Abstract
Background
Directly observed therapy (DOT) programs for HIV treatment have demonstrated feasibility, acceptability, and improved viral suppression, but few have been rigorously tested. We describe a randomized controlled trial testing the efficacy of an antiretroviral DOT program in methadone maintenance clinics. Our objective was to determine if DOT is more efficacious than self-administered antiretroviral therapy for reducing HIV viral load, improving adherence, and reducing drug resistance among opioid dependent drug users receiving methadone treatment.
Methods
Participants were randomized to treatment as usual (TAU) or antiretroviral DOT for the 24-week intervention. TAU participants received standard adherence counseling, and DOT participants received standard adherence counseling plus directly observed antiretroviral therapy, which was delivered at the same time as they received daily methadone. Assessments occurred at baseline, weekly for 8 weeks, and then monthly for 4 months. Our primary outcomes were between group changes from baseline to the end of the intervention in: HIV viral load, antiretroviral adherence, and number of viral mutations.
Results
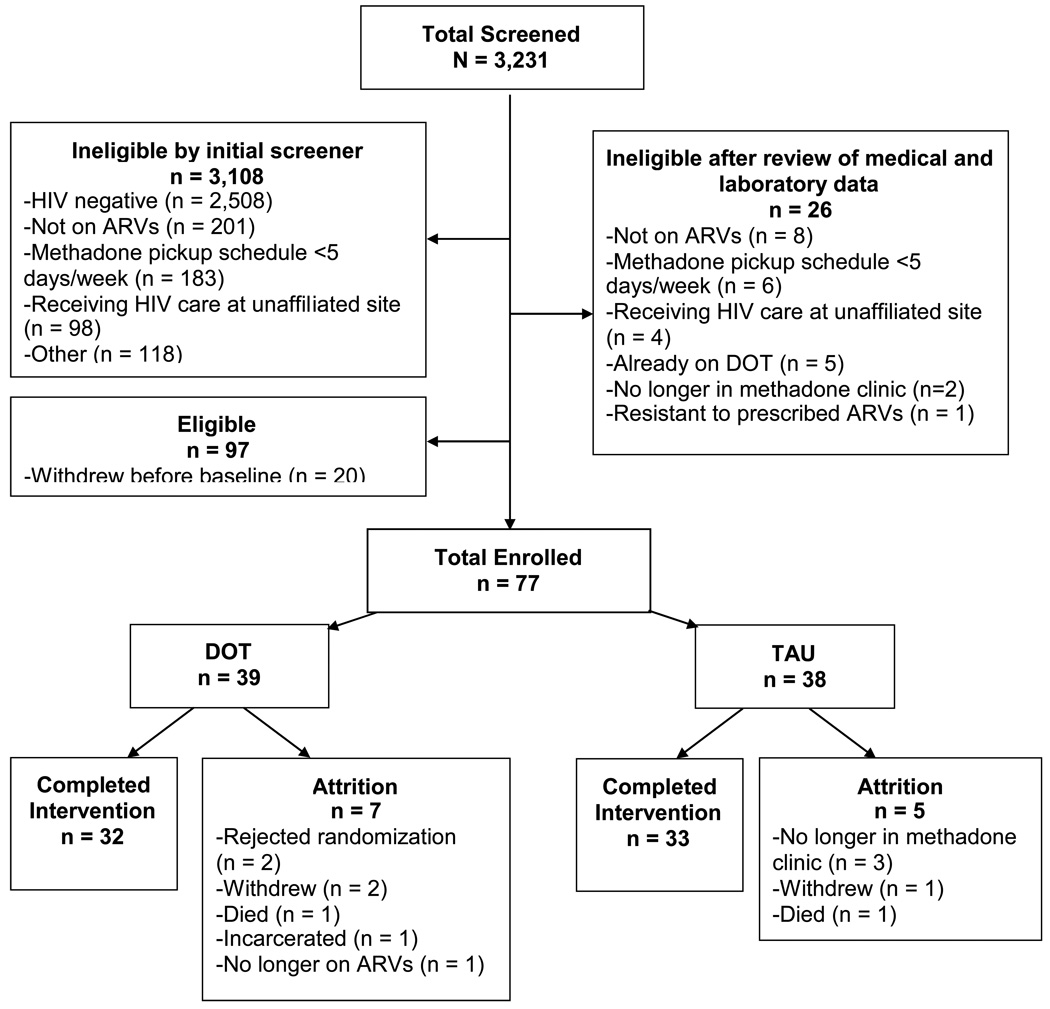
Between June 2004 and August 2007, we screened 3,231 methadone maintained patients and enrolled 77; 39 participants were randomized to DOT and 38 to TAU. 65 completed the 24-week intervention.
Conclusions
Our trial will allow rigorous evaluation of the efficacy of directly observed antiretroviral therapy delivered in methadone clinics for improving adherence and clinical outcomes. This detailed description of trial methodology can serve as a template for the development of future DOT programs and can guide protocols for studies among HIV-infected drug users receiving methadone for opioid dependence.
