterone (Ohta et al. 1992), and cortisol (Yasuda et al. 1992) are able to increase POP activity in the peripheral tissues (Figure 1).
The differences between POP protein and POP coding mRNA distribution may also be due to posttranslational modification of POP (Figure 1). It still remains unclear how the POP enzyme is transported from these synthesis sites to the protein expression areas, if it is transported at all. The sequence analyses of POP protein (Venäläinen et al. 2004) and its gene (Kimura et al. 1999), although very partial in the promoter region, indicate that POP is mainly a cytosolic protein. Tenorio-Laranga et al. (2008) have demonstrated that POP is indeed associated to membranes and suggested that palmitoylation may be the anchoring mechanism (Figure 1). That could explain the trafficking of POP across the cell and its membrane interactions. However, the efforts to confirm POP palmitoylation in vitro or in vivo have been unsuccessful.
Another interesting fact is that phosphotyrosine proteome studies have reported a phosphorylated form of POP. Phosphorylation is believed to take place at the Tyr71, but it is not known which kinase is responsible or what the consequences of this kind of a modification are (Luo et al. 2008).
Conclusively, there are several chemical and other factors that can affect POP activity, possibly even protein expression (Figure 1). Moreover, differences between the expression profiles of POP determined by enzyme activity, mRNA, and immunohistochemistry clearly point to a strict endogenous regulation of POP, i.e., via endogenous inhibitor and/or protein trafficking. Interestingly, POP trafficking between the cytosol and the nucleus occurs even though there is no nuclear transport signal in the POP sequence. Nevertheless, more studies on regulatory systems of POP, especially characterization of endogenous inhibitor, are clearly needed.
Insights Into the Physiological Functions of POP Protein Based on Its Distribution
Catalytic Functions
POP has traditionally been implicated in the metabolism of short, under-30-mer, proline-containing peptides, such as SP, TRH, and AVP (Walter 1976; Kato et al. 1980a; Yoshimoto et al. 1981; Garcia-Horsman et al. 2007), which are believed to be involved in memory and learning (Nillni and Sevarino 1999; Hasenohrl et al. 2000; Rose and Moore 2002). Changes in these neuropeptide levels have been reported in several neurodegenerative diseases (Beal et al. 1987; Mantle et al. 1996). Moreover, POP and its effects on neuropeptide metabolism have been widely studied in vitro and also, to some extent, in vivo. In some studies, POP inhibitors have been able to restore the neuropeptide levels in the brain in vivo (Toide et al. 1995b; Bellemere et al. 2003,2005). However, in these studies, generally only acute treatment with POP inhibitor caused a significant increase in neuropeptide levels, whereas chronic treatment failed to do so. Hence, it seems likely that the role of POP in hydrolysis of its “traditional” substrates in vivo may be overestimated.
One argument against the view that hydrolysis of neuropeptides is the main function of POP in the brain is the different cellular localizations of POP and its substrates (Brandt et al. 2007; Myöhänen et al. 2008a). Neuropeptides are released outside the cell directly from the storage vesicles, whereas POP is thought to be mainly an intracellular enzyme, though perhaps having some putative membrane-bound form (Tenorio-Laranga et al. 2008). How is it possible for an intracellular enzyme to metabolize neuropeptides released outside the cell? One could speculate that membrane-bound POP may be able to cleave neuropeptides in the vicinity of the cell membrane, assuming that it is orientated extracellularly. The location of the active site of the membrane-bound form is, however, not known. Moreover, even though intracellular receptors for SP (Baude and Shigemoto 1998; Levesque et al. 2007) and TRH (Sun et al. 2003; Cook and Hinkle 2004) have been found, these are more likely to be the result of internalization during receptor recycling during a desensitization event. In that situation, the normal stimulating ligands are not able to activate the receptors in the intracellular space (Hökfelt et al. 2003; Sun et al. 2003; Cook and Hinkle 2004). This contradiction may be the reason for inconsistent effects of POP inhibitors on neuropeptide levels in the brain (Männistö et al. 2007). Moreover, when we studied the spatial association of POP, SP, and its NK-1 receptor in the rat brain, a rather poor colocalization was found (Table 4). Only in the cerebellar Purkinje cells was the colocalization moderate (Table 4; Myöhänen et al. 2008a). Furthermore, POP containing GABAergic neurons only rarely colocalized with SP, although SP and GABA were commonly found in the same cells (Myöhänen et al. 2008a) and GABAergic cells are known to utilize SP as a neurotransmitter (McGinty 2007). Therefore, it seems that POP may have some other functions beyond the cleavage of SP and possibly other neuropeptides. One alternative explanation could be an involvement of POP in the processing of the immature forms of proline-containing peptides, because POP has been detected in the RER and Golgi apparatus (Table 3; Myöhänen et al. 2008b).
Although there are only a few studies that have examined the involvement of POP in peptide hydrolysis in the peripheral tissues, some associations have been found. Elevated POP activity has been reported in inflammation (Kamori et al. 1991; Kakegawa et al. 2004), where a general mediator is SP. Furthermore, SP is highly expressed in the lymphocytes and T cells, where enzymatic POP activity and expression are also high (Shirasawa et al. 1994; Vanhoof et al. 1994; Goossens et al. 1996). However, there are no in vivo studies demonstrating that POP is able to hydrolyze SP in the peripheral tissues. There is an intense expression of TRH in the pancreas, where TRH is synthesized in the insulin-producing β cells (Leduque et al. 1989). Although the presence of POP in the pancreas has not been consistently reported (Yoshimoto et al. 1982; Fuse et al. 1990; Salers 1994), its possible involvement in controlling the level of pancreatic TRH in rats during development was evaluated by Salers et al. (1991). These workers reported that even though POP degraded TRH in vitro, it failed to do so in vivo. Interestingly, the activity of POP is decreased in atria and increased in ventricles as a consequence of hypertension such as that seen in nephrectomized rats with left renal artery obstruction (Cicilini et al. 1994). Moreover, a clear correlation was observed between angiotensin-converting enzyme and POP activity in patients suffering from renovascular hypertension (Goossens et al. 1996). POP may regulate these actions via the cleavage of kidney neuropeptides, such as AVP (Lee et al. 2003). However, there is no direct proof that POP is involved in AVP hydrolysis in vivo or that the changes of POP activity in hypertension are caused by altered AVP cleavage.
Because POP inhibitors have been relatively ineffective in modulating neuropeptide levels in vivo and there is a lack of other direct in vivo results showing that POP significantly hydrolyzes bioactive peptides (Männistö et al. 2007), functions for POP other than direct neuropeptide cleavage should be seriously considered. Di Daniel et al. (2009) showed, using POP knock-out mice, that effects caused by lack of the POP gene can be restored by both normal POP and catalytically inactive POP proteins. Moreover, POP was shown to be able to form protein–protein interactions with GAP-43 in vitro (Di Daniel et al. 2009). Protein–protein interaction between POP and α-synuclein has also been proposed previously (Brandt et al. 2008). This clearly suggests that non-hydrolytic functions for POP exist and might mediate some of the physiological effects of POP.
