Abstract
The behavioral and motivational changes that result from use of abused substances depend upon activation of neuronal populations in the reward centers of the brain, located primarily in the corpus striatum in primates. To gain insight into the cellular mechanisms through which abused drugs reinforce behavior in the primate brain, changes in firing of neurons in the ventral (VStr, nucleus accumbens) and dorsal (DStr, caudate-putamen) striatum to “natural” (juice) vs. drug (intravenous (IV) cocaine) rewards were examined in four rhesus monkeys performing a visual Go-Nogo decision task. Task-related striatal neurons increased firing to one or more of the specific events that occurred within a trial represented by: 1) Target stimuli (Go trials) or 2) Nogotarget stimuli (Nogo trials), and 3) Reward delivery for correct performance. These three cell populations were further subdivided into categories that reflected firing exclusively on one or the other type of signaled reward (juice or cocaine) trial (20–30% of all cells), or, a second subpopulation that fired on both (cocaine and juice) types of rewarded trial (50%). Results show that neurons in the primate striatum encoded cocaine rewarded trials similar to juice rewarded trials, except for 1) increased firing on cocaine rewarded trials, 2) prolonged activation during delivery of IV cocaine infusion, 3) differential firing in ventral (VStr cells) vs. dorsal (DStr cells) striatum cocaine rewarded trials. Reciprocal activations of antithetic subpopulations of cells during different temporal intervals within the same trial suggest a functional interaction between processes that encode drug and natural rewards in the primate brain.
Keywords: Go-Nogo task, cocaine vs. juice rewards, neuronal firing correlates, dorsal/ventral-striatum, nonhuman primates, drug abuse
Introduction
A major factor studied extensively with respect to the neurobiological basis of drug abuse and addiction has been the difference between the rewarding properties of abused substances and natural appetitive/nutritional rewards (Carelli et al 2000, Dackis and O’Brien 2001, Vezina 2004). Understanding the relationship of drug induced alterations in normal brain activity to drug seeking behavior in animals (Kantak et al 2002, Sun and Rebec 2005) is necessary in order to develop effective agents that can control addiction in humans (Risinger et al 2005). It has also been postulated that drug seeking behavior engages the same motivation-related brain circuits in the striatum and other regions (Gass and Olive 2008), including the same dopaminergic (DA) projections, that are activated by natural rewards (Marinelli and White 2000, Kalivas and McFarland 2003). Insight into the neural changes related to drug addiction, relapse and abstinence has been gained by monitoring activity in these reward circuits in animals actively engaged in drug seeking behaviors (Bowman et al. 1996, Bradberry et al 2000, Bradberry 2008, Rebec 2006, Vezina 2004, Hollander and Carelli 2007). It is likely that drugs such as cocaine can “tap into” existing brain reward circuitry therefore, as information on neuronal processes within these areas accumulates (Samejima et al 2005, Morris et al 2006, and Lau and Glimcher 2008) it is important that neuronal encoding associated with stimuli that represent drug vs. natural rewards be directly compared to provide insight into a potential basis for cocaine addiction in humans (Volkow et al. 2006, Cooper and Knutson 2008).
To examine the neural basis of the difference between drugs vs. natural rewards, recordings were obtained from the caudate-putamen and nucleus accumbens in the respective dorsal (DStr) and ventral (VStr) regions of striatum in rhesus monkeys (n=4) performing a Go-Nogo task for intravenous (IV) cocaine or a squirt of a sweet juice liquid as rewards delivered on separate trials within the same session. The results indicate that: a) neurons in VStr and DStr encode the same events within the Go-Nogo task, however the firing tendencies for events in different classifications were not similar in the two areas, b) event-related cell firing in both areas was also segregated with respect to firing to stimuli that signaled either juice or cocaine reward delivery on a given trial, and 3) both VStr and DStr cells consistently exhibited more robust firing on cocaine vs. juice rewarded trials irrespective of event-classification.
Methods
All animal procedures for NHPs were reviewed and approved by the Institutional Animal Care and Use Committee of Wake Forest University, in accordance with U.S. Department of Agriculture (USDA), International Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), and National Institutes of Health guidelines.
Apparatus
Four adult male rhesus monkeys (Macaca mulatta) weighing 4.7–9.3kg were acclimated to primate chairs and trained to respond to a visual display screen using a video tracking device attached to the wrist for detection of arm movements which were displayed by displacement of a 3 cm diameter filled circle (cursor) on the screen (Hampson et al. 2004). Arm movements were “tracked” by two-dimensional cursor displays on the same screen where stimulus objects were presented and responses (cursor movements) were required to perform the task (Figure 1).
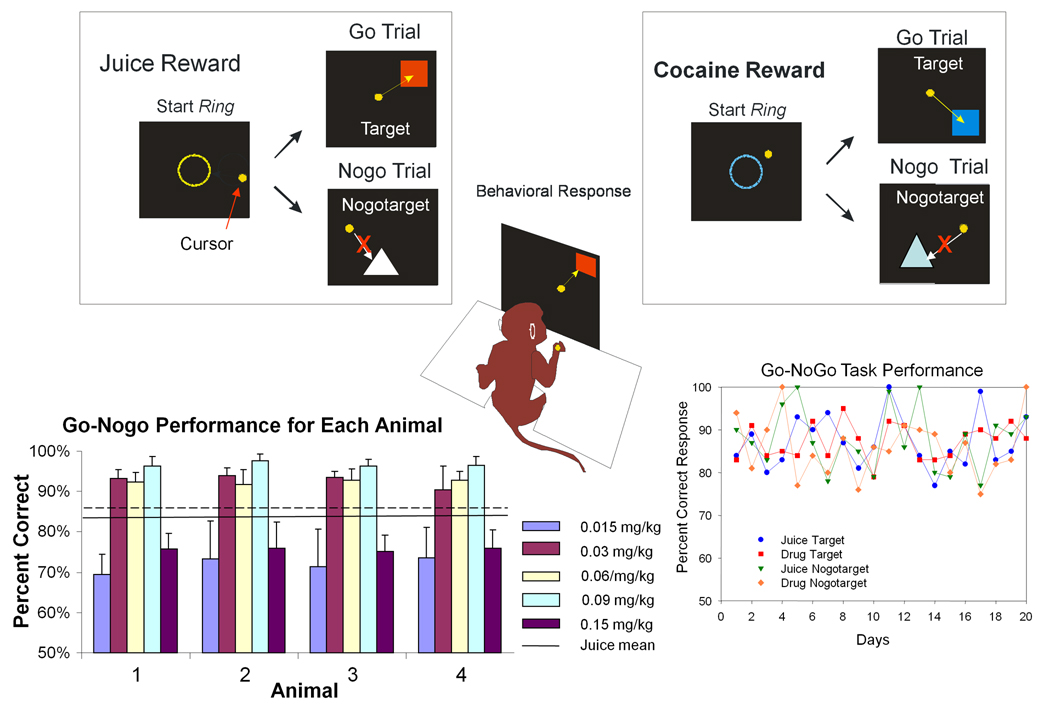
Figure 1. Illustration of Juice and Cocaine Reward Trials and Behavioral Performance in Go-Nogo Task.
Upper: Illustration of Go-Nogo task for Juice (left) and Cocaine (right) rewarded trials. Start Ring, Target and Nogotarget stimuli are shown for each type of trial. Trial initiated by presentation of Start Ring the color of which specified the type of reward (Juice or Cocaine) for the trial. Placement of cursor inside the Start Ring initiated presentation of Target or Nogotarget images. The ‘Go’ response required placement of cursor in Target image for 500 ms after which reward delivery was initiated. The ‘Nogo’ response required withholding cursor outside Nogotarget image for 5.0 s, after which reward delivery was initiated. Any contact of cursor with Nogotarget image terminated trial immediately with no reward and screen blanked for 10 sec. Correct performance on signaled juice reward trials operated a valve to deliver 0.5 mls of a fruit-flavored juice solution via a chair mounted sipper tube near the animals’ mouth. Correct performance on signaled cocaine reward trials operated a peristaltic pump for a 10 sec. intravenous (IV) infusion of cocaine hydrochloride (dose range 0.015–0.15 mg/kg). Lower Left: Performance (mean ±SEM percent correct) on cocaine reward trials for each of the four animals at each dose of IV cocaine show typical inverted U-shape dose-effect curves for each animal across the dose range employed (0.015–0.15 mg/kg). Overall mean (solid line) and SEM (dashed line) for juice rewarded trials averaged across the same sessions shown for cocaine dose-response performance. Lower Right: Performance (mean percent correct) over 20 daily sessions for a single animal for each of the 4 different types of trials Go (Target) cocaine or juice; and Nogo (Nogotarget) cocaine or juice.
Behavioral Task
Animals were trained to perform a Go-Nogo task consisting of presentation of specific images on the screen to which the monkeys were trained to respond by either: 1) moving the cursor into the image (Target) on Go trials, or 2) withholding the cursor for 5.0 s from placement in a different (Nogotarget) image on Nogo trials. Animals were motivated to respond for highly preferred juice rewards by withholding the daily allocation of fluid until after behavioral test sessions. Such restrictions ranged between 2–4 hrs from change to “daytime” in home cage light-dark cycle. Hydration levels were monitored on a weekly basis according to standard ACUC protocols for classification of dehydration. Trials were initiated by placing the cursor completely inside a 15 cm diameter circle (“Start Ring”) displayed in the center of the screen, after which (1.5 s) either the Target or Nogotarget image was presented at one of nine different locations on the screen. The type of impending reward on a given trial was signaled (Figure 1) by: 1) the color of the Start Ring (yellow = juice reward, blue = cocaine reward), and 2) the color and shape of the Target and Nogotarget images that signaled the type of required behavioral response (Go or Nogo). A “Go” response to a Target image required cursor positioning within the image boundaries for at least 0.5 s, after which was delivered either: 1) a squirt of juice (via sipper tube mounted next to the animal’s mouth) or 2) an IV injection of cocaine hydrochloride (via indwelling catheter) from a computer activated peristaltic pump. “Nogo” trials required withholding the cursor outside the Nogotarget image boundary after presentation for at least 5.0 s, after which either juice or cocaine (depending on the type of start ring and image) was non-contingently delivered automatically in the same manner as after a correct response on Go trials described above. The inter-trial interval (ITI) varied between 3.0 and 10.0 s for juice rewarded trials and for cocaine trials that followed juice trials. However, a 10 sec ITI automatically followed cocaine trials due to the duration of the IV infusion pump operation. The probability of juice vs. cocaine rewarded trials varied randomly during the session with the exception that a minimum 2.0 min was imposed between cocaine rewarded trials. Animals received a maximum of 20–25 cocaine rewarded trials per session. Failure to respond appropriately under either type of trial contingency (Go or Nogo) initiated a 10.0 sec “timeout” period where no reward was delivered and the screen was blanked until start of the next trial. On Target (Go) trials if a response did not occur it was scored as an error and on Nogotarget (Nogo) trials if the cursor touched the image during the 5.0 s display period the trial was scored as an error. Five dose levels of cocaine (0.015, 0.03, 0.06, 0.09, 0.15 mg/kg/injection) were employed at various times during the study, with only one dose level administered for all trials within a session. Assessments of neuronal firing were combined over the above dose levels for this report to provide sufficient numbers of active neurons to accurately determine firing differences between cocaine vs. juice rewarded trials. Neuronal dose-effects and related findings are the subject of another report from this same study (Opris et al. 2009, in preparation).
