Abstract
High blood flow through baboon polytetrafluorethylene aorto-iliac grafts increases neointimal vascular smooth muscle cell (SMC) death, neointimal atrophy, and cleavage of versican to generate the DPEAAE neoepitope, a marker of ADAMTS-mediated proteolysis. In this study, we have determined the effect of high blood flow on transcript abundance in the neointima for ADAMTS1, -4, -5, -8, -9, -15, and -20. We found that after 24 hr of flow, the mRNA for ADAMTS4 was significantly increased, whereas that for the other family members was unchanged. Because vascular SMC death is markedly increased in the graft after 24 hr of high flow, we next examined the possibility that the ADAMTS4 induction and the cell death are causally related. The addition of Fas ligand to SMC cultures increased both ADAMTS4 mRNA and cell death ∼5-fold, consistent with the idea that ADAMTS4-dependent cleavage of versican may be partly responsible for cell death and tissue atrophy under these conditions. (J Histochem Cytochem 57:889–897, 2009)
Keywords: ADAMTS, intimal atrophy, flow, proteoglycan, smooth muscle cells
Versican is a major component of the extracellular matrix (ECM) of atherosclerotic lesions and the growing vascular intimas of restenotic arteries, transplant arteriosclerotic arteries, stented arteries, vein grafts, balloon-injured rat and monkey arteries (Lin et al. 1996; Kalish et al. 2004; Kenagy et al. 2006), and healing tissues in general (Wight and Merrilees 2004). Thus, versican is a significant component of the transitional ECM formed after injury, which may be permissive for smooth muscle cell (SMC) proliferation and migration (Evanko et al. 1999). Of particular interest, intimal tissue formed after balloon- or stent-mediated injury regresses over time and is associated with a loss of versican (Matsuura et al. 1996; Asakura et al. 1998; Imanaka-Yoshida et al. 2001; Farb et al. 2004). The loss of the water binding properties of the glycosaminoglycan chains of versican would have a major impact on lesion volume. This relationship between versican and intimal growth or regression is also observed in a baboon polytetrafluoroethylene (PTFE) graft model in which a neointimal layer either grows or regresses in response to changes in blood flow (Kenagy et al. 2005). Thus, increasing flow in a graft with an established neointimal layer induces neointimal atrophy starting within 1 day (Berceli et al. 2002). This atrophy involves the death of cells and the loss of ECM. We have previously demonstrated that within days, there is an increase in the levels of the serine proteases urokinase and plasmin and of a discrete cleavage product of versican (G1-DPE), which can be generated by members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motif) family, ADAMTS1, -4, -5, and -9 (Sandy et al. 2001; Kenagy et al. 2002,2005; Somerville et al. 2004). In this study, we have investigated whether the vascular atrophy and cell death associated with high flow in this baboon model are accompanied by induction of one or more of the ADAMTSs (1, 4, 5, 8, 9, 15, or 20), which are capable of degrading versican.
Materials and Methods
Baboon Graft Neointimal Regression Model
Male baboons (Papio cynocephalus anubis) weighing ∼10 kg were used. Bilateral aorto-iliac PTFE grafts (5-cm length, 4-mm diameter, 60-μm internodal distance; W.L. Gore, Flagstaff, AZ) were placed as previously described (Mattsson et al. 1997; Berceli et al. 2002). Animals were anesthetized with ketamine (10 mg/kg, intramuscularly) and maintained on isoflurane inhalation anesthetic during all operative procedures. After 8 weeks, a superficial femoral artery-to-vein fistula (10-mm length) was created on one side to increase blood flow. The contralateral graft was maintained as a normal flow control. Midstream graft blood velocities, measured using duplex ultrasonography as previously described (Geary et al. 1994), were increased acutely ∼4-fold (Berceli et al. 2002). Animals were euthanized with sodium pentobarbital (160 mg/kg, intravenously). Grafts were obtained after 1 day of high flow; midgraft portions were fixed in buffered 10% formalin for histology, and the remaining portions were frozen for preparation of RNA. Ten baboons were grafted for this study, but three developed thrombosis before fistula placement, and the RNA preparations of another two were degraded. Therefore, five were left for analysis by microarray. Archived specimens (fixed specimens from five baboons with 1 day of high flow and four baboons with 4 days of high flow) and data (Western blots of extracts of graft intimas from three baboons) from previous experiments were also used (Berceli et al. 2002; Hsieh et al. 2006). Animal care and procedures were conducted at the University of Washington Regional Primate Research Center in accordance with state and federal laws and under protocols approved by the University of Washington Institutional Animal Care and Use Committee and the Regional Primate Research Center, and complied with the “Guide for the Care and Use of Laboratory Animals” issued by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC, National Academic Press, 1996.