Cell Proliferation/differentiation
The differences in the subcellular expression of POP in the brain, cell lines, and peripheral tissues may depend on the ability of the cells to proliferate (Tables 2 and 3). It is well known that mature brain tissue generally does not undergo proliferation, whereas certain peripheral tissues and cell lines are continuously dividing. We tried to determine whether the expression of POP in the nuclei would be connected with the active moment of cell proliferation by studying the colocalization of nuclear POP protein and cell proliferation marker Ki-67 (Myöhänen et al. 2008c). However, only partial colocalization was seen. Furthermore, the nuclear localization of POP supports a role for this enzyme in cell division and/or differentiation, because POP activity is also high in early development (Matsubara et al. 1998; Agirregoitia et al. 2003,2007) and in cancer cells (Goossens et al. 1996). The expression of POP protein was also significantly increased in cancerous tumors (Liu et al. 2008; Myöhänen TT and Männistö PT, unpublished data). In neuronal primary cultures, POP was expressed in the nuclei of the cells early in development but no longer when the cells had matured (Moreno-Baylach et al. 2008). High levels of POP activity were seen in the testis, where the POP protein was exclusively present in the developing spermatids, confirming the results of Kimura et al. (2002) in the mouse testis.
Moreover, recent studies using POP knock-out mice showed that the lack of POP decreases the growth of neuronal growth cones by 1.8-fold, compared with wild-type mouse (Di Daniel et al. 2009). These actions may be mediated via protein–protein interactions with GAP-43, which is involved in growth cone morphology and axonal growth (Oestreicher et al. 1997). In addition to other results, this also indicates the role of POP in the regulation of cell growth events.
In proliferating tissues, such as some peripheral tissues and immortal cell cultures, nuclear POP may have novel roles, such as modification of nuclear transport (Schulz et al. 2005; Puttonen et al. 2006), or other catalytic functions such as prolyl cis-trans isomerization, as observed with bacterial POP (Lu et al. 2007; Ikura et al. 2008). However, recent studies have shown that mammalian POP does not possess this capability (Brandt et al. 2008), and prolyl isomerase activity of bacterial POP is only 5% of the activity of FKBP12, a conventional prolyl isomerase (Ikura et al. 2008). Notably, studies using POP knock-out mice have shown that the effect of POP on the growth cone is dependent not on catalytic activity but rather on protein–protein interactions (Di Daniel et al. 2009).
On the basis of previous cell culture results and recent studies on POP knock-out mice, we conclude that it seems very likely that POP is involved in cell proliferation and growth. Moreover, this action may not be associated with catalytic activity, but POP tight-binding inhibitors are able to affect to cell proliferation and growth by blocking the active site. Protein–protein interaction with GAP-43 might explain the effects of POP on cell growth, but it does not explain the cytosolic–nuclear trafficking of POP during maturation and the effects of POP on cell proliferation. Therefore, more-detailed studies of POP's actions in cell proliferation are needed.
POP and Its Associations With Neurotransmitters
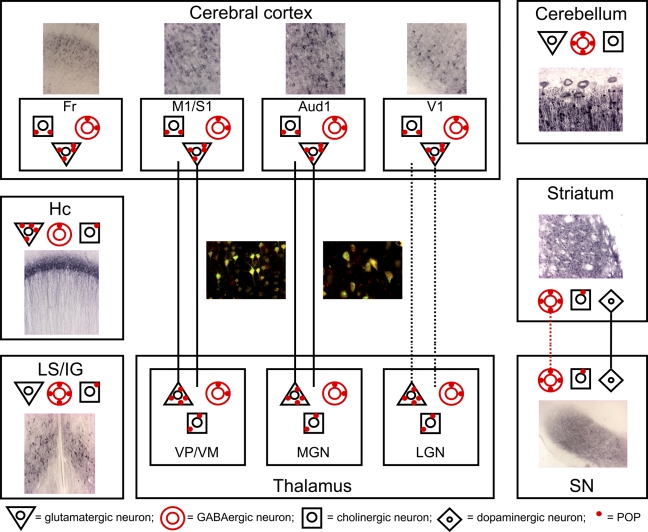
POP has been associated with a wide range of neurotransmitters. Administration of POP inhibitors has caused elevated acetylcholine (ACh) activity in the cortex of old rats (Toide et al. 1997). Changes in POP activity have been observed after administration of an NMDA antagonist (Ahmed et al. 2005; Arif et al. 2007) and in the nigrostriatal path after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration (Krupina et al. 2006). These findings point to the participation of POP in cholinergic and glutamatergic neurotransmission in the brain. We recently studied spatial associations of POP and several neurotransmitters, such as GABA, Ach, and dopamine (Figure 2; Table 4; Myöhänen et al. 2008b). Generally, POP was present in both GABAergic and cholinergic systems but not in dopaminergic neurons, despite the intensive POP immunostaining in the nigrostriatal pathway. Furthermore, nigral lesions with 6-hydroxydopamine did not evoke any changes in POP immunoreactivity (Myöhänen et al. 2008b).
Figure 2.
Localization of POP in different neuronal cell types in the central nervous system. Based on the neuroanatomy and colocalization studies, POP is present in glutamatergic, γ-aminobutyric acid (GABA)ergic, and cholinergic systems. Red dots indicate the presence of POP. Moreover, POP is evidently located in the thalamocortical/corticothalamic projection neurons between VP/VM and M1/S1 and Aud1/MGN. POP is also believed to be localized in the projection neurons between V1-cortex and LGN and GABAergic neurons of the nigrostriatal path (dashed line). Aud, primary auditory cortex; Fr, frontal cortex; Hc, hippocampus; LS/IG, lateral septum/indusium griseum; M1/S1, primary motor/somatosensory cortex; MGN, medial geniculate nucleus; LGN, lateral geniculate nucleus; SN, substantia nigra; VP/VM, ventrobasal/ventromedial thalamic nuclei; V1, primary visual cortex.
One tempting possibility to account for the interactions of POP with the neurotransmitter pathways is that several of these systems use neuropeptides (i.e., POP substrates) as modulators. GABAergic interneurons contain several neuropeptides, such as SP and somatostatin (Emson and Lindvall 1979; Kawaguchi and Kondo 2002; Garcia-Horsman et al. 2007), and there is an abundant colocalization of POP and GABA in the cerebral cortex (Table 3; Myöhänen et al. 2008b). In the striatum, POP was reported to be present mainly in the GABAergic neurons but not in either dopaminergic neurons or cholinergic neurons, stressing a role for POP in the inhibitory regulation of the nigrostriatal pathway (Paxinos 2004). Interestingly, these GABAergic neurons indeed use SP as a neurotransmitter (Besson et al. 1990; McGinty 2007). SP also participates in the “direct nigrostriatal pathway” by slowly modifying the GABAergic signaling activated by loss of dopaminergic input, as occurs in Parkinson's disease (Skidgel and Erdös 2006; Standaert and Young 2006).
Moreover, SP can modulate the cellular activity of cerebellar GABAergic Purkinje cells through parallel fibers (Inagaki et al. 1982; Del Fiacco et al. 1988; Nakaya et al. 1994). POP is clearly present in these cells, moderately colocalized with SP (Myöhänen et al. 2008a). However, POP, SP, and GABA are poorly colocalized in other brain areas (Myöhänen et al. 2008a). Therefore, it seems unlikely that POP is involved to any significant extent in SP-modulated GABAergic neurotransmission.
