Abstract
The purpose of this study was to clarify a previously controversial issue concerning glutamate (Glu) immunoreactivity (IR) in the inner segment (IS) of photoreceptors by using in vivo cryotechnique (IVCT) followed by freeze substitution (FS), which enabled us to analyze the cells and tissues reflecting living states. Eyeballs from anesthetized mice were directly frozen using IVCT. The frozen tissues were processed for FS fixation in acetone containing chemical fixatives, and embedded in paraffin. Deparaffinized sections were immunostained with an anti-Glu antibody. The strongest Glu-IR was obtained in the specimens prepared by FS with paraformaldehyde or a low concentration of glutaraldehyde, whereas no Glu-IR was obtained without the chemical fixatives. The Glu was immunolocalized in the IS, outer and inner plexiform and ganglion cell layers. Thus, the immunolocalization of Glu in the IS was clearly demonstrated using IVCT. (J Histochem Cytochem 57:883–888, 2009)
Keywords: glutamate, photoreceptor, inner segment, immunohistochemistry, in vivo cryotechnique, freeze substitution
It has been difficult to obtain stable immunoreactivity (IR) of an amino acid, glutamate (Glu), in paraffin-embedded tissue sections of eyeballs prepared by perfusion fixation and alcohol dehydration, probably owing to a technical diffusion artifact and/or antigen masking. Using a bioassay measurement of cultured photoreceptor cells from the guinea pig retina, mitochondria in the inner segment (IS) of photoreceptors were reported to produce Glu (Poitry-Yamate et al. 1995; Tsacopoulos et al. 1998). It was also proposed that metabolic lactate is taken up into the cytoplasmic matrix of the photoreceptors. Part of the carbon skeleton of lactate-pyruvate enters the tricarboxylic acid cycle as citrate in mitochondria located in the IS, and is converted to α-ketoglutarate and then to Glu (Tsacopoulos et al. 1998). However, with immunohistochemical approaches, a controversial issue has arisen as to whether the Glu is immunostained in the IS. In some cases, enucleated eyes of the goldfish (Marc et al. 1990), cat (Pourcho and Owczarzak 1991), chicken (Kalloniatis and Fletcher 1993; Sun and Crossland 2000), rat (Fletcher and Kalloniatis 1996), and monkey (Kalloniatis et al. 1996) were fixed by immersion fixation, resulting in positive Glu-IR in the IS. However, the Glu-IR in the IS was not detected by perfusion fixation (Sasoh et al. 1998,2006); therefore, it was concluded that the localization and/or expression of Glu was probably due to postmortem changes induced by ischemia.
To overcome such contradictory Glu-IR results in the IS, the application of in vivo cryotechnique (IVCT) was assumed to be useful because it can instantly immobilize all biological materials in a living state in tiny ice crystals (Ohno et al. 1996). By using common freeze-substitution (FS) fixation for specimens with IVCT, we have already demonstrated the immunohistochemical merit of locating soluble serum proteins in living animal tissues (Zea-Aragon et al. 2004; Ohno et al. 2006; Zhou et al. 2007; Saitoh et al. 2008), which are easily lost during the preparation steps. In addition, IVCT also enabled us to visualize rapid changes within seconds in living animal bodies, such as molecular conformation of rhodopsin phosphorylation in the living mouse retina (Terada et al. 2006) or attachment of proteins to ischemia-reactive drugs in the living mouse liver (Terada et al. 2007). With IVCT-FS, it was possible to retain biological molecules in the photoreceptor layer of mouse eyeballs without obvious ice crystal formation at the light microscopic level (Terada et al. 2006). Therefore, in this study, we focused on Glu-immunolocalization in the IS of eyeballs prepared with IVCT-FS.