Array and Quantitative PCR (qPCR) Methods
Total RNA was prepared from paired 1-day high- and normal-flow graft neointimas of five baboons by using solution D-phenol-chloroform-isopropanol (Hsieh et al. 2006). Quality was determined using the Agilent 2100 Bioanalyzer (Santa Clara, CA). The Illumina sentrix human ref-8 system (Illumina, Inc.; San Diego, CA) microarrays were used according to the manufacturer's instructions. In brief, total RNA was used for cDNA synthesis, followed by an amplification/labeling step to synthesize biotin-labeled cRNA using the Ambion MessageAmp kit (Foster City, CA). Then cRNA was fragmented and hybridized with the Sentrix BeadChip in a hybridization oven at 55C. After hybridization, the Sentrix BeadChips were washed with Wash E1 buffer (Illumina, Inc.) solution and then blocked for 5 min in 4 ml of 1% blocker casein in PBS, Hammarsten grade (Pierce Biotechnology, Inc.; Rockford, IL). Array signals were developed by 10-min incubation in 2 ml of 1 μg/ml Cy3-streptavidin (Amersham Biosciences; Buckinghamshire, UK) solution and 1% blocking solution. The BeadChips were washed a second time in diluted Wash E1 buffer for 5 min, dried immediately after removal by centrifugation, and scanned using the Illumina BeadArray Reader GX. The summarized bead intensities were quantile normalized without the control probes and paired by animal. Boxplots of the unnormalized signal and density distributions and MA plots (made using the log2 [signal of array/median signal of all arrays]) were analyzed. The normalized intensities were then analyzed using Significance Analysis of Microarrays software (http://www-stat.stanford.edu/~tibs/SAM/) without a threshold for fold change. Complete data from the microarray experiment will be presented in a separate manuscript.
qPCR was performed using the 7500 Fast real-time PCR system (Applied Biosystems; Foster City, CA) according to the manufacturer's instructions. Standard, not fast, cycling conditions were used (40 cycles of 95C for 15 sec and 60C for 1 min). Taqman primers and probes (ADAMTS1: assay ID Hs00199608_m1 based on RefSeq NM_006988.3; ADAMTS4: assay ID Hs00192708_m1 based on RefSeq NM_005099.4; ADAMTS5: assay ID Hs00199841_m1 based on RefSeq NM_007038.3) and Multiscribe reverse transcriptase were purchased from Applied Biosystems, and master mix (2× SensiMix) from Quantace (Bioline USA Inc.; Taunton, MA).
Human Aortic Organ Culture
Abdominal aortic specimens from organ donors were obtained anonymously (all procedures were in compliance with and approved by the University of Washington Human Subjects Review Board) and kept in University of Wisconsin solution at 4C until used. Samples were placed in high-glucose Dulbecco's modified Eagles medium (DMEM) with 20 mM Hepes, pH 7.4, and loose connective tissue was removed from the adventitia. Explants were made from aorta that had an endothelial surface that was smooth, white, and free of raised atherosclerotic lesions. One full thickness explant of 1 cm2 was placed in 5 ml of DMEM with 20 mM Hepes. In a series of experiments, four 6-mm-diameter discs, which were made using a skin biopsy punch, of full thickness explants were used in each well. Samples of conditioned medium (0–3 and 4–7 days) were frozen, and tissue at 0 and 7 days was fixed in 10% neutral buffered formalin or frozen.
Cell Death
SMCs were cultured from the thoracic aortas of baboons from prior studies as described (Hsieh et al. 2006). SMCs were seeded at 200,000/35-mm dish in 10% fetal bovine serum DMEM overnight. Cells were then changed to serum-free DMEM, at which time 50 ng/ml Fas ligand (FasL, a fusion protein of soluble FasL with a FLAG tag; Alexis Biochemicals, San Diego, CA), 2 μg/ml anti-FLAG (to cross-link FasL), and 1.5 μg/ml cycloheximide were added. After 6 and 24 hr, cells were collected for analysis of cell death (Annexin V-FITC Apoptosis Detection Kit I from BD Pharmingen; San Diego, CA) or for extraction of RNA (Qiagen RNeasy mini kit; Valencia, CA) for qPCR of ADAMTS4.
Immunohistochemical Staining
Immunohistochemical staining of versican (2B1, Seikagaku; Associates of Cape Cod, Inc., East Falmouth, MA), ADAMTS4 (VMAH) (Gao et al. 2002), and G1-DPE (Sandy et al. 2001) was performed using the avidin-biotin-peroxidase method (Vector Laboratories; Burlingame, CA) as previously described (Kenagy et al. 2005). Murine or rabbit IgG was used as a negative control as appropriate. Scoring of staining intensity as higher, lower, or the same between the paired high- and normal-flow grafts was performed blinded. For double labeling for ADAMTS4 and cell death, sections were first stained with antibody to ADAMTS4 as previously described (Kenagy et al. 2005), followed by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) as previously described (Kenagy and Clowes 2000). Images were acquired using a DP70 CCD color camera (RGB, 12 bits/channel; Olympus America, Inc.; Center Valley, PA) using DP70 controller/manager software v3.02 with one-touch color balancing.