Surgery
All surgical procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Wake Forest University and performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. Animals were sedated with ketamine (10 mg/kg), intubated, and maintained throughout surgery with isoflurane (1–3% to effect) and oxygen as needed. Craniotomy sites were selected to overlie the stereotaxic coordinates (23.0 ± 1.0 mm anterior to interaural line; 4.0 ± 0.5 mm lateral to midline) for Dorsal (DStr) and Ventral (VStr) Striatum (Caudate Nucleus and Nucleus Accumbens respectively) as certified by individual MRIs for each monkey (Szabo and Cowan, 1984). Recording access cylinders (Crist Instruments) were fixed to the skull with screws and dental cement above each craniotomy site to allow daily placement of microelectrodes into the brain (Hampson et al. 2004). Each animal received antibiotics (cefazolin, 25 mg/kg) for 7 days after surgery, and acetaminophen (10 mg/kg) and/or buprenorphine (0.7 mg/kg) as needed for pain. Animals were allowed to recover from the procedure for 5–10 days before resuming behavioral training and testing. Vascular access ports (Norfolk Medical Products, Skokie, IL) for drug infusions were implanted in a separate surgery session. The port was positioned subcutaneously in the mid-scapular region with the catheter inserted into the femoral vein, and threaded for a distance calculated to terminate near the vena cava. The port and cannula were flushed daily with heparinized saline (5 ml).
Cocaine Administration and Dose
Correct performance on signaled cocaine rewarded trials operated a peristaltic pump for 10 sec. intravenous (IV) administration of cocaine hydrochloride. The doses of cocaine used as rewards for correct performance (0.015, 0.03, 0.06, 0.09, and 0.15 mg/kg/injection in saline) were changed daily and were within the range for published dose-effect curves for cocaine self-administration and administration during cognitive performance in nonhuman primates (Bradberry et al. 2000, Jentsch et al. 2002, Nader et al. 2006, Bradberry 2008). Upon successful completion of a trial, cocaine was immediately infused intravenously (IV) over a 10.0 s interval via infusion pump into the vascular access port (0.15 ml/sec, 1.5 ml/injection). Cocaine trials were spaced at least 2.0 min apart and interleaved randomly with juice rewarded trials.
Electrode Positioning and Recording
Recording procedures in monkeys were as described elsewhere (Hampson et al., 2004). Single neuron action potentials were recorded using tungsten electrodes (1–5 MΩ impedance) mounted in an Alpha-Omega EPS-8 multiple electrode positioning system. Electrodes were positioned independently on a daily basis in tracks that traversed the DStr (caudate nucleus; AP 23, ML 4, DV 18–28 mm) and VStr (nucleus accumbens; AP23, ML 4, DV 29–33 mm) stereotaxic coordinates, confirmed by individual MRIs, using a multi-electrode microdrive (Alpha Omega, Inc.) mounted prior to each session that allowed recording of 1–4 individual neurons simultaneously along the above dorsal-ventral tract traversed at slightly different lateral coordinates in each recording session. Recording loci were monitored within the session via stereotaxic coordinates and distances from landmark white-matter structures where single unit activity was consistently absent during electrode advancement along a selected vertical track. A representative range of recording tracks is shown in Figure 8B. The electrode tracks ranged from 20.5–22.5 mm anterior/posterior relative to Interaural zero; 3.6–5.2 mm from midline (right side) and 19.0 to 28.0 mm from brain surface for DStr (dorso/ventral) and 31.0 to 33.0 mm for VStr (dorso/ventral). At the completion of the recording session electrodes were removed, the cranial cylinders disinfected and sealed and the animal returned to its home cage. Single as well as multiple simultaneously identified recordings from DStr and VStr neurons were isolated and stored via waveform and timestamp in relation to specific events (perievent histograms) within trials in the Go-Nogo task.
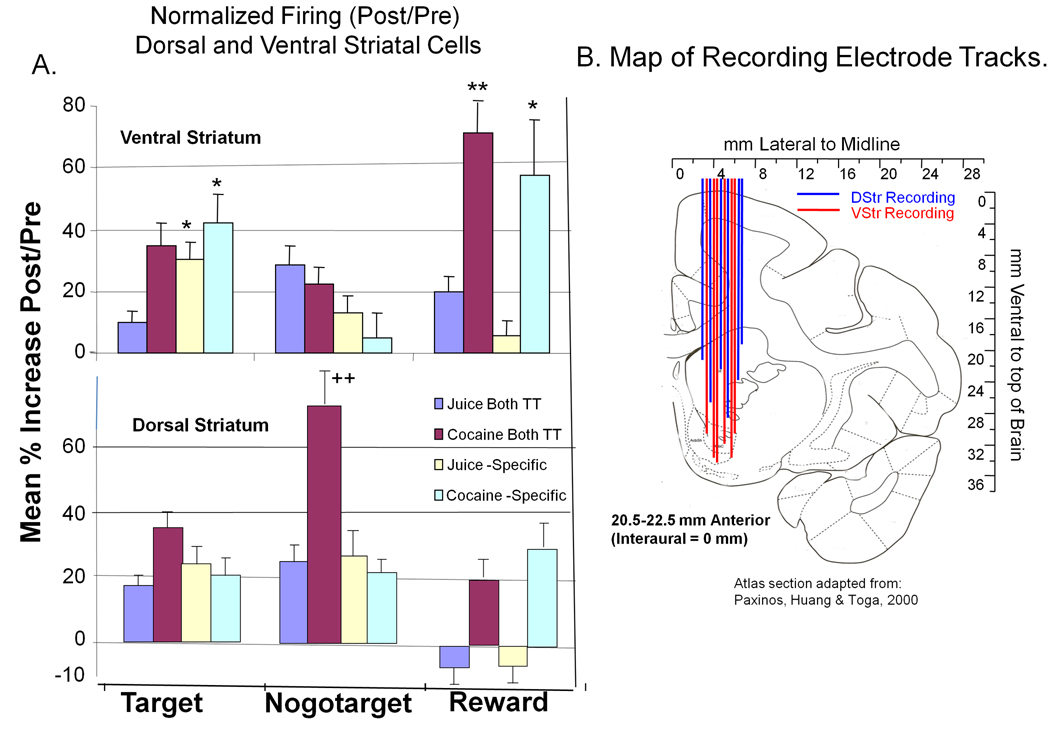
Figure 8. Normalized Firing Changes across all Categories of Events and Reward with Locations of Recording Tracks for VStr and DStr Neurons.
A: Mean (± SEM) normalized percent increase (post/pre event) in cell firing for VStr (top) and DStr (bottom) neurons in all three event categories and the four subcategories related to reward specificity. Scores of 0% indicate no change in pre vs. post event firing rate. Within each event (Target, Nogotarget and Reward) subclasses include normalized firing ratios for Juice-specific, Cocaine-specific and Both TT categories of firing. Asterisks: (VStr>DStr) *p<0.01, **p<0.001 (Student t-test); Pluses (DStr>VStr): ‡p<0.001, (Student t-test). B: Diagram of vertically oriented electrode tracks used for recording DStr and VStr neurons as mapped on the Paxinos et al (2000) monkey brain atlas as confirmed by individual MRIs in each animal. Some tracks allowed sampling from both areas (VStr and DStr) in the same ventral excursion. Range of penetrations in the anterior-posterior (AP) plane was restricted to ±1.5–2.0 mm relative to midpoint of the recording cylinder indicated in Atlas coordinates as 20.5–22.5 mm AP.
Statistical Analysis
Single-neuron waveforms were discriminated and analyzed with a multineuron acquisition system (Plexon Systems Inc) and NeuroExplorer software. Standard scores [z = (peak firing rate to event – pre-event 1.0s baseline firing rate) ÷ (SD of baseline firing rate)] were calculated for each neuron for each of the six events in the task to determine relevance of firing. Only neurons that showed significant firing changes (z > 3.09, P < 0.001) in perievent histograms derived from specific events according to the responses to particular images (Target or Nogotarget) or type of reward (juice or cocaine) were retained for analysis. Neurons exhibiting other firing properties (i.e. negative z scores) were not frequently observed and exhibited much higher firing rates than the mean values of DStr and VStr cells. Single neuron activity recorded from each brain region is presented as perievent histograms (PEHs) related to Target and Nogotarget images as well as firing associated with Reward (juice or cocaine) delivery. Comparison of juice and cocaine trial events involved assessment of differences in mean firing rates for successive 250 ms time bins displayed as mean (±SEM) firing rate histograms around each of the 3 task events. Cells encountered during pre-session recording that did not respond to trial events (< 15%) were not utilized. It was also possible for a given neuron to show significant activation to more than one event or reward condition within a session, however, further breakdown to that level of categorization was not included in the current study in order to provide sufficient statistical representation of proportions of cells that fired on a given trial event to compare on cocaine vs. juice rewarded trials.
Results
Behavioral Performance on Juice and Cocaine Rewarded Trials
Because of the implication of the dorsal (DStr) and ventral (VStr) striatum in cocaine addiction in animals and humans (Volkow and Fowler, 2000; Kalivas and McFarland 2003, Risinger et al. 2005), this study employed a simple Go-Nogo task to test the influence of cocaine vs. juice rewards on the encoding of behavioral contingencies by striatal neurons under circumstances where the task was well-learned and difference in behavioral performance on cocaine vs. juice rewarded trials was not a major contributing factor. Firing rates of DStr and VStr neurons were recorded in animals performing a simple Go-Nogo task (Figure 1) which manipulated three different parameters: 1) the type of reward (cocaine or juice) signaled by the Start Ring; 2) type of response required on a given trial (Go for Target images or non-responding for Nogotarget images), and 3) the method of reward delivery (IV cocaine vs. oral juice) for correct responses. The graph at the lower left in Figure 1 shows the dose-dependence of correct responding to cocaine Target images for each animal to illustrate the typical inverted U-shaped relation to performance over the 0.015–0.15 mg/kg dose range employed (Bradberry et al 2000). Figure 1 shows that there were no major differences in overall performance on Go trials across animals at each of the dose levels used in the task. The graph at the lower right illustrates the average day-to-day performance of a single animal on the four different types of trials in the task, Go or Nogo for juice and cocaine reward. There were no major differences in performance on Go vs. Nogo trials either within or across animals, consistent with the intended simplicity of the task to minimize behavioral influences on striatal neuron firing tendencies.