Keywords: Directly observed therapy, HIV, medication adherence, methadone, randomized trial, substance abuse treatment, antiretroviral therapy
INTRODUCTION
Optimal antiretroviral adherence is necessary to minimize replication of drug resistant virus and achieve the clinical benefits of HIV treatment. Current and former drug users have disproportionately worse HIV treatment outcomes compared to non-drug users, in part because of poor medication adherence (1–3). Though numerous adherence-improving interventions have been evaluated in randomized trials, very few have addressed HIV-infected drug users (4).
Programs providing directly observed therapy (DOT) for tuberculosis have been shown to improve medication adherence and clinical response, and to reduce the incidence of drug resistant infection (5;6). Since these same goals apply to HIV treatment, extending the tuberculosis DOT model to antiretroviral therapy for HIV is reasonable. However, treatment for tuberculosis is time limited and dosing can be two or three times weekly for much of the treatment duration, while antiretroviral therapy for HIV is long-term and requires treatment with once or twice daily dosing. Because of the requirement for chronic daily treatment, antiretroviral DOT programs have been implemented in community settings using outreach workers (7;8), or in settings with infrastructures allowing frequent contact, such as prisons (9) or methadone maintenance clinics (10;11).
Methadone maintenance clinics are a particularly promising setting for DOT programs because federal regulations mandate patients to receive their daily methadone dose at the clinic, and the majority of doses are directly observed by nurses. Prior observational studies of methadone-clinic-based antiretroviral DOT programs suggest that they are feasible, acceptable, and improve rates of viral suppression (10;12). However we know of no randomized clinical trials evaluating antiretroviral DOT administered on-site in a methadone clinic.
We developed the Support for Treatment Adherence Research through Directly Observed Therapy (STAR*DOT) trial, a randomized controlled trial, to test the efficacy of directly observed antiretroviral therapy provided on-site at a methadone clinic during a 24-week study period. Our objective was to determine if DOT is more efficacious than self-administered antiretroviral therapy for reducing HIV viral load, improving antiretroviral adherence, and reducing HIV drug resistance, among antiretroviral naïve or experienced opioid dependent methadone-maintained drug users. In this paper, we provide a detailed methodological description of the STAR*DOT trial, which may serve as a template for investigators designing DOT trials. In addition, we discuss pertinent safety and confidentiality issues that can guide protocols for studies among HIV-infected drug users receiving methadone for opioid dependence. We also report baseline characteristics of the study population.
METHODS
Study design
The STAR*DOT trial was a randomized controlled trial in which methadone-maintained participants were randomly assigned to one of two groups: treatment as usual (TAU) or directly observed therapy (DOT) for treatment of HIV. Participants in the TAU (control) group received standard adherence support (described below), including HIV adherence counselor, and self-administered their antiretroviral medications. Participants in the DOT (intervention) group received standard adherence support plus directly observed antiretroviral therapy, which was delivered at the methadone window five or six days per week depending on the participants’ methadone pick-up schedule. The study design was flexible to accommodate changes in methadone and antiretroviral regimens and other causes of unusual dosing regimens (e.g., thrice weekly dialysis dosing). The primary outcomes were changes from baseline to the end of the intervention in HIV viral load, adherence rate, and number of drug resistant mutations.
Study setting
The STAR*DOT trial was conducted in the Division of Substance Abuse (DoSA), a network of twelve methadone maintenance clinics administered by the Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. Together, these affiliated clinics provide care for approximately 3,500 opioid dependent patients, of whom 10–15% is HIV-infected. These clinics were the sites for study recruitment, delivery of the intervention, and all research visits.
Substance abuse treatment at each DoSA clinic is delivered by a multidisciplinary staff comprised of nurses, substance abuse counselors, a social worker, a part-time psychiatrist, and a medical team consisting of a physician and at least one physician assistant or nurse practitioner. The medical team provides comprehensive medical care including HIV care. Patients are free to choose whether to receive HIV and other medical care at the methadone clinic or elsewhere.
Study inclusion and exclusion criteria
Potential STAR*DOT trial participants were eligible for inclusion if they were HIV-infected; were prescribed antiretroviral therapy; received HIV medical care at their methadone clinic or a site that was closely affiliated with the DoSA network; attended the methadone clinic either five or six days per week to receive methadone (hereafter, five or six day pick-up schedule); were on a stable dose of methadone for two weeks prior to the baseline visit; and were genotypically sensitive to their prescribed antiretroviral regimen. Participants were excluded if they were unable or unwilling to provide informed consent, were already receiving antiretroviral DOT, or if their primary HIV care provider did not agree to their participation in the study. Participants who discontinued methadone therapy during the study period were no longer eligible.
Protection of human subjects
Approvals, confidentiaility, and data safety and monitoring
The trial was approved by the Committee on Clinical Investigations of the Albert Einstein College of Medicine and the Institutional Review Board of Montefiore Medical Center. All participants gave written informed consent. In addition, we asked participants to sign a New York State release of confidential HIV information, which allowed the trial’s Medical Director (KMB) to communicate directly with participants’ HIV providers. To further protect trial participants, we obtained a certificate of confidentiality from the National Institutes of Health, which protects participants from being identified in any civil, social, criminal, or legislative dealings at the Federal, State or local level.
