Abstract
The transmembrane isoform of agrin (Tm-agrin) is the predominant form expressed in the brain but its putative roles in brain development are not well understood. Recent reports have implicated Tm-agrin in the formation and stabilization of filopodia on neurites of immature central and peripheral neurons in culture. In maturing central neurons, dendritic filopodia are believed to facilitate synapse formation. In the present study we have investigated the role of Tm-agrin in regulation of dendritic filopodia and synaptogenesis in maturing cultures of hippocampal neurons. We did this by infecting the neurons with an RNAi lentivirus to deplete endogenous agrin during the developmental period when filopodia density on the dendritic arbor was high, and synapse formation was rapid. We found that dendritic filopodia density was markedly reduced, as was synapse density along dendrites. Moreover, synapse formation was more sharply reduced on dendrites of infected neurons contacted by uninfected axons than on uninfected dendrites contacted by infected axons. The results are consistent with a physiological role for Tm-agrin in the maturation of hippocampal neurons involving positive regulation of dendritic filopodia and consequent promotion of synaptogenesis, but also suggest a role for axonal agrin in synaptogenesis.
Introduction
Synapse formation between axons and dendrites in the central nervous system (CNS) is initiated by physical interaction between the out-growing axon of the presynaptic neuron and the dendritic arbor of the post-synaptic neuron. Motile dendritic filopodia that contact and recruit passing axons facilitate this interaction (Ziv and Smith, 1996). Filopodia also play a role in shaping axonal and dendritic arbors (Chang and De Camilli, 2001; Niell et al., 2004). After initiating synaptogenesis, some dendritic filopodia may differentiate into the more stable spines (reviewed in Yuste and Bonhoeffer, 2004). Dendritic spines are the post-synaptic sites of most excitatory synapses in the CNS (Harris, 1999; Hering and Sheng, 2001). At nascent stages of synaptogenesis, many filopodia are still observable on the dendritic arbor, and are still highly motile (Dailey and Smith, 1996; Dunaevsky et al., 1999; Lendvai et al., 2000). As synapses develop and mature, the number of filopodia declines, while those still present become more stable and more spines are established. In mature, highly developed neurons, dendritic spines still show significant plasticity. They change in size, shape and number in response to stimuli leading to both long term potentiation (LTP) and long term depression (LTD), thought to be the physical basis of memory formation (Collin et al., 1997) (and reviewed in Luscher et al., 2000).
Various cell surface molecules are known to influence spine properties in response to external signals by mediating cell adhesion, affecting Rho-family GTPase activity or altering Ca2+ fluxes, ultimately modulating actin dynamics (Ethell and Pasquale, 2005). Some of these molecules provide linkages between the presynaptic and post-synaptic membranes, whereas others connect these membranes to the extracellular matrix (ECM). Although the roles of the ECM in synapse development and function are not completely understood, some ECM components and their cell surface receptors are known to regulate synaptic plasticity (Dityateva et al., 2003).
Agrin is a large proteoglycan (McMahan et al., 1992; Tsen et al., 1995) that is expressed as secreted and transmembrane forms (Burgess et al., 2000; Neumann et al., 2001). The most extensively studied and established role of agrin is in development of the neuromuscular junction. Agrin secreted by motor neurons is required for stable post-synaptic differentiation of skeletal muscle cells, most notably the aggregation of acetylcholine receptors (Gautam et al., 1996). The transmembrane form of agrin (Tm-agrin) is predominantly expressed in neurons of the central nervous system (CNS) (Burgess et al., 2000; Neumann et al., 2001), but its functions there are not yet well established (Neumann et al., 2001; Smith and Hilgenberg, 2002). Synaptogenesis was inhibited by anti-sense reduction of agrin expression in hippocampal neuron cultures (Ferreira, 1999; Bose et al., 2000) and was reduced in sympathetic neuron cultures from agrin knock-out mice (Gingras et al., 2002). These mice also showed impaired synaptogenesis in the sympathetic ganglia in vivo (Gingras et al., 2007), suggesting an in vivo role for agrin in neuron-to-neuron synapse formation. A recent report suggests that agrin secreted by glial cells plays a role in hippocampal neuron synapse formation in culture (Tournell et al., 2006). Early results with agrin knock-out mice did not support a role for agrin in synapse formation or maintenance in the CNS (Li et al., 1999; Serpinskaya et al., 1999). However, a recent study (Ksiazek et al., 2007) has indicated an in vivo role for agrin in the formation or stabilization of excitatory synapses in cortical neurons of young mice. It is widely supposed that the absence of gross abnormalities in synaptic development in the CNS of agrin-null mice may be due to the presence of functionally redundant molecules that can compensate for the loss of agrin (Serpinskaya et al., 1999).
Recently it was reported that the clustering of the endogenous membrane-bound agrin or over-expression of Tm-agrin leads to increased formation of filopodia-like processes along developing axons and dendrites of central and peripheral neurons (Annies et al., 2006; McCroskery et al., 2006). Moreover, suppression of agrin expression in immature hippocampal neurons reduces the number of filopodia by destabilizing them and reducing their formation (McCroskery et al., 2006). All of these results suggest that Tm-agrin has a role in positively regulating neuronal filopodia. Thus, the regulation of filopodia could contribute to agrin’s putative role in regulation of neurite outgrowth and synaptogenesis.
In the present study, we have suppressed Tm-agrin expression during the period of rapid synaptogenesis in maturing hippocampal cultures in order to investigate the role of Tm-agrin in regulating dendritic filopodia and to determine the possible consequences to synapse formation and dendritic arbor development. We demonstrate that this suppression results in a large reduction in the density of filopodia along dendrites, a modest increase in dendrite length and branching, and a severe reduction in the number of synapses formed. The reduction in synapses appears to depend more on the loss of agrin in the post-synaptic dendrite than in the presynaptic axon.
Experimental Methods
Rat hippocampal neuron cultures and transfection
Hippocampal neuron cultures were prepared as previously described (Neuhuber and Daniels, 2003) with slight modifications. Hippocampi from E19-20 rat embryos were incubated for 15 min at 37 °C in 10 ml Earle’s balanced salt solution containing 100 units papain (Worthington/Technicon, Bad Vibel, Germany) followed by trituration in Eagle’s minimal essential medium (MEM) (Invitrogen, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT).