Ventral Striatal (VStr) Cell Activity on Juice vs. Cocaine Rewarded Trials
Figure 2 shows single neuron activity recorded in the VStr of animals performing the Go-Nogo task on either juice or cocaine rewarded trials (Figure 1). Figure 2A depicts the firing of 3 different VStr neurons recorded during performance in the Go-Nogo task. The perievent histograms and raster displays in the left column (Juice) show firing of each cell to one of the 3 main trial events (Target, Nogotarget and Reward) on juice rewarded trials. In the right column (Cocaine) are shown firing correlates of the same cells to the same trial events when cocaine was signaled or delivered as the reward for successful performance. As can be seen the same type of phasic cell firing was recorded for a particular event but in some cases (top and bottom) on both types of rewarded trial (cocaine or juice).
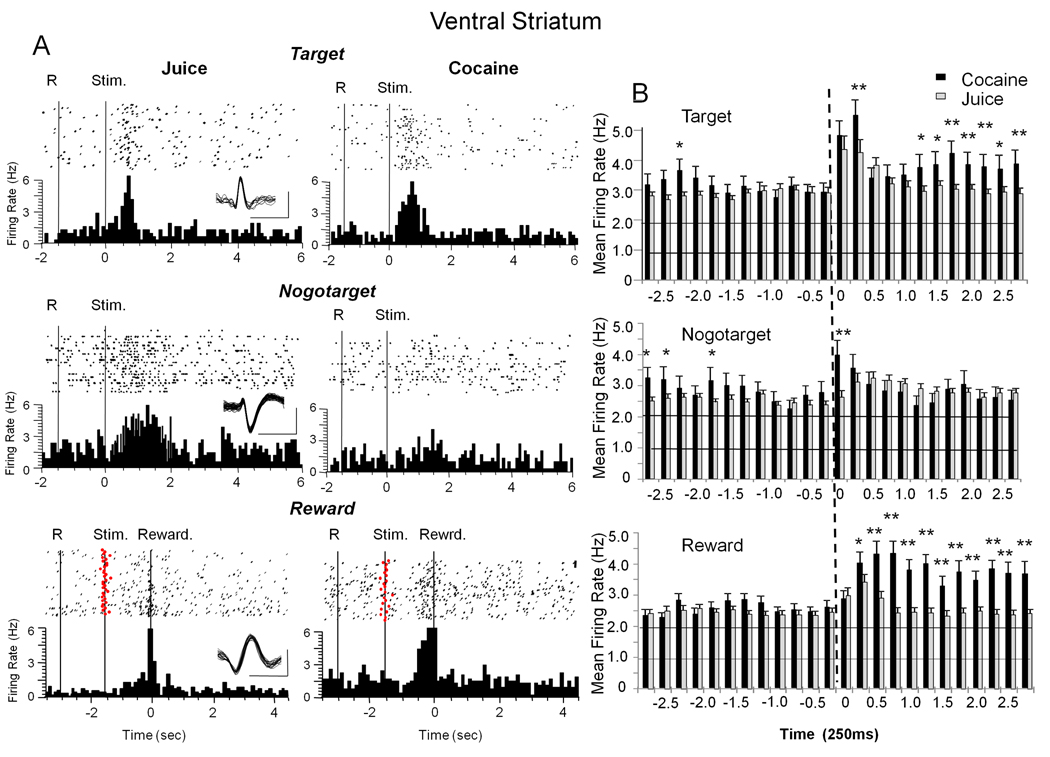
Figure 2. Firing of Ventral Striatal (VStr) Cells to Events in Signaled Cocaine and Juice Rewarded Trials.
A: Single trial raster displays (dots) and summed perievent histograms (PEHs) illustrate firing patterns of individual VStr cells during the three events in the Go-Nogo task. At each level the rasters (dots = neural spike occurrences) and PEHs depict firing synchronized to the indicated trial event (Target, Nogotarget, Reward). The three levels show a different VStr cell firing to one of the three types of trial events (Target, Nogotarget, Reward) on either a juice (left column) or cocaine (right column) rewarded trial. Superimposed waveforms are shown as insets for each isolated neuron (scale bar: 1 ms, 250 µV). Solid vertical lines indicate behavioral events: R=Start Ring response; Stim = presentation of Target, Nogotarget images. Reward (lower row) indicates firing synchronized to juice delivery (squirt in mouth) or initiation of cocaine administration via IV infusion pump following successful trial completion. Red dots show onset of Target images (stim) synchronized to reward delivery (0 s, dotted vertical line). B: Histograms show mean (± SEM) firing rate in 250 ms bins for VStr cells over ± 2.75s classified on the basis of one of the three Go-Nogo task events (0 s, dotted vertical line) shown in A. black bars: cocaine reward trials; gray bars: juice reward trials. All asterisks = cocaine > juice trials: Target *p<0.01 F(1,5950) > 8.40, **p<0.001 F(1,5950) > 12.72; Nogotarget *p<0.01, F(1,5302) > 7.96, **p<0.001 F(1,5302) >14.49; Reward *p<0.01, F(1,6214) > 10.41, **p<0.001, F(1,6214) > 21.11.
Figure 2B shows mean (±SEM) firing rate histograms (± 2.75 s) of VStr cells for the three trial events illustrated in Figure 2A that occurred on cocaine (black bars) or juice (grey bars) trials. A total of 133 VStr cells responded to one or more of the 3 events in the Go-Nogo task in the following proportions: Target 60.2%, Nogotarget 51.1% and Reward 64.6%, which was not significantly different with respect to a given event category. Significant changes in mean firing rate are shown in Figure 2B (upper) for Target events on cocaine trials in the 0.5 – 2.75s time interval (F(13,5950) = 2.91, p<0.01) following Target image presentation and on juice trials over a shorter time period (0.25–0.75s, F(6,5950) = 5.96, p<0.01). Asterisks in this and other Figures with firing rate histograms indicate significant differences between cocaine vs. juice firing at the indicated 250 ms intervals. Significant changes in mean firing rate to Nogotarget events (Figure 2B middle) at 0.25s after presentation on cocaine (F (6, 5302) = 4.99, p<0.001) and juice trials (F (6,5302) = 3.22 p<0.01) are also shown in the same format. The most pronounced distinction between cocaine and juice trials was following reward delivery (Figure 2B lower) where cocaine trials were associated with significantly increased firing relative to juice trials for at least 2.5s after a correct response (cocaine 0.5 – 2.75s vs. pre 1.0s, F(14,6214) = 6.72. p < 0.001, juice 0.25–0.75s vs. pre 1.0s F(6,6214) = 3.03 p<0.01). Importantly, when summed over all three task-related events (Target, Nogotarget and Reward), mean firing was significantly greater on cocaine vs. juice trials in the post-event firing period (F(14,1876) = 5.02, p<0.001), but mean firing rates prior to the same events were not significantly different (F12,1876) = 2.48) between reward conditions. A major basis for this difference in VStr cell firing on juice vs. cocaine trials was the increased discharge at 0.5–1.5 s following Target and Reward events (F(1,4197) > 11.48, **p< 0.001, asterisks in Figure 2B) which corresponded to the time of IV delivery of cocaine via the operation of the infusion pump (see Methods).
Subpopulations of VStr Cells Firing to Different Rewards within each Event
Figure 3A illustrates firing of individual VStr cells to Target presentation on juice (left) or cocaine (right) signaled trials with discharge patterns that reflect a further sub-categorization of reward related firing within each event. This differentiation segregated cells on the basis of increased firing specific only to juice trials (Juice-specific, upper left); or specific only to cocaine trials (Cocaine-specific middle), while a third sub-category showed cells that fired on both trial types cocaine and juice (Both TT lower) rewarded trials. Cells that fired to the Target image in the Juice-specific (37.7% of total cells) or Cocaine-specific (33.0%) subcategories did not show increased discharge to the Target image on the opposite trial type (Figure 3A upper and middle), while cells classified as Both TT (40.2%) discharged to Target image presentations on cocaine and juice trials (Figure 3A lower). Figure 3B shows mean (± SEM) firing rate histograms for all VStr cells in each of the 3 reward subcategories for the Target event (n=128) segregated by type of signaled reward (juice vs. cocaine). Firing was significantly increased for 1.0 sec following Target presentation (F(6,2038) = 5.14, p< 0.01) in cells that fired only on juice reward trials (Juice-specific). Interestingly, activity in Cocaine-specific VStr cells that fired to Target images only on cocaine trials was increased for 0.5s (F(4,2038) = 16.18, p< 0.001) after presentation, returned to background firing level, then increased again at 1.75 – 2.75s after presentation (F(1,2038) > 2.89, p < 0.01 each bin), the time interval corresponding to infusion pump delivery of IV cocaine. Comparison of firing increases between the two cell groups revealed no significant differences in the first 0.5s following Target presentation, however there was significantly higher firing in the later time period for the Cocaine-specific vs. Juice-specific cell groups (1.5 – 2.75 s, F(1,2038) > 11.14, p< 0.001 asterisks in Figure 3B). Superimposed on the histograms in Figure 3B are the distribution of latencies (gray bars) for behavioral (Go) responses to the Target image for cocaine and juice trials respectively, indicating no significant differences in behavioral response latency as a function of type of reward and, also showing that the late prolonged discharge in Cocaine-specific cell firing was not due to delayed behavioral responses.