Materials and Methods
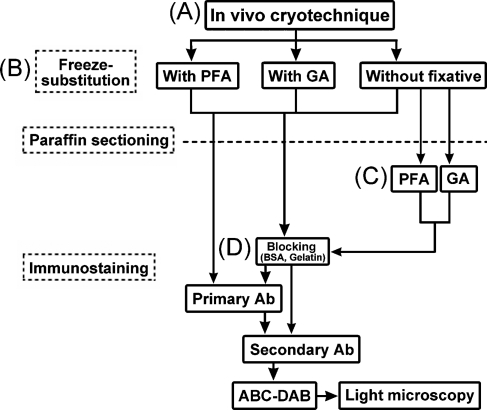
The present study was approved by the Animal Use Committee at the University of Yamanashi, and performed in accordance with the guidelines governing animal experiments within the institution. The whole protocol of this experiment is flow-charted in Figure 1.
Figure 1.
A flow diagram of the preparation steps for the mouse eyeball tissues, as prepared by the in vivo cryotechnique (IVCT) (A) and freeze-substitution (FS) fixation for the glutamate (Glu) immunostaining. During the FS, paraformaldehyde, glutaraldehyde, or no fixative was added to acetone (B). Some thin sections of eyeball tissues without the chemical fixative during FS were treated with paraformaldehyde or glutaraldehyde (C). Before immunoreaction of the primary antibody, a common blocking treatment with bovine serum albumin (BSA) or fish gelatin was performed on the sections (D). PFA, paraformaldehyde; GA, glutaraldehyde; Ab, antibody; ABC-DAB, horseradish-avidin-biotin complex and diaminobenzidine reactions.
Dot-blot Analysis for Bovine Serum Albumin (BSA), BSA-conjugated Glu, and Glu Against the Anti-Glu Antibody
The antibody used for the immunohistochemistry in this study was a commercially available anti-Glu antibody (cat #G6642; Sigma-Aldrich Corp., St. Louis, MO), which was developed in rabbits using purified Glu conjugated to keyhole limpet hemocyanin (KLH) as the immunogen. According to the manufacturer's instructions, it reacts with Glu-KLH, Glu-BSA, KLH, and l-Glu, but not with BSA, using a dot-blot immunobinding assay. In this study, we reconfirmed the specific IR with dot-blot analysis, as described below. One microliter of BSA (10 mg/ml; Sigma) was placed on a nitrocellulose membrane (Millipore; Billerica, MA). BSA-dotted membranes were treated with 0.1% glutaraldehyde at room temperature for 1 hr, then some were treated with Glu (2 mg/ml; Sigma) at room temperature for 1 hr. All were treated with 5% fish gelatin (cat #G7765; Sigma) at room temperature for 2 hr. Then they were incubated with the same rabbit anti-Glu antibody that was used for immunohistochemistry (1:30,000 in dilution) at 4C overnight, followed by a biotinylated anti-rabbit IgG antibody (1:3000; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 1 hr and a peroxidase-labeled avidin-biotin complex (ABC) (1:3000; Pierce, Rockford, IL) at room temperature for 30 min. The immunoreaction products were visualized with a metal-enhanced diaminobenzidine (DAB) reaction (Pierce). As a positive immunocontrol, the BSA dot on the nitrocellulose membrane was treated with fish gelatin, immunostained with a sheep anti-BSA antibody (Bethyl Laboratories; Montgomery, TX), and visualized in the same way as described above.
To evaluate the antibody IR against Glu alone, a dot-blot analysis on glass slides was performed, as described previously (Terada et al. 2006). Briefly, ionized amine residue-coated glass slides (MAS glass slide; Matsunami Glass Ind. Ltd, Osaka, Japan) were treated with glutaraldehyde, and then Glu (Sigma) was additionally attached. The Glu-attached glass slides were subsequently treated with BSA, the primary anti-Glu antibody, and a secondary AlexaFluor 488–conjugated anti-rabbit IgG antibody (Invitrogen Corp., Carlsbad, CA). For the immunocontrol study, the primary antibody was omitted. The treated samples were observed in a fluorescence microscope (BX-61; Olympus, Tokyo, Japan).
