Abstract
Much has been learned about the pathophysiological state that underlies the development of increased total body volume and edema in left ventricular failure. Very little, however, is known about the mechanism underlying systemic hypervolemia in patients with isolated right ventricular dysfunction. In this manuscript, we describe our randomized clinical trial to assess the relationship between severity of pulmonary arterial hypertension and neurohormonal activation, total plasma volume and renal function. We assess the role of aldosterone and vasopressin in volume retention in patients with pulmonary arterial hypertension with right ventricular failure. As understanding of the pathogenesis of left ventricular failure has been associated with improved therapies, the better understanding of the mechanisms of isolated right ventricular cardiac failure will also lead to improved patient care.
Keywords: Aldosterone, vasopressin, spironolactone, right ventricular failure and pulmonary arterial hypertension
INTRODUCTION
Mechanism of Body Volume Regulation
A paradox exists in understanding cardiac failure. Specifically, patients with heart failure exhibit increased total blood volume and yet their kidneys are retaining sodium and water. The explanation is that the normal kidney does not respond to changes in total blood volume, but rather responds to what has been termed effective arterial blood volume. In recent years this term had been better defined (1–4). In general terms, approximately 85% of circulating blood volume is in the low-pressure venous side of the circulation, whereas only 15% is in the high-pressure arterial circulation. The integrity of the arterial circulation depends on cardiac output and systemic vascular resistance and is modulated by arterial stretch baroreceptors in the carotid sinus, aortic arch, and afferent arteriole of the glomerulus (5). Thus, despite an increase in total blood volume, arterial underfilling can occur secondary to a decrease in cardiac output in low-output heart failure or systemic arterial vasodilation in high-output heart failure. With arterial underfilling secondary to either condition, arterial baroreceptor–mediated activation of the neurohumoral axis occurs. The resultant increase in sympathetic nervous system (SNS) activity and renin-angiotensin-aldosterone system (RAAS) lead to sodium retention, and the increase in the non-osmotic release of arginine vasopressin (AVP) is associated with water retention and hyponatremia in advanced left ventricular failure. Hyponatremia is a known risk factor for increased mortality in both right and left cardiac failure patients (6, 7).
There is also evidence that increased plasma AVP concentration with left ventricular failure stimulates V1 vasopressin receptors on blood vessels, which contributes, along with angiotensin II and the sympathetic nervous system, to increase systemic vascular resistance in low-cardiac output failure (8). This arterial baroreceptor–mediated neurohumoral activation maintains arterial pressure, but at the expense of renal vasoconstriction and sodium and water retention. These arterial baroreceptor pathways override any of the low-pressure reflexes in the atria during left ventricular failure. For example, normally an increase in transmural atrial pressure normally suppresses AVP and stimulates atrial natriuretic peptide (ANP), which lead to increased sodium and water excretion (9). With advanced left ventricular failure, however, left atrial pressure rises, yet sodium and water retention occurs. This indicates that activation of the arterial stretch receptors in cardiac failure predominates over any atrial-renal reflex. There is also evidence that these normal atrial reflexes are blunted in patients with left ventricular failure (10). An increase in the ventricular synthesis of brain natriuretic peptide (BNP) also occurs in left ventricular failure and may attenuate the degree of renal sodium and water retention by both suppressing the RAAS and SNS (11).
Right Ventricular Failure
Less is known about the mechanism underlying systemic hypervolemia in patients with pulmonary arterial hypertension and isolated right ventricular dysfunction, in whom left ventricular function is normal. The neurohumoral axis has been rarely studied in PAH with right ventricular failure in humans. The present clinical study will focus on PAH. Induction of early experimental models of right ventricular failure by graded valvular damage showed a decrease in renal blood flow, preserved glomerular filtration rate (GFR), and intense sodium and water retention (12). The underlying neurohormonal state in this model was not examined. In spite of the presence of pulmonary artery baroreceptors, investigators have concluded that when cardiac output is kept constant, pulmonary arterial distention has no direct effect on renal hemodynamics (13, 14). Thus, the renal hemodynamic changes and the retention of sodium and water observed in patients with PAH may be mediated by systemic, rather than pulmonary, arterial baroreceptors. One single study of patients with PAH showed increases in plasma endothelin, ANP, and norepinephrine concentrations, but not in the plasma renin concentrations. Plasma aldosterone and AVP concentrations were not assessed (15).