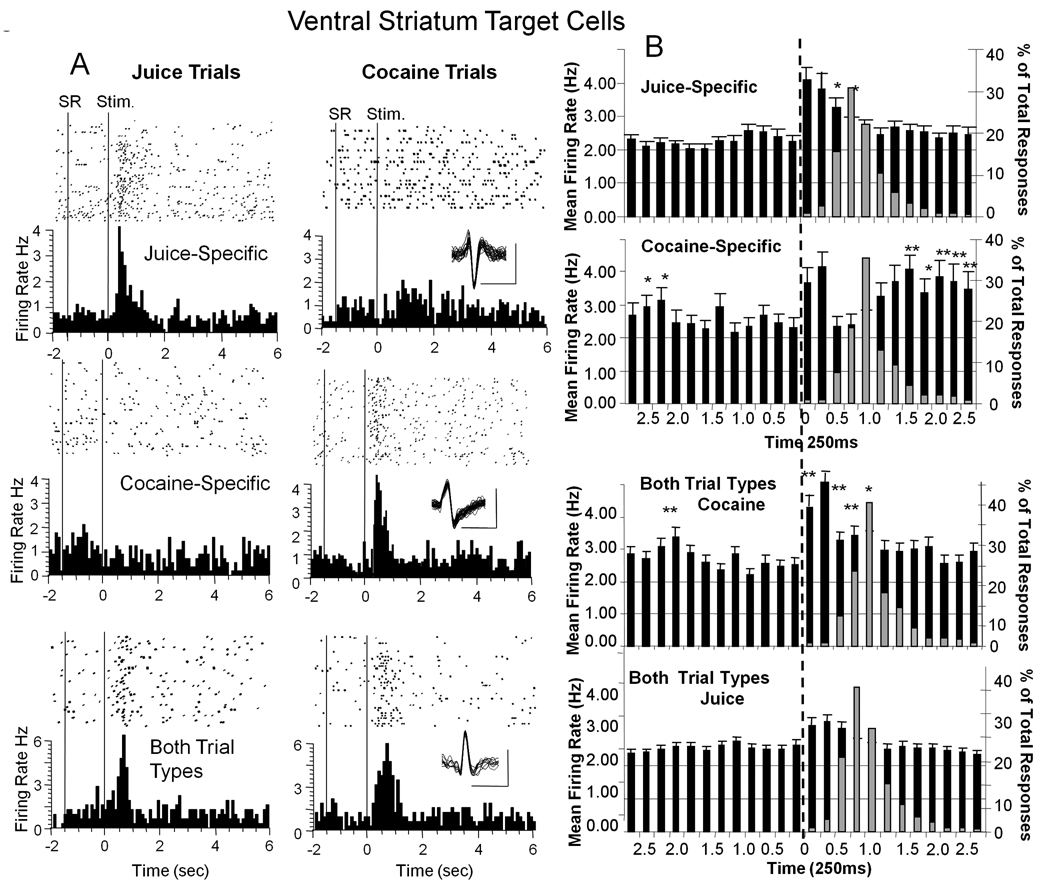
Figure 3. Classification of Target VStr Cell Firing into Reward Subcategories.
A: Single trial raster displays and PEHs for Target VStr neurons classified with respect to firing on juice or cocaine rewarded trials. Top: Juice-specific cell fires only to Target stimulus presentation on signaled juice reward trials (left). Middle: Cocaine-specific cell fires only to Target image presented on signaled cocaine reward trials (right). Bottom: VStr Target cell that fired to both types of trial, juice and cocaine (Both TT). Stim = presentation of Target image. B: Mean (± SEM) firing rate histograms summed over all VStr Target cells classified in the categories shown in A for ± 2.75 sec relative to Target presentation (time 0 s, dotted vertical line). Juice-specific and Cocaine-specific VStr cell firing is shown for the respective juice and cocaine reward signaled trials (upper and middle); Firing of VStr cells in Both Trial Types (Both TT) subcategory is shown for cocaine and juice reward trials in the lower two panels. Superimposed gray bars after Target image presentation (vertical dotted lines) indicate distribution of % of behavioral response latencies (across all animals) to Target image on the same time base for the respective trial types. Asterisks: Cocaine-specific > Juice-specific *p<0.01 F(1,2038) > 9.10, **p<0.001 F(1,2038) > 11.14. Both Trial Types: cocaine > juice trials **p< 0.001 F(1,1943) > 11.36, F(1,1950) > 3.56, p < 0.01.
The lower two histograms in Figure 3B depict the third sub-category where VStr Target cell firing significantly increased within 1.0 s after image presentation on both juice (Juice trials-Both F(6,1943) = 2.78, p< 0.05) and cocaine reward trials (Cocaine trials-Both F(7,1943) > 16.83, p<0.001). However, higher firing rates occurred to presentation of the cocaine Target image (F(1,1943) > 11.36, p< 0.01 asterisks in Figure 3B) even though the same VStr cells were recorded in both circumstances. It is important to note that unlike Cocaine-specific cells there was no secondary or prolonged increase in firing on cocaine trials during IV cocaine delivery for VStr cells that responded to Target images on both types of trial (Figure 3B Both TT, cocaine trials). In addition cocaine trials produced a brief transient increase in firing (F(1,1943) > 6.13, p < 0.01) in the pre-period before Target presentation in the Cocaine-specific and in the Both TT subgroup (cocaine trials) classifications, which may have reflected increased firing to the Start Ring, signaling the onset of a cocaine trial (Figure 1).
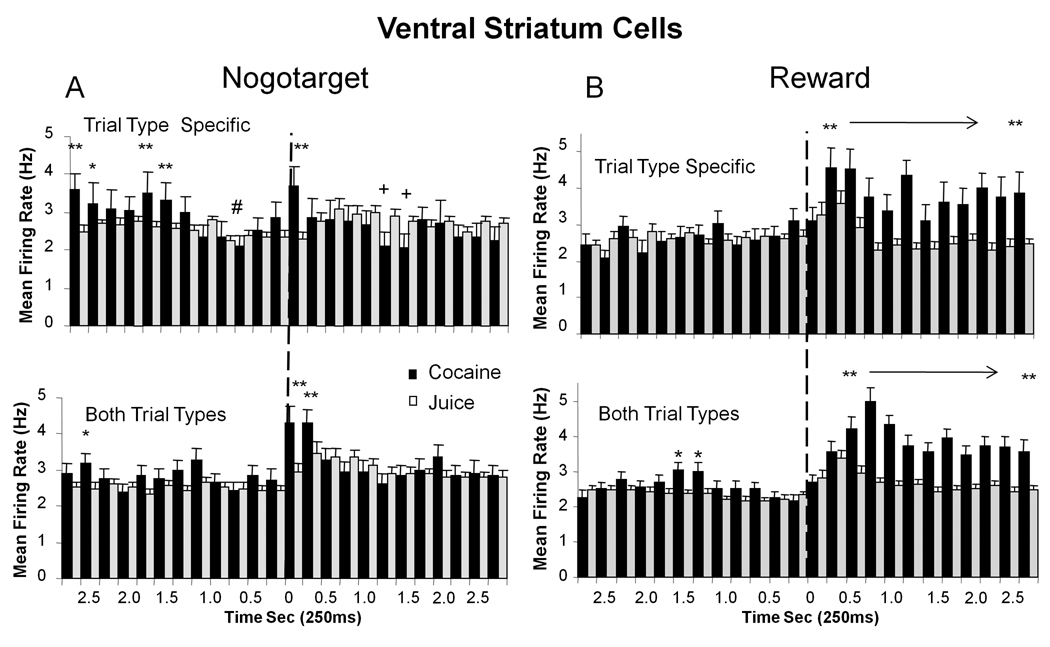
The specificity of VStr cell discharges in the other two event categories, Nogotarget and Reward, was similar to that in the above detailed description for Target events, in that reward related firing was also segregated with respect to Juice-specific, Cocaine-specific, or Both TT subcategories. Figure 4 (left) shows mean (±SEM) firing rate histograms of VStr cells to Nogotarget (Nogo) image presentation on Juice-specific (53.1% of total cells) or Cocaine-specific (21.8%) trials (top) as well as for cells (lower left) that fired on Both TT (31.1%). Nogotarget event firing for Juice-specific and Cocaine-specific cells showed only minor increases in cell discharge immediately following (0.25s) Nogotarget presentation for either reward condition (Figure 4A), however this was coupled with significantly increased firing in the pre-event (-2.75 to −1.5 s) period (F(1,2231) > 11.73, p<0.001) in the Cocaine-specific sub-category (Figure 4, top left). In addition, there was also a significant decline in cell firing in the same sub-category (F(4,2231)=12.55, p< 0.001) immediately prior to Nogotarget presentation. In contrast, cells in the Nogotarget subcategory that increased firing on both cocaine and juice trials (Both TT) exhibited significantly increased firing on cocaine trials (F(5,1487) = 5.72, p< 0.01) in the 1.0 s interval following image presentation (F(1,1487) > 14.53, p <0.001) compared to when the same cells fired on juice trials (Figure 4A, lower left, grey bars).
Figure 4. Segregation of VStr Nogotarget and Reward Firing into Reward Subcategories.
Mean (±SEM) firing rate histograms of VStr neurons with discharge synchronized to Nogotarget (left) and Reward (right) events (Figure 2) subdivided into cocaine and juice reward categories as in Figure 3 for Target event firing. A. Combined histograms for Nogotarget, Juice-specific (gray bars) and Cocaine-specific (black bars) cell firing (upper) and similar data for different groups of cells that fired to Nogotarget events on Both Trial Types (Both TT) cocaine and juice rewarded trials. Asterisks: Cocaine-specific > Juice-specific: *p<0.01, **p<0.001, F(1,2231) > 7.73; Pluses: Juice-specific > Cocaine-specific: +p<0.01, F(1,2231) > 8.31. Decrease in Cocaine-specific firing at 1.0 s prior to Nogotarget presentation: #p <0.001, F(4,2231) =12.5. Asterisks Both TT: cocaine > juice trials, *p<0.01, **p <0.001, F(1,1487) > 6.58. B.Reward, Upper histograms show Juice-specific (grey bars) and Cocaine-specific (black bars) cell firing for ± 2.75 s relative to event onset (0 s, dotted lines). Cocaine-specific > Juice-specific for Reward at 0.5–2.75 s interval (**p< 0.001, F(1,1967) > 16.37). Both TT cell firing on cocaine and juice rewarded trials is shown in lower two histograms; cocaine > juice trials for Reward event at 0.75–2.75 s (*p<0.01, **p<0.001, F(1,4030) > 6.88). Dotted lines indicate occurrence of Nogotarget and Reward events (0 s).
A more distinct difference between cocaine vs. juice trials was exhibited by VStr cell firing to Reward events, shown in Figure 4B. Cells that fired on Juice-specific (38.2% of total cells) or Cocaine-specific (30%) trials each yielded significant firing increases (F(4,2015) =3.54 p <0.001; F(22,2015) =2.41 p <0.001, respectively) commencing 0.5s after Reward delivery. Reward event discharges were significantly higher for Cocaine-specific cells (F(1,1967) > 16.37, p< 0.001, asterisks in Figure 4B) persisting for at least 2.75 s which was consistent with the extended duration of the IV pump delivery of cocaine rewards (10.0 sec). A similar set of firing increases was obtained in VStr cells that fired to Reward events (64%) on juice (F(9,1967) = 2.65, p<0.01, post 0.50–0.75s) and cocaine (F(22,1967) = 4.13 p<0.001, 0.75–2.75s) trials (Both TT), with cocaine trials again showing significantly higher and more prolonged firing rates (F(1,4030) >16.58, p<0.001,asterisks in Figure 4B, lower). The unique tendency for VStr cell firing to segregate into the above event categories and reward sub-categories of firing suggests that reward-dependent identification of individual trial events dominated VStr encoding of information while animals performed the Go-Nogo task.