Western Analysis
Frozen portions of the baboon graft neointima, which was easily stripped from the PTFE graft material with forceps, were pulverized, extracted (0.05 M Tris, pH 7.5, 0.01 M CaCl2, 2.0 M guanidine HCl, and 0.2% Triton X-100), and dialyzed (0.1 M Tris, 5 mM EDTA, 0.2% Triton X-100, pH 7.4); 5 μg protein was loaded per lane for SDS-PAGE (8%), and Western blotting was performed. Frozen human aortic organ culture tissue was weighed and extracted as described (Sandy et al. 2001). The same proportion of each culture (tissue extract and conditioned medium were identical in terms of wet weight, dimensions of tissue used, and medium per culture, and the tissue was free of atherosclerotic lesions) was loaded in each lane. Blots were developed using chemiluminescent reagents (Pharmacia; Piscataway, NJ) and quantified using ImageQuant (GE Healthcare; Piscataway, NJ). The specificity of detection was confirmed by showing that the signals on the ADAMTS4 blots were eliminated by preadsorption of the antibody with a 10 μM solution of the immunizing peptide (data not presented).
Results
ADAMTS Proteinases in Baboon PTFE Graft Neointima
We previously observed a 70-kDa ADAMTS-mediated cleavage product of V1 versican (G1-DPE) in graft neointima that was markedly increased after 4 days of high blood flow (Kenagy et al. 2005). Therefore, we investigated the expression of ADAMTS enzymes that have been shown to degrade versican or aggrecan, namely ADAMTS1, -4, -5, -8, -9, -15, and -20 (Sandy et al. 2001; Somerville et al. 2004; Cross et al. 2005; Nicholson et al. 2005). By microarray, ADAMTS4 was the only family member that was induced in the neointima 1 day after the switch to high blood flow (1.45 ± 0.07 high:normal flow ratio; Table 1). Expression of the other relevant ADAMTSs in normal-flow intimas was less than 50% the level of ADAMTS4. Finally, qPCR, showed that levels of ADAMTS4 were 2.73 ± 1.03-fold normal flow (n=4).
Table 1.
Microarray analysis of the effect of high blood flow on ADAMTS gene expression in polytetrafluoroethylene graft intimas
| ADAMTS | High flow | Normal flow | High/normal ratio |
|---|---|---|---|
| ADAMTS1 | 95.0 ± 2.4 | 88.1 ± 2.2 | 1.08 ± 0.05 |
| ADAMTS4 | 289.4 ± 21.9 | 199.4 ± 9.0 | 1.45 ± 0.07a |
| ADAMTS5 | 108.6 ± 1.5 | 100.2 ± 2.2 | 1.09 ± 0.03 |
| ADAMTS8 | 97.3 ± 2.0 | 101.8 ± 2.2 | 0.96 ± 0.04 |
| ADAMTS9 | 83.7 ± 3.8 | 79.4 ± 2.4 | 1.06 ± 0.06 |
| ADAMTS15 | 94.6 ± 3.6 | 82.2 ± 3.5 | 1.16 ± 0.09 |
| ADAMTS20 | 83.4 ± 2.9 | 81.8 ± 2.8 | 1.02 ± 0.02 |
q<.0001 by SAM analysis.
Values are the mean ± SEM of standard numerical values of gene expression by microarray analysis and of their ratios obtained from analysis of paired normal- and high-flow graft intimas from five animals.
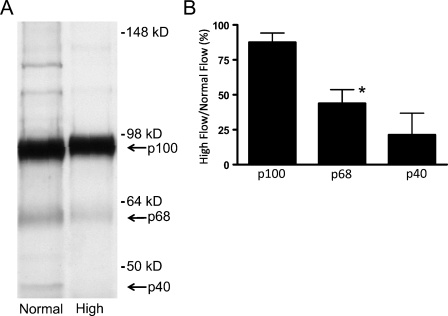
Because G1-DPE is increased after 4 days of high flow (Kenagy et al. 2005) but not after 1 day (immunohistochemical data not presented), we performed Western analysis for ADAMTS4 on graft neointima 4 days after the switch to high blood flow. We observed a major band at ∼90 kDa and minor bands at ∼60 kDa and ∼40 kDa (Figure 1A). These forms were previously shown to represent the proform, the N-terminally activated form, and the N- and C-terminally activated forms of ADAMTS4, respectively; they have been termed p100, p68, and p40 (Flannery et al. 2002; Gao et al. 2004). Although the abundance of p100 was not markedly altered after 4 days of high flow, the abundance of p68 and p40 was reduced to 44% and 21% of normal flow levels, respectively (Figure 1B). The identity of the minor bands at ∼120–140 kDa in the normal flow samples is not known, although they have been seen previously in cell culture supernatants and tissue extracts (Gao et al. 2002). ADAMTS5 was not detected with an antibody shown to detect ADAMTS5 immunohistochemically (Plaas et al. 2007).