IVCT for Living Mouse Eyeballs
Ten adult C57BL/6 mice weighing 20–30 g were used. Under pentobarbital anesthesia, IVCT was performed on the left eyeballs with natural light coming through windows (1355 ± 23 lx) (Figure 1A), by pouring 50 ml liquid isopentane-propane cryogen (−193C) cooled in liquid nitrogen. The low temperature was maintained by pouring extra liquid nitrogen on the eyeballs (Terada et al. 2006). The mice were always under normal physiological conditions, with their hearts beating (Ohno et al. 1996). The frozen eyeballs were obtained using a dental drill in liquid nitrogen, and processed for the next FS step.
FS Fixation With Various Chemical Fixatives for Paraffin Embedding
The frozen retinal tissues were processed for FS in acetone containing 2% paraformaldehyde, and 0.14%, 1.4%, or 14% glutaraldehyde, or no chemical fixative (Figure 1B), which was prepared using a molecular sieve (type 3A; Nakarai Tesque, Kyoto, Japan) for complete dehydration (Ohno et al. 2006). The frozen eyeballs were freeze-substituted at −80C for 24 hr and then put into a deep freezer at −30C and −10C for 2 hr each. Finally, they were placed in a refrigerator at 4C for 2 hr. They were warmed at room temperature for 1 hr, washed in pure acetone, immersed in xylene, and embedded in paraffin wax, as reported previously (Terada et al. 2007). The paraffin-embedded specimens were processed for the immunohistochemical step.
Immunostaining of Glu on Paraffin Sections
The paraffin-embedded eyeball tissues were cut at 5 μm thickness and routinely deparaffinized with xylene, a graded series of ethanol, and phosphate-buffered saline (PBS). Some thin sections were stained with hematoxylin-eosin (HE) for pure morphology. For other tissue sections without fixative during FS, 2% paraformaldehyde or 1.25% glutaraldehyde in 0.1 M PB was used for 1 hr at room temperature (Figure 1C). Some sections were treated by microwaving for 3 min in citrate buffer (pH 6.0). For immunostaining, they were pretreated with 5% BSA or 5% fish gelatin (Sigma) in PBS (Figure 1D), and with a rabbit polyclonal anti-Glu antibody at 1:10,000–30,000 dilutions (cat #G6642; Sigma) at 4C overnight. They were additionally treated with a biotinylated anti-rabbit IgG antibody (Vector) at room temperature for 1 hr, then a peroxidase-labeled ABC (Pierce) at room temperature for 1 hr, and visualized with metal-enhanced DAB (Pierce). Finally, they were incubated in 0.04% osmium tetroxide solution for 30–60 sec to increase and protect the contrast of imunoreaction products, and observed under a light microscope.
Results
Immunolocalization of Glu in Retinas Reflecting Their Living State
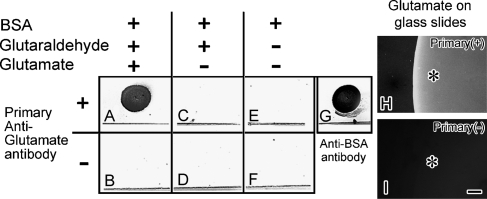
First, to evaluate the IR against Glu-BSA, BSA or Glu alone of the commercially available anti-Glu antibody (cat #G6642; Sigma) dot-blot analysis was performed (Figure 2). The antibody reacted to the Glu-BSA (Figure 2A); however, it did not react to BSA itself (Figures 2C and 2E). In addition, IR was also obtained against Glu alone attached to glass slides with glutaraldehyde (Figure 2H), but it was not detected without the primary antibody (Figure 2I). These findings confirmed that the antibody reacts to Glu-BSA or Glu alone, but not to BSA itself.
Figure 2.