If the neurons were to be transfected we used the Nucleofector System (Amaxa Biosystems, Gaithersburg, MD) according to the protocol provided by the manufacturer. Briefly, 2×106 neurons were re-suspended in 100 μl Rat Neuron Nucleofector Solution (Amaxa) with 5 μg expression construct. The cell suspension was electroporated using the O-03 program (Amaxa). After transfection, the cell suspension was diluted with MEM supplemented with 10% horse serum, penicillin (100 units/ml), and streptomycin (100 μg/ml).
All neurons were plated on coverslips coated with a mixture of 66 μg/ml poly-D-lysine (PDL; Sigma, St. Louis, MO) and 6.6 μg/ml mouse laminin (Invitrogen). This substrate promoted maturation in low-density cultures without glial conditioned medium (Howard et al., 2003). The coverslips were placed in a 10 cm petri dish, and the cells plated at a density of 2 ×105 per dish in initial studies or later, at 1 ×105 per dish to provide better separation between neurons. Neurons for protein or RNA collection were plated onto identically coated 6-well plates at a density of 1-2 ×106 per well.
Three hours after initial plating, the medium was removed and gently replaced with Neurobasal medium plus B27 supplements (Invitrogen), penicillin, and streptomycin (referred to here as Neurobasal +). Cultures were maintained at 37° with 10% CO2. After 3 days in culture each coverslip was transferred to a separate well in a 6-well dish. Each week, half of the medium was removed and very gently replaced with fresh Neurobasal +.
Generation of siRNA lentivirus
We used the identical shRNA sequence (number 3) described in the experimental methodology section of our previous publication (McCroskery et al., 2006) that knocked down transmembrane agrin expression by >95%. This sequence targets all agrin isoforms, but since hippocampal neurons almost exclusively expressed the transmembrane form (see Results) this was the affected isoform in our experiments. The sequence was cloned into the lentiviral expression plasmid pNUTS (kind gift of Dr. Vittorio Sartorelli), containing a U6 promoter and a separately expressed ubiquitin promoter-driven eGFP to indicate transduction. The pNUTS plasmid generates a replication-deficient lentiviral vector when co-transfected with,the packaging vectors (ViraPower packaging mix; Invitrogen) into HEK 293FT packaging cells with Lipofectamine 2000 following the Virapower protocols (Invitrogen). After 72 h the culture supernatants were centrifuged at 1000 X g for 15 min at 4° to pellet cell debris. The supernatant was then filtered through a Millex-HV 0.45 μm PDVF filter (Millipore). The virus was concentrated 10-15x by centrifugation in a Centricon plus-20 filter (Millipore) following the manufacturer’s instructions. Aliquots were stored at -80 °C. Both the Tm-agrin and control shRNA virus preparations were titered according to the Virapower protocol and found to contain ∼5 ×107 infectious units/μl.
Western blot analysis
The construct, DsRed-Agrin4,19 (TM-agrin) was generated from a full-length GFP-Tm-agrin construct designated Full-length (TM)GFP(N0)-agrin4,19 (Neuhuber and Daniels 2003) by cloning in-frame into the pDsRed-Monomer-C1 Vector (Clontech Laboratories, Inc. Mountain View, CA), generating a construct with DsRed at the 5′ end of full-length Tm-agrin. This plasmid was transfected into hippocampal neurons using the Amaxa system. After two weeks the neurons were infected with either VsiAgrin or VsiMock and were incubated for a further 1, 3 or 7 days. To collect protein, the cells were scraped into ice-cold lysis buffer containing 0.1% NP-40, 50 mM Tris (pH7.5), 250 mM NaCl, 5 mM EDTA, and protease inhibitors. After 10 min incubation on ice, the lysate was passed through a 22 gauge needle 6 times. Insoluble material was removed by centrifugation at 20,000 × g for 3 min at 4°. Protein concentration of extracts was evaluated by the BCA assay (Pierce Biotechnology, Rockford, IL). Equal amounts of protein were separated on 3-8% Tris-acetate SDS-PAGE gels (Novex, San Diego, CA) and transferred to polyvinylidene difluoride membranes. The lower halves of the gels were excised to monitor protein loading and stained with Coomassie blue for imaging with the Odyssey infrared imaging system (Li-Cor Biotechnology, Lincoln, NE). After incubation in blocking buffer (Tris buffered saline plus 0.01% Tween 20 and 5% dry non-fat milk) for 2 hr at room temperature, blots were probed with a rabbit dsRed antibody (1:5,000; A6455, Clontech) overnight at 4°C. Membranes were incubated with an Alexa 680-conjugated anti-rabbit IgG secondary antibody (1:5000; Molecular Probes), and the signal was visualized on the Odyssey infrared imaging system.
Semi-quantitative RT-PCR
To assess the level of endogenous agrin messenger RNA in hippocampal neurons, total RNA was isolated from neurons using Trizol (Invitrogen). The neurons were harvested after 14 days in culture (before infection) and 1, 3 or 7 days after infection with the siAgrin or siMock lentivirus, or with no infection at the same time points. RNA was isolated from 3 different neuron preparations for each condition. Three μg of total RNA was reverse transcribed using Superscript III by the manufacturer’s protocol using random hexamers (Invitrogen). To determine which isoforms of agrin were expressed in the hippocampal cultures, PCR primers specific to either form were used (Neumann et al., 2001); Tm-agrin specific sense: 5′ TGGTGCCTCC ATGCTGGTTCG, Sc-agrin specific sense: 5′ AGCCCACAAGAATGAGTTGC, common anti-sense 5′AAGCCACATAACATTC CCCTGC. Annealing temperature for both sets was 55°. For a positive control 3 μg of total RNA from the neuroblastoma X glioma cell line NG108-15 was reverse transcribed as described above. This cell line is known to express agrin (Pun and Tsim, 1997) and RT-PCR in the present study revealed that both secretory and transmembrane forms are expressed. After electrophoresis, bands were scanned and relative expression levels were determined with a densitometer (Bio-Rad, Hercules, CA). PCR with primers specific for β-actin and GAPDH (not shown) were used to normalize RNA levels.