The STAR*DOT trial was a low-risk behavioral intervention that was highly integrated with usual clinical care; for this reason we did not create an independent data safety and monitoring board. Instead we established a data safety and monitoring plan, which required interim analyses every 12 months to determine whether sufficient risks or benefits had developed that would warrant cessation of the trial.
Specific safety protocols
We developed three safety protocols related to antiretroviral therapy, and one protocol to detect participants reporting high levels of psychological distress. Documentation of each safety-related encounter was stored in participants’ permanent files.
The first protocol was designed to prevent unanticipated lapses in antiretroviral therapy for participants in the DOT arm. Because the process of preparing DOT pilltrays was complex (described in detail below), we maintained a stockpile in our DoSA central pharmacy that contained a one-month supply of all approved antiretroviral medications. Stockpile medications were used if pilltray delivery to individual methadone clinics was delayed for any reason.
Second, because antiretroviral medications can increase metabolism of methadone (13;14), we regularly assessed opioid withdrawal. At every research visit we administered the subjective opioid withdrawal scale (SOWS) as part of a larger computerized interview. At the end of the interview, the computer program displayed an alert if the participant reported clinically significant opioid withdrawal symptoms. We also used the objective opioid withdrawal scale (OOWS) at every visit, which is a 13-item interviewer-administered scale that assesses withdrawal signs (e.g., yawning or rhinorrhea). If the interviewer noted more than two signs of opioid withdrawal they advised the participant to speak with their medical provider.
The third antiretroviral related protocol was designed to minimize exposure to non-prescribed antiretroviral medications. Though allergic reactions to antiretroviral medications are rare, we used unique pill bottle monitoring caps and pillboxes for each participant and cleaned pill counting trays and other supplies with alcohol prior to each use.
Finally, we made an a priori ethical decision to consistently review the responses to the Brief Symptom Inventory (BSI), used to measure psychological distress. To achieve this, we programmed the computerized survey to display an alert if a participant endorsed being distressed by “thoughts of ending your life”, “thoughts of death or dying”, or “having urges to beat, injure or harm someone”. If alerted, interviewers followed a detailed protocol that involved a brief assessment, and, if necessary, immediate referral to the clinic medical provider.
Study implementation
Prior to implementation, the Principal Investigator (JHA) and Medical Director (KMB) explained the STAR*DOT trial at regularly scheduled meetings for all DoSA network physicians. In addition, prior to initiating recruitment at each DoSA clinic, the Medical Director, Project Director, and interviewers attended a clinic staff meeting to explain trial procedures and discuss their potential impact on clinic flow and nursing responsibilities. The Medical Director also provided a 30-minute DOT training for the nursing staff at each clinic.
Patient recruitment, enrollment, and reimbursement
The initial steps in participant recruitment included a brief screening survey, informed consent, and in depth verification of eligibility using medical record and laboratory data and discussion with providers. Potential trial participants were first screened using a 13-question survey administered by interviewers to willing patients at all DoSA network clinics. The interviewers approached patients in clinic waiting areas, and administered brief screening surveys in nearby private rooms. To preserve the confidentiality of HIV-infected patients, we embedded HIV-related questions with questions about other chronic medical conditions.
Potential trial participants who were eligible after the brief screener were invited to the second step of recruitment, in which interviewers explained trial procedures in detail and discussed the required informed consents. If patients consented to be in the trial the interviewers drew blood for genotypic resistance testing and scheduled an appointment two weeks later for a baseline assessment. At this visit, participants were asked for authorization to discuss the trial, including the resistance results, with their HIV providers.
The final step in the recruitment process involved confirmation of eligibility by medical record review and discussion with the patient’s HIV provider. These steps were completed by the Medical Director (KMB), who also reviewed genotypic testing results to confirm sensitivity to prescribed antiretroviral regimens. If the patient had not authorized the Medical Director to discuss the study with their provider, the Medical Director explained the results to the patient and encouraged them to contact their HIV provider. Patients who were resistant to their prescribed regimen were ineligible for the trial.
Participants were reimbursed $25 for each research visit and $5 extra for each visit involving a blood draw. Recruitment began in June 2004 and ended in August 2007. Participants are followed for. Follow-up of all patients, including the additional year beyond the 24-week intervention, was completed in February 2009.
Randomization
Prior to recruitment, random-number tables were used to allocate subjects to the DOT intervention arm or the TAU control arm. Randomization was stratified by antiretroviral experience (versus naïve) and by once daily (versus twice daily) dosing, because of the influence of these two factors on adherence and virologic outcome (15;16). Four distinct randomization lists were created: (1) antiretroviral-naïve, once-daily, (2) antiretroviral-naïve, twice-daily, (3) antiretroviral-experienced, once-daily, and (4) antiretroviral-experienced, twice-daily. To ensure comparison groups of roughly equal size, we randomized by blocks within each of these four strata. Blocks were of variable number to minimize the chance of upcoming group assignment (TAU or DOT) being anticipated by interviewers.