Dot-blot analysis against variously prepared BSA with an anti-Glu antibody on nitrocellulose membranes (A–G). BSA-dotted membranes were treated with glutaraldehyde/Glu (A,B) or glutaraldehyde (C,D), or without treatment. BSA was assumed to be a binding dot pattern at the upper area of the line in each square. It was blocked with gelatin and incubated with (A,C,E) or without (B,D,F) the anti-Glu antibody. (G) Immunopositive control to show the binding of BSA as a dot pattern on the membrane with the anti-BSA antibody. Only after treatment with both glutaraldehyde and Glu (A), the immunoreactivity (IR) is obtained. (H,I) Glu-IR by attaching Glu to glass slides and immunostaining with (H) or without (I) the primary anti-Glu antibody (Primary) at higher magnification under a fluorescence microscope. Asterisks in (H) and (I) indicate the spot areas where the Glu was attached to glass slides. Bar = 50 μm.
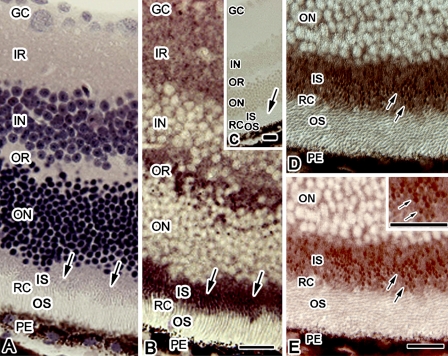
Morphology for the eyeball tissues with IVCT-FS was evaluated by HE staining, and the rod and cone photoreceptors were observed as well-preserved areas without obvious ice crystals at the light microscopic level (Figure 3A). The Glu-IR was obtained in thin sections of the eyeball specimens with IVCT followed by FS with paraformaldehyde (Figure 3B). The Glu was immunolocalized in the IS, outer and inner plexiform layers, and ganglion cell layer, judged by HE staining of an adjacent section. Although the level of Glu has been reported to be highly detected in a subpopulation of amacrine cells and ganglion cells (Sun et al. 2007a,b), the Glu was dispersively immunolocalized in the inner and outer plexiform layers and ganglion cell layer in the tissues with IVCT-FS. This was probably due to the distance from the initial frozen surface causing larger ice crystal formation. At a higher-magnified view of the sections, in which photoreceptor cell layers were obliquely sectioned (Figures 3D and 3E), Glu-IR was observed as tiny dot patterns (arrows in Figure 3E), probably indicating that the Glu immunostaining was inside the photoreceptor cells. From these results, Glu was assumed to localize in the IS.
Figure 3.
Hematoxylin-eosin staining (A) and Glu immunostaining (B) images in adjacent sections of eyeball tissue obtained by IVCT followed by FS with paraformaldehyde. (C) Immunocontrol without the primary anti-Glu antibody. Arrows indicate the inner segment (IS) of photoreceptors. (D,E) Highly magnified view of diffraction interference contrast (DIC) (D) and Glu-immunostaining (E) images of a retina, in which photoreceptor cells in the outer segment (OS) and IS are obliquely sectioned. The Glu immunostaining is observed as tiny dot patterns (arrows in E) in the IS. Inset in E shows a higher magnified view of an area of the IS. GC, ganglion cell layer; IR and OR, inner and outer plexiform layers; IN and ON, inner and outer nuclear layers; RC, photoreceptor cell layer; PE, pigment epithelium. Bar = 20 μm.