Immunohistochemistry and imaging
Neuron cultures were fixed with freshly prepared 4% paraformaldehyde, 4% sucrose in 0.1 M sodium phosphate buffer for 30 min. The fixative was pre-warmed to 37°C and allowed to cool to room temperature during the fixation period. Immunofluorescence labeling was performed essentially as described (Neuhuber and Daniels, 2003). The fixed cells were permeabilized for 30 min at RT in 0.05% saponin in Dulbecco’s phosphate buffered saline (DPBS) with 10% normal goat serum and 1% BSA to block non-specific antibody binding. Cultures to be labeled with antibody to PSD-95 were incubated for 3 min in 0.05% Triton X-100 in DPBS before blocking. Primary antibodies used were mouse monoclonal antibody to PSD-95 (1:2000) (DakoCytomation, Inc., Carpinteria, CA), mouse monoclonal antibody to MAP2 (1:2000) (Chemicon International, Temecula, CA), rabbit antiserum to γ-actin (1:1000) (kindly provided by Dr. J. Bulinsky); rabbit antiserum to synaptotagmin I (V216; 1:1000) (kindly provided by Dr. T. Sudhof) and chicken monoclonal antibody to GFP (1:1500) (Aves Labs, Inc., Tigard, Or). Primary antibodies were detected with anti-mouse Alexa Fluor 488 or 555 (1:800); anti-rabbit Alexa Fluor 555 (1:800), Cy5 (1:200) or Alexa Fluor 647 (1:500) and anti-chicken Alexa Fluor 488 (1:800). All fluorescent secondary antibodies were obtained from Molecular Probes (Invitrogen, Carlsbad, CA), except for anti-rabbit Cy5, which was obtained from Jackson Immunoresearch Labs (West Grove, PA). Possible background staining was identified by using secondary antibodies only. Neurons were imaged using a 40x, 1.3 N.A. oil immersion objective on a Zeiss Axioplan microscope (Zeiss Inc, Oberkochen, Germany). Digital images were acquired with a CCD camera (C4742-95, Hamamatsu Photonics, Hamamatsu, Japan). All images were acquired with MetaMorph software (Universal Imaging Corp, Downingtown, PA), which was also used to perform measurements of neurite lengths.
Quantification and statistical analysis
For this study we differentiated between spines and filopodia by shape and length, defining a spine to be more round or mushroom shaped and less than 3 μm long, and filopodia narrower and between 3 and 10 μm long. If an elongate protrusion also showed MAP2 staining we considered it to be a branch. To determine the density of dendritic filopodia and spines, we measured the lengths of all dendrites of a given neuron and manually counted the filopodia or spines (provided that they clearly emanated from the dendrites of that particular neuron) to calculate the number per 100 μm. Synapse density on dendrites was determined similarly, the criterion for a synapse was co-localization of puncta of PSD-95 and synaptotagmin labeling. Images to be used for quantitation of filopodia or synapses, using a particular combination of labels, were acquired under constant conditions for a given fluorescence channel. Adjustments of brightness and contrast and color balance adjustments for overlays to be used for quantitation were also kept constant. One-tailed t-tests were conducted to evaluate the significance of differences in mean values of filopodia, spine or synapse density using Instat Graphpad software. Mean values were considered to be statistically different for P values < 0.05. Results are presented as mean ± standard error (SEM).
Results
Density of filopodia, spines and synapses in maturing hippocampal neurons
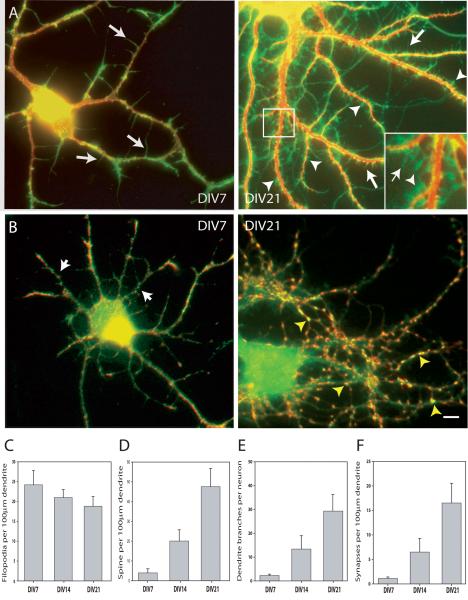
Our strategy to study the role of Tm-agrin in regulating dendritic filopodia, arbor development and synaptogenesis was to knock down agrin expression in hippocampal neuron cultures specifically during a period of rapid synapse formation and a decrease in dendritic filopodia density. This was done by use of a defective lentivirus carrying an shRNA specific to Tm-agrin, which we previously used to efficiently knock down agrin expression by transfection at the time of plating the hippocampal neurons (McCroskery et al., 2006). Thus it was first necessary to determine the appropriate time to infect with the lentivirus to knock down agrin expression in our culture system. Our primary goal was to quantify two related processes in maturing cultured neurons: change in filopodia number on dendrites and synapse formation. To detect and count filopodia and spines on the dendrites, the cultures were immuno-stained for MAP2 to highlight the dendritic arbor, and for γ-actin, to highlight actin-based protrusions. Figure 1A shows representative images of hippocampal neurons cultured for 7 or 21 days. Quantitative data were taken at DIV 7, 14 and 21 from 3 separate experiments (n=100 dendrites at least 100 μm long from at least 25 separate neurons at DIV 7, 14 or 21). As previously reported for dendritic arbors of hippocampal neurons (Ziv and Smith, 1996), our cultures displayed an abundance of filopodia (long arrows), which decreased in density as the neurons matured between one and three weeks in culture (Figure 1C). This decrease in filopodia density was correlated with an increase in the density (Figure 1D) of spines (arrowheads in 1A) and an increased number of branches (Figure 1E).
Figure 1. Changes in the relative number of filopodia, spines, dendrite branches and synapses during development in culture.
(A) Fixed hippocampal neurons were immuno-stained for γ-actin (green), to visualize filopodia and spines and MAP2 (red) to highlight the dendritic arbor. Examples shown here are at 7 days in vitro (DIV) and 21 DIV. Arrows indicate examples of filopodia; arrowheads indicate examples of spines. The inset in the right-hand panel is an ∼ 3-fold enlargement of the boxed area. (B) Fixed hippocampal neurons were immuno-stained for PSD-95 (green), to visualize post-synaptic sites and synaptogamin (red) to highlight the pre-synaptic boutons. Overlapping puncta of PSD-95 and synaptotagmin (yellow) were considered to be synapses. Examples shown here are at 7 DIV and 21 DIV. Yellow arrowheads indicate examples of synapses. Arrows indicate examples or PSD-95 puncta without overlapping synaptotagmin puncta. Bar = 10 μm. (C) Graph illustrating the number of filopodia /100μm dendrite length comparing DIV 7, 14 and 21 hippocampal neurons (n=100 dendrites at least 100 μm long, from at least 25 separate neurons at DIV7, 14 or 21; 3 separate experiments). As the neurons mature, the density of filopodia declines. (D-F) Graphs illustrating the number of spines /100μm dendrite length (D), dendritic branches per neuron (E) and the number of synapses /100μm dendrite length (F), comparing DIV 7, 14 and 21 hippocampal neurons. In C, D and F, n=100 dendrites at least 100 μm long from at least 25 separate neurons at DIV 7, 14 or 21; 3 separate experiments. In E, n= at least 25 neurons at DIV 7, 14 or 21; 3 separate experiments). As the neurons mature, the density of synapses increases 15 fold, similar to the increase observed with spines, while branching of the dendritic arbor increases markedly. All data bars represent the mean ± SEM.