Baseline assessment
The baseline assessment consisted of a survey administered using Audio Computer-Assisted Self-Interview (ACASI) technology. During the interview the participant simultaneously read the question on the computer screen and listened to it read aloud through headphones. Participants also had blood drawn and gave a urine sample for illicit drug toxicology testing. Following completion of the baseline assessment, participants received their randomization assignment to either the DOT or the TAU arm.
Intervention and control conditions
DOT intervention: dispensing of antiretrovirals by clinic nurses
For participants in the DOT intervention arm, DoSA clinic nurses dispensed and observed ingestion of daily antiretroviral medications at the usual methadone dispensing locations. To expedite this process and minimize nursing burden, all DOT antiretroviral medications were prepared in advance in individualized pilltrays. Pilltrays were labeled by interviewers, and filled by a centralized DoSA pharmacist, in a process described below. Each pilltray contained seven removable single dose pillboxes that contained all of the different medications taken together in one dose. Participants on once daily regimens had one pilltray while twice daily regimens required two pilltrays: one containing the morning doses and one containing the evening doses. Depending on methadone pick-up schedule and dosing frequency, certain doses could not be observed (i.e., Sunday or evening doses). In these instances, participants were given single dose pillboxes, or “take home doses”, for each unobservable dose and were asked to return the pillbox to the nurses at the next clinic visit, whether or not they had taken the pills.
DOT intervention: preparing pilltrays for use in the methadone clinics
Preparing pilltrays for use in the DOT intervention required communication between the Medical Director (KMB), HIV primary care providers, designated community pharmacy, interviewers, and centralized DoSA pharmacist. First, for each participant, the Medical Director verified each antiretroviral medication by speaking directly with his or her HIV provider. She then called in prescriptions to a single designated community pharmacy that delivered the medications by messenger directly to the DoSA central pharmacy. HIV primary care providers were asked to suspend writing prescriptions for DOT participants for the 24-week intervention period, and to contact the Medical Director directly if a participant changed antiretroviral medications.
For each participant, interviewers created pilltrays, labeling each of the seven pillboxes with participant identifying data, antiretroviral medication name and dose, dosing directions, and any specific instructions (e.g., take with food). Interviewers then delivered labeled, empty pilltrays to the centralized DoSA pharmacist, who filled the pilltrays with the appropriate medications for each participant. Pilltrays were delivered from the centralized DoSA pharmacy to the individual methadone clinics every two weeks, along with scheduled methadone deliveries.
TAU control condition
Participants in the TAU control condition continued to receive HIV medical care and ad hoc support from their primary care provider (e.g., pillboxes to use at home, discussion of adherence barriers). In addition, HIV adherence counseling (described below) was provided in each DoSA clinic by trained adherence counselors. Finally, to provide objective measures of adherence, participants in the TAU arm underwent pill counts at all research visits, and were also administered a MEMS cap at the baseline visit, which they retained for the duration of the trial.
Adherence counseling
Formal manualized adherence counseling was provided in each DoSA clinic by para-professional adherence counselors trained to provide six 30–40 minute sessions (17). The first two sessions focused on motivational interviewing techniques to assess adherence obstacles and develop treatment goals. The following four sessions were tailored to the individual patient and focused on cognitive-behavioral skills building. All participants in both arms were referred for adherence counseling.
Visit schedule and measures
Frequency and length of research visits during the 24-week intervention period was the same for all study participants (Table 1). We standardized the visit schedule to control for any improvement in adherence that might result from study participation alone {Braunholtz 2001}. We used four measures to assess antiretroviral adherence: self-report, pill count, Medication Event Monitoring Systems (MEMS), and nursing records of directly observed doses.
Table 2.
Schedule and content of study visits
| Study Arm | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Audio Computer Assisted Self interview ACASI) | Both | Fulla | Shortb | Short | Short | Short | Short | Short | Short | Full | Short | Short | Short | Full |
| MEMS Download and Usage Questionnairec | TAU | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Pill Count | Both | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Nursing DOT records | DOT | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Urine toxicologyd | Both | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Opiate withdrawal | Both | X | X | X | X | X | X | X | X | X | X | X | X | X |
| CD4+ T-cells | Both | X | X | X | X | |||||||||
| HIV viral load | Both | X | X | X | X | |||||||||
| Genotypic viral resistance | Both | X | X | X | ||||||||||
| Blood for storage | Both | X |
Full ACASI survey includes measures of: antiretroviral adherence1, opiate withdrawal2, recent drug and alcohol use3,4, beliefs about dug interactions5, attitudes toward HIV medications5, adherence self efficacy3,5, medication side effects4,5, HIV-related quality of life6,coping7, intimate partner violence and prior child abuse8, addiction severity9, alcohol dependence10, service utilization/hospitalizations11, psychological distress and lonliness12, and social support13,14.
Short ACASI survey includes measures of antiretroviral adherence1, opiate withdrawal2, and recent drug and alcohol use3,4.
MEMS usage questionnaire (interviewer-administered) assess periods of time during which the participant took mediation from another container and did not use the MEMS cap.