Figure 1.
(A) Effect of increased blood flow on ADAMTS4 in intimal extracts of 4-day normal and high blood flow polytetrafluoroethylene (PTFE) grafts. This Western blot shows results from graft intimas of one representative baboon. (B) Immunoblot analysis of ADAMTS4 expression in graft intimal extracts. Data are expressed as the ratio of high- to normal-flow graft values (% mean ± SEM; *p<.02 high vs normal flow; n=2–3). Although the major form of ADAMTS4, the proform p100, is not altered by high flow, p68 and p40 are decreased.
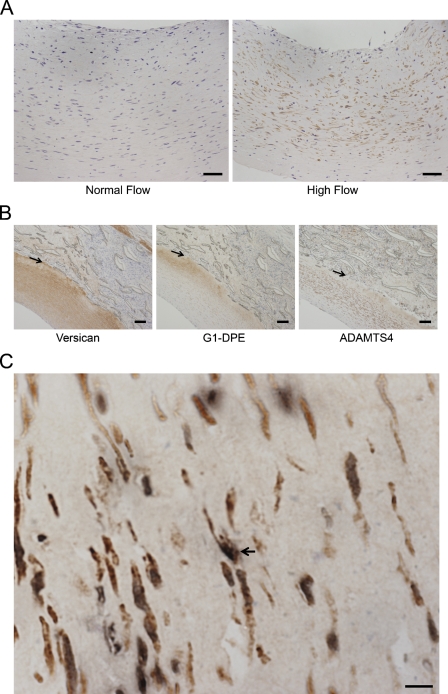
Immunostaining of the graft neointima for ADAMTS4 demonstrated that it was also increased in the 1-day high-flow graft neointimas in 7 of 10 paired grafts (Figure 2A), which is consistent with increased ADAMTS4 mRNA observed at 1 day. Most staining was observed in the deeper half of the intima. Although versican was observed throughout the intima, the majority of G1-DPE was detected deep in the intima (Figure 2B), as previously observed (Kenagy et al. 2005). The deep intima is also where most cell death occurs (Berceli et al. 2002), and we observed dying cells (TUNEL-positive) that were also positive for ADAMTS4 (Figure 2C). There was no difference in ADAMTS4 evident at 4 days (data not presented), which is consistent with the lack of change at 4 days in the most abundant form of ADAMTS4, p100 (Figure 1).
Figure 2.
(A) Photomicrograph of ADAMTS4 staining in baboon PTFE graft neointima after 1 day of normal (left panel) or high (right panel) blood flow. The lumen is at the top, and all tissue visible is the neointima. High flow increased ADAMTS4 immunostaining. (B) Photomicrographs of versican (2B1; left panel), G1-DPE (middle panel), and ADAMTS4 (left panel) in a high-flow PTFE graft neointima. Arrows mark the neointima–PTFE border. (C) Double immunostaining of a high-flow PTFE graft neointima for ADAMTS4 (brown) and TUNEL (black). The relatively acellular right side is near the PTFE boundary. The arrow indicates an example of a double-labeled cell. Bars: A = 50 μm; B = 100 μm; C = 10 μm.
The decreased levels of active ADAMTS4 in the graft neointima after 4 days of high blood flow occurs at about the time that G1-DPE is increased (Kenagy et al. 2005) and may be the result of the loss of enzyme from the tissue subsequent to protease activation and versican cleavage. C-terminal truncation has been shown to decrease binding of ADAMTS4 to heparin and the ECM (Flannery et al. 2002; Kashiwagi et al. 2004), and there is a potential heparin binding site (R206PRRAKR212) in the prodomain adjacent to the cleavage site (R212-F213). Thus, tissue levels of the active forms (Figure 1) might be an underestimate, owing to diffusion from the tissue. Because of the obvious difficulty of addressing this issue in vivo, we examined ADAMTS4 distribution in an organ culture system of human aorta.