To evaluate the number of synapses on dendrites we immuno-stained for the post synaptic marker PSD-95, seen as small puncta on dendrites (short arrows in 1B DIV7), and the pre-synaptic marker synaptotagmin, seen as puncta along axons. A synapse was defined as a site where PSD-95 and synaptotagmin puncta overlapped or were touching (yellow arrowheads in Figure 1B, DIV 21). Quantitative data were taken at DIV 7, 14 and 21 from 3 separate experiments (n=100 dendrites at least 100 μm long from at least 25 separate neurons at DIV 7, 14 or 21). The number of synapses per 100 μm of dendrite increased 15-fold between DIV 7 and 21 (Figure 1F), in parallel with the increase in spines (Figure 1D). The greatest absolute increase in synapse density appeared to occur between DIV 14 and 21. Therefore we decided to infect with the lentivirus at DIV14 and assay possible effects of Tm-agrin suppression at DIV 21. We also decided not to further evaluate spine formation. Since it was difficult to conclusively distinguish short spines from filopodia in DIV21 neurons, all protrusions less than 3 μm long were ignored in subsequent assays.
Agrin knock-down in mature hippocampal neuron cultures
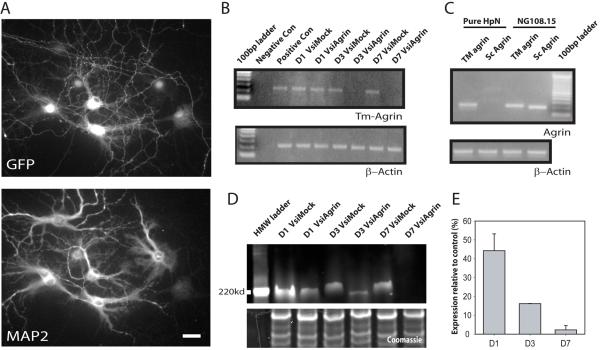
In order to introduce our shRNA constructs into hippocampal neurons in mature cultures with high efficiency, the same shRNA sequence (called siAgrin) used previously (McCroskery et al., 2006) was cloned into the pNUTS vector to generate a defective lentivirus. The final shRNA-expressing virus was termed VsiAgrin. The nonsense control sequence identical to that used previously (McCroskery et al., 2006) was cloned into pNUTS to make VsiMock. Figure 2A shows a hippocampal culture infected with VsiAgrin at 14 DIV and then fixed 3 days later, demonstrating efficiency of infection. Immunofluorescent staining with an antibody against GFP (to identify infected neurons) and MAP2 (to identify all neurons), showed that the virus consistently infected > 95% of the cells (as did VsiMock, not shown). To demonstrate that the virus effectively down-regulates agrin, we measured endogenous agrin messenger RNA. Figure 2B shows one of 3 separate semi-quantitative RT-PCR assays comparing the relative mRNA levels of agrin in hippocampal neurons that had been infected with VsiAgrin after 0, 1, 3 or 7 days. Agrin expression was reduced after one day in VsiAgrin-infected cultures and was visually undetectable after 3 or 7 days, suggesting an estimated reduction in agrin mRNA levels of >95%. VsiMock did not affect agrin mRNA levels. We also used semi-quantitative RT-PCR to determine whether the hippocampal neurons in our cultures expressed primarily Tm-agrin, as suggested previously by in situ hybridization in mouse brain (Stone and Nikolics, 1995), and whether this was the form of agrin message suppressed by VsiAgrin. RNA was obtained from cultures of hippocampal neurons kept free of contamination with glia or any other dividing cell types by the addition of 2.5 μM cytosine arabinoside to the culture medium. Figure 2C shows that only the transmembrane form of agrin was detected in the highly enriched cultures of DIV 21 hippocampal neurons, while both the transmembrane and secreted forms were detected in the RNA from cultures of neuroblastoma-glioma cell line NG108-15, which was previously shown to express agrin (Pun and Tsim, 1997).
Figure 2. Demonstration of effectiveness of the shRNA-producing lentivirus in suppression of Tm-agrin expression in hippocampal neurons.
(A) Immunofluorescence image of infected hippocampal neurons, immuno-stained for GFP to indicate viral infection and MAP2 to distinguish neurons. The lentivirus infected >95% of neurons, albeit with variable expression of GFP. Bar = 50 μm. (B) Semi-quantitative RT-PCR demonstrates the effectiveness of the shRNA (VsiAgrin) in knocking down endogenous Tm-agrin message expression. Densitometry of the bands in 3 separate experiments showed that the Tm-agrin message was reduced by ∼15% at 1 day post infection (D1), and by almost 100% at D3 and D7, while the mock shRNA (VsiMock) did not effect expression. RT-PCR for β-actin was used to demonstrate that equal amounts of cDNA were used. (C) Semi-quantitative RT-PCR from RNA isolated from either hippocampal neurons or from a neuroblastoma-glioma hybrid cell line, NG108-15, using isoform-specific oligos shows that hippocampal neurons in culture express only the transmembrane form of agrin. (D) To demonstrate the loss of Tm-agrin protein after suppression of the message, we over-expressed dsRed monomer-Tm-agrin in hippocampal neurons, and after 14 DIV infected with either VsiAgrin or VsiMock, then determined Tm-agrin protein levels via Western blots using a dsRed antibody (example shown here). The lower box shows the Coomassie stained lower half of same gel used for Tm-agrin blotting, demonstrating equal protein loading. The small apparent discrepancy in mobility between the VsiAgrin- and VsiMock-treated protein bands probably reflects differences in glycosylation level as well as the differences in concentration of Tm-agrin protein caused by VsiAgrin treatment. (E) Quantitative analysis of blots taken from 4 different experiments show that the relative DsRed Tm-agrin protein levels had dropped by 45% at D1, 85% at D3, and almost 100% at D7, compared to VsiMock. Bars show the mean ± SEM.