Neuronal Firing in Dorsal Striatum (DStr) on Juice vs. Cocaine Rewarded Trials
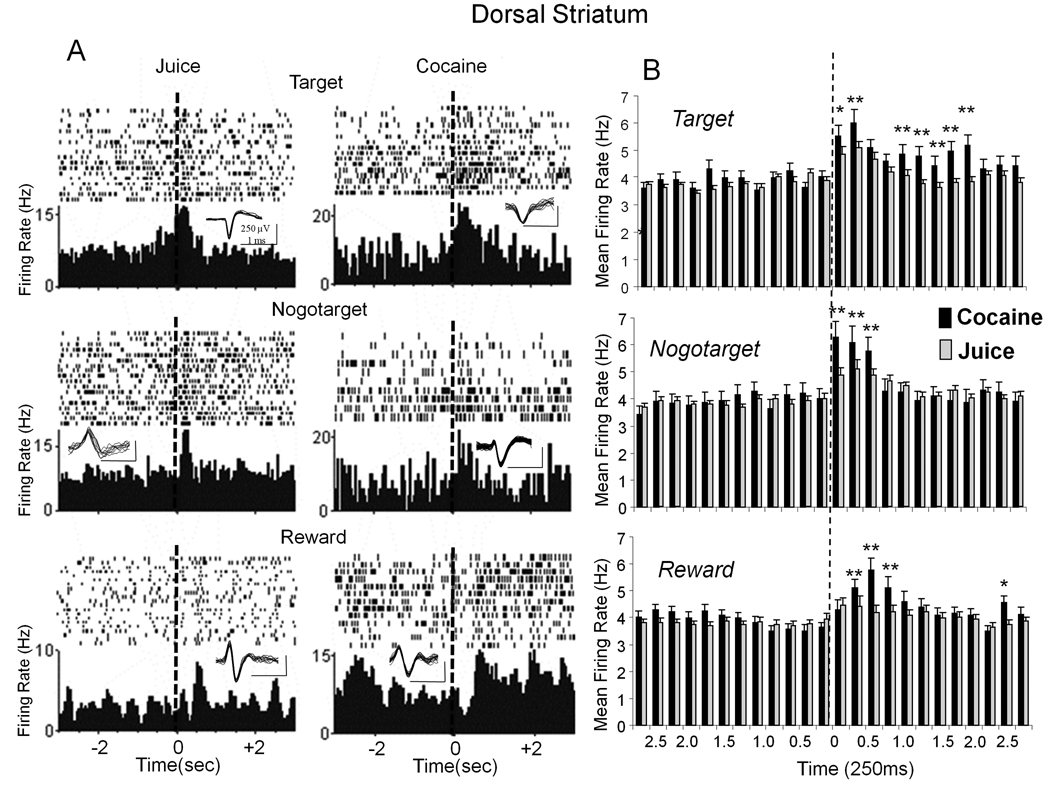
Recordings in the same animals of presumed medium spiny neurons (Shidara et al. 1998, Samejima et al. 2005) in the dorsal striatum (DStr) were analyzed as VStr cells above and segregated with the same classification schemes. The overall mean (±SEM) baseline firing rate of DStr cells (Figure 5B) was significantly higher than VStr cells (3.83 ± 0.29 Hz vs. 2.60 ± 0.21 Hz, respectively, p<0.001) however, phasic changes in firing rates with respect to Go-Nogo trial events and type of reward were similar. In addition there were slight differences compared to VStr cells with respect to the percentage of total recorded DStr cells (n=81) that fired in one or more of the 3 event categories during performance of the task, Target (69.1%), Nogotarget cells (55.5%), and Reward (57.4%). Figure 5A shows six individual DStr neurons that exhibited increased firing on signaled juice or cocaine rewarded trials during Target, Nogotarget and Reward events as shown in Figure 2 for VStr cells. Figure 5B depicts mean (± SEM) firing rate histograms for DStr cells recorded during each of the three events on juice (gray bars) and cocaine (black bars) rewarded trials. Firing was significantly increased in the percentage of DStr cells (26.4%) that fired to the Target image on cocaine trials (F(11,4174) = 5.27, p < 0.001) and in the percentage of DStr cells (33.9%) that fired on juice trials ( F(7,4174) = 2.78, p<0.01) with cocaine trials showing significantly higher rates (F(1,4174) > 11.34, p<0.001) following Target image presentation (top, Figure 5B asterisks). Unlike VStr cells, firing of DStr cells to Nogotarget events was increased markedly on juice (F(5,3886)= 8.02, p<0.01, 44.8%) and cocaine (F(5,3886) = 32.9, p<0.001, 34.1%) rewarded trials, with firing also significantly higher (F(1,3886) > 17.98, p<0.001) on cocaine trials (asterisks, Figure 5B middle). Firing relative to the pre-reward delivery baseline was significantly elevated for Reward events on juice and cocaine trials (F(6,3910) > 3.35, p<0.01) with the percentage of DStr cells nearly the same (40.8% and 34%, respectively), with slightly higher firing (F1,4419 > 9.60, p<0.001) on cocaine trials but only within 0.5–0.75s interval following reward delivery (asterisks in Figure 5B, lower).
Figure 5. Firing of Dorsal Striatal (DStr) Cells to Go-Nogo Events on Signaled Cocaine and Juice trials.
A: Perievent histograms and single trial raster displays illustrate typical firing patterns of six individual DStr cells during the same trial events shown for VStr cells in Figure 2A. Top: Middle and Lower raster displays and PEHs depict examples of 6 different DStr neurons that fired specifically to the indicated events (Target, Nogotarget or Reward) on either a juice (left column) or cocaine (right column) trial. Rasters and histograms constructed at ± 3.0 sec around the indicated event (“0” s, dotted line). Neural spike waveforms isolated for each cell indicated above each PEH (scale bar: 1.0 ms, 250 µV). B: Mean (± SEM) firing rate histograms for DStr cells classified as firing to Target, Nogotarget, and Reward events (“0” s dotted line, ± 2.75 s) on juice (grey bars) and cocaine (black bars) rewarded trials. Asterisks: *p<0.01, **p<0.001; Target F(1,4174) >11.34, Nogotarget F(1,3886) >17.98, Reward F(1,3910) >11.53.
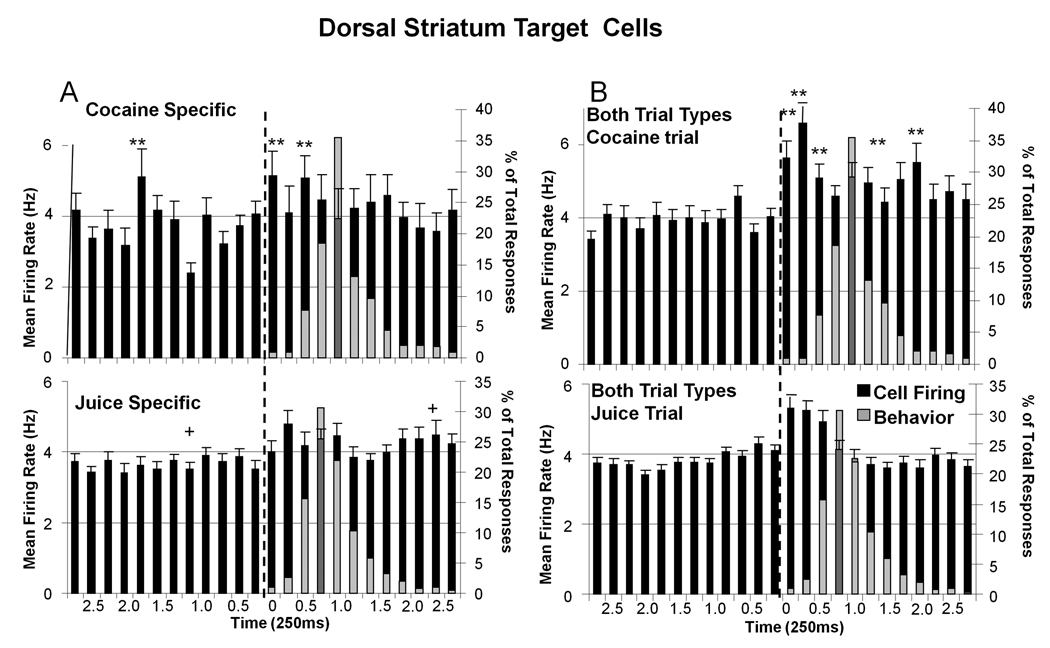
Figure 6A shows the same sub-categorization as in Figure 3 for DStr cell firing to Target images on Juice-specific (33.8%) or Cocaine-specific (26.4%) trials. DStr cells that fired to the Target event on both types of rewarded trials (Both TT 49.6%) are shown in Figure 6B. Firing of Cocaine-specific DStr cells (Figure 6A) was significantly reduced 1.0 s prior to Target presentation (F(7,934) = 3.76, p<0.01) but increased significantly (F(1,934) > 10.90 p<0.001) in the 0.75 s period following Target image presentation relative to cells that fired only on juice trials (Figure 6A Juice-specific). Juice-specific DStr cells showed small increases in firing to Target images 0.5s (F(2,934) = 4.12, p<0.01) following Target presentation (Figure 6A lower). In marked contrast, DStr cells that fired on cocaine and juice rewarded trials (Both TT) showed robust and sustained firing increases (Figure 6B) for 1.0 s after Target presentation on cocaine (F(11,1151)= 4.02, p<0.001, 0.25–2.0s post-Target) and juice trials (F(6,1151) = 3.46, p<0.01), with firing on cocaine trials significantly higher and of longer duration (F(1,1151) > 11.38, p< 0.001, asterisks in Figure 6B).
Figure 6. DStr Target Cell Firing Segregated into Reward Subcategories.
Mean (± SEM) firing rate histograms for DStr Target cells (0 s ± 2.75 sec) classified into the same 3 reward categories shown in Figure 3 for VStr Target cells. A: DStr Target cells that fired on only cocaine (Cocaine-specific, top) or juice (Juice-specific, lower) signaled reward trials. Asterisks (cocaine >juice): *p<0.01, **p<0.001, F(1,934) > 10.90; Plus (juice >cocaine): +p<0.01, F(1,934) > 4.42. Superimposed light gray bars indicate the same behavioral latency data following Target image presentations as shown in Figure 3 (dark gray bars represent neural firing where the latency bar would have obscured the black bar). B: DStr Target cells that fired on Both Trial Types, cocaine trials (top) or juice trials (lower). Asterisks (cocaine > juice): **p<0.001, F(1,1151) > 11.38. Superimposed gray bars indicate the same distribution (% of total) of behavioral responses for each type of trial as shown in A.