Urine tested for methadone, opiates, cocaine, benzodiazepines, barbituates, amphetamines, cannabinoids, and phencyclidine
Laboratory samples
Interviewers drew blood for HIV viral load and CD4+ T-cell counts at baseline and weeks 8, 16, 20, and 24. At baseline, with consent, we collected two storage samples to maintain in a repository. We performed toxicology testing for illicit drugs on either urine or oral fluid at every research visit. To examine the incidence of drug resistance mutations, we tested participants for genotypic viral resistance at the pre-baseline eligibility visit, and for those with an HIV viral load greater than 500 copies/ml at weeks 8, 20, and 24.
Psychosocial measures
Psychosocial domains and instruments are listed in Table 1. The full ACASI interview took between two and four hours to complete, and was administered at baseline, and at weeks 12, and 24. An abbreviated ACASI survey was administered at all other research visits during the 24-week intervention period.
Sample size and power calculations
We estimated a 30% difference between the DOT and TAU arms in the proportion of subjects achieving the main outcome of an undetectable HIV viral load (<75 copies/ml). We calculated the sample size needed to detect this effect size with 80% or more power and a two-sided significance level of 0.05. Based on our prior work in the same population, (19;20) we assumed a maximum attrition rate of 40%.
Because HIV viral load was assessed at four post-randomization time points, we considered a range of within-subject or intraclass correlation coefficients (ICC from 0.4 to 0.6) (21). The ICC represents the variance in the outcome for each subject over the four time points. Higher ICC (indicating low variance within subjects over time) requires more participants to detect a difference between groups compared to lower ICC (indicating high variance within subjects over time). Based on the estimated effect size of 30%, the sample size required for each group was 24 for an ICC of 0.4, 27 for an ICC of 0.5, and 30 for an ICC of 0.6. We therefore planned to recruit 50 participants per group, which would provide adequate power even with the most conservative ICC of 0.6 and the maximum attrition rate of 40%.
Planned statistical analyses
The three primary outcome variables will be between-group changes from baseline to the end of the intervention in: HIV viral load, adherence, and number of drug resistance mutations. All analyses will be conducted according to the intention-to-treat principle that analyzes all participants in their original randomization arm. We will first test whether important baseline measures (e.g., depression, cocaine use) are balanced between the two arms using t-tests or Chi-square tests. Unbalanced baseline measures will be included in all analyses to adjust for potential confounding effects.
We hypothesize that over the course of the 24-week intervention period the proportion of participants with an undetectable HIV viral load (analyzed dichotomously) will be higher in the DOT group than in the TAU group. We will build logistic mixed-effects models to contrast rates of undetectable HIV viral load over the course of the trial between the DOT and TAU arms. Mixed-effects models are valid even if attrition is associated with observed covariates and outcomes. They are also unlikely to require imputation techniques for missing data, and have greater statistical power than analyses of completers only.
Though adherence is assessed using several measures in this trial, during the 24-week intervention period, adherence for both DOT and TAU participants is assessed by self-report and pill count. Therefore, these two measures will be used to assess the effect of the DOT intervention on adherence. To account for inherent social desirability biases in self-reported adherence, for the majority of analyses we will dichotomize this variable as 100% vs. <100% (22). A logistic mixed-effects model will be used to analyze repeatedly measured self-reported adherence. Pill count adherence will be treated as a continuous estimate and will be analyzed using linear mixed-effects models to compare mean pill counts between study arms over the course of the trial.
We hypothesize that fewer new drug resistance mutations will develop in the DOT arm compared to the TAU arm. We will restrict this analysis to participants with genotype results for both baseline and week 24 and no change in antiretroviral regimen during the 24-week intervention period. These restrictions are to isolate the development of new drug resistance mutations for participants on stable antiretroviral therapy. Major drug resistance mutations will be defined according to the 2008 consensus statement from the International AIDS Society (23). The outcome for this analysis will be total number of drug resistance mutations, which is calculated as the number of unique mutations at baseline subtracted from the number of mutations at the end of intervention (week 24). To test between-group difference in the total number of drug resistance mutations, we will apply analysis of covariance that controls for potential confounders.
RESULTS
Screening and baseline interviews occurred between June 2004 and September 2007. All STAR*DOT trial participants had completed the 24-week intervention by February 2008.
A total of 3,231 participants were screened. Of these, 97 were eligible and 77 enrolled in the study (Figure 1). The main reasons for exclusion after the initial screener and review of medical and laboratory data were HIV negative (77%) or not on antiretroviral therapy (29%). Of the 77 enrollees, 39 were randomized to the DOT arm and 38 to the TAU arm. The overall retention rate after 24 weeks was 84%. By study arm, retention rates were 82% for DOT and 87% for TAU. Sixty-five participants completed the 24-week intervention period.
Figure 1.
Flow chart of study recruitment, enrollment and completion.
The sample was 41% male, 35% Hispanic, and 31% Black with a mean age of 47 (Table 2). Rates of self-reported substance use in the 30 days prior to the baseline interview were: 26% for heroin, 29% for cocaine, 36% for crack, and 15% for marijuana. The median duration of methadone maintenance was 8 years [interquartile range (IQR) 3–19], and the median dose of methadone was 100 mg (IQR 90–140).