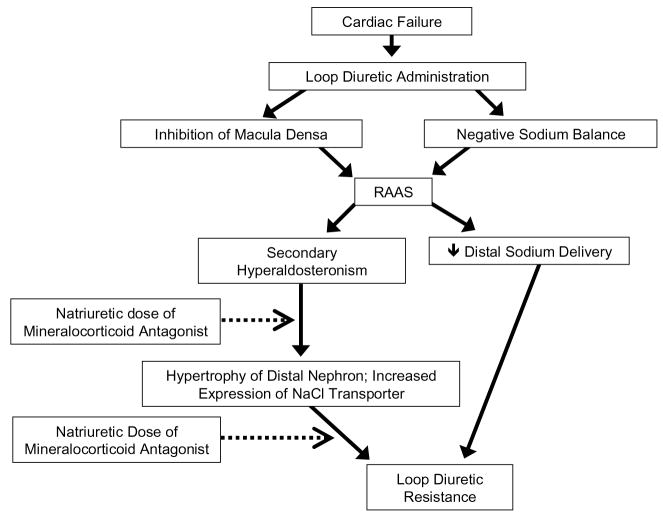
Survival in patients with PAH and right ventricular failure correlates inversely with right ventricular function and right atrial pressure (RAP). These right-sided parameters are influenced by volume overload secondary to increase in sodium and water retention which worsen survival. As in virtually every edematous disorder, secondary hyperaldosteronism may be involved in right ventricular failure associated with PAH. Loop diuretics have been a mainstay for the treatment of volume overload in both left and right-sided cardiac failure for the past four decades. However, their use worsens this secondary hyperaldosteronism and may lead to diuretic resistance (Figure 1)(16–18). Mineralocorticoid antagonists have been shown to afford cardiovascular protection in patients who had left-sided heart failure receiving angiotensin-converting enzyme inhibitors. In the Randomized Aldactone Evaluation Study (RALES), improved survival in patients with left ventricular failure was demonstrated using dosages of spironolactone (25 to 50 mg/d), which did not alter urinary sodium excretion (19). In earlier studies in left ventricular failure, natriuretic doses of mineralocorticoid antagonists have been shown to be effective and reverse the loop diuretic resistance without causing hyperkalemia (20, 21). In cirrhosis with portal hypertension and splanchnic vasodilatation, another arterial underfilling state, mineralocorticoid antagonists are the primary diuretics used to treat the sodium retention and ascites. The effective dosage of spironolactone correlates directly with the plasma aldosterone concentration, thus patients with marked hyperaldosteronism may require a daily dose up to 400 mg (22, 23). Whether the natriuretic doses of mineralocorticoid antagonists are beneficial for PAH patients with intact left ventricular function but with right ventricular failure is not known.
Figure 1.
Mechanisms of diuretic resistance with potential benefit of mineralocorticoid antagonism. Dotted line indicates inhibition.
The analysis of systemic and renal hemodynamics and activation of the neurohormonal axis (SNS, RAAS, AVP) in patients with PAH with right ventricular failure has not heretofore been undertaken. Moreover, the response to mineralocorticoid and vasopressin receptor antagonists has not been investigated in PAH patients with sodium and water retention. On this background we intend to study patients with PAH with right ventricular failure, who do not have left ventricular dysfunction, to understand the pathophysiology of renal sodium and water retention. This study will be undertaken in three parts with specific objective: for the first part (cross-sectional) is to examine the association between severity of pulmonary arterial hypertension and degree of neurohormonal activation; second part (acute) is to evaluate the effect of spironolactone and conivaptan on the rate and amount of sodium and water excretion respectively; and third part (cohort) is to evaluate the effect of chronic administration of spironolactone on functional and hemodynamic parameters of pulmonary arterial hypertension.