We next determined how effectively and how rapidly the infection with VsiAgrin would reduce the amount of cellular Tm-agrin protein, taking into consideration the unknown rate of protein turnover. In our hands, detection of endogenous agrin protein in hippocampal neurons by use of commercial antibodies has proven unreliable. Instead, we transfected monomeric DsRed-Tm-agrin (DsRed fused 5′ to full length Tm-agrin) into hippocampal neurons and, after 14 DIV, infected with either VsiAgrin or VsiMock. Protein was collected 1, 3 or 7 days later. Figure 2D shows a representative Western blot of dsRed-Tm-agrin in neuron cultures. The reduction in protein levels followed that of mRNA levels, but with a lag presumably corresponding to the time required for turnover of pre-existing dsRed-Tm-agrin. The amount of protein was sharply reduced by 3 days after infection and was undetectable by 7 days (Figure 2E), indicating that Tm-agrin turnover is fast enough to allow an effective knock-down of expression in cultures between 14 and 21 DIV.
Tm-agrin knock-down moderately increases dendritic length and branching
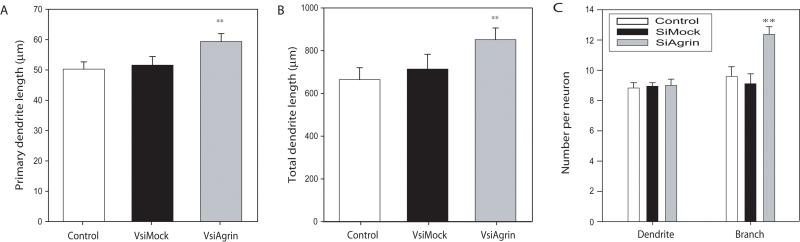
In order to have well separated neurons, the effects of VsiAgrin infection on maturing hippocampal neurons described below were assayed in cultures plated at 1.2 × 105 cells per well (“low density”), half the density used for the time course experiments described above (Figure 3A and B). Measurements of dendritic arbors in DIV 21 cultures that had been infected at 14 DIV revealed a small, but significant, increase in the mean length of the primary dendrite (the longest dendrite), and the mean total length of all dendrites per neuron in VsiAgrin-infected cultures compared to VsiMock and uninfected cultures (18 ± 3% and 26 ± 1%, respectively). The mean number of dendrites originating from the soma was comparable in uninfected neurons, VsiMock and VsiAgrin-infected neurons. However, there was a 28± 2% (p<0.01) increase in the number of dendrite branches per neuron in VsiAgrin-infected neurons compared to VsiMock neurons (Figure 3C).
Figure 3. Knock-down of Tm-agrin leads to moderately increased dendritic length and branching.
Hippocampal neurons were cultured for 2 weeks, then infected with VsiMock, VsiAgrin or left uninfected. At 7 days post infection the cultures were fixed, immuno-stained for MAP2 and imaged. (A and B) Measurements of fixed cells at DIV21, 7 days post infection, showed that VsiAgrin-infected neurons had moderate increases in primary dendrite length and the total length of dendrites per neuron (18± 3% and 26± 1% respectively) compared to both the uninfected control and VsiMock neurons. (C) Cells infected with VsiAgrin showed no difference in the number of primary dendrites per neuron but had significantly more branches per neuron (28± 2%) compared to either VsiMock or uninfected control neurons. (n=150 neurons, 6 separate experiments). Bars show the mean ± SEM. Asterisks indicate values significantly different from VsiMock and uninfected control.
Tm-agrin knock-down decreases dendritic filopodia density
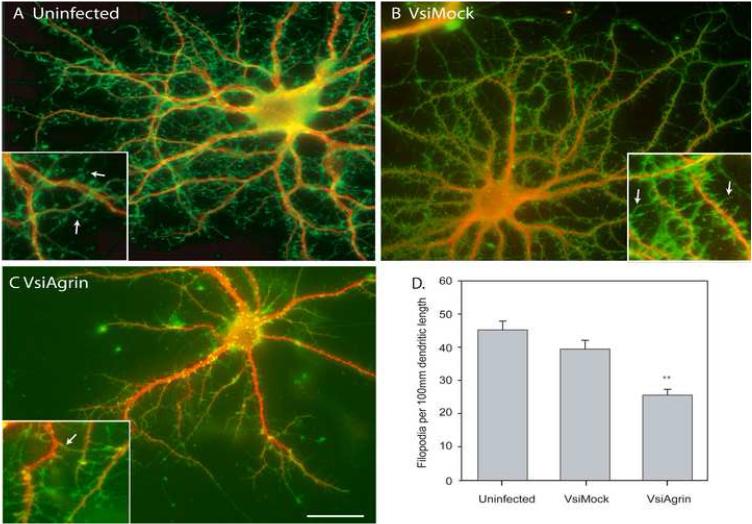
Low-density cultures of hippocampal neurons were infected with either VsiAgrin or VsiMock virus after 14 DIV or left uninfected, then fixed 7 days later. After immuno-staining for both MAP2 and γ-actin, individual neurons were imaged and assayed (n= 75 neurons from 3 separate isolations and infections). Figure 4 shows representative images of DIV 21 neurons that were not infected (Figure 4A), infected with VsiMock (Figure 4B) or VsiAgrin (Figure 4C). As can been seen in Figure 4D, knocking down Tm-agrin in hippocampal neurons reduced dendritic filopodia density by 43± 5% compared to non-infected neurons and by 37± 4% compared to VsiMock-infected neurons (p<0.05). There was no statistically significant reduction of filopodia density in neurons that were infected with VsiMock. These results indicate that Tm-agrin positively regulates filopodia density on the dendrites of maturing hippocampal neurons, as it does on the neurites of immature ones (McCroskery et al., 2006).
Figure 4. Knock-down of Tm-agrin in mature hippocampal neurons reduces filopodia density.
Hippocampal neurons fixed at DIV21, 7 days post infection, were immuno-stained for γ-actin (green) to visualize filopodia and spines, and MAP2 (red) to highlight the dendritic arbor. (A), (B) and (C) are examples of uninfected, VsiMock-infected and VsiAgrin-infected neurons, respectively. Inserts are magnified 2.5X. Arrows indicate examples of filopodia. Bar = 50 μm. (D) Quantitative assay of filopodia density shows that VsiAgrin-infected neurons had a significantly lower number of filopodia per 100μm dendritic length than control or VsiMock-infected neurons. VsiMock filopodia density was not significantly different from the uninfected control (n=75 neurons, 3 separate experiments). Bars show the mean ± SEM. Asterisks indicate value significantly different from VsiMock and uninfected control.