Table 3.
Baseline characteristics of study sample (n=77)
| Age, mean (sd) | 47 (6.9) |
| Gender, n (%) | |
| Male | 41 (53) |
| Race, n (%) | |
| White | 10 (13) |
| Black | 31 (40) |
| Other | 36 (47) |
| Ethnicity, n (%) | |
| Hispanic | 35 (45) |
| Non-Hispanic | 42 (55) |
| Education, n (%) | |
| High school (partial or completed) | 58 (75) |
| College (partial or completed) | 19 (25) |
| Marriage status, n (%) | |
| Married / living with partner | 34 (44) |
| Widowed / separated / divorced | 22 (29) |
| Single | 21 (27) |
| Employment, n (%) | |
| Employed | 2 (3) |
| Unable to work / unemployed / other, n (%) | 75 (97) |
| Type of Health Insurance, n (%)a | |
| Medicaid | 69 (90) |
| Medicare | 16 (21) |
| Private insurance | 6 (8) |
| Self-reported illicit drug use in the past 30 days, n (%) | |
| Heroin | 20 (26) |
| Cocaine | 22 (29) |
| Crack | 27 (35) |
| Marijuana | 11 (14) |
| Amphetamines | 3 (4) |
| Years of methadone maintenance, median (interquartile range [IQR]) (n=48) | 10 (5–16) |
| Median methadone dose mg, median (IQR) (n=57) | 120 (90–180) |
| Years living with HIV, median (IQR) | 13 (9–17) |
| Years of ARV treatment, n (%) | |
| <1 year | 18 (25) |
| 1 – 5 years | 34 (46) |
| >5 years | 21 (29) |
| Number of pills in regimen, n (%)b | |
| 1 pill | 8 (10) |
| 2 pills | 39 (51) |
| >= 3 pills | 30 (39) |
| Number of doses per day, n (%) | |
| One dose per day | 21 (27) |
| More than one dose a day | 56 (73) |
| Resistance, n (%)c | |
| Not resistant | 62 (81) |
| Resistant | 15 (19) |
| Major mutations, n (%) | |
| NRTId | 5 (6) |
| NNRTIe | 6 (8) |
| PIf | 7 (9) |
| Viral Load (copies/ mL), n (%) (n=73) | |
| <75 | 35 (45) |
| 75 – 400 | 5 (6) |
| 401 – 10,000 | 20 (26) |
| 10,001 – 100,000 | 10 (13) |
| > 100,000 | 3 (4) |
| Absolute CD4+ T cell count cells/mm3, median (IQR) (n=74) | 345 (151–494) |
| Seven-day self-reported adherence , n (%) (n=76) | |
| 100% | 55 (72) |
| < 100% | 21 (28) |
Categories not mutually exclusive
Participants reporting <3 pills were prescribed combination pills containing 2 or 3 medications
At least one major mutation based on the 2008 consensus of the International AIDS Society-USA Drug Resistance Mutations Group; reference #
NRTI; nucleoside reverse transcriptase inhibitor
NNRTI; non-nucleoside reverse transcriptase inhibitor
PI; protease inhibitor
Median duration of HIV infection was 13 years (IQR 9–17), and 100% of participants were antiretroviral treatment experienced. Viral load was undetectable for 45% the sample and the median CD4+ T-cell count was 345 cells/mm3 (IQR 151–494). At baseline, 19% of the sample was resistant to any antiretroviral medication. A minority of participants (10%) was prescribed antiretroviral medications in one combination pill, 51% was prescribed two combination pills, and 39% was prescribed three or more pills. Antiretroviral dosing schedules were once daily for 21 participants (27%), twice daily for 54 participants (70%), and three times daily for 1 participant (3%).
Our intervention was feasible and easily implemented in a busy clinical setting. Staff at the DoSA clinics worked collaboratively with the STAR*DOT team and participants were enrolled from all 12 clinics (median 6 participants per clinic). Health care providers communicated with the study Medical Director and nurses at each clinic recorded daily events on study calendars. Health care providers at an additional 4 unaffiliated clinics were responsive when contacted by the study Medical Director to discuss their patients’ eligibility. Overall, 34 providers assisted with implementing the study. One provider did not agree to have their patient in the study because they felt that the patient’s attendance at the methadone clinic was so poor that their HIV treatment might be jeopardized if they were randomized to DOT.
The DOT program was also acceptable to patients. Most patients who were eligible chose to participate. Only two participants refused their randomization, which was DOT in both cases.
The core components of our DOT program did not require additional funding over existing clinic operating budgets. We did not provide salary support for medical providers, nurses or adherence counselors, and antiretroviral medications were paid for by patients’ existing insurance plans. We did supply pillboxes used for DOT, and we paid the DoSA central pharmacist a stipend to fill the pillboxes; however, these devices are widely available and inexpensive. Funding for the research aspects of this DOT program (e.g., salaries for research staff, computers for interviews, and MEMS caps) was provided by a grant from the National Institutes of Health (NIDA R01 DA015302).