Repeated administration of POP inhibitors has increased M3-muscarinic receptor mRNA levels (Katsube et al. 1996) and cholinergic activity in the cortex of old rats (Toide et al. 1997). The mechanisms to explain the actions of POP on the cholinergic system are not known, but neuropeptide (e.g., TRH) cleavage could be one of the mechanisms involved (Toide et al. 1993). Moreover, POP was partially colocalized with the cortical system but not with the hippocampal or medial septal cholinergic systems. Because the two latter areas are crucial in cognitive cholinergic functions (Paxinos 2004; Myöhänen et al. 2008b), it is most unlikely that the effects of POP on memory and learning are mediated via these cholinergic systems.
The cortical spiny pyramidal cells and hippocampal CA1 pyramidal neurons, which are very rich in POP, are mostly glutamatergic (DeFelipe et al. 2002; Paxinos 2004). Furthermore, changes in POP activity were recently observed in the rat cortex and hippocampus after administration of an NMDA antagonist to induce schizophrenia-like symptoms (Ahmed et al. 2005; Arif et al. 2007). These findings can be interpreted as supporting the participation of POP in glutamatergic neurotransmission in the brain, possibly by modifying the levels of POP substrates such as TRH (Kasparov et al. 1994).
Moreover, POP was highly colocalized with IP3R1 in hippocampal CA1 pyramidal cells (Myöhänen et al. 2008a). In these cells, IP3 has been associated with long-term potentiation (LTP), an important phenomenon in memory and learning (Lynch and Voss 1991; Bliss and Collingridge 1993; Khodakhah and Armstrong 1997; Jun et al. 1998; Fujii et al. 2004; Taufiq et al. 2005). Interestingly, LTP is induced by the activation of NMDA–glutamate receptors (Bliss and Collingridge 1993; Fujii et al. 2004), and therefore, on the basis of the proposed involvement of POP in IP3 signaling (Williams and Harwood 2000; Schulz et al. 2002; Williams et al. 2002; Cheng et al. 2005), one could hypothesize that the memory effects of POP are mediated by changes in LTP in the hippocampus. Further support for this hypothesis is found in POP knock-out studies (Di Daniel et al. 2009), which demonstrated the association between POP and GAP-43, in which the latter is connected with hippocampal LTP (Ramakers et al. 1995; Namgung et al. 1997) and the phosphoinositide system (Laux et al. 2000; Caprini et al. 2003).
We conclude that although there is no direct evidence that POP is involved in neuronal neurotransmitter systems, administration of POP inhibitors has affected levels of neurotransmitters, and substances affecting neurotransmitters have been able to change POP activity. In addition, POP was colocalized with several neurotransmitters in the CNS, and therefore, we suggest that POP has a function in neurotransmitter systems. However, this mechanism is more likely to be indirect than direct, because a direct effect on neurotransmitters would require extracellular expression of POP. Interestingly though, Klegeris et al. (2008) have recently reported that POP is secreted under stimulation of THP-1 cells. However, the immunohistochemical data do not provide any sign of extracellular POP.
Moreover, knock-out studies have further supported the role of POP in phosphoinositode signaling and our hypothesis that POP may be involved with memory and learning functions via hippocampal LTP rather than via neuropeptide metabolism.
Other Functions
Thalamocortical Signaling
Recently, POP was shown in a neurotracing study to be abundantly present in the thalamocortical and corticothalamic neurons (Figure 2; Myöhänen et al. 2009). These results suggest that POP may be involved in the regulation of thalamocortical neurotransmission in several thalamocortical loops, possibly as a regulator of levels of neuropeptides such as somatostatin, TRH, and orexin (Myöhänen et al. 2009).
Moreover, POP was present to the same extent in both inhibitory and excitatory neurons in the thalamus, but the coexpression of POP with IP3R1 in thalamus was even more striking. IP3 signaling in the thalamus has been rather poorly characterized, but it may be involved in the regulation of thalamocortical rhythms (de la Vega et al. 1996), which are one of the foundation stones of consciousness (Tancredi et al. 2000; Manning et al. 2004). Moreover, VPA evidently modifies these spontaneous rhythms (Nowack et al. 1979; Mares et al. 1992; Zhang et al. 1996), although not via the GABAergic system (Zhang et al. 1996). POP is linked to IP3 regulation also via VPA (Cheng et al. 2005).
Collectively, these findings support a role for POP in thalamocortical neurotransmission, possibly through the regulation of IP3 signaling. However, functional data are needed to verify this hypothesis.
Motor Functions
The high POP protein levels and enzyme activity in the nigrostriatal systems of both rat and human (Table 2; Irazusta et al. 2002; Agirregoitia et al. 2005; Myöhänen et al. 2007,2008b) may point to a role for POP in motor functions (Paxinos 1990,2004; Mantle et al. 1996). Furthermore, the high expression of POP in cerebellar Purkinje cells and thalamocortical/corticothalamic projection neurons (Figure 2; Table 4; Myöhänen et al. 2009) may be evidence for an involvement in the control of movement (Thach et al. 1992; Paxinos 2004).
In the nigrostriatal pathway, POP is present primarily in the GABAergic cells, not in the dopaminergic or cholinergic cells (Table 4; Myöhänen et al. 2008b). The GABAergic neurons of the nigrostriatal pathway are the most-dominant striatopallidal and striatonigral fibers, and they inhibit the activities of the globus pallidus and substantia nigra (Paxinos 2004). This indicates a role for POP in the regulation of inhibitory neurotransmission of the nigrostriatal pathway. However, the minor colocalization of POP, GABA, and SP in the striatum (Myöhänen et al. 2008a) indicates that POP either affects some other neuropeptide system, such as enkephalin, or may have some other yet-unidentified regulatory function in the nigrostriatal pathway.
Nevertheless, there is no direct in vivo evidence supporting the role of POP in motor functions. Interestingly though, POP knock-out mice showed increased locomotor activity (Di Daniel et al. 2009). Also, various memory models, such as the Morris water maze and the radial maze, where POP inhibitors have shown beneficial effects (Toide et al. 1997; Miyazaki et al. 1998; Shinoda et al. 1999), actually measure the increased speed of movement. On the basis of abundant POP expression in the nigrostriatal path, motor cortex, and thalamic motor nuclei and the novel data on POP-deficient mice, we propose a role for POP in locomotor regulation, although the mechanism behind this action needs to be determined.
Summary
POP has been widely studied since its discovery in 1971, and various POP inhibitors have been developed as experimental drugs for use in memory disorders. However, the true physiological function of this enzyme has remained a mystery. On the basis of recent findings, it seems probable that in the CNS, POP may not be exclusively important in metabolizing in vivo its best-characterized in vitro substrates. Instead, we propose that POP may also hydrolyze different types of substances, possibly even degradation products of mature proteins or peptide precursors. Moreover, non-catalytic functions may be an interesting novel explanation for POP's actions, including protein–protein interactions. However, there is a huge need for more-detailed studies concerning non-hydrolytic roles and functions of POP in the CNS, especially in the peripheral tissues.