Tm-agrin knock-down decreases dendritic synapse density
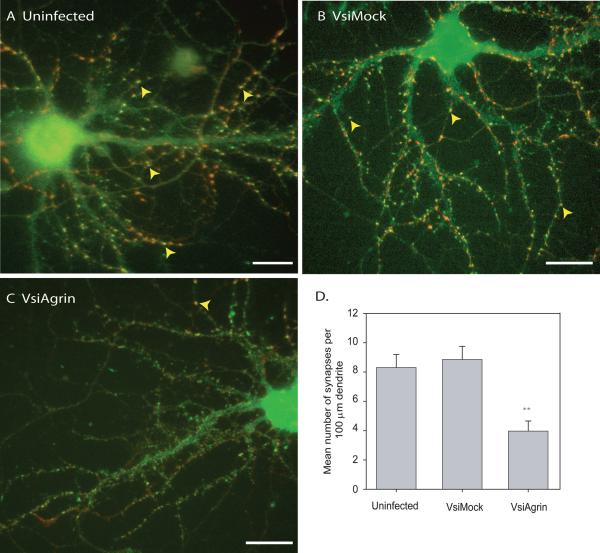
In order to determine the effect of agrin knock-down on synaptogenesis in maturing cultures, infected and control neurons were immuno-stained for PSD-95 and synaptotagmin in an experiment of the same design as used to examine the effect on filopodia density (above). The cultures used for the synapse assay were taken from the same isolations as those for the filopodia assay. Individual neurons were imaged and assayed (n= 75 neurons from 3 separate isolations and infections) for dendritic synapse density as described above. Representative images of DIV 21 neurons that were not infected (Figure 5A) infected with VsiMock (Figure 5B) or VsiAgrin (Figure 5C) are shown. Down-regulation of Tm-agrin by the shRNA reduced dendritic synaptic density by 52± 4% compared to uninfected neurons (p<0.01) and 55± 4% compared to VsiMock-infected neurons (p<0.01). Thus, the reduction of dendritic filopodia density by Tm-agrin suppression was paralleled by a similar reduction in dendritic synapse formation.
Figure 5. Knock-down of Tm-agrin in mature hippocampal neurons reduces synapse density.
Hippocampal neurons fixed at DIV21, 7 days post infection, were immuno-stained for PSD-95 (green) to visualize post-synaptic puncta and synaptogamin (red) to label presynaptic boutons. All dendrites were measured and all synapses were counted as described above. Examples of hippocampal neurons from (A) uninfected control, (B) VsiMock-infected and (C) VsiAgrin-infected cultures are shown. Yellow arrowheads indicate examples of overlapping puncta of PSD-95 and synaptotagmin that were counted as synapses. (D) Quantitative assay of synapse density in each group demonstrates that the VsiAgrin-infected neurons had a significantly lower number of synapses per 100μm dendritic length compared to control or VsiMock-infected neurons. Synapse density of VsiMock-infected neurons was not significantly different from uninfected control (N=75 neurons, 3 separate experiments). Bars show the mean ± SEM. Asterisks indicate value significantly different from VsiMock and uninfected control. Bar = 50 μm.
Depletion of dendritic Tm-agrin expression reduces dendritic synapse density more than depletion of axonal Tm-Agrin expression
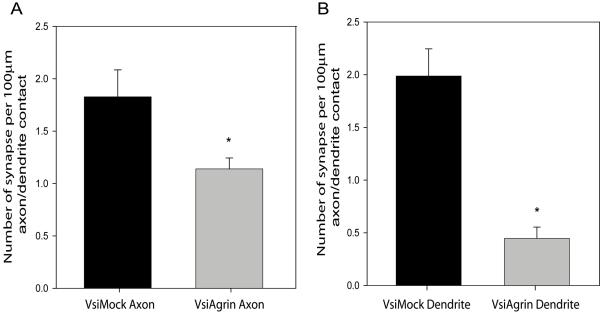
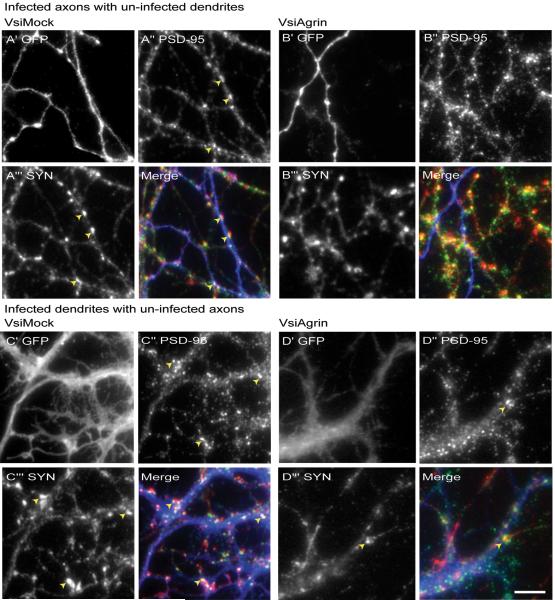
Reducing agrin expression decreased synapse density by over 50% (Figure 5D). This reduction was correlated with a similar decrease in dendritic filopodia density, consistent with the hypothesis that the reduction in synapse formation resulted from lower filopodia density. However, Tm-agrin expression was reduced in axons as well as dendrites. To estimate the relative role of pre- or post-synaptic agrin on synaptogenesis, we used a reduced concentration of shRNA lentivirus to infect only about 40% of the neurons, and then examined segments of infected axons interacting with uninfected dendrites or segments of infected dendrites interacting with uninfected axons. To obtain quantitative data from these experiments, we selected low-density regions of the cultures where we could find segments of single axons unambiguously contacting single dendrites, and assayed synapse density only along such segments. The results shown in Figure 5 included dendrites contacted by more than one axon, where more synapses would form. For this reason, the VsiMock-treated synapse densities in Figure 7 are lower than those shown in Figure 5. Figure 6A shows an example of axons from a VsiMock-infected neuron, pseudo-colored blue, penetrating an uninfected dendritic network. Synapse formation was unaffected, with numerous synaptotagmin (red) and PSD-95 (green) puncta overlapping. Conversely a VsiAgrin-infected axon (Figure 6B) interacting with an uninfected dendritic network shows few overlapping puncta, although many PSD-95 (green) puncta are present. In contrast, several synapses are seen where the same dendritic arbor is contacted by axons of uninfected neuron(s), showing that the dendrites of this neuron were competent to form synapses. Figure 7A shows that synaptic density along VsiAgrin-infected axons was reduced by 38± 2% (p<0.001; n=16 segments from 8 neurons, 3 separate experiments) relative to VsiMock-infected axons .