DISCUSSION
STAR*DOT represents the first randomized controlled trial of a directly observed antiretroviral program in a methadone program. Our trial is consistent with prior work on the feasibility of DOT programs in methadone clinics (10;11;24), and extends this research by randomizing participants to a DOT intervention or a treatment-as-usual control condition. Outcomes from the STAR*DOT trial will contribute to current knowledge by evaluating the efficacy of DOT for reducing HIV viral load, improving adherence, and reducing drug resistance.
Our trial has several strengths. First, because our goal was to design a DOT program that could potentially be implemented in a busy methadone clinic without increasing staff burden or affecting clinic flow, we asked methadone clinic nurses to administer antiretroviral doses selected by patients’ primary care providers. The major difference between our grant-funded DOT program and a program without additional funding is the specially prepared pilltrays for observed and take-home antiretroviral doses. Ad hoc DOT programs in our system exist for non-HIV medications (e.g., benzodiazepines), and in these cases nurses dispense daily doses directly from patients’ medication bottles stored in the clinic. If antiretroviral DOT in our setting proves efficacious, future studies should assess the feasibility and efficacy of a DOT program using only available resources.
In addition, we used multiple methods to measure adherence at multiple time points. Adherence is a complex behavior, and lack of a gold standard makes accurate measurement challenging (25). Combining available adherence measures to minimize the error associated with each individual method has been suggested as a strategy for improving adherence assessment (26–29). In addition to varying by measurement method, adherence rates in the same individual vary over time (30). To obtain more rigorous adherence estimates and examine changes in adherence over time, we assessed adherence by multiple methods and at numerous time points.
Our trial is designed to evaluate the efficacy of antiretroviral DOT for patients in methadone clinics regardless of clinical status. We did not target patients failing therapy and in fact, almost half our sample had an undetectable viral load at baseline. This decision was based on a belief that adherence is dynamic over time and patients therefore need ongoing support. Adherence interventions should be designed to both improve and maintain adherence behaviors. Because we did not target those failing therapy, the findings may be more generalizable to clinic-based populations of HIV+ methadone patients.
Limitations to this trial should be noted. Because antiretroviral dosing schedule was not an eligibility criterion, DOT participants on twice daily regimens received a lower dose of the intervention than those on once daily medications. However, the percent of observed doses has not been associated with virologic failure in other research (12), suggesting that even minimal participation in a DOT program may improve antiretroviral adherence. The effect of DOT programs on adherence with non-observed, or “take-home” doses is an area that merits further research.
Further, several aspects of the study design may have had unintended adherence improving effects in the TAU arm. These include frequent visits with interviewers, participation in adherence counseling, and use of a MEMS cap. To minimize a differential effect between groups, we balanced the two study arms regarding the amount of exposure to interviewers and referred all participants for adherence counseling. If present, an unintended adherence improving effect in the TAU group from using MEMS caps would bias results toward the null hypothesis and therefore strengthen any significant findings.
Lastly, several secular changes over the past few years may have affected our trial. First, co-formulated antiretroviral medications are increasingly available and markedly decrease pill burden. This change may improve adherence, but is unlikely to have a differential effect by study arm. Since we planned this trial, a small number of DOT efficacy studies from other sites have been published, allowing for a more precise estimation of effect size. Three studies reported slightly smaller effect sizes (25%) for achieving an undetectable HIV viral load than the one on which we based our power calculation (30%) (11;31;32). Although we enrolled fewer participants than expected, because of the high retention rate our study remains adequately powered to detect an effect size of 25% even with a conservative ICC estimate of 0.6.
In conclusion, results of the STAR*DOT trial will advance our knowledge of the efficacy of directly observed antiretroviral therapy for HIV-infected methadone maintained drug users for achieving HIV treatment success. If our results provide evidence that DOT in methadone clinics is an effective structural intervention, protocols can be developed to implement and evaluate DOT programs in similar settings. Data from this study will also advance our understanding of antiretroviral adherence in methadone-maintained drug users over time, and of the measurement characteristics of commonly used adherence measures.
Acknowledgments
This work was funded by National Institutes of Health grants R01 DA015302 awarded to Dr. Arnsten, K23 DA021087 awarded to Dr. Berg, and a Center for AIDS Research grant (P30 AI51519) awarded to the Albert Einstein College of Medicine of Yeshiva University.
The authors thank Nina Cooperman, Joseph Hecht, Maite Villanueva, Metta Cantlo, Amanda Carter, Megha Ramaswamy, Francesca Parker, Laxmi Modali, Hillel Cohen, and Moonseong Heo for assistance with protocol development, pharmacy coordination, data collection and management, and statistical consultation. The trial was dependent on the cooperation of the medical providers, nurses, and patients at the Albert Einstein College of Medicine and Montefiore Medical Center Division of Substance Abuse. We also thank the Center for AIDS Research Albert Einstein College of Medicine, and the staff at Melrose Pharmacy, Bendiner & Schlesinger, Inc., and Bio-Reference laboratories, Inc.