POP is not clearly associated with any specific neurotransmitter pathways in the CNS but instead seems to be involved in multiple neuronal systems, both inhibitory and excitatory. However, it is not known how POP interacts with these systems or how these systems affect POP. These problems should be studied at the cellular level, and the extracellular secretion of POP should also be considered. The association between POP and IP3 is convincing, and recent studies with a POP knock-out strain have provided further support for this connection. This might explain the beneficial effects of POP inhibitors on memory tasks and may link POP with hippocampal memory effects, such as LTP, and on the other hand, with the regulation of thalamocortical/corticothalamic signalling. Further studies are needed.
POP seems to have different roles in the peripheral tissues than in the CNS, particularly in cell proliferation and possibly even in differentiation, probably owing to clear nuclear localization. More studies are needed to clarify the nuclear functions of the POP protein. Possible POP substrates in the nucleus also need to be identified, and the effects of POP and POP inhibitors on cell proliferation/differentiation and even on tumor growth should be explored.
Acknowledgments
These studies were supported by EU FP7-HEALTH-2007-B, proposal No. 223077 (NEUROPRO) funding, grants from the Academy of Finland (No. 210758), University of Helsinki Research Foundation, Sigrid Juselius Foundation (to PTM). T.T.M. is supported by Orion-Farmos Research Foundation.
We thank Ewen MacDonald, PhD, for his comments about the linguistic form of the paper.
References
- Agirregoitia N, Casis L, Gil J, Ruiz F, Irazusta J (2007) Ontogeny of prolyl endopeptidase and pyroglutamyl peptidase I in rat tissues. Regul Pept 139:52–58 [DOI] [PubMed] [Google Scholar]
- Agirregoitia N, Gil J, Ruiz F, Irazusta J, Casis L (2003) Effect of aging on rat tissue peptidase activities. J Gerontol A Biol Sci Med Sci 58:B792–797 [DOI] [PubMed] [Google Scholar]
- Agirregoitia N, Laiz-Carrion R, Varona A, Rio MP, Mancera JM, Irazusta J (2005) Distribution of peptidase activity in teleost and rat tissues. J Comp Physiol [B] 175:433–444 [DOI] [PubMed] [Google Scholar]
- Ahmed MM, Arif M, Chikuma T, Kato T (2005) Pentylenetetrazol-induced seizures affect the levels of prolyl oligopeptidase, thimet oligopeptidase and glial proteins in rat brain regions, and attenuation by MK-801 pretreatment. Neurochem Int 47:248–259 [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Wada T, Nagai M, Kojima F, Harada S, Takeuchi T, Takahashi H, et al. (1990) Deficiency of kallikrein-like enzyme activities in cerebral tissue of patients with Alzheimer's disease. Experientia 46:94–97 [DOI] [PubMed] [Google Scholar]
- Arif M, Chikuma T, Ahmed MM, Yoshida S, Kato T (2007) Suppressive effect of clozapine but not haloperidol on the increases of neuropeptide-degrading enzymes and glial cells in MK-801-treated rat brain regions. Neurosci Res 57:248–258 [DOI] [PubMed] [Google Scholar]
- Bai R, Edler MC, Bonate PL, Copeland TD, Pettit GR, Luduena RF, Hamel E (2009) Intracellular activation and deactivation of tasidotin, an analogue of dolastatin 15: correlation with cytotoxicity. Mol Pharmacol 75:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär JW, Rahfeld JU, Schulz I, Gans K, Ruiz-Carrillo D, Manhart S, Rosche F, et al. (2006) Prolyl endopeptidase cleaves the apoptosis rescue peptide humanin and exhibits an unknown post-cysteine cleavage specificity. Adv Exp Med Biol 575:103–108 [DOI] [PubMed] [Google Scholar]
- Baude A, Shigemoto R (1998) Cellular and subcellular distribution of substance P receptor immunoreactivity in the dorsal vagal complex of the rat and cat: a light and electron microscope study. J Comp Neurol 402:181–196 [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Mazurek MF (1987) Neuropeptides in Alzheimer's disease. J Neural Transm Suppl 24:163–174 [PubMed] [Google Scholar]
- Bellemere G, Morain P, Vaudry H, Jegou S (2003) Effect of S 17092, a novel prolyl endopeptidase inhibitor, on substance P and alpha-melanocyte-stimulating hormone breakdown in the rat brain. J Neurochem 84:919–929 [DOI] [PubMed] [Google Scholar]
- Bellemere G, Vaudry H, Morain P, Jegou S (2005) Effect of prolyl endopeptidase inhibition on arginine-vasopressin and thyrotrophin-releasing hormone catabolism in the rat brain. J Neuroendocrinol 17:306–313 [DOI] [PubMed] [Google Scholar]
- Bellemere G, Vaudry H, Mounien L, Boutelet I, Jegou S (2004) Localization of the mRNA encoding prolyl endopeptidase in the rat brain and pituitary. J Comp Neurol 471:128–143 [DOI] [PubMed] [Google Scholar]
- Besson MJ, Graybiel AM, Quinn B (1990) Co-expression of neuropeptides in the cat's striatum: an immunohistochemical study of substance P, dynorphin B and enkephalin. Neuroscience 39:33–58 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39 [DOI] [PubMed] [Google Scholar]
- Brandt I, Gerard M, Sergeant K, Devreese B, Baekelandt V, Augustyns K, Scharpe S, et al. (2008) Prolyl oligopeptidase stimulates the aggregation of alpha-synuclein. Peptides 29:1472–1478 [DOI] [PubMed] [Google Scholar]
- Brandt I, Scharpe S, Lambeir AM (2007) Suggested functions for prolyl oligopeptidase: a puzzling paradox. Clin Chim Acta 377:50–61 [DOI] [PubMed] [Google Scholar]
- Brandt I, Vriendt KD, Devreese B, Beeumen JV, Dongen WV, Augustyns K, Meester ID, et al. (2005) Search for substrates for prolyl oligopeptidase in porcine brain. Peptides 26:2536–2546 [DOI] [PubMed] [Google Scholar]
- Breen G, Harwood AJ, Gregory K, Sinclair M, Collier D, St Clair D, Williams RS (2004) Two peptidase activities decrease in treated bipolar disorder not schizophrenic patients. Bipolar Disord 6:156–161 [DOI] [PubMed] [Google Scholar]
- Caprini M, Gomis A, Cabedo H, Planells-Cases R, Belmonte C, Viana F, Ferrer-Montiel A (2003) GAP43 stimulates inositol trisphosphate-mediated calcium release in response to hypotonicity. EMBO J 22:3004–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavasin MA, Rhaleb NE, Yang XP, Carretero OA (2004) Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 43:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM (1990) Processing of angiotensin peptides by NG108-15 neuroblastoma x glioma hybrid cell line. Peptides 11:375–380 [DOI] [PubMed] [Google Scholar]
- Cheng L, Lumb M, Polgar L, Mudge AW (2005) How can the mood stabilizer VPA limit both mania and depression? Mol Cell Neurosci 29:155–161 [DOI] [PubMed] [Google Scholar]