Glu-IR in Retinas Prepared With Glutaraldehyde During FS
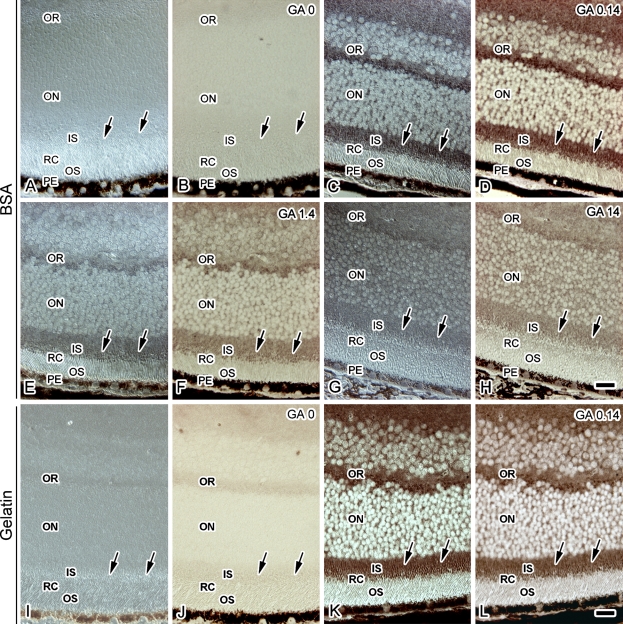
It was thought that the efficiency of paraformaldehyde linking of amino acids was not as high as that of glutaraldehyde. Therefore, to evaluate the effect of glutaraldehyde on the intensity of Glu-IR during the FS step, various concentrations of glutaraldehyde were added to the FS solution (Figure 4). The tissue sections were treated with BSA before the primary anti-Glu antibody incubation (Figures 4A–4H). In the samples prepared with 0.14% glutaraldehyde, the Glu immunolocalization was similar to that obtained with paraformaldehyde in the FS solution (Figure 4D). However, the Glu-IR became weaker with increasing glutaraldehyde concentrations (Figures 4F and 4H). This result also indicated that Glu is localized in the IS, probably reflecting the living state.
Figure 4.
DIC (A,C,E,G,I,K) and Glu immunostaining (B,D,F,H,J,L) images of the eyeball tissue sections treated with BSA (A–H) or fish gelatin (I–H) in samples with IVCT followed by FS with various concentrations of glutaraldehyde [0% (GA 0; A,B,I,J), 0.14% (GA 0.14; C,D,K,L), 1.4% (GA 1.4; E,F), and 14% (GA 14; G,H)]. Arrows indicate the IS of photoreceptors. Note that strong IR is detected with IVCT in eyeball tissues that were freeze-substituted with 0.14% glutaraldehyde, sectioned, and treated with BSA (D) or fish gelatin (L). IR and OR, inner and outer plexiform layers; IN and ON, inner and outer nuclear layers; RC, photoreceptor cell layer; IS and OS, inner and outer segments of RC; PE, pigment epithelium. Bar = 20 μm.
After treatment with paraformaldehyde or glutaraldehyde on the thin sections of the retinal tissue samples that were freeze-substituted with acetone alone, Glu-IR was not detected (data not shown), indicating that the chemical fixation during FS is necessary to retain the Glu molecules in the tissue samples. In addition, microwave treatment of the samples prepared by FS with paraformaldehyde or glutaraldehyde did not change the Glu-IR (data not shown).
Finally, to examine whether BSA is effective as a carrier or blocking protein for the following primary antibody reaction, sections were treated with fish gelatin, which was expected to effectively inhibit nonspecific binding of the antibodies, before the primary anti-Glu antibody incubation (Figures 4I–4L). In sections of the specimen prepared with IVCT followed by FS without chemical fixatives (Figure 4J), Glu-IR was not obtained with the fish gelatin pretreatment. In contrast, the Glu-IR was clearly detected with the fish gelatin pretreatment in sections of the specimen prepared with IVCT followed by FS with glutaraldehyde (Figure 4L). Thus, BSA is probably effective as a blocking reagent, and it became clear that the paraffin sections of the retinal specimens with IVCT-FS retained many Glu molecules, which can be more clearly immunostained by the anti-Glu antibody.