HYPOTHESIS
Secondary hyperaldosteronism and the non-osmotic release of AVP are the major factors in sodium and water retention in PAH with right ventricular failure. Natriuretic doses of mineralocorticoid antagonist and aquaretic doses of V2 receptor antagonist will attenuate the sodium and water retention, respectively, and be associated with clinical improvement. The specific outcomes for each part are as follows:
1. Cross-sectional Study
• Primary
Estimate the correlation between severity of pulmonary hypertension and neurohumoral activation, renal blood flow and total plasma volume.
Severity of pulmonary hypertension is assessed functionally by cardiac index (CI), RAP, plasma BNP, 6 minute walk test, and WHO functional classification.
Neurohormonal activation is defined as (a) SNS: serum levels of epinephrine and norepinephrine, (b) RAAS: plasma renin activity, angiotensin II, aldosterone, (c) Plasma AVP, (d) Plasma BNP.
• Secondary
Examine the correlations between mean pulmonary artery pressure (mPAP), pulmonary vascular resistance; and neurohumoral activation, renal function and total plasma volume.
2. Acute Study
• Primary
Effect of conivaptan on electrolyte-free water excretion
Effect of spironolactone on sodium excretion
• Secondary
Estimate the correlation between responses to these drugs and severity of PAH.
3. Cohort Study
• Primary
Effect of 6 months administration of spironolactone on composite outcome of CI, BNP and RAP.
• Secondary
Descriptive assessment of the effect of 6 months of spironolactone compared to usual care on:
Occurrence of an adverse event defined as occurrence of PAH-related death, hospitalization or early study discontinuation due to worsening PAH, need for initiation of new PAH-specific therapy, lung transplantation, or atrial septostomy.
Change in 6 minute walk distance, mean pulmonary artery pressure, and pulmonary vascular resistance.
Sodium and water balance: measured as change in body weight and sodium excretion.
Need for increased dose of loop diuretics or addition of another class of diuretics.
RESEARCH DESIGN AND METHODS
1. Organization of the Study
This study is an investigator initiated project and is conducted at the Clinical Translational Research Center (CTRC) at University Hospital, Aurora, Colorado. The initial study protocol was developed in 2007, and received approval by the Colorado Multiple Institutional Review Board (COMIRB) in November, 2008 after revisions. A data safety monitoring board has been established to review the progress of participants and any adverse events on quarterly basis. The eligible patients are identified from Pulmonary Hypertension Clinic at University Hospital, Aurora, Colorado. Once patients meet the inclusion and exclusion criteria, they are informed of the study, and offered the opportunity to participate.
2. Inclusion and Exclusion Criteria
Inclusion and Exclusion Criteria are shown in Table 1. Eligible subjects of all ethnic groups, of both sexes, range in age from 18 to 75 years, and have a documented history of WHO Group 1 advanced PAH, excluding patients with portal hypertension. Patients were considered to have Group 1 advanced PAH when they have mPAP >35 mmHg at rest, pulmonary capillary wedge pressure (PCWP) <15 mmHg, and pulmonary vascular resistance (PVR) >5 wood units. They also need to have right ventricular failure defined by RAP >7 mmHg along with either dilated right ventricle, or absence of inferior vena cava collapse or plasma BNP >100 pg/ml. Normal left ventricular function needs to be present as assessed by echocardiogram, multiple gated acquisition (MUGA) cardiac scan, or invasive left ventriculography. Female patients of childbearing age must be practicing effective birth control.
Table 1.
Inclusion/Exclusion Criteria
| Inclusion Criteria |
|
| Exclusion criteria |
|
3. Overview
The proposed research includes baseline assessment of PAH patients’ neurohumoral axis, systemic and renal hemodynamics. The second part consists of evaluating their response to acute treatment with conivaptan and spironolactone. The third part of the research is an assessment of the response to chronic (6 months) administration of spironolactone. A total of 26 PAH patients will be enrolled from the outpatient Pulmonary Hypertension Clinic, with the expectation that at least 20 will provide complete data at the end of the 6-month intervention.
For the acute and cohort studies, patients will be randomly assigned to group 1 (intervention) and group 2 (usual care) within each stratum of disease etiology (idiopathic PAH/non-idiopathic PAH) and background prostacycline therapy (yes/no) in permuted blocks as they are consented. This strategy reduces the confounding due to heterogeneity of the population.