- Cicilini MA, Ramos PS, Vasquez EC, Cabral AM (1994) Heart prolyl endopeptidase activity in one-kidney, one clip hypertensive rats. Braz J Med Biol Res 27:2821–2830 [PubMed] [Google Scholar]
- Cook LB, Hinkle PM (2004) Fate of internalized thyrotropin-releasing hormone receptors monitored with a timer fusion protein. Endocrinology 145:3095–3100 [DOI] [PubMed] [Google Scholar]
- Cunningham DF, O'Connor B (1997) Proline specific peptidases. Biochim Biophys Acta 1343:160–186 [DOI] [PubMed] [Google Scholar]
- Cunningham DF, O'Connor B (1998) A study of prolyl endopeptidase in bovine serum and its relevance to the tissue enzyme. Int J Biochem Cell Biol 30:99–114 [DOI] [PubMed] [Google Scholar]
- Daly DJ, Maskrey P, Pennington RJ (1985) Characterization of proline endopeptidase from skeletal muscle. Int J Biochem 17:521–524 [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI (2002) Microstructure of the neocortex: comparative aspects. J Neurocytol 31:299–316 [DOI] [PubMed] [Google Scholar]
- de la Vega MT, Nunez A, Montano JA (1996) Carbachol stimulates inositol phosphate formation in rat thalamus slices through muscarinic M3-receptor activation. Neurosci Lett 213:29–32 [DOI] [PubMed] [Google Scholar]
- Del Fiacco M, Perra MT, Quartu M, Rosa MD, Zucca G, Levanti MC (1988) Evidence for the presence of substance P-like immunoreactivity in the human cerebellum. Brain Res 446:173–177 [DOI] [PubMed] [Google Scholar]
- Di Daniel E, Glover CP, Grot E, Chan MK, Sanderson TH, White JH, Ellis CL, et al. (2009) Prolyl oligopeptidase binds to GAP-43 and functions without its peptidase activity. Mol Cell Neurosci 41:373–382 [DOI] [PubMed] [Google Scholar]
- Dresdner K, Barker LA, Orlowski M, Wilk S (1982) Subcellular distribution of prolyl endopeptidase and cation-sensitive neutral endopeptidase in rabbit brain. J Neurochem 38:1151–1154 [DOI] [PubMed] [Google Scholar]
- Emson PC, Lindvall O (1979) Distribution of putative neurotransmitters in the neocortex. Neuroscience 4:1–30 [DOI] [PubMed] [Google Scholar]
- Fujii S, Sasaki H, Mikoshiba K, Kuroda Y, Yamazaki Y, Mostafa Taufiq A, Kato H (2004) A chemical LTP induced by co-activation of metabotropic and N-methyl-D-aspartate glutamate receptors in hippocampal CA1 neurons. Brain Res 999:20–28 [DOI] [PubMed] [Google Scholar]
- Fülop V, Bocskei Z, Polgar L (1998) Prolyl oligopeptidase: an unusual beta-propeller domain regulates proteolysis. Cell 94:161–170 [DOI] [PubMed] [Google Scholar]
- Fuse Y, Polk DH, Lam RW, Reviczky AL, Fisher DA (1990) Distribution and ontogeny of thyrotropin-releasing hormone degrading enzymes in rats. Am J Physiol 259:E787–791 [DOI] [PubMed] [Google Scholar]
- Garcia-Horsman JA, Männistö PT, Venäläinen JI (2007) On the role of prolyl oligopeptidase in health and disease. Neuropeptides 41:1–24 [DOI] [PubMed] [Google Scholar]
- Goossens F, De Meester I, Vanhoof G, Scharpe S (1996) Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem 34:17–22 [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P (2001) Substance p. Int J Biochem Cell Biol 33:555–576 [DOI] [PubMed] [Google Scholar]
- Harwood AJ, Agam G (2003) Search for a common mechanism of mood stabilizers. Biochem Pharmacol 66:179–189 [DOI] [PubMed] [Google Scholar]
- Hasenohrl RU, Souza-Silva MA, Nikolaus S, Tomaz C, Brandao ML, Schwarting RK, Huston JP (2000) Substance P and its role in neural mechanisms governing learning, anxiety and functional recovery. Neuropeptides 34:272–280 [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Bartfai T, Bloom F (2003) Neuropeptides: opportunities for drug discovery. Lancet Neurol 2:463–472 [DOI] [PubMed] [Google Scholar]
- Huston JP, Hasenohrl RU (1995) The role of neuropeptides in learning: focus on the neurokinin substance P. Behav Brain Res 66:117–127 [DOI] [PubMed] [Google Scholar]
- Ikura T, Kinoshita K, Ito N (2008) A cavity with an appropriate size is the basis of the PPIase activity. Protein Eng Des Sel 21:83–89 [DOI] [PubMed] [Google Scholar]
- Inagaki S, Sakanaka M, Shiosaka S, Senba E, Takagi H, Takatsuki K, Kawai Y, et al. (1982) Experimental and immunohistochemical studies on the cerebellar substance P of the rat: localization, postnatal ontogeny and ways of entry to the cerebellum. Neuroscience 7:639–645 [DOI] [PubMed] [Google Scholar]
- Irazusta J, Larrinaga G, Gonzalez-Maeso J, Gil J, Meana JJ, Casis L (2002) Distribution of prolyl endopeptidase activities in rat and human brain. Neurochem Int 40:337–345 [DOI] [PubMed] [Google Scholar]
- Irazusta J, Silveira PF, Gil J, Varona A, Casis L (2001) Effects of hydrosaline treatments on prolyl endopeptidase activity in rat tissues. Regul Pept 101:141–147 [DOI] [PubMed] [Google Scholar]
- Ishino T, Ohtsuki S, Homma K, Natori S (1998) cDNA cloning of mouse prolyl endopeptidase and its involvement in DNA synthesis by Swiss 3T3 cells. J Biochem (Tokyo) 123:540–545 [DOI] [PubMed] [Google Scholar]
- Jalkanen AJ, Puttonen KA, Venäläinen JI, Sinervä V, Mannila A, Ruotsalainen S, Jarho EM, et al. (2007) Beneficial effect of prolyl oligopeptidase inhibition on spatial memory in young but not in old scopolamine-treated rats. Basic Clin Pharmacol Toxicol 100:132–138 [DOI] [PubMed] [Google Scholar]
- Jiang CH, Tsien JZ, Schultz PG, Hu Y (2001) The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci USA 98:1930–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun K, Choi G, Yang SG, Choi KY, Kim H, Chan GC, Storm DR, et al. (1998) Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem 5:317–330 [PMC free article] [PubMed] [Google Scholar]
- Kakegawa H, Matano Y, Inubushi T, Katunuma N (2004) Significant accumulations of cathepsin B and prolylendopeptidase in inflammatory focus of delayed-type hypersensitivity induced by Mycobacterium tuberculosis in mice. Biochem Biophys Res Commun 316:78–84 [DOI] [PubMed] [Google Scholar]
- Kamori M, Hagihara M, Nagatsu T, Iwata H, Miura T (1991) Activities of dipeptidyl peptidase II, dipeptidyl peptidase IV, prolyl endopeptidase, and collagenase-like peptidase in synovial membrane from patients with rheumatoid arthritis and osteoarthritis. Biochem Med Metab Biol 45:154–160 [DOI] [PubMed] [Google Scholar]
- Kasparov S, Pawelzik H, Zieglgansberger W (1994) Thyrotropin-releasing hormone enhances excitatory postsynaptic potentials in neocortical neurons of the rat in vitro. Brain Res 656:229–235 [DOI] [PubMed] [Google Scholar]
- Kato A, Fukunari A, Sakai Y, Nakajima T (1997) Prevention of amyloid-like deposition by a selective prolyl endopeptidase inhibitor, Y-29794, in senescence-accelerated mouse. J Pharmacol Exp Ther 283:328–335 [PubMed] [Google Scholar]