ADAMTS4 in Aortic Organ Culture
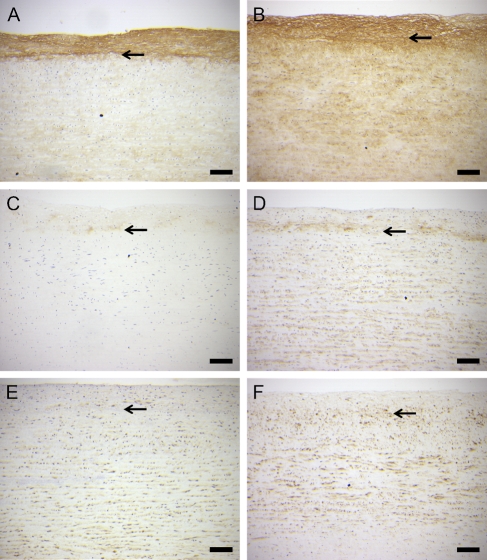
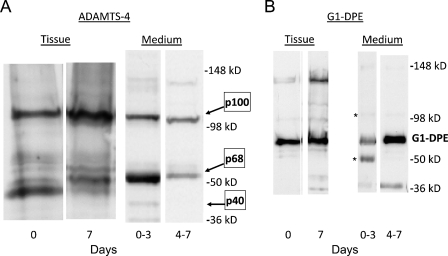
Immunostaining of both versican (Figures 3A and 3B) and G1-DPE (Figures 3C and 3D) was increased in the aortic organ cultures by day 7. On the other hand, there was no consistent change in ADAMTS4 staining with increasing time in culture (Figures 3E and 3F). Western analysis of tissue showed that the cultured human aorta predominantly contained the p100 and p68 forms of ADAMTS4 (Figure 4A), which were at similar levels at day 0 (the ratio of p100 to p68 was 0.75 ± 0.21; mean ± SEM; n=4). However, the ratio of p100 to p68 in the tissue at 7 days was 11.7 ± 6.2. This increase in the p100-to-p68 ratio appears to be explained by the larger amount of p68 than p100 lost to the culture medium during the first 3 days (Figure 4A, right panel). More total ADAMTS4 was released into the medium during the first 3 days than during the following days (Figure 4A, right panel), whereas more G1-DPE was released during the later period (Figure 4B, right panel). The tissue contained less ADAMTS4 than the medium because more total tissue was loaded per lane (20%), compared with the medium (5%). These data suggest de novo synthesis and C-terminal truncation followed by diffusion into the medium.
Figure 3.
Immunohistochemical staining of human aortic organ culture samples for versican (A,B), the versican ADAMTS cleavage fragment G1-DPE (C,D), and ADAMTS4 (E,F) at time 0 (A,C,E) and day 7 of incubation in vitro (B,D,F). Culture of arterial samples increased immunostaining of versican and G1-DPE, but immunostaining of ADAMTS4 was not consistently different from fresh tissue. The arrow indicates the internal elastic lamina. Bar = 200 μm.
Figure 4.
Western blots of human aortic organ culture tissue and medium for ADAMTS4 (A) and the versican ADAMTS cleavage fragment (G1-DPE) (B). The amount of medium loaded per lane is 5% of the total, and the tissue extract loaded is 20%. Asterisk: ADAMTS4 bands remaining from previous blot of membrane in A.
Induction of ADAMTS4 Occurs When SMC Death Is Increased
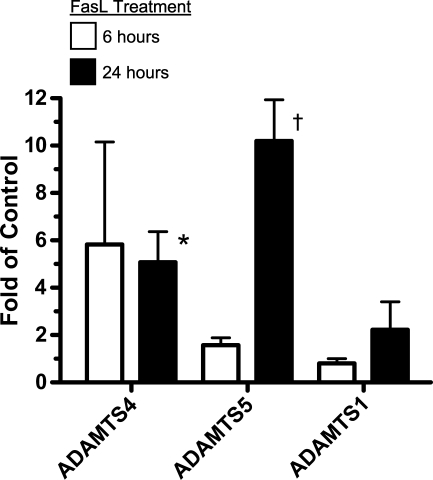
The induction of ADAMTS4 mRNA by high flow in the graft intima by 1 day (Table 1) coincides with the increased SMC death shown previously (Hsieh et al. 2006). Significantly, dying SMCs (Berceli et al. 2002), G1-DPE product (Kenagy et al. 2005), and ADAMTS4 protein (Figure 2) are all found most abundantly in the deep neointima. In addition, in the aortic organ culture experiments ADAMTS4 and G1-DPE were most notably observed near the cut edges of the explants in the 7-day but not in the freshly fixed tissue (data not presented). Because we have previously observed that dying SMCs are only seen at the cut edges of aortic explants (Kenagy and Clowes 2000), these results are consistent with an association between cell death and synthesis of ADAMTS4. Therefore, to determine whether death induces ADAMTS4 expression, we tested the effect of FasL on ADAMTS4 mRNA levels. We observed that treatment of cultured SMCs with FasL increased both the apoptotic SMC population (Figure 5) and message levels for ADAMTS4 ∼5-fold at both 6 hr and 24 hr (Figure 6). To determine the specificity of this effect, levels of ADAMTS1 and ADAMTS5, which are known to degrade versican, were also determined. ADAMTS5, but not ADAMTS1, was also induced by treatment with FasL.