1. Cross-sectional Study
All 26 patients have right heart catheterization for the baseline pulmonary hemodynamics. The effective renal blood flow (RBF) and the total plasma volume are measured by 99mTc-mercaptoacetyltriglycine (MAG3) renography and 51chromium labeled autologous red blood cells respectively. A 6-minute walk test is done in all the patients to assess the functional capacity. Blood is drawn after patients seated comfortably at least for 15 minutes for basic metabolic panel, complete blood count, serum osmolarity, plasma epinephrine, norepinephrine (NE), renin activity (PRA), aldosterone (PA), angiotensin (Ang II), AVP, and BNP. The above tests and procedures are done to evaluate the association between PAH severity and the degree of neurohormonal activation.
2. Acute Studies
Ten of 26 PAH patients, are admitted to the inpatient clinical translational research center (CTRC). The patients are selected randomly in pairs from the same stratum initial randomization list, so that in each pair, one will be group 1 and one group 2, and as similar as possible. Patients are instructed to receive a diet containing 87 mEq (2.0 gm) of sodium and 50 meq of potassium per day at least 2–3 days prior to admission. They continue to take all their outpatient medications including diuretics and oxygen supplement. There should not have been any changes in the dose of any medication over prior 2 weeks. Two studies will evaluate the effect of conivaptan on water excretion (study 2a) followed by the acute effect of spironolactone on sodium excretion (study 2b). Patients receive a standard diet consisting of 2.0 gm of sodium and 2.0 liters of fluids per 24 hours during the CTRC admission.
On day 1 of admission, the tests and procedures mentioned in the cross-sectional study are done in the inpatient setting in these 10 patients. Over next 2 days of admission, a cross-over study of the effect of a single dose of conivaptan on electrolyte-free water excretion is conducted. Treatment order will be randomly assigned. On study day 2, after the breakfast, five patients receive conivaptan 20 mg intravenously over 30 minutes in addition to usual care, and the other 5 receive usual care. All 10 patients receive a water load of 15 ml/kg orally over 30 minutes. Urine volume is collected over next 3 hours to calculate the percent of the water load excreted over this period of time. Other than brief standing to void, subjects remain sitting throughout the 3 hour period. Blood sample is withdrawn for serum sodium, AVP and osmolarity before the conivaptan administration and again at 60 minutes after water load. Urine osmolality, sodium and potassium concentrations are measured before conivaptan administration and again at 1 and 2 hours after water load. The study is repeated on the following day (study day 3), but patients cross over to receive the intervention (conivaptan or usual care) that they did not receive on the previous day. The difference in urine volume and electrolyte-free water excretion in the conivaptan versus usual care conditions will be the outcome measure.
Following completion of the water loading study (end of study day 3) the same 10 PAH subjects receive spironolactone 200 mg orally plus usual care or usual care only (5 per group), based on the randomization scheme described above. The following day (study day 4), these patients are given a saline excretion test. Two hours after breakfast, with the subjects seated comfortably, blood sample is drawn for basic metabolic panel, osmolarity, PRA, PA, Ang II, AVP and NE along with urine sodium, potassium and osmolarity in all the 10 patients. Then, an intravenous infusion of 3% NaCl at rate of 0.1ml/kg/minutes is infused for 30 minutes. Urine is collected over next 4 hours to calculate the percent of sodium excretion. Blood sample is withdrawn again at 4 hour.
The results of this test will be compared across the groups (spironolactone vs. usual care). The sodium loading experiment is not done as a cross-over to avoid loading each patient twice. Patients are observed for 7–8 hours after saline load for any adverse events and then are discharged to be followed in the outpatient clinic in 1 week. Just before discharge, those 5 patients, who had received spironolactone a day prior, are started on 50 mg of spironolactone orally daily (group 1) and the other 5 patients are on usual care (group 2).