Discussion
From our results with IVCT-FS, the IS of photoreceptors was clearly immunopositive for Glu. It is well known that levels of Glu production are different among cells in retinal layers. Several enzymes for Glu metabolism, including Glu dehydrogenase, phosphate-activated glutaminase, aspartate aminotransferase, and ornithine aminotransferase, were already reported to have a high level of activity in the IS (Ross and Godfrey 1997; Endo et al. 1999). Glu-IR was obtained in some restricted areas in retinal tissue samples with IVCT, probably reflecting the normal in vivo metabolic conditions of the IS without anoxia or ischemia.
Glu-IR has been reported to be artificially changed by some chemical fixation procedures. Using perfusion fixation, no Glu-IR was detected in the IS (Sasoh et al. 1998,2006). Indeed, in the paraffin-embedded mouse eyeball samples with perfusion fixation followed by alcohol dehydration, the Glu-IR in the retina was inconsistent (data not shown). However, in the samples with IVCT-FS, it was strongly detected in the IS, as shown in Figure 3. This discrepancy in immunohistochemical findings was probably caused by diffusion loss of Glu by cell membrane modification, which could be due to mechanical stress of flow pressures with perfusion fixation and also the effects of anoxia. We have previously documented the diffusion artifacts with perfusion fixation in the mouse spleen to visualize immunoglobulin-producing cells (Saitoh et al. 2008). For this reason, immersion fixation may be a better way to preserve Glu in comparison to perfusion fixation, and IVCT is very helpful in examining Glu immunolocalization. It is difficult to confirm how much Glu in tissues was lost during the FS step with chemical fixatives such as paraformaldehyde and glutaraldehyde. We assume that the immunolocalization with IVCT-FS reflects the original distribution for the following two reasons. First, if the diffusion artifact of Glu had strongly appeared, Glu immunolocalization would have been distributed in much wider areas, such as the outer segment of photoreceptors. Second, the Glu immunolocalization in the samples treated with glutaraldehyde during the FS step showed a distribution pattern similar to that with paraformaldehyde, indicating retention of Glu by the chemical fixatives during FS. The intensity of the IR was changed with different concentrations of glutaraldehyde, as expected. This may be due to alteration of cross-linked tissue structures hindering some Glu reactivity against BSA and/or antibodies in the paraffin sections.
Without any tissue treatment for antigen retrieval, such as microwaving or detergent solutions, strong IR was often obtained in sections from the paraffin-embedded specimens with IVCT-FS, as described before (Saitoh et al. 2008). Some tiny ice crystals were probably helpful for the penetration of blocking reagents and antibodies in the deparaffinized thin sections, as previously reported (Ohno et al. 2006). From our results, it is obvious that the BSA treatment was affected as a blocking reagent, but not as a carrier protein, judging from the specific IR against Glu alone with the dot-blot analysis (Figure 2) and the same immunostaining pattern with the BSA treatment as was seen with the fish gelatin treatment (Figure 4).
A more precise study of ultrastructural immunolocalization of Glu in the samples with IVCT is our further concern with electron microscopy, reflecting the living morphological findings (Ohno et al. 1996; Ohno et al. 2007). In a previous study, the IS was reported to have a special mitochondrial metabolic cycle, closely related to Glu (Tsacopoulos et al. 1998). To date, the highest concentration of Glu in the IS has been unclear in cell organelles of animal retinas. Mitochondria are strong candidates to localize Glu because they are densely packed in the IS. In conclusion, IVCT is useful for studying the immunocytochemical distribution of small molecular Glu in living animal retinas.