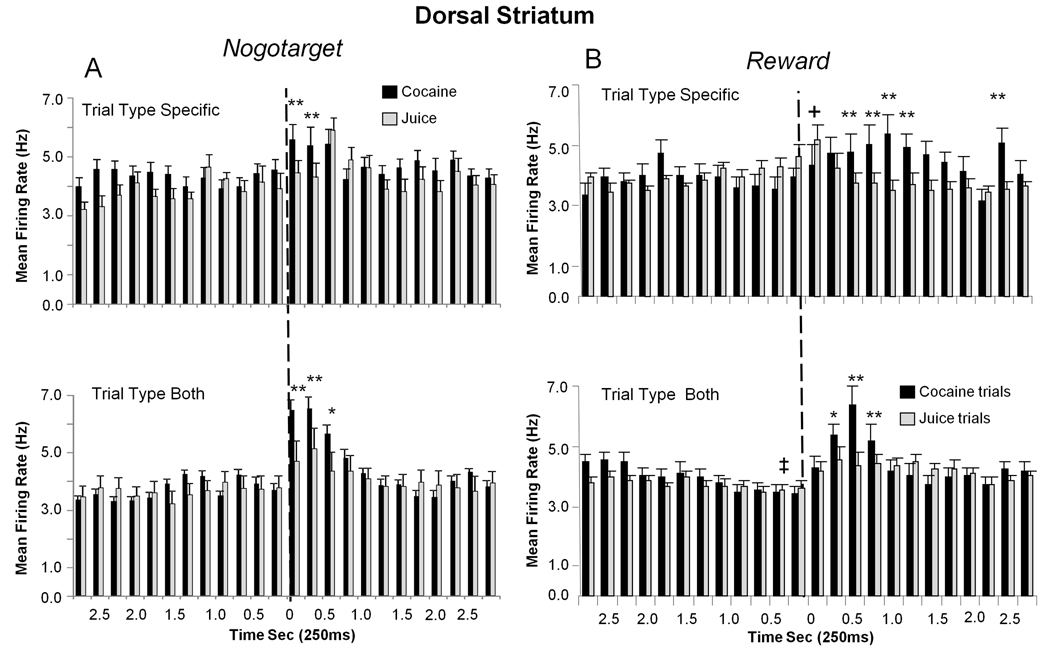
DStr Nogotarget and Reward cell firing shown in Figure 7 was divided into the same 3 sub-categories as shown in Figure 4 for VStr cells. Nogotarget firing in DStr cells classified as Juice-specific (34.1%) or Cocaine-specific (40.8%) showed significantly increased discharges in the 0.25–0.75s interval after Nogotarget image presentation (Juice-specific F(6,863) = 2.02, p<0.05; Cocaine-specific F(6,863) = 2.44, p<0.05), with Cocaine-specific cell firing significantly higher (F(1,863) > 14.06, p<0.001 asterisks, Figure 7A). However DStr cells that showed elevated firing on both types of rewarded trial (Both TT 35.8%, juice; F(5,647) = 3.07 p<0.01, cocaine; F(6,647) = 12.70, p<0.001), also showed significantly higher firing rates on cocaine trials (F(1,647) > 23.03, p<0.001, Figure 7A).
Figure 7. Segregation of DStr Nogotarget and Reward Firing into Reward Subcategories.
Mean (± SEM) firing rate histograms of DStr neurons with discharge synchronized to Nogotarget and Reward events (dotted line at 0 s) subdivided into cocaine and juice trial categories as in Figure 4 for VStr Target cells. A. DStr cells that fired to Nogotarget events. Upper: Juice-specific (gray bars) and Cocaine-specific (black bars) cell firing on the respective trial types. Asterisks (cocaine > juice): **p<0.001 (F(1,863) > 14.06). Lower: Combined firing rate histograms for Nogotarget cells that fired on Both Trial Types (Both TT): juice (gray bars) or cocaine (black bars) trials. Asterisks (cocaine > juice): **p<0.001, F(1,647) > 23.03. B. DStr cells that fired to Reward events. Upper: Juice-specific and Cocaine-specific cell activity shown in mean (± SEM) firing rate histograms for the respective trial types. Asterisks (cocaine > juice): **p<0.001, F(1,1030) > 15.04, Plus (juice > cocaine): +p<0.01, F(1,1030) > 6.87. Lower: Mean firing rate histograms for DStr Reward cells that fired on Both Trial Types. cocaine trials (black bars) and juice trials (grey bars). Asterisks (cocaine > juice): *p<0.01, **p<0.001, F(1,911) > 9.46, Plus (cocaine & juice trials both showed decreased firing relative to −2.5s interval): ‡p< 0.001, F(4,911) > 7.17.
DStr cells classified as firing to Reward events (Figure 7B) were segregated into Juice-specific (44.8 %) and Cocaine-specific (34.1%) subcategories and showed significantly elevated firing after reward delivery (Juice-specific, F(1,1030) = 4.47 p< 0.05, 0.25s post-delivery; Cocaine-specific, F(6,1030) = 3.70, p<0.001, 0.5–1.5s post-delivery) with Cocaine-specific cells in this category showing significantly higher firing (F(1,1030) > 15.04, p<0.001, asterisks in Figure 7B upper,). In DStr cells that fired on juice and cocaine trials (Reward, Both TT 33.3%) rates were significantly elevated for 0.5 s following reward delivery (juice trials, F(6,911) = 2.99, p<0.01; cocaine trials F(6,911) = 15.20, p<0.001, 0.25–0.75s) with cocaine trials showing larger increases than juice trials (F(1,911) > 11.5, p<0.001 asterisks in Figure 7B). However as noted by the (‡) symbol in Figure 7B, on juice and cocaine trials firing in this subclass of DStr cells declined significantly (F(4,911) > 7.17, ‡p< 0.001) in the 1.0s interval immediately prior to reward delivery (dotted line) which was unique with respect to other event sub-classifications in the task (Figure 3–Figure 6).
Relative Firing Differences Between VStr and DStr Cells
Figure 8 summarizes the changes for VStr and DStr cells in the 12 inter-related (event and trial-type) sub-classifications based on normalized firing rates that can be compared directly across each category. VStr cells were more responsive than DStr cells for Target events on Juice-specific and Cocaine-specific trials (p< 0.01, t > 2,99) and for cocaine Reward events irrespective of reward specificity sub-classification (Figure 8, Ventral Striatum; Reward Cocaine-specific, Reward Cocaine Both TT, p<0.01, t > 2.66). In contrast DStr cells exhibited significantly higher normalized rates of discharge to Nogotarget events (Nogotarget Cocaine Both TT, p<0.001, t > 3.45), but again only on cocaine rewarded trials. Figure 8 also shows that DStr cells actually showed slight decreases in normalized firing following delivery of juice rewards as indicated by the negative percentage increase.
Figure 8B shows the distribution of recording tracks in the monkey striatum from the Paxinos et al (2003) atlas cited in Methods. The tracks were designed to traverse both DStr and VStr in the same electrode recording session. Using this approach DStr cells were recorded before VStr cells in most cases but each region was regularly sampled within the same behavioral test session in most cases and there were no differences with respect to the order in which cells in each region were recorded with respect to firing correlates. The mediolateral extent of most recording tracks was determined by the anatomic location of VStr, but some DStr recording tracks were not immediately above Nucleus Accumbens. The anterior-posterior extent of the recording tracks was determined by the cranial well on the animal’s skull which allowed traversals of 1.5 to 2.0 mm.
Dynamics of Reward Encoding By Different Subclasses of VStr Target Cells
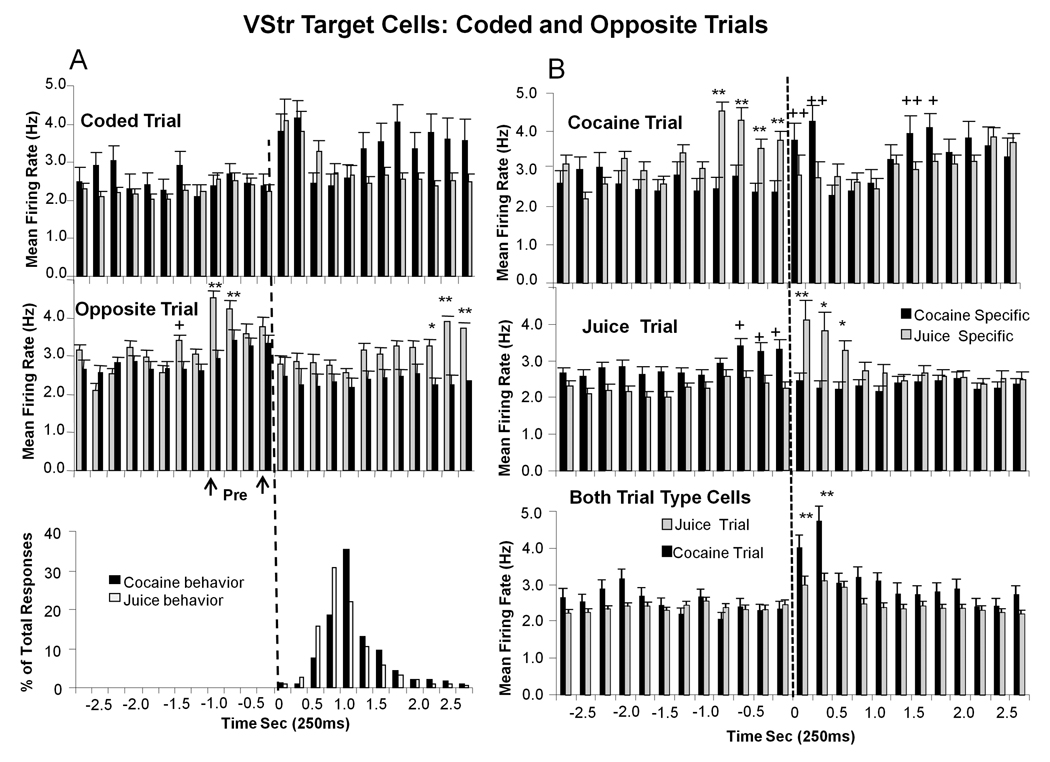
Differential encoding of trial events as a function of type of signaled reward (juice or cocaine) was not the sole function of the VStr cells that fired to Target events in the Go-Nogo task. This was revealed by the types of discharge patterns that occurred when the opposite type of trial was presented (i.e. juice trial for Cocaine-specific cells, or cocaine trial for Juice-specific cells) during the session. This is illustrated in Figure 9A by comparisons of mean (± SEM) firing rate histograms for VStr Juice-specific (gray bars) and Cocaine-specific (black bars) cells on trials where the reward for that cell group was signaled by the Target image (i.e. “coded trial”), with firing in the same cell groups synchronized to presentation of Target images that signaled the “opposite” type of reward (Figure 9A “opposite trial”). While there were no significant increases in discharge following presentation of the opposite Target image, there was increased firing in the cell groups prior to presentation of Target image that coded the opposite trial type (Figure 9A pre-arrows). Figure 9A (‘Pre’) shows a significant increase in firing for both cell groups in the 1.0 s period immediately prior to presentation of the Target image for the opposite trial (Juice-specific F(8,2038) = 6.30, p<0.001; Cocaine-specific F(8,2038) = 3.40, p<0.01), with significantly higher firing rate for Juice-specific cells (F(1,2038) > 6.52, −1.0-0s, asterisks in Figure 9A, opposite trial). Secondly, there was a significant elevation in firing in Juice-specific cells commencing 1.25 s after Target image presentation on the opposite (cocaine signaled) reward trial (F(4,2038) = 5.96, p<0.001) which corresponded to the time of cocaine delivery via the infusion pump (Figure 3,Figure 4,Figure 6,Figure 7). Finally, the distribution of behavioral “Go” response latencies after Target image presentation for cocaine and juice rewarded trials is shown at the bottom of Figure 9A to indicate the absence of increased firing during behavioral responding by the “inappropriate” cell groups on the opposite trial.