3. Cohort Study
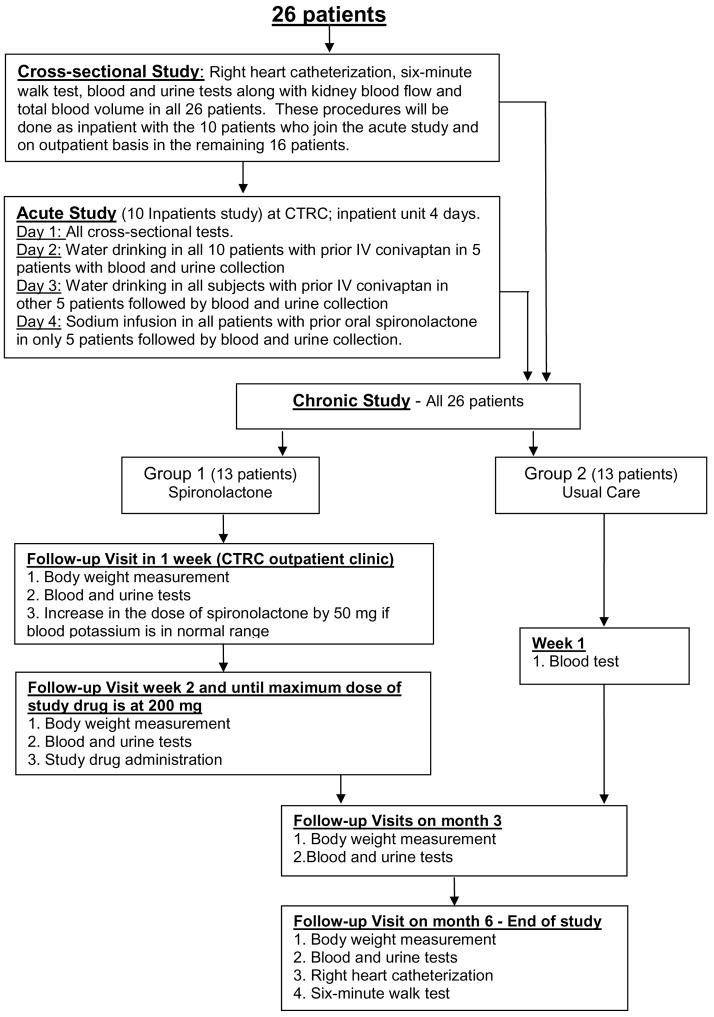
The 10 patients from the acute study continue with or without the spironolactone treatment as assigned and become part of the cohort study. The remaining 16 patients are seen in CTRC outpatient clinic. The participants flow from one study to another has been demonstrated in Figure 2. They get all the tests and procedures mentioned in cross-sectional study as outpatient. Then they begin treatment with spironolactone 50 mg orally (group 1, n=8) or usual care (group 2, n=8). All group 1 patients and the participants of the acute study are seen at CTRC clinic within 1 week. They have history and physical examination done. Basic metabolic panel is checked and if serum potassium and renal function remains stable, the dose of spironolactone is increased to 100 mg po daily in group 1 patients. Group 1 patients return in 1 week for blood work to have basic metabolic panel, body weight, and urine Na/K ratio to document renal affect of spironolactone. Dose of spironolactone is up titrated to maximum of 200 mg/day in increments of 50 mg/week with a weekly check on serum potassium and renal function. Group 2 patients do not have any further visit till month 3. Afterwards all patients will be seen in clinic at month 3 and 6.
Figure 2.
Participants flow from one study to another.
4. Stopping Rules
These rules are based on the occurrence of adverse events which are defined as below:
Reportable Adverse Events
Cross sectional study: adverse events from right heart catheterization
Bleeding
Infection
Blood clot
Ventricular arrhythmias
Acute Study: adverse events from saline and water loading, conivaptan and spironolactone
Weight change
Worsening of dyspnea
Increase in peripheral edema by 1 point on exam.
Increase in jugular venous pressure by 2 cm.
Decrease in renal function defined as serum creatinine increase of 1.5 times baseline or 25% eGFR decrease from baseline.
Prolonged hospitalization
Chronic Study:
Hyperkalemia: Persistent serum potassium concentration of >5.5mEq/l for more than 2 weeks in spite of dietary K restriction, short term use of kayexalate, decreased dose of spironolactone, increased furosemide dose and failure of 2 attempts to resume spironolactone at any dose if it was discontinued.
Renal dysfunction related to spironolactone: Serum creatinine increase of 1.5 times baseline or 25% eGFR decrease from baseline. Dr Bansal and Dr Schrier will be responsible to decide if renal dysfunction is related to spironolactone.