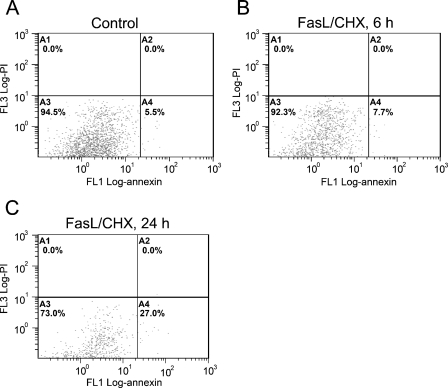
Figure 5.
Fluorescent-activated cell sorting analysis of control (A) smooth muscle cells (SMCs) or those treated with FasL for 6 hr (B) or 24 hr (C) and stained with propidium iodide (y axis) and annexin V (x axis). Annexin V–positive, propidium iodide–negative SMCs (A4) increased significantly by 24 hr.
Figure 6.
Induction of ADAMTS4 in baboon SMCs by FasL. Results of quantitative PCR are reported as the ratio of FasL-treated to control values and are the mean ± SEM of three to four independent experiments. Open and closed bars are 6 hr and 24 hr of treatment, respectively. *p<.05 vs control, †p=.073 vs control.
Discussion
Our data are consistent with the idea that cell death–promoting signals, such as increased blood flow, stimulate ADAMTS4 transcription and that ADAMTS4 cleaves versican at Glu441-Ala442, promoting neointimal atrophy. Within 1 day of the switch to high blood flow in the baboon PTFE graft, there is increased SMC death (Hsieh et al. 2006) and increased ADAMTS4 mRNA but no change in the other ADAMTS enzymes capable (ADAMTS1,-4, -5, and -9) or potentially capable (ADAMTS8, -15, and -20) of cleaving versican (Sandy et al. 2001; Somerville et al. 2004; Cross et al. 2005; Nicholson et al. 2005). Induction of SMC death using FasL increases levels of ADAMTS4 message. However, unlike increased blood flow in vivo, this effect is not entirely specific, in that ADAMTS5, but not ADAMTS1, was also induced by FasL. In addition, while we did observe dying SMCs that expressed ADAMTS4 (Figure 2C), over 50% of neointimal SMCs are immunopositive for ADAMTS4, but less than 10% of the cells are TUNEL positive (Berceli et al. 2002; Hsieh et al. 2006). This suggests the possibility that dying SMCs may secrete secondary factors that act in a paracrine manner. An additional possibility is that TGFβ, which can be secreted by SMCs that engulf dying SMCs (Fries et al. 2005), induces ADAMTS4 in surrounding SMCs (Moulharat et al. 2004).
The signaling pathways for induction of ADAMTS4 apparently shared by FasL and high blood flow/shear stress have not been investigated. One pathway may involve the transcription factor nuclear factor of activated T-cell (NFAT), which is activated by shear stress and Fas ligand (Sun et al. 2006; Celil Aydemir et al. 2007). NFAT activates ADAMTS4 transcription (Thirunavukkarasu et al. 2006) and regulates SMC migration and proliferation (Liu et al. 2005). It is interesting that shear stress increases SMC apoptosis in vitro via endogenous FasL/Fas interactions (Apenberg et al. 2003), raising the possibility that this pathway operates in the graft. This is supported by observations that FasL induces MCP-1 in SMCs in vitro (data not presented; Schaub et al. 2000), that this induction is partially mediated by IL-1α (Schaub et al. 2000), and that IL-1α increases ADAMTS4 mRNA in cartilage (Patwari et al. 2005). However, increased blood flow does not increase MCP-1 (data not presented), suggesting that this Fas/IL-1 pathway is not operative in the graft neointima.