Figure 9. Firing of VStr Target Neurons on the Opposite Type of Rewarded Trial.
A. Top: Mean (± SEM) firing rate histograms of Juice-specific VStr cells (grey bars) and Cocaine-specific cells (black bars) on trials in which the Target image signaled the respective type of reward (Coded Trial, cf. Figure 3B). Middle: Mean (± SEM) firing rate histograms for the same two groups of cells on trials in which the Target image signaled the “other” (i.e. opposite) type of reward (Opposite Trial). Anticipatory firing of Juice-specific and Cocaine-specific cell groups occurred 1.0 sec prior to image presentation on Opposite trials (“pre”), but declined to baseline at the time of presentation of the opposite Target image (0 s vertical dotted line). Note re-escalation of Juice-specific cell firing coincident with time of cocaine delivery (1.5–2.75 s, see also B Top). Asterisks (juice > cocaine): *p <0.01, **p <0.001, F(1,2038) > 6.52. Bottom: Distribution of behavioral responses for juice and cocaine rewarded trials (same data as in Figure 3&Figure 6). B. Top: Depiction of firing of Cocaine-specific (black bars) and Juice-specific (gray bars) cells on cocaine (top) and juice (middle) reward trials. Juice-specific cells did not respond to cocaine trial Target presentations but increased firing when Cocaine-specific cells fired during cocaine delivery (see Figure 9A Middle). Asterisks (juice > cocaine: *p <0.01, **p <0.001, F(1,2038) > 6.52. Plus (cocaine > juice): +p <0.001 ++p< 0.0001, F(1,2038) > 8.73. Middle: A similar anticipatory firing (−0.75-0s) occurred in Cocaine-specific cells during juice rewarded trials (Juice Trial). Pluses (cocaine > juice): +p < 0.01, F(1,2038) > 8.33; Asterisks (juice > cocaine): *p < 0.01, **p < 0.001, F(1,2038) > 8.26. Bottom: Mean (± SEM) firing for VStr Target cells that fired on either juice (gray bars) or cocaine (black bars) trials (Both TT) did not show complimentary anticipatory firing prior to Target presentation on the opposite reward trial. There was also no extended firing in this cell group (Both Trial Types) during cocaine delivery as in Juice-specific cells. However there was a marked difference in firing to the Target image on cocaine vs. juice trials. Asterisks (cocaine > juice): **p<0.001, F(1,3886) = 9.96.
The mean firing rate histograms In upper and middle panels of Figure 9B show the simultaneous discharge patterns of both subpopulations of VStr cells (Juice-specific and Cocaine-specific) on the same trials (upper-cocaine trial; middle-juice trial). In each case firing was increased approximately 1.0 s prior to when the Target image was presented in the subpopulations encoding the opposite trial type. Firing in the inappropriate subpopulation decreased to background level as the appropriate cell group tuned to that Target image increased firing in association with behavioral responding (Figure 9A, bottom). It can also be seen that firing increased again 1.5s after Target image presentation in each ‘specific’ subpopulation, but only on cocaine trials (Figure 9B. top) and not on juice trials (Figure 9B middle). Comparison with VStr cells that responded on both types of rewarded trial (Both TT) showed no differential discharge patterns on the opposite type of trial (Figure 3B), firing only increased after presentation of the Target image in these cells (Figure 9B).
Discussion
VStr and DStr neurons were recorded in behaving NHPs to determine how cell activity represented specific events when cocaine vs. juice was the signaled or obtained reward during performance of a simple Go-Nogo task. The results support other findings in NHPs showing striatal mechanisms of task differentiation and reward selectivity (Apicella et al 1992, Shidara et al. 1998, Tremblay et al 1998, Cromwell and Schultz 2003, Samejima et al. 2005, Lee et al 2006, Lau and Glimcher 2008) as well as differential responsiveness to cocaine vs. natural rewards (Bowman et al. 1996). However new insight into the neural correlates of how cocaine and juice rewards were compatibly encoded within VStr and DStr is provided via determination of a complex hierarchical classification scheme composed of trial-specific neuronal firing patterns for cocaine vs. juice rewards for the same behaviors. Such a scheme is consistent with several new proposals regarding 1) the basis for activation of neuronal firing related to drug seeking in rats, NHPs and humans (Vezina 2004, Rebec and Sun 2005, Risinger et al 2005, Hollander and Carelli 2007, Wakazono and Kiyatkin 2008) and 2) the encoding of reward value by the striatum (Zink et al. 2004, Di Pietro et al. 2006, Ding and Hikosaka 2006, Grace et al. 2007, Lee et al 2006) and other brain regions (Takeda and Funahashi 2006, Simmons and Richmond 2008, Mogami et al. 2007, Belova et al. 2007).
The classification scheme demonstrated here must, however be qualified by several factors inherent in the comparison of neuronal firing on cocaine vs. juice trials, the most important of which are mode of delivery, and potency. As indicated above differences with respect to the extended duration of discharge following cocaine reward delivery (Figure 4) may reflect conditioned or anticipatory firing relative to the timecourse of drug infusion. Secondly, although similar with respect to overall average performance levels (Figure 1), variances in cocaine reward “value” or potency on individual trials could have also contributed to firing differences between cocaine vs. juice rewards (Figure 8). Both of the above factors impose inherent discontinuities with respect to direct comparisons of natural and drug-related reward properties, however, the segregation of cocaine reward effects into the same subcategories as those exhibited for juice rewarded events, cannot be explained by these two factors. Therefore, the demonstration that striatal neurons encode drug and natural rewards via the same classification scheme supports the suggestion that normal ‘unadulterated’ brain-reward processes are activated by abused drugs such as cocaine.
Differences Between VStr and DStr Cell Firing on Cocaine vs. Juice Trials
Figure 8 indicates the differences in firing tendencies between VStr and DStr cells with respect to specific events in the Go-Nogo task. Prior reports have indicated different functional roles for dorsal and ventral striatum in addition to showing differential sensitivity to cocaine exposure (Apicella et al 1992, Shidara et al. 1998, Bradberry et al. 2000, Porrino et al. 2007, Meredith et al. 2007, Takahashi et al. 2007, Bradberry 2008). Functional distinctions in the current study showed DStr cells were biased to fire more to on Nogo trials to Nogotarget images, whereas VStr cells were more prone to respond to on Go trials to Target images and following Reward (i.e. cocaine) delivery. Other distinguishing features between DStr and VStr included differences in baseline firing rates (Figure 3–Figure 7), absence of prolonged discharge during intravenous cocaine delivery in DStr cells (Figure 7, Reward-Both), and reduced sensitivity of VStr Cocaine-specific and Juice-specific cell firing to Nogotarget events (Figure 4&Figure 8). Such differences support prior and current proposals of an underlying scheme in which dorsal and ventral striatum provide differential encoding of reward contingent motor responses (Apicella et al 1991, Rebec 2006, Gdowski et al 2007, Atallah et al. 2007, Takahashi et al. 2007, Seger 2008).
The above distinctions with respect to classifications of task specific firing into sub-categories likely reflect expression of different cortico-striate inputs to medium spiny and possibly other types of striatal neurons (Hemby et al 2005, Haruno and Kawato 2006, Lee et al 2006, Lau and Glimicher 2007). However, categorization of reward specific firing likely involves well characterized changes in dopaminergic inputs to striatal neurons (Vezina 2004, Calabresi et al. 2007, Shultz, 2007) as has been shown in a variety of contexts (Everitt et al. 1999, Tobler et al. 2005, Morris et al. 2006, Grace et al. 2007) including human reward seeking (Risinger et al. 2005, Sinha et al. 2005, Volkow et al. 2006). Recently such dopaminergic influences (Carelli 2004) were demonstrated on a time scale that would account for the trial-to-trial differences in firing rates of the VStr and DStr cells reported here (Roitman et al. 2008).
The operation of the reciprocal striato-nigral circuitry in primates (Haber et al., 2000) is likely involved in food-related rewards and cocaine drug seeking in a manner similar to that demonstrated in rodent studies (Rebec 2006). Belin and Everitt (2008) recently demonstrated a possible prioritization of activation during cocaine-seeking in rodents, dependent on the permissive actions of the ventral striatum for eventual expression of drug-seeking by dopamine regulated dorsal striatal output to motor systems. Within this context, an “Actor/Critic” model (Joel et al 2002) has been suggested by Schoenbaum and associates (Takahashi et al 2007, Takahashi et al 2008) to incorporate predictive encoding within ventral striatal (‘critic’) neurons for dorsal striatal (‘actor’) motor networks, which can account for deficits in cocaine sensitized rats. The current finding in NHPs of a differential distribution of firing in VStr (Target, Reward) vs. DStr (Nogotarget) during cocaine rewarded events (Figure 8) is consistent with the above proposals in rats of differential roles played by each striatal region in maintaining correct behavioral performance.