Serious Adverse Events
Cross-sectional Study involving right heart catheterization
Stroke
Pulmonary Emboli
Life threatening ventricular arrhythmias
Acute Study
Weight change of more than 5 kg
Chronic Study
Life threatening hyperkalemia: Serum K >6.5 meq/L with EKG changes of hyperkalemia.
Renal dysfunction requiring dialysis
Individual Stopping Criteria
Occurrence of any serious adverse event during cross sectional study will exclude patient from undergoing the second right heart catheterization at the end of the study.
Weight gain of more than 5 kg at any point of time during acute study will exclude the patient from the acute study.
Occurrence of any serious adverse event during chronic study will discontinue the patient from the chronic study.
Study Stopping Criteria
Occurrence of any serious adverse event during cross sectional study in ≥ 3 patients will lead to discontinuation of the right heart catheterization procedure.
Occurrence of any serious adverse event during acute study in ≥ 3 patient will lead to discontinuation of acute part of the study.
Occurrence of any serious adverse event during chronic study in ≥ 3 patients will cause the discontinuation of the chronic study.
As the adverse events in the three parts of the studies are entirely unrelated to each other, only that part of the study will be stopped if SAE occurs in 3 or more patients, not the entire study.
The DSMB panel will review the results of the baseline studies after 3 patients have completed the CTRC inpatient stay, and again after all 7 and 10 patients. Three month results (serum potassium, renal function, change in body weight and sodium excretion, BNP, WHO functional class etc) will be summarized after 10 and after 20 complete the outpatient visits.
5. Risk Management
Saline and Water Load
These loadings can cause worsening of peripheral edema, and rarely shortness of breath, ascites, pleural or pericardial effusion. This may occur in less than 1 in 100. Saline and water loading have been done previously in patients with right and left side cardiac failure without any consequences, when they were already edematous and also off diuretics (24–27). In our study, patients continue to take diuretics. We administer half of the dose of saline compared to the dose given previously in patients with pulmonary hypertension with right ventricular failure. These procedures are performed under investigator’s close supervision. One faculty member from the Pulmonary Hypertension Group is also be available on pager 24 hours a day and 7 days a week for the coverage of these patients.
In case of volume overload patients will be given intravenous furosemide. Patients requiring intravenous furosemide will be excluded from the acute study.
Spironolactone
The most common adverse effect of spironolactone is hyperkalemia. With the high dose it can occur more frequently. To avoid this problem, spironolactone is increased by 50 mg/week from 50 to 200 mg/d. The plasma potassium concentration is carefully monitored at least weekly till the stable dose is achieved. Dietary potassium restriction is standard. The patients continue their loop diuretics and may have their dose increased so that their kaliuretic effect will balance the potential increase in potassium caused by spironolactone. We also do not enroll patients receiving ACE inhibitor or ARBs as they can contribute to hyperkalemia.
The plan for management of hyperkalemia is as follows:
-Serum potassium 5.5–6.0 meq/l: Kayexalate will be started, and spironolactone dose will either be decreased or discontinued depending on their dose at that time.
-Serum potassium more than 6.0 meq/l: An on site EKG will be done to determine cardiac rhythm. In case of any arrhythmias, patient will be hospitalized and managed by standard treatment. In absence of arrhythmias, kayexalate will be started and spironolactone dose will either be decreased or discontinued depending on their dose at that time.
In either scenario, patient will come back to clinic for follow up and repeat blood draw in 3 days and again in a week and based on serum potassium at day 7, spironolactone in same or reduced dose will be reinstituted. Patients will again be followed in 1 week. Patients will also be reinstructed for low potassium diet.
Renal dysfunction is the other side effect of spironolactone which occurs because of diuresis and weight loss. Patients are monitored for their renal function along with serum potassium concentration at least on a weekly basis. In case of renal dysfunction, a proper approach to acute kidney injury will be applied with assessment of volume status, concurrent medications and urine analysis. If spironolactone associated diuresis is thought to be the causative factor for renal dysfunction, its dose will be reduced or discontinued. With any sign of hypovolemia, a mild saline challenge may be offered. Spironolactone can cause gynecomastia in few patients. Patients suffering from intolerable gynecomastia will be switched to mineralocorticoid receptor blocker, eplerenone.