- Kato T, Nakano T, Kojima K, Nagatsu T, Sakakibara S (1980a) Changes in prolyl endopeptidase during maturation of rat brain and hydrolysis of substance P by the purified enzyme. J Neurochem 35:527–535 [DOI] [PubMed] [Google Scholar]
- Kato T, Okada M, Nagatsu T (1980b) Distribution of post-proline cleaving enzyme in human brain and the peripheral tissues. Mol Cell Biochem 32:117–121 [DOI] [PubMed] [Google Scholar]
- Katsube N, Sunaga K, Chuang DM, Ishitani R (1996) ONO-1603, a potential antidementia drug, shows neuroprotective effects and increases m3-muscarinic receptor mRNA levels in differentiating rat cerebellar granule neurons. Neurosci Lett 214:151–154 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S (2002) Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31:277–287 [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Armstrong CM (1997) Induction of long-term depression and rebound potentiation by inositol trisphosphate in cerebellar Purkinje neurons. Proc Natl Acad Sci USA 94:14009–14014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Matsui H, Takahashi T (2002) Expression and localization of prolyl oligopeptidase in mouse testis and its possible involvement in sperm motility. Zoolog Sci 19:93–102 [DOI] [PubMed] [Google Scholar]
- Kimura A, Yoshida I, Takagi N, Takahashi T (1999) Structure and localization of the mouse prolyl oligopeptidase gene. J Biol Chem 274:24047–24053 [DOI] [PubMed] [Google Scholar]
- Klegeris A, Li J, Bammler TK, Jin J, Zhu D, Kashima DT, Pan S, et al. (2008) Prolyl endopeptidase is revealed following SILAC analysis to be a novel mediator of human microglial and THP-1 cell neurotoxicity. Glia 56:675–685 [DOI] [PubMed] [Google Scholar]
- Koshiya K, Kato T, Tanaka R, Kato T (1984) Brain peptidases: their possible neuronal and glial localization. Brain Res 324:261–270 [DOI] [PubMed] [Google Scholar]
- Krupina NA, Zolotov NN, Bogdanova NG, Orlova IN, Khlebnikova NN, Kryzhanovskii GN (2006) Activities of prolyl endopeptidase and dipeptidyl peptidase IV in brain structures of rats with dopamine deficiency-dependent MPTP-induced depressive syndrome. Bull Exp Biol Med 142:554–556 [DOI] [PubMed] [Google Scholar]
- la Cour T, Kiemer L, Mølgaard A, Gupta R, Skriver K, Brunak S (2004) Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel 17:527–536 [DOI] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P (2000) GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol 149:1455–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduque P, Bulant M, Dubois PM, Nicolas P, Vaudry H (1989) Processing of thyrotropin-releasing hormone prohormone (pro-TRH) in the adult rat pancreas: identification and localization of pro-TRH-related peptides in beta-cells of pancreatic islets. Endocrinology 125:1492–1497 [DOI] [PubMed] [Google Scholar]
- Lee CR, Watkins ML, Patterson JH, Gattis W, O'connor CM, Gheorghiade M, Adams KF Jr (2003) Vasopressin: a new target for the treatment of heart failure. Am Heart J 146:9–18 [DOI] [PubMed] [Google Scholar]
- Leprince J, Cosquer D, Bellemère G, Chatenet D, Tollemer H, Jégou S, Tonon MC, et al. (2006) Catabolism of the octadecaneuropeptide ODN by prolyl endopeptidase: identification of an unusual cleavage site. Peptides 27:1561–1569 [DOI] [PubMed] [Google Scholar]
- Levesque M, Wallman MJ, Parent R, Sik A, Parent A (2007) Neurokinin-1 and neurokinin-3 receptors in primate substantia nigra. Neurosci Res 57:362–371 [DOI] [PubMed] [Google Scholar]
- Liu JM, Kusinski M, Ilic V, Bignon J, Hajem N, Komorowski J, Kuzdak K, et al. (2008) Overexpression of the angiogenic tetrapeptide AcSDKP in human malignant tumors. Anticancer Res 28:2813–2817 [PubMed] [Google Scholar]
- Lu KP, Finn G, Lee TH, Nicholson LK (2007) Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol 3:619–629 [DOI] [PubMed] [Google Scholar]
- Luo W, Slebos RJ, Hill S, Li M, Brabek J, Amanchy R, Chaerkady R, et al. (2008) Global impact of oncogenic Src on a phosphotyrosine proteome. J Proteome Res 7:3447–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA, Voss KL (1991) Presynaptic changes in long-term potentiation: elevated synaptosomal calcium concentration and basal phosphoinositide turnover in dentate gyrus. J Neurochem 56:113–118 [DOI] [PubMed] [Google Scholar]
- Manning JP, Richards DA, Leresche N, Crunelli V, Bowery NG (2004) Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience 123:5–9 [DOI] [PubMed] [Google Scholar]
- Männistö PT, Venäläinen JI, Jalkanen AJ, Garcia-Horsman JA (2007) Prolyl oligopeptidase: a potential target for the treatment of cognitive disorders. Drug News Perspect 20:293–305 [DOI] [PubMed] [Google Scholar]
- Mantle D, Falkous G, Ishiura S, Blanchard PJ, Perry EK (1996) Comparison of proline endopeptidase activity in brain tissue from normal cases and cases with Alzheimer's disease, Lewy body dementia, Parkinson's disease and Huntington's disease. Clin Chim Acta 249:129–139 [DOI] [PubMed] [Google Scholar]
- Mares P, Pohl M, Koryntova H (1992) Influence of phenytoin and valproate on thalamocortical evoked potentials and their paired-pulse potentiation. Physiol Res 41:475–478 [PubMed] [Google Scholar]
- Matsubara Y, Ono T, Tsubuki S, Irie S, Kawashima S (1998) Transient up-regulation of a prolyl endopeptidase activity in the microsomal fraction of rat liver during postnatal development. Eur J Biochem 252:178–183 [DOI] [PubMed] [Google Scholar]
- McGinty JF (2007) Co-localization of GABA with other neuroactive substances in the basal ganglia. Prog Brain Res 160:273–284 [DOI] [PubMed] [Google Scholar]
- Mentlein R, von Kolszynski M, Sprang R, Lucius R (1990) Proline-specific proteases in cultivated neuronal and glial cells. Brain Res 527:159–162 [DOI] [PubMed] [Google Scholar]
- Miyazaki A, Toide K, Sasaki Y, Ichitani Y, Iwasaki T (1998) Effect of a prolyl endopeptidase inhibitor, JTP-4819, on radial maze performance in hippocampal-lesioned rats. Pharmacol Biochem Behav 59:361–368 [DOI] [PubMed] [Google Scholar]