ADAMTS4 mRNA was increased after 1 day of high blood flow (Table 1), but G1-DPE was increased after 4 days (Kenagy et al. 2005). In day 4 tissue, we found decreased p68 and p40 but no major change in p100 ADAMTS4 (Figure 1). This suggests at least two possibilities: (1) The activity of ADAMTS4 is primarily regulated by enzyme activation, and the active enzyme is rapidly released from the tissue after versican cleavage, whereas G1-DPE remains bound to matrix through its known hyaluronan binding properties; or (2) ADAMTS4 is not the ADAMTS responsible for versican cleavage at Glu441-Ala442. With regard to the first possibility, ADAMTS4 is present in the baboon intima primarily as the proform p100. This is consistent with activation by cell surface or extracellular proprotein convertases (Pratta et al. 2003; Longpre et al. 2009). It is interesting that cytokine treatment of cartilage explants increases the ADAMTS-specific aggrecan neoepitope NITEGE without a change in message for ADAMTS1, -2, -3, -4, or -5, which led two groups to suggest the importance of posttranscriptional regulation of ADAMTS4 activity (Flannery et al. 1999; Pratta et al. 2003). An additional activation mechanism would be loss of inhibitory factors such as TIMP-3, which effectively inhibits ADAMTS4 (Kashiwagi et al. 2001). However, we did not observe changes in message levels of TIMP-3 or of TIMP- 1, -2, and -4 (data not presented; Kenagy et al. 2002). Finally, compared with p100, p68 is preferentially released from cultured aortic tissue, consistent with the hypothesis that active ADAMTS4 is rapidly lost from tissue in vivo. Full-length p100 may bind to heparan sulfate proteoglycans in the tissue via heparin binding sites in the C-terminal spacer domain (Flannery et al. 2002; Kashiwagi et al. 2004) and a potential heparin binding site (R206PRRAKR212) in the prodomain. Regarding the second possibility, we have not successfully detected ADAMTS1 or ADAMTS5 in the baboon graft neointima by immunostaining or Western blot. For the other verified versicanase, ADAMTS9 (Somerville et al. 2003), the very low levels of detectable mRNA were not altered by increased blood flow. In addition, Demircan et al. (2005) found that active ADAMTS9 was only detectable in a chondrocytic cell line after induction of ADAMTS9 mRNA, suggesting that ADAMTS9 is regulated primarily through increased transcription.
The regulation of the cleavage and loss of versican is of interest from the perspective of the possibility of treating stenotic vein grafts or restenotic stented arteries by pharmacological induction of intimal atrophy rather than additional surgery (Min et al. 2008). Because versican is a major component of these intimal lesions, the loss of versican would have a major impact on lesion volume, particularly through the water binding properties of versican. The chondroitin sulfate chains of versican also have an inhibitory effect on elastin fiber formation, which will impact the structural integrity of the lesion (Huang et al. 2006). In addition, cleavage of versican by ADAMTS4 may produce fragments with various biological activities. G1-DPE does not contain glycosaminoglycan chains, but could remain bound to the matrix via hyaluronate. The effect of this cleavage product on SMC and endothelial cell function is not known, but the G1 globular domain (which is 87 amino acids shorter than G1-DPE) and G3 domains of versican have been reported to stimulate astrocytoma and 3T3 cell proliferation and migration (Kenagy et al. 2006).
Acknowledgments
Supported by grants from the National Institutes of Health, U.S. Public Health Service (HL30946, RR00166, and HL07828), the Shriners Hospitals (to JDS), and the Arthritis Foundation (to JDS).
We thank Jun Xue, Hangjun Duan, and David Pritchard for assistance with Illumina arrays and Lynn Amon for normalization of arrays. We also thank Drs. Jorge Reyes, Andre Dick, Austin Spitzer, and other members of the Division of Transplantation of the University of Washington Department of Surgery for procuring the human aortic tissue.
References
- Apenberg S, Freyberg MA, Friedl P (2003) Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine Fas/FasL pathway. Biochem Biophys Res Commun 310:355–359 [DOI] [PubMed] [Google Scholar]
- Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, Hori M, et al. (1998) Remodeling of in-stent neointima, which became thinner and transparent over 3 years: serial angiographic and angioscopic follow-up. Circulation 97:2003–2006 [DOI] [PubMed] [Google Scholar]
- Berceli SA, Davies MG, Kenagy RD, Clowes AW (2002) Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg 36:1248–1255 [DOI] [PubMed] [Google Scholar]
- Celil Aydemir AB, Lee S, Won KD, Gardner TR, Prince D, Mok AJ, Young-In LF (2007) Nuclear factor of activated T cell mediates proinflammatory gene expression in response to mechanotransduction. Ann N Y Acad Sci 1117:138–142 [DOI] [PubMed] [Google Scholar]
- Cross NA, Chandrasekharan S, Jokonya N, Fowles A, Hamdy FC, Buttle DJ, Eaton CL (2005) The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate 63:269–275 [DOI] [PubMed] [Google Scholar]
- Demircan K, Hirohata S, Nishida K, Hatipoglu OF, Oohashi T, Yonezawa T, Apte SS, et al. (2005) ADAMTS-9 is synergistically induced by interleukin-1beta and tumor necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum 52:1451–1460 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN (1999) Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19:1004–1013 [DOI] [PubMed] [Google Scholar]
- Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, Wight TN, et al. (2004) Extracellular matrix changes in stented human coronary arteries. Circulation 110:940–947 [DOI] [PubMed] [Google Scholar]
- Flannery CR, Little CB, Hughes CE, Caterson B (1999) Expression of ADAMTS homologues in articular cartilage. Biochem Biophys Res Commun 260:318–322 [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, Mackie SA, et al. (2002) Autocatalytic cleavage of ADAMTS-4 (aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem 277:42775–42780 [DOI] [PubMed] [Google Scholar]