Figure 7. Depletion of Tm-agrin in dendrites reduces synapse density more than depletion of Tm-agrin in axons.
Synapse density was assayed in cultures such as shown in Figure 6, along segments of dendrites where it was clear that the only contact was between an infected axon and uninfected dendrite or vice versa. (A) Synaptic density along contacts with VsiAgrin-infected axons was reduced by 38± 2% relative to VsiMock-infected axons (p<0.01; n=16 segments from 8 neurons, 3 separate experiments), whereas (B) Synaptic density along VsiAgrin-infected dendrites was reduced by 79± 1% relative to VsiMock-infected dendrites (p<0.0001; n=14 segments from 8 neurons, 3 separate experiments). Synaptic density with VsiAgrin-infected axons was significantly different from synaptic density with VsiAgrin-infected dendrites (p<0.0001). Bars show mean ± SEM. Asterisks indicate values significantly different from VsiMock control.
Figure 6. Depletion of Tm-agrin in dendrites or axons alone reduces synapse density.
Hippocampal neuron cultures were infected with a reduced concentration of shRNA lentivirus to obtain an infection rate of approximately 40%. This allowed the identification of regions of contact between axons and dendrites where either the axon or the dendrite, but not both, emanated from an infected neuron. In each 4-part panel, X’ shows GFP immuno-staining (blue in merge, indicting virus infected axons or dendrites), X” shows PSD-95 (green in merge) and X”’ shows synaptotagmin (SYN) red in merge) in the same field. Yellow arrowheads indicate examples of counted synapses. (A) Example of axon(s) from a VsiMock-infected neuron penetrating an uninfected dendritic network. Synapse formation is unaffected, with numerous PSD-95 and synaptotagmin puncta overlapping along the regions of contact between infected axons and uninfected dendrites. (B) In contrast, a VsiAgrin-infected axon interacting with an uninfected dendritic network shows relatively few overlapping puncta, although many PSD-95 (green in merge) puncta are present. In the same field many synapses are seen where dendrites from the same uninfected neuron are contacted by axons of an uninfected neuron. (C) Part of the dendritic arbor of a VsiMock-infected neuron innervated by axons from uninfected neurons, displaying a typical density of synapses. (D) In contrast, the dendritic arbor of a VsiAgrin-infected neuron displays a low density of synapses. Bar = 10 μm.
Figure 6C shows part of the dendritic arbor of a VsiMock-infected neuron innervated by axons from uninfected neurons, displaying a typical density of synapses. In contrast, the dendritic arbor of a VsiAgrin-infected neuron (Figure 6D) displays a low density of synapses. Synaptic density along VsiAgrin-infected dendrites was reduced by 79± 1% relative to VsiMock-infected dendrites (p<0.0001) (Figure 7B). Synaptic density with VsiAgrin-infected axons was significantly different from synaptic density with VsiAgrin-infected dendrites (p<0.0001; n=14 segments from 8 neurons, 3 separate experiments). These results suggest that dendritic Tm-agrin plays a more important role in synapse formation in this system than does axonal Tm-agrin, but both appear to play a significant role.
Discussion
We have used a lentivirus to efficiently introduce a specific RNAi sequence into maturing cultured hippocampal neurons, yielding an overall reduction of agrin message by what appears to be more than 95% at 3 days after infection. PCR indicated that the cultured neurons expressed the transmembrane form of agrin (Tm-agrin) rather than the secretory form, consistent with previous reports for CNS neurons (Neumann et al., 2001). Tm-agrin protein was markedly reduced by 3 days post-infection and virtually eliminated by 7 days. The lag in reduction of agrin protein relative to reduction of message was presumably related to the rate of turnover of the protein. One striking result of decreased agrin expression was a 43% decrease in dendritic filopodia density at 7 days after infection with the siAgrin lentivirus. This is consistent with our previous results (McCroskery et al., 2006) and those of Annies et al (2006) indicating that Tm-agrin positively regulates the formation and stability of filopodia on the neurites of immature neurons. The present results extend the role of agrin in regulation of filopodia to the dendrites of maturing hippocampal neurons.
In contrast to previous reports of decreases in dendrite length (Ferreira, 1999; Ksiazek et al., 2007) and branching (Mantych and Ferreira, 2001) when agrin expression was inhibited or absent, we found a small increase in total dendrite length and in the number of dendrite branches. The increase in branches was surprising in itself since there was a 43% reduction in filopodia in the same neurons and filopodia are considered to be a precursor to de novo branch formation (Szebenyi et al., 1998). It is likely that some destabilization of the F-actin core of filopodia is required for the invasion of microtubules (Kornack and Giger, 2005) in transition to a branch, thus the reduction in agrin in this system may cause the small net increase observed in the number of branches, by destabilizing the actin filaments of filopodia. The differences between the present and previously published results with respect to morphology of the dendritic arbor might be ascribed to differences in the systems used and in developmental stage. Some of the previous studies involved cultures containing astroglia conditioned medium or astroglia, sources of soluble agrin (Ferreira, 1999), which can increase extension and branching of dendrites in week-old cultures (Mantych and Ferreira, 2001). The other (Ksiazek et al., 2007) involved in vivo development, where glia play an important role. In contrast, cultures used in our studies contained defined culture medium and few glial cells. It remains to be determined whether Tm-agrin in neurons and soluble agrin from glia or other sources, such as the proteolytic cleavage of Tm-agrin (Reif et al., 2007) play different roles in neuronal morphogenesis in vivo. In relation to this, it has recently been shown that activity-dependent formation of dendritic filopodia in hippocampal slices of young adult mice requires the proteolytic cleavage of agrin by neurotrypsin (Matsumoto-Miyai et al., 2009). It is not yet known whether proteolytic cleavage of agrin is involved in filopodia regulation or synaptogenesis during development, as opposed to plasticity in adults.
A role for agrin in mammalian CNS synapse formation has been supported by in vitro studies (Ferreira, 1999, Bose et al., 2000) and more recently by a study in vivo (Ksiazek et al., 2007). In the latter study, the density of synaptic spines and excitatory synapses on cortical pyramidal neuron dendrites was reduced ∼ 30% in young agrin null mice that were rescued from perinatal death by targeted expression of agrin in motor neurons. Our present finding that synaptic density on the dendrites of cultured hippocampal neurons was reduced ∼40% by infection with an agrin specific RNAi expressing lentivirus during a period of rapid synapse formation is consistent with these earlier findings.