- Morain P, Lestage P, De Nanteuil G, Jochemsen R, Robin JL, Guez D, Boyer PA (2002) S 17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies. CNS Drug Rev 8:31–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Baylach MJ, Felipo V, Männistö PT, Garcia-Horsman JA (2008) Expression and traffic of cellular prolyl oligopeptidase are regulated during cerebellar granule cell differentiation, maturation, and aging. Neuroscience 156:580–585 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Kääriäinen TM, Jalkanen AJ, Piltonen M, Männistö PT (2009) Localization of prolyl oligopeptidase in the thalamic and cortical projection neurons: a retrograde neurotracing study in the rat brain. Neurosci Lett 450:201–205 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Männistö PT (2008a) Spatial association of prolyl oligopeptidase, inositol 1,4,5-triphosphate type 1 receptor, substance P and its NK-1 receptor in the rat brain: an immunohistochemical study. Neuroscience 153:1177–1189 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT (2008b) Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. J Comp Neurol 507:1694–1708 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT (2008c) Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol 130:993–1003 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Tupala E, Garcia-Horsman JA, Miettinen R, Männistö PT (2007) Distribution of immunoreactive prolyl oligopeptidase in human and rat brain. Neurochem Res 32:1365–1374 [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N (1994) Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347:249–274 [DOI] [PubMed] [Google Scholar]
- Namgung U, Matsuyama S, Routtenberg A (1997) Long-term potentiation activates the GAP-43 promoter: selective participation of hippocampal mossy cells. Proc Natl Acad Sci USA 94:11675–11680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni EA, Sevarino KA (1999) The biology of pro-thyrotropin-releasing hormone-derived peptides. Endocr Rev 20:599–648 [DOI] [PubMed] [Google Scholar]
- Nowack WJ, Johnson RN, Englander RN, Hanna GR (1979) Effects of valproate and ethosuximide on thalamocortical excitability. Neurology 29:96–99 [DOI] [PubMed] [Google Scholar]
- Oestreicher AB, De Graan PN, Gispen WH, Verhaagen J, Schrama LH (1997) B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog Neurobiol 53:627–686 [DOI] [PubMed] [Google Scholar]
- Ohta N, Takahashi T, Mori T, Park MK, Kawashima S, Takahashi K, Kobayashi H (1992) Hormonal modulation of prolyl endopeptidase and dipeptidyl peptidase IV activities in the mouse uterus and ovary. Acta Endocrinol (Copenh) 127:262–266 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Homma K, Kurata S, Komano H, Natori S (1994) A prolyl endopeptidase of Sarcophaga peregrina (flesh fly): its purification and suggestion for its participation in the differentiation of the imaginal discs. J Biochem (Tokyo) 115:449–453 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Homma K, Kurata S, Natori S (1997a) Molecular cloning of cDNA for Sarcophaga prolyl endopeptidase and characterization of the recombinant enzyme produced by an E. coli expression system. Insect Biochem Mol Biol 27:337–343 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Homma K, Kurata S, Natori S (1997b) Nuclear localization and involvement in DNA synthesis of Sarcophaga prolyl endopeptidase. J Biochem (Tokyo) 121:1176–1181 [DOI] [PubMed] [Google Scholar]
- O'Leary RM, Gallagher SP, O'Connor B (1996) Purification and characterization of a novel membrane-bound form of prolyl endopeptidase from bovine brain. Int J Biochem Cell Biol 28:441–449 [DOI] [PubMed] [Google Scholar]
- O'Leary RM, O'Connor B (1995) Identification and localisation of a synaptosomal membrane prolyl endopeptidase from bovine brain. Eur J Biochem 227:277–283 [DOI] [PubMed] [Google Scholar]
- Paxinos G (1990) The Human Nervous System. 1st ed. San Diego, Academic Press
- Paxinos G (2004) The Rat Nervous System. 3rd ed. San Diego, Elsevier Academic Press
- Polgar L (1994) Prolyl oligopeptidases. Methods Enzymol 244:188–200 [DOI] [PubMed] [Google Scholar]
- Puttonen KA, Lehtonen S, Raasmaja A, Männistö PT (2006) A prolyl oligopeptidase inhibitor, Z-Pro-Prolinal, inhibits glyceraldehyde-3-phosphate dehydrogenase translocation and production of reactive oxygen species in CV1-P cells exposed to 6-hydroxydopamine. Toxicol In Vitro 20:1446–1454 [DOI] [PubMed] [Google Scholar]
- Ramakers GM, De Graan PN, Urban IJ, Kraay D, Tang T, Pasinelli P, Oestreicher AB, et al. (1995) Temporal differences in the phosphorylation state of pre- and postsynaptic protein kinase C substrates B-50/GAP-43 and neurogranin during long-term potentiation. J Biol Chem 270:13892–13898 [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, et al. (2000) Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA 97:12880–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ (1994) Families of serine peptidases. Methods Enzymol 244:19–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ (2008) MEROPS: the peptidase database. Nucleic Acids Res 36:D320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Moore FL (2002) Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol 23:317–341 [DOI] [PubMed] [Google Scholar]
- Rossner S, Schulz I, Zeitschel U, Schliebs R, Bigl V, Demuth HU (2005) Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer's disease. Neurochem Res 30:695–702 [DOI] [PubMed] [Google Scholar]
- Salers P (1994) Evidence for the presence of prolyl oligopeptidase and its endogenous inhibitor in neonatal rat pancreatic beta-cells. Regul Pept 50:235–245 [DOI] [PubMed] [Google Scholar]
- Salers P, Ouafik LH, Giraud P, Dutour A, Maltese JY, Oliver C (1991) Evidence for pyroglutamyl peptidase I and prolyl endopeptidase activities in the rat insulinoma cell line RINm 5F: lack of relationship with TRH metabolism. Mol Cell Biochem 106:15–24 [DOI] [PubMed] [Google Scholar]
- Schulz I, Gerhartz B, Neubauer A, Holloschi A, Heiser U, Hafner M, Demuth HU (2002) Modulation of inositol 1,4,5-triphosphate concentration by prolyl endopeptidase inhibition. Eur J Biochem 269:5813–5820 [DOI] [PubMed] [Google Scholar]
- Schulz I, Zeitschel U, Rudolph T, Ruiz-Carrillo D, Rahfeld JU, Gerhartz B, Bigl V, et al. (2005) Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J Neurochem 94:970–979 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Matsuo A, Toide K (1996) Pharmacological studies of a novel prolyl endopeptidase inhibitor, JTP-4819, in rats with middle cerebral artery occlusion. Eur J Pharmacol 305:31–38 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Miyazaki A, Toide K (1999) Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on spatial memory and on cholinergic and peptidergic neurons in rats with ibotenate-induced lesions of the nucleus basalis magnocellularis. Behav Brain Res 99:17–25 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Toide K, Ohsawa I, Kohsaka S (1997) Specific inhibitor for prolyl endopeptidase suppresses the generation of amyloid beta protein in NG108-15 cells. Biochem Biophys Res Commun 235:641–645 [DOI] [PubMed] [Google Scholar]