Patients with spironolactone related hyperkalemia and renal dysfunction will remain initially in the study. However, if patients fail two attempts to resume the spironolactone in any dose, they will be terminated from getting the intervention (spironolactone) but will continue to be followed in the study, and will be included in intention to treat analysis at the end of the study.
6. Statistics
Sample Size Calculation and Data Analysis
Cross-sectional Study
Data for 26 subjects at baseline affords a reasonable level of power to identify clinical parameters that correlate with disease severity. A sample of 26 gives 80% power to detect univariate correlations of at least 0.52 in absolute value and 50% power for correlations of at least 0.39; that is, a correlation of 0.39 will be significant at the 0.05 level.
Acute Studies
The subsets of 10 patients selected for the acute studies provide > 80% power to detect differences of 1.2 SD in the spironolactone study (2-sample t test) and as small as 1 SD in the conivaptan study. These effects are smaller than those observed in the left heart failure population. Hensen (20) administered 97 mmol/day to CHF patients; prior to spironolactone (200 mg p.o. bid) administration, urinary Na+ excretion was 76±19.6 (mean ± SD) mmol/day and on the second day of spironolactone administration Na+ excretion was 131± 32 meql/day, an effect size of 1.77SD. Udelson et al (28) administered 20 mg IV conivaptan or placebo to CHF patients; during the 12-hour study period, change in urine output and urine osmolality were −11.3±100 mL/hr and −2.2± 165 mOsmol/kg (mean ± SD) in the control group and 152.2±100 mL/hr and −249.8± 160 mOsmol/kg in the conivaptan group, effect sizes of 1.35 SD and 1.7 SD and respectively. These tests have not been reported in the PAH population. The data from the crossover study will be analyzed using a mixed effect model with subjects within sequence as a random effect, and treatment as fixed effects for the continuous endpoints.
Expected Improvement Rate in Cohort Study
Our primary goal is to demonstrate that intervention with spironolactone is beneficial to PAH patients with right heart failure. If all 3 of the primary measures, CI, BNP and RAP, improve more in the spironolactone group than the placebo group we can reject the null hypothesis of no difference between groups at the .125 significance level, based on the exact binomial probability for 3 successes in 3 trials when the true probability for success is .5. Another way to state this is that we will reject the null hypothesis at the p=0.125 level.
7. Significance
Right ventricular failure patients are dying of volume overload. Yet, the mechanisms causing increased sodium and water retention in these patients are not clear. A better understanding of the mechanisms and role of mineralocorticoid antagonist and vasopressin antagonist in patients with right ventricular failure will positively impact their care in future.
Acknowledgments
This research is supported by National Institute of Health grant to Colorado Clinical Translational Science Institute at University of Colorado, Denver and research grant by Astellas Pharma US.
Grant Support: 1. Astellas Pharma US VAPR-8D02
2. NIH grant to Colorado Clinical Translational Science Institute at University of Colorado Denver
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shweta Bansal, Instructor of Medicine, Division of Renal Disease and Hypertension, University of Colorado Denver, Aurora, Colorado, USA.
David Badesch, Professor, Division of Pulmonary Sciences/Critical Care Med, University of Colorado Denver, Aurora, Colorado, USA.
Todd Bull, Associate Professor, Division of Pulmonary Sciences/Critical Care Med, University of Colorado Denver, Aurora, Colorado, USA.
Robert W. Schrier, Professor, Division of Renal Disease and Hypertension, 12700 E. 19th Avenue, Box C281, P.O. Box 6511, Aurora, CO 80045, Phone 303-724-4837, Fax 303-724-4868, e-mail: Robert.schrier@ucdenver.edu.