- Fries DM, Lightfoot R, Koval M, Ischiropoulos H (2005) Autologous apoptotic cell engulfment stimulates chemokine secretion by vascular smooth muscle cells. Am J Pathol 167:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson VP, Jin S, Zuo FR, Sandy JD (2004) ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem 279:10042–10051 [DOI] [PubMed] [Google Scholar]
- Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD (2002) Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem 277:11034–11041 [DOI] [PubMed] [Google Scholar]
- Geary RL, Kohler TR, Vergel S, Kirkman TR, Clowes AW (1994) Time course of flow-induced smooth muscle cell proliferation and intimal thickening in endothelialized baboon vascular grafts. Circ Res 74:14–23 [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Kenagy RD, Mulvihill, ER, Jeanette JP, Wang X, Chang CM, Yao Z, et al. (2006) Bone morphogenetic protein 4: potential regulator of shear stress-induced graft neointimal atrophy. J Vasc Surg 43:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, et al. (2006) Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res 98:370–377 [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Matsuura R, Isaka N, Nakano T, Sakakura T, Yoshida T (2001) Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angioplasty: clinical significance of early tenascin-C expression. Virchows Arch 439:185–190 [DOI] [PubMed] [Google Scholar]
- Kalish JA, Willis DJ, Li C, Link JJ, Deutsch ER, Contreras MA, Quist WC, et al. (2004) Temporal genomics of vein bypass grafting through oligonucleotide microarray analysis. J Vasc Surg 39:645–654 [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H (2004) Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem 279:10109–10119 [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K (2001) TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem 276:12501–12504 [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Clowes AW (2000) Blockade of smooth muscle cell migration and proliferation in baboon aortic explants by interleukin-1β and tumor necrosis factor-α is nitric oxide-dependent and nitric oxide-independent. J Vasc Res 37:381–389 [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Davies MG, Berceli SA, Hawkins SM, Wight TN, Clowes AW (2002) Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol 22:400–404 [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Lara S, Sandy JD, Clowes AW, Wight TN (2005) Accumulation and loss of extracellular matrix during shear stress-mediated intimal growth and regression in baboon vascular grafts. J Histochem Cytochem 53:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy RD, Plaas AH, Wight TN (2006) Versican degradation and vascular disease. Trends Cardiovasc Med 16:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Wilson JE, Roberts CR, Horley KJ, Winters GL, Costanzo MR, McManus BM (1996) Biglycan, decorin, and versican protein expression patterns in coronary arteriopathy of human cardiac allografts: distinctness as compared to native atherosclerosis. J Heart Lung Transplant 15:1233–1247 [PubMed] [Google Scholar]
- Liu Z, Zhang C, Dronadula N, Li Q, Rao GN (2005) Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem 280:14700–14708 [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R (2009) Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol 41:1116–1126 [DOI] [PubMed] [Google Scholar]
- Matsuura R, Isaka N, Imanaka-Yoshida K, Yoshida T, Sakakura T, Nakano T (1996) Deposition of PG-M/versican is a major cause of human coronary restenosis after percutaneous transluminal coronary angioplasty. J Pathol 180:311–316 [DOI] [PubMed] [Google Scholar]
- Mattsson EJR, Kohler TR, Vergel SM, Clowes AW (1997) Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol 17:2245–2249 [DOI] [PubMed] [Google Scholar]
- Min SK, Kenagy RD, Clowes AW (2008) Induction of vascular atrophy as a novel approach to treating restenosis. A review. J Vasc Surg 47:662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulharat N, Lesur C, Thomas M, Rolland-Valognes G, Pastoureau P, Anract P, De Ceuninck F, et al. (2004) Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage 12:296–305 [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Malik SB, Logsdon JM Jr, Van Meir EG (2005) Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P, Gao G, Lee JH, Grodzinsky AJ, Sandy JD (2005) Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage 13:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, et al. (2007) Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage 15:719–734 [DOI] [PubMed] [Google Scholar]
- Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC (2003) Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum 48:119–133 [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, et al. (2001) Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 276:13372–13378 [DOI] [PubMed] [Google Scholar]
- Schaub FJ, Han DK, Liles WC, Adams LD, Coats SA, Ramachandran RK, Seifert RA, et al. (2000) Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med 6:790–796 [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Apel ED, Lewis RM, Wang LW, Sanes JR, Leduc R, et al. (2004) ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem 279:35159–35175 [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, et al. (2003) Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem 278:9503–9513 [DOI] [PubMed] [Google Scholar]
- Sun M, Ames KT, Suzuki I, Fink PJ (2006) The cytoplasmic domain of Fas ligand costimulates TCR signals. J Immunol 177:1481–1491 [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu K, Pei Y, Moore TL, Wang H, Yu XP, Geiser AG, Chandrasekhar S (2006) Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun 345:197–204 [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ (2004) Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res 94:1158–1167 [DOI] [PubMed] [Google Scholar]