- Shirasawa Y, Osawa T, Hirashima A (1994) Molecular cloning and characterization of prolyl endopeptidase from human T cells. J Biochem 115:724–729 [DOI] [PubMed] [Google Scholar]
- Skidgel RA, Erdös EG (2006) Histamine, bradykinin, and their antagonists. In Brunton L, Lazo J, Parker K, Buxton I, Blumenthal D, eds. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 11th ed. New York, McGraw-Hill Medical Publishing Division, 629–651
- Soeda S, Yamakawa N, Ohyama M, Shimeno H, Nagamatsu A (1985) An inhibitor of proline endopeptidase: purification from rat brain and characterization. Chem Pharm Bull (Tokyo) 33:24445–24451 [PubMed] [Google Scholar]
- Soeda S, Yamakawa N, Shimeno H, Nagamatsu A (1986) Effects of polyamines on proline endopeptidase activity in rat brain. J Neurochem 46:1304–1307 [DOI] [PubMed] [Google Scholar]
- Standaert DG, Young AB (2006) Treatment of central nervous system degenerative disorders. In Brunton L, Lazo J, Parker K, Buxton I, Blumenthal D, eds. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 11th ed. New York, McGraw-Hill Medical Publishing Division, 527–545
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, et al. (2002) Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99:4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lu X, Gershengorn MC (2003) Thyrotropin-releasing hormone receptors: similarities and differences. J Mol Endocrinol 30:87–97 [DOI] [PubMed] [Google Scholar]
- Szeltner Z, Rea D, Juhasz T, Renner V, Fulop V, Polgar L (2004) Concerted structural changes in the peptidase and the propeller domains of prolyl oligopeptidase are required for substrate binding. J Mol Biol 340:627–637 [DOI] [PubMed] [Google Scholar]
- Tancredi V, Biagini G, D'Antuono M, Louvel J, Pumain R, Avoli M (2000) Spindle-like thalamocortical synchronization in a rat brain slice preparation. J Neurophysiol 84:1093–1097 [DOI] [PubMed] [Google Scholar]
- Taufiq AM, Fujii S, Yamazaki Y, Sasaki H, Kaneko K, Li J, Kato H, et al. (2005) Involvement of IP3 receptors in LTP and LTD induction in guinea pig hippocampal CA1 neurons. Learn Mem 12:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio-Laranga J, Venäläinen JI, Männistö PT, Garcia-Horsman JA (2008) Characterization of membrane-bound prolyl endopeptidase from brain. FEBS J 275:4415–4427 [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15:403–442 [DOI] [PubMed] [Google Scholar]
- Toide K, Fujiwara T, Iwamoto Y, Shinoda M, Okamiya K, Kato T (1996) Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on neuropeptide metabolism in the rat brain. Naunyn Schmiedebergs Arch Pharmacol 353:355–362 [DOI] [PubMed] [Google Scholar]
- Toide K, Iwamoto Y, Fujiwara T, Abe H (1995a) JTP-4819: a novel prolyl endopeptidase inhibitor with potential as a cognitive enhancer. J Pharmacol Exp Ther 274:1370–1378 [PubMed] [Google Scholar]
- Toide K, Okamiya K, Iwamoto Y, Kato T (1995b) Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on prolyl endopeptidase activity and substance P- and arginine-vasopressin-like immunoreactivity in the brains of aged rats. J Neurochem 65:234–240 [DOI] [PubMed] [Google Scholar]
- Toide K, Shinoda M, Fujiwara T, Iwamoto Y (1997) Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on spatial memory and central cholinergic neurons in aged rats. Pharmacol Biochem Behav 56:427–434 [DOI] [PubMed] [Google Scholar]
- Toide K, Shinoda M, Takase M, Iwata K, Yoshida H (1993) Effects of a novel thyrotropin-releasing hormone analogue, JTP-2942, on extracellular acetylcholine and choline levels in the rat frontal cortex and hippocampus. Eur J Pharmacol 233:21–28 [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Ishiura S, Sugita H (1990) Regulation of prolyl endopeptidase activity by the intracellular redox state. J Biol Chem 265:21448–21453 [PubMed] [Google Scholar]
- Vanhoof G, Goossens F, Hendriks L, De Meester I, Hendriks D, Vriend G, Van Broeckhoven C, et al. (1994) Cloning and sequence analysis of the gene encoding human lymphocyte prolyl endopeptidase. Gene 149:363–366 [DOI] [PubMed] [Google Scholar]
- Venäläinen JI, Juvonen RO, Männistö PT (2004) Evolutionary relationships of the prolyl oligopeptidase family enzymes. Eur J Biochem 271:2705–2715 [DOI] [PubMed] [Google Scholar]
- Walter R (1976) Partial purification and characterization of post-proline cleaving enzyme: enzymatic inactivation of neurohypophyseal hormones by kidney preparations of various species. Biochim Biophys Acta 422:138–158 [DOI] [PubMed] [Google Scholar]
- Walter R, Shlank H, Glass JD, Schwartz IL, Kerenyi TD (1971) Leucylglycinamide released from oxytocin by human uterine enzyme. Science 173:827–829 [DOI] [PubMed] [Google Scholar]
- Williams RS (2005) Pharmacogenetics in model systems: defining a common mechanism of action for mood stabilisers. Prog Neuropsychopharmacol Biol Psychiatry 29:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ (2002) A common mechanism of action for three mood-stabilizing drugs. Nature 417:292–295 [DOI] [PubMed] [Google Scholar]
- Williams RS, Harwood AJ (2000) Lithium therapy and signal transduction. Trends Pharmacol Sci 21:61–64 [DOI] [PubMed] [Google Scholar]
- Yamakawa N, Shimeno H, Soeda S, Nagamatsu A (1994) Regulation of prolyl oligopeptidase activity in regenerating rat liver. Biochim Biophys Acta 1199:279–284 [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Mizutani S, Kurauchi O, Kasugai M, Narita O, Tomoda Y (1992) Induction by cortisol of aminopeptidases production from the human placenta in tissue culture. Horm Metab Res 24:110–114 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Kado K, Matsubara F, Koriyama N, Kaneto H, Tsura D (1987) Specific inhibitors for prolyl endopeptidase and their anti-amnesic effect. J Pharmacobiodyn 10:730–735 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Simmons WH, Kita T, Tsuru D (1981) Post-proline cleaving enzyme from lamb brain. J Biochem 90:325–334 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Tsukumo K, Takatsuka N, Tsuru D (1982) An inhibitor for post-proline cleaving enzyme; distribution and partial purification from porcine pancreas. J Pharmacobiodyn 5:734–740 [DOI] [PubMed] [Google Scholar]
- Zhang YF, Gibbs JW III, Coulter DA (1996) Anticonvulsant drug effects on spontaneous thalamocortical rhythms in vitro: valproic acid, clonazepam, and alpha-methyl-alpha-phenylsuccinimide. Epilepsy Res 23:37–53 [DOI] [PubMed] [Google Scholar]