References
- 1.Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol. 2007;18:2028–31. doi: 10.1681/ASN.2006111302. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119:S47–53. doi: 10.1016/j.amjmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–85. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (2) N Engl J Med. 1988;319:1127–34. doi: 10.1056/NEJM198810273191705. [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW. Body fluid volume regulation in health and disease: a unifying hypothesis. Ann Intern Med. 1990;113:155–9. doi: 10.7326/0003-4819-113-2-155. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O’Connor CM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–8. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 7.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–9. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liard JF, Spadone JC. Hemodynamic effects of antagonists of the vasoconstrictor action of vasopressin in conscious dogs. J Cardiovasc Pharmacol. 1984;6:713–9. doi: 10.1097/00005344-198407000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47:1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 10.Koepke JP, DiBona GF. Blunted natriuresis to atrial natriuretic peptide in chronic odium-retaining disorders. Am J Physiol. 1987;252:F865–71. doi: 10.1152/ajprenal.1987.252.5.F865. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Lowes BD, Ferguson DA, Odom J, Kim JK, Robertson AD, et al. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail. 1998;4:37–44. doi: 10.1016/s1071-9164(98)90506-1. [DOI] [PubMed] [Google Scholar]
- 12.Barger AC, Yates FE, Rudolph AM. Renal hemodynamics and sodium excretion in dogs with graded valvular damage, and in congestive failure. Am J Physiol. 1961;200:601–8. doi: 10.1152/ajplegacy.1961.200.3.601. [DOI] [PubMed] [Google Scholar]
- 13.Ledsome JR, Kan WO. Reflex changes in hindlimb and renal vascular resistance in response to distention of the isolated pulmonary arteries of the dog. Circ Res. 1977;40:64–72. doi: 10.1161/01.res.40.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Coleridge JC, Kidd C. Reflex effects of stimulating baroreceptors in the pulmonary artery. J Physiol. 1963;166:197–210. doi: 10.1113/jphysiol.1963.sp007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–5. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 16.He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension. 1995;26:137–42. doi: 10.1161/01.hyp.26.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(−)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol. 2001;12:1335–41. doi: 10.1681/ASN.V1271335. [DOI] [PubMed] [Google Scholar]
- 18.Ellison DH, Velazquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest. 1989;83:113–26. doi: 10.1172/JCI113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 20.Hensen J, Abraham WT, Durr JA, Schrier RW. Aldosterone in congestive heart failure: analysis of determinants and role in sodium retention. Am J Nephrol. 1991;11:441–6. doi: 10.1159/000168356. [DOI] [PubMed] [Google Scholar]
- 21.van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993;71:21A–28A. doi: 10.1016/0002-9149(93)90241-4. [DOI] [PubMed] [Google Scholar]
- 22.Gatta A, Angeli P, Caregaro L, Menon F, Sacerdoti D, Merkel C. A pathophysiological interpretation of unresponsiveness to spironolactone in a stepped-care approach to the diuretic treatment of ascites in nonazotemic cirrhotic patients. Hepatology. 1991;14:231–6. [PubMed] [Google Scholar]
- 23.Perez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84:961–8. [PubMed] [Google Scholar]
- 24.Stewart AG, Waterhouse JC, Billings CG, Baylis PH, Howard P. Hormonal, renal, and autonomic nerve factors involved in the excretion of sodium and water during dynamic salt and water loading in hypoxaemic chronic obstructive pulmonary disease. Thorax. 1995;50(8):838–45. doi: 10.1136/thx.50.8.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charloux A, Chaouat A, Piquard F, Brandenberger G, Weitzenblum E, Geny B. Renal hyporesponsiveness to brain natriuretic peptide: both generation and renal activity of cGMP are decreased in patients with pulmonary hypertension. Peptides. 2006;27:2993–9. doi: 10.1016/j.peptides.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Stewart AG, Waterhouse JC, Billings CG, Baylis P, Howard P. Effects of angiotensin converting enzyme inhibition on sodium excretion in patients with hypoxaemic chronic obstructive pulmonary disease. Thorax. 1994;49:995–8. doi: 10.1136/thx.49.10.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldsmith SR, Francis GS, Cowley AW., Jr Arginine vasopressin and the renal response to water loading in congestive heart failure. Am J Cardiol. 1986;58:295–9. doi: 10.1016/0002-9149(86)90065-2. [DOI] [PubMed] [Google Scholar]
- 28.Udelson JE, Smith WB, Hendrix GH, Painchaud CA, Ghazzi M, Thomas I, et al. Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104:2417–23. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- 29.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]