References
- Endo S, Ishiguro S, Tamai M (1999) Possible mechanism for the decrease of mitochondrial aspartate aminotransferase activity in ischemic and hypoxic rat retinas. Biochim Biophys Acta 1450:385–396 [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis K (1996) Neurochemical architecture of the normal and degenerating rat retina. J Comp Neurol 376:343–360 [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher EL (1993) Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol 336:174–193 [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Marc RE, Murry RF (1996) Amino acid signatures in the primate retina. J Neurosci 16:6807–6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Liu WLS, Kalloniatis M, Raiguel SF, Van Haesendonck E (1990) Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci 10:4006–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Terada N, Ohno S (2006) Histochemical analyses of living mouse liver under different hemodynamic conditions by “in vivo cryotechnique”. Histochem Cell Biol 126:389–398 [DOI] [PubMed] [Google Scholar]
- Ohno N, Terada N, Saitoh S, Ohno S (2007) Extracellular space in mouse cerebellar cortex revealed by in vivo cryotechnique. J Comp Neurol 505:292–301 [DOI] [PubMed] [Google Scholar]
- Ohno S, Terada N, Fujii Y, Ueda H, Takayama I (1996) Dynamic structure of glomerular capillary loop as revealed by an in vivo cryotechnique. Virchows Arch 427:519–527 [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M (1995) Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci 15:5179–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG, Owczarzak MT (1991) Connectivity of glycine immunoreactive amacrine cells in the cat retina. J Comp Neurol 307:549–561 [DOI] [PubMed] [Google Scholar]
- Ross CD, Godfrey DA (1997) Distribution of activities of aspartate aminotransferase isoenzymes and malate dehydrogenase in guinea pig retinal layers. J Histochem Cytochem 35:669–674 [DOI] [PubMed] [Google Scholar]
- Saitoh S, Terada N, Ohno N, Ohno S (2008) Distribution of immunoglobulin-producing cells in immunized mouse spleens revealed with “in vivo cryotechnique”. J Immunol Methods 331:114–126 [DOI] [PubMed] [Google Scholar]
- Sasoh M, Ma N, Ito Y, Esaki K, Uji Y (2006) Changes in localization of amino acids in the detached cat retina. Ophthalmic Res 38:74–82 [DOI] [PubMed] [Google Scholar]
- Sasoh M, Ma N, Yoshida S, Semba R, Uji Y (1998) Immunocytochemical localization of glutamate in normal and detached cat retina. Invest Ophthalmol Vis Sci 39:786–792 [PubMed] [Google Scholar]
- Sun D, Vingrys AJ, Kalloniatis M (2007a) Metabolic and functional profiling of the normal rat retina. J Comp Neurol 505:92–113 [DOI] [PubMed] [Google Scholar]
- Sun D, Vingrys AJ, Kalloniatis M (2007b) Metabolic and functional profiling of the ischemic/reperfused rat retina. J Comp Neurol 505:114–130 [DOI] [PubMed] [Google Scholar]
- Sun H, Crossland WJ (2000) Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine, and GABA immunoreactivity in the chick retina. Anat Rec 260:158–179 [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Ohguro H, Li Z, Ohno S (2006) Immunohistochemical detection of phosphorylated rhodopsin in light-exposed retina of living mouse with in vivo cryotechnique. J Histochem Cytochem 54:479–486 [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Saitoh S, Ohno S (2007) Immunohistochemical detection of hypoxia in mouse liver tissues treated with pimonidazole using “in vivo cryotechnique”. Histochem Cell Biol 128:253–261 [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S (1998) Trafficking of molecules and metabolic signals in the retina. Prog Retin Eye Res 17:429–442 [DOI] [PubMed] [Google Scholar]
- Zea-Aragon Z, Terada N, Ohno N, Fujii Y, Baba T, Ohno S (2004) Effects of anoxia on serum immunoglobulin and albumin leakage through blood-brain barrier in mouse cerebellum as revealed by cryotechniques. J Neurosci Methods 138:89–95 [DOI] [PubMed] [Google Scholar]
- Zhou H, Ohno N, Terada N, Saitoh S, Fujii Y, Ohno S (2007) Involvement of follicular basement membrane and vascular endothelium in blood-follicule barrier formation of mice revealed by “in vivo cryotechnique”. Reproduction 134:307–317 [DOI] [PubMed] [Google Scholar]