For additional details regarding adherence measurement protocols contact the corresponding author.
Footnotes
Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255-66.
Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse.1987;13(3):293–308.
Purcell DW, Metsch LR, Latka M, et al. Interventions for seropositive injectors – research and evaluation: an integrated behavioral intervention with HIV –positive injection drug users to address medical care, adherence, and risk reduction. J Acquir Immune Defic Syndr. 2004;1(37):suppl 2:S110-8.
Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381.
Arnsten JH, Li X, Mizuno Y et al. Factors associated with antiretroviral therapy adherence and medication errors among HIV infected injection drug users. J Acquir Immune Defic Syndr. 2007;46 (Suppl 2): S64-S71.
Wu AW, Revicki DJ, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res. 1997;6:481–493.
Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. J Pers Soc Psychol. 1989;56:267–283.
Zachary, MJ, Schecter, CB, Kaplan, ML, Mulvihil MN. Provider evaluation of a multifaceted system of care to improve recognition and management of pregnant women experiencing violence. Womens Health Issues. 2002;12(1):5–15.
McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33.
Maisto, SA, Saitz, R. Alcohol use disorders: screening and diagnosis. Am J Addict. 2003;12(1):S12-S25.
Weaver MR, Conover CJ, Proescholdbell RJ, Arno PS, Ang A, Ettner SL et al. Utilization of mental health and substance abuse care for people living with HIV/AIDS, chronic mental illness, and substance abuse disorders. J Acquir Immune Defic Syndr.. 2008;47(4):449–458.
Derogatis, LR, Spencer PM. The Brief Symptom Inventory (BSI): Administration, Scoring and Procedure Manual – 1. Baltimore, MD: John Wiley; 1982.
Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66(1):20–40.
Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nurs Res. 1981;30(5):264–269.
Reference List
- 1.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: substance use, health care and psychological States. J Health Psychol. 2005;10(4):545–558. doi: 10.1177/1359105305053422. [DOI] [PubMed] [Google Scholar]
- 3.Gatt S, Sammut R. An exploratory study of predictors of self-care behaviour in persons with type 2 diabetes. Int J Nurs Stud. 2008 doi: 10.1016/j.ijnurstu.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2003;11(6):185–198. [PubMed] [Google Scholar]
- 5.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus Statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279(12):943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 6.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330(17):1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 7.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38 Suppl 5:S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 8.Mitty JA, Macalino GE, Bazerman LB, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39(5):545–550. [PubMed] [Google Scholar]
- 9.Babudieri S, Aceti A, D'Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284(2):179–180. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Conway B, Prasad J, Reynolds R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38 Suppl 5:S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 11.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38 Suppl 5:S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care STDS. 2007;21(8):564–574. doi: 10.1089/apc.2006.0192. [DOI] [PubMed] [Google Scholar]
- 13.Tossonian HK, Raffa JD, Grebely J, et al. Methadone dosing strategies in HIV-infected injection drug users enrolled in a directly observed therapy program. J Acquir Immune Defic Syndr. 2007;45(3):324–327. doi: 10.1097/QAI.0b013e318061b5fd. [DOI] [PubMed] [Google Scholar]
- 14.McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11(4):271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 15.Molina JM, Journot V, Morand-Joubert L, et al. Simplification therapy with once-daily emtricitabine, didanosine, and efavirenz in HIV-1-infected adults with viral suppression receiving a protease inhibitor-based regimen: a randomized trial. J Infect Dis. 2005;191(6):830–839. doi: 10.1086/428091. [DOI] [PubMed] [Google Scholar]
- 16.Horberg M, Silverberg M, Hurley L, Delorenze G, Quesenberry C. Influence of prior antiretroviral experience on adherence and responses to new highly active antiretroviral therapy regimens. AIDS Patient Care STDS. 2008;22(4):301–312. doi: 10.1089/apc.2007.0101. [DOI] [PubMed] [Google Scholar]
- 17.Cooperman NA, Arnsten JH, Klein RS. Current sexual activity and risky sexual behavior in older men with or at risk for HIV infection. AIDS Educ Prev. 2007;19(4):321–333. doi: 10.1521/aeap.2007.19.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesney MA, Ickovics JR, et al. Chambers DB Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 19.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19(11):1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 2002. [Google Scholar]
- 22.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-Report Measures of Antiretroviral Therapy Adherence: A Review with Recommendations for HIV Research and Clinical Management. AIDS and Behavior. 2006 doi: 10.1007/s10461-006-9078-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the Drug Resistance Mutations in HIV-1: Spring 2008. Top HIV Med. 2008;16(1):62–68. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 24.Clarke S, Keenan E, Ryan M, Barry M, Mulcahy F. Directly observed antiretroviral therapy for injection drug users with HIV infection. AIDS Read. 2002;12(7):305–306. [PubMed] [Google Scholar]
- 25.Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S149–S155. doi: 10.1097/01.qai.0000243112.91293.26. S149–S155. [DOI] [PubMed] [Google Scholar]
- 26.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 28.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 29.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 30.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16(16):2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 31.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45(6):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21(11):1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]