Segregation of Task Events and Type of Reward in Ventral Striatum
Since the VStr and DStr encoded the three Go-Nogo trial events with nearly the same percentages of cells firing for each event, the major distinction within cell populations recorded in each region was the intensity of cell firing with respect to the type of reward (cocaine vs. juice) that was signaled or delivered (Figure 2–Figure 7). This is consistent with prior studies that determined that striatal neurons encode relevant behavioral events in terms of different types or features of rewards within the task (Apicella et al 1992, Shidara et al. 2002, Samejima et al. 2005, Lau and Glimcher 2007, Williams and Eskandar 2006, Simmons and Richmond 2008). An important report by Bowman et al (1996) showed selective firing of striatal neurons to anticipated cocaine rewarded trials in NHPs performing a delay task, and that the firing of cocaine responsive cells did not predict the activity of cells that fired to natural rewards. Because of these and other prior reports (Carelli et al 2000, Robinson et al. 2001, Crownwell and Schultz 2003, Rebec 2006), the current finding of differentiated firing of striatal neurons to delivery of different types of rewards (i.e. Juice-specific and Cocaine-specific cells) was expected to some extent. However, the fact that cells that fired on either type of rewarded trial (Figure 8, VStr Reward-Both TT cells) also gave more robust discharges on cocaine trials, suggests that reward-related firing may have been independent for some striatal cell types (Bowman et al 1996). Comparisons of Target, Nogotarget and Reward events (Figure 3–Figure 7), showed that cocaine rewarded trials produced significantly higher and in some cases more prolonged, firing rates in VStr and DStr than on juice trials. This finding suggests that all striatal neuronal firing is not strictly segregated into either one reward category or another (Carelli et al 2000), but may have overlapping functional circuits regulated by other contingencies such as “detection” of reward associated events irrespective of type of reward (Schultz 2007). The fact that firing associated with delivery of reward on cocaine trials was significantly prolonged relative to juice trials (Reward Figure 4&Figure 6), most likely reflects a conditioned association to the delay between successful responding (or not responding on Nogo trials) and the delayed arrival of IV cocaine delivered via the infusion pump (Everitt et al. 1999, Dackis and O’Brien 2001, Sun and Rebec 2006, Hollander and Carelli 2007). However, it is interesting that behavioral latencies to respond on Go trials to Target images were not different for cocaine or juice rewarded trials (Figure 9C). Therefore the prolonged or secondary discharge in VStr and DStr Cocaine-specific cell firing (Figure 3&Figure 6) suggests a relationship to “anticipation” (Tremblay et al 1998, Lau and Glimcher 2008) of the subsequent psychostimulant effects of IV delivered cocaine (Bradberry 2008, Negus et al 2007), whose time course of action after systemic delivery has recently been characterized by Wakazono and Kiyatkin (2007).
Striatal Reward Encoding Scheme: SubClassification of Task-Related Events
Additional insight into the encoding scheme related to the segregation of VStr and DStr cell firing with respect to type of reward was provided by data shown in Figure 9 regarding classified cell group firing on the opposite type of rewarded trial. The unexpected increase in firing prior to, but not following, presentation of the “inappropriate” Target image (Figures 9A middle, “Pre”), suggests that cells that encoded the opposite reward for the same event were reciprocally engaged by the Start Ring on trials in which, for these cell groups, the opposite type of reward was signaled. However, it is clear that these same two VStr (Target) cell groups suppressed firing to the Start Ring when it signaled presentation of the Target image for the appropriate reward- to which firing increased (Figure 9B upper & middle). Such temporal reciprocity of firing between the two exclusive cell groups (Juice-specific and Cocaine-specific) would tentatively provide a relative enhancement of the instantaneous neural signal appropriate for a given Target event. A similar scheme of reciprocal categorization of task-relevant information has recently been suggested by Seger (2008) as an interactive feature of different “corticostriatal loops” responsible for performance of well-established (i.e. integrated) motor behaviors, such as employed here. Finally, in contrast, this type of complementary firing was (necessarily) not present in the third subpopulation of VStr cells that fired to Target events on either type of rewarded trial (Figure 9B Both TT).
Summary
There are three main aspects of the above findings that are critical to interpretation of how the rewarding effects of abused drugs such as cocaine could gain control of VStr and DStr neural activity. First, task-dependent stimulus, response and reward events were integrated into a very detailed and specific scheme with the information content for any trial segregated into: a) isolated representation of particular trial event, b) representation of that same event for a specific type of reward condition and c) differential activation at the time of reward delivery. This dichotomy with respect to reward specificity is unique in that it reflects a segregation of signaled trial types by striatal neurons that were: 1) exclusive for a given event for a specific type of reward (Juice or Cocaine-specific cell groups), or 2) inclusive of that event under either reward condition (Both trial type cell groups). Secondly, in agreement with prior reports in NHPs (Bowman et al 1996) and rodents (Carelli 2004, Deadwyler et al. 2004, Hollander and Carelli 2007), firing of striatal neurons when cocaine was the signaled or delivered reward exceeded firing to the same event on natural reward trials (Figure 2–Figure 7). This was demonstrated by the fact that even if cell firing occurred for both types of rewards (Both TT category); higher discharge rates were nearly always associated with cocaine rewarded trials (Figure 3–Figure 5, Figure 7). Third, the distribution of overall cell firing to events exclusive for each type of rewarded trial (20–30% of total cells), or inclusive for the same events on each type of rewarded trial (approximately 50%), was similar in DStr and VStr. While the finding of reward exclusivity in striatal cell firing is similar to prior reports in NHPs (Bowman et al. 1996), the finding of a larger proportion of cells that responded to either type of reward (Both TT) has not been reported previously in primates. However the prior report by Bowman et al. (1996) of a complete lack of predictability in firing between the two groups of reward specific neurons was duplicated here in the Cocaine-specific and Juice-specific cell groups. The lack of significant differences in the percentages of cells responding on cocaine vs. juice rewarded trials suggests that cocaine associated trial events activated the same functional cell types in VStr and DStr but to a higher degree than natural rewards (Takehashi et al 2008, Belin and Everitt 2008). While a test of reward potency of cocaine vs. juice trials is the subject of another report (Opris et al 2009, In preparation) such differences are consistent with other reports of striatal cell discharge in NHPs that showed higher phasic firing rates associated with higher reward value (Tobler et al, 2005, Samejima et al 2005, Simmons and Richmond 2008, Lau and Glimcher, 2008). It is therefore likely that drugs that are abused such as cocaine “tap into” existing brain reward circuitry and utilize encoding and other processing features in the same manner as natural rewards. This implies that the similarities in firing categorization for juice vs. cocaine rewarded events reported here may be one potential basis for cocaine, and possibly other types of drug, addiction in humans (Gottfried et al. 2003, Zink et al, 2004, Volkow et al. 2006, Cooper and Knutson 2008).
Acknowledgment
We thank Charles West, Stephanie Hodge, Ashley Morgan, Santos Ramirez, Josh Long, Christopher K. Craig, Lucy Fasano, Vernell Collins and Michael Todd for their assistance on this project. This work was supported by National Institutes of Health Grants DA06634, DA023573 and DA00119, to S.A.D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apicella P, Scarnati E, Ljungberg T, Shultz W. Neuronal activity in monkey striatum related to expectation of predictable environmental events. J. Neurophysiol. 1992;68:945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Paniagua DL, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat. Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine Seeking Habits Depend upon Dopamine-Dependent Serial Connectivity Linking the Ventral with the Dorsal Striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine. J. Neurophys. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett B, Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J. Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus ‘‘natural’’ (water and food) reward. J. Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47:180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J. Neurophysiol. 2003;89(5):2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J. Subst. Abuse Treatment. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hayashizaki S, Cheer J, Hampson RE. Reward, memory and substance abuse: functional neuronal circuits in the nucleus accumbens. Neurosci. Biobehav. Rev. 2004;27:703–711. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of Reward Modulation in the Frontal Eye Field and Caudate of the Macaque. J. Neurosci. 2006;2126(25):6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Europ J. Neurosci. 2006;24:3285–3297. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. N Y Acad. Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism1 Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdowski MJ, Miller LE, Bastianen CA, Nenonene EK, Houk JC. Signaling patterns of globus pallidus internal segment neurons during forearm rotation Brain Res. 2007;1155:56–69. doi: 10.1016/j.brainres.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Yukiori G, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends. Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: A possible mechanism for encoding information into memory. Proc. Natl. Acad. Sci. USA. 2004;101:3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J. Neurochem. 2005;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli Increase cocaine seeking and activate accumbens core neurons after abstinence. J. Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Kato K, Inoue M, Mikami A. Neurons in the macaque orbitofrontal cortex code relative preference of both rewarding and aversive outcomes. Neurosci. Res. 2007;57:434–445. doi: 10.1016/j.neures.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Joel D, Niva Y, Ruppin E. Actor–critic models of the basal ganglia: new anatomical and computational perspectives Neural Networks. 2002;15:535–547. doi: 10.1016/s0893-6080(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology. 2002;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci. 2007;27(52):14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58(3):451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Seitz AR, Assad JA. Activity of Tonically Active Neurons in the Monkey Putamen During Initiation and Withholding of Movement. J Neurophysiol. 2006;95:2391–2403. doi: 10.1152/jn.01053.2005. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J. Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the striatum and its subdivisions. Brain Struct. Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action Nat. Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Mogami T, Tanaka K. Reward association affects neuronal responses to visual stimuli in macaque TE and perirhinal cortices. J. Neurosci. 2006;26:1057–6770. doi: 10.1523/JNEUROSCI.4924-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, the inferior temporal cortex and the striatum. J. Cog. Neurosci. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "Agonist" Medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. San Diego, CA: Academic Press; 2003. The Rhesus Monkey Brain Stereotaxic Coordinates. [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJR. The Effects of Cocaine: A Shifting Target over the Course of Addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal Substrates of relapse to coaine-seeking behavior: role of prefrontal cortex. J Exp. Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV. Behavioral Electrophysiology of Psychostimulants Neuropsycho-pharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli Nat. Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their role in generalization, response selection, and learning via feedback. Neurosci. Biobehav. Rev. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Aigner TG, Richmond BJ. Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. J. Neurosci. 1998;18:2613–2625. doi: 10.1523/JNEUROSCI.18-07-02613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JM, Richmond BJ. Dynamic changes in representations of preceding and upcoming reward in monkey orbitofrontal cortex. Cereb. Cortex. 2008;18:93–103. doi: 10.1093/cercor/bhm034. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of cynomolgus monkey (Macacca Fascicularis) J. Comp. Neurol. 1984;222:265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J. Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Front. Integr. Neurosci. 2007;1:1–11. doi: 10.3389/neuro.07/011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Schoenbaum G, Niv Y. Silencing the critics: understanding the effects of cocaine sensitization on dorsolateral and ventralstriatum in the context of an Actor/Critic model Front. Integr. Neurosci. 2008;2:86–99. doi: 10.3389/neuro.01.014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda SI, Funahashi S. Reward-period activity in primate dorsolateral prefrontal and orbitofrontal neurons is affected by reward schedules. J. Cog. Neurosci. 2006;18:212–226. doi: 10.1162/089892906775783679. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. J. Neruphysiol. 1998:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–8239. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Telang F, Fowler JS, Logan J, Childress A, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakazono Y, Kiyatkin EA. Electrophysiological evaluation of the time-course of dopamine uptake inhibition induced by intravenous cocaine at a reinforcing dose. Neurosci. 2008;6:824–835. doi: 10.1016/j.neuroscience.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat. Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GC. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]