The authors of these previous studies have suggested that agrin from the synapse-forming neurons (Ksiazek et al., 2007) or from glia (Tournell et al., 2006) may act upon receptors such as MuSK (Garcia-Osta et al., 2006), or the alpha3 subunit of the Na+/K+- ATPase (Hilgenberg et al., 2006) to organize or stabilize synapses. Another possible target for agrin is the agrin-binding putative MuSK coreceptor Lrp4, which is also expressed in brain (Kim et al., 2008; Zhang et al, 2008). The results of the present study suggest an additional pathway through which Tm-agrin may promote synaptogenesis. We show that the reduction in dendritic synapse density resulting from infection with the siAgrin lentivirus is correlated with a similar reduction in the density of dendritic filopodia, which are believed to facilitate synaptogenesis by recruiting the passing axons and in some instances are converted into stable dendritic spines (Konur and Yuste, 2004; Johnson and Ouimet, 2006). Moreover, in experiments where only ∼ 40% of neurons were infected with the siAgrin lentivirus, we showed that suppression of Tm-agrin in dendrites alone is twice as effective in reducing dendritic synapse density as suppression in the axons alone. Therefore, the present results suggest a physiological role of Tm-agrin in the promotion of synaptogenesis through positive regulation of dendritic filopodia. However, we also observed that dendritic synapse density was significantly reduced when siAgrin-infected axons contacted uninfected dendrites. This is consistent with the idea that multiple pathways involving agrin expressed by both presynaptic and post-synaptic neurons are involved in the regulation of synapse formation or stabilization.
The signal transduction pathway by which Tm-agrin regulates filopodia is poorly understood. A recent study has shown that the induction of filopodia by antibodies that cluster Tm-agrin is dependent on the formation of lipid rafts and requires the activation of Fyn and MAP kinase (Ramseger et al., 2009). The authors suggest that Tm-agrin may act as a receptor or co-receptor to activate this pathway. Studies on the induction of filopodia by transfection of muscle cells (Uhm et al., 2001) or neurons (McCroskery et al., 2006) with Tm-agrin constructs indicate that induction of filopodia is not dependent on the Z-site inserts of the C-terminal moiety of agrin that are required for activation of MuSK (Glass et al., 1996) or to the alpha3 subunit of the Na+/K+-ATPase (Hilgenberg et al., 2006) and in fact requires only the N-terminal moiety of the molecule. Thus regulation of filopodia by endogenous Tm-agrin may not depend on binding to either MuSK or to the alpha3 subunit of the Na+/K+-ATPase. Induction of filopodia by transfection of agrin in SH-SY5Y neuronal cells is dependent on the activation of the Rho family GTPase Cdc42 (McCroskery et al., 2006), which plays a well-known role in stimulation of actin filament formation (reviewed in Ridley, 2006), and appears to be crucial for the induction of acetylcholine receptor aggregation on myotubes by agrin (Weston et al., 2000; Weston et al., 2003). Interestingly, it has been shown that receptor-mediated activation of Cdc42 can result in activation of the MAP kinase pathway (Kang et al., 2008). This may tie the observations of (McCroskery et al. 2006) to those of Ramseger et al. (2009). However, the upstream signaling pathway regulated by Tm-agrin leading to Cdc42 activation is not known. The N-terminal moiety of agrin contains binding sites for heparan sulfate and chondroitin sulfate glycosamino glycans (GAGs), which can interact with a variety of glycoproteins and growth factors (Cotman et al., 1999; Kim et al., 2003; Winzen et al., 2003). These GAG chains appear to be important for the activation of Cdc42 in Tm-agrin-transfected cells and the induction of filopodia in neurons (unpublished data). It is thus of interest that reduction in the expression of the membrane-bound glycoprotein M6a (Alfonso et al., 2005) or the transmembrane proteoglycan syndecan 2, which is expressed in the developing hippocampus (Ethell and Yamaguchi, 1999; Lin et al., 2007) results in a reduction of filopodia and synapses in hippocampal neuron cultures (Lin et al., 2007).
In summary, this study has provided evidence for a role of Tm-agrin in the regulation of CNS synapse formation through its effects on dendritic filopodia. This suggests a novel pathway whereby Tm-agrin and other membrane glycoproteins or proteoglycans may regulate synaptogenesis in the CNS.
Acknowledgements
This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso J, Fernandez ME, Cooper B, Flugge G, Frasch AC. The stress-regulated protein M6a is a key modulator for neurite outgrowth and filopodium/spine formation. Proc Natl Acad Sci U S A. 2005;102:17196–17201. doi: 10.1073/pnas.0504262102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annies M, Bittcher G, Ramseger R, Loschinger J, Woll S, Porten E, Abraham C, Ruegg MA, Kroger S. Clustering transmembrane-agrin induces filopodia-like processes on axons and dendrites. Mol Cell Neurosci. 2006;31:515–524. doi: 10.1016/j.mcn.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bose CM, Qiu D, Bergamaschi A, Gravante B, Bossi M, Villa A, Rupp F, Malgaroli A. Agrin controls synaptic differentiation in hippocampal neurons. J Neurosci. 2000;20:9086–9095. doi: 10.1523/JNEUROSCI.20-24-09086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, De Camilli P. Glutamate regulates actin-based motility in axonal filopodia. Nat Neurosci. 2001;4:787–793. doi: 10.1038/90489. [DOI] [PubMed] [Google Scholar]
- Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Halfter W, Cole GJ. Identification of extracellular matrix ligands for the heparan sulfate proteoglycan agrin. Exp Cell Res. 1999;249:54–64. doi: 10.1006/excr.1999.4463. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityateva G, Hammond M, Thiel C, Ruonala MO, Delling M, Siebenkotten G, Nix M, Dityatev A. Rapid and efficient electroporation-based gene transfer into primary dissociated neurons. J Neurosci Methods. 2003;130:65–73. doi: 10.1016/s0165-0270(03)00202-4. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol. 1999;144:575–586. doi: 10.1083/jcb.144.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A. Abnormal synapse formation in agrin-depleted hippocampal neurons. J Cell Sci. 1999;112(Pt 24):4729–4738. doi: 10.1242/jcs.112.24.4729. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. J Neurosci. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Gingras J, Rassadi S, Cooper E, Ferns M. Agrin plays an organizing role in the formation of sympathetic synapses. J Cell Biol. 2002;158:1109–1118. doi: 10.1083/jcb.200203012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras J, Rassadi S, Cooper E, Ferns M. Synaptic transmission is impaired at neuronal autonomic synapses in agrin-null mice. Dev Neurobiol. 2007;67:521–534. doi: 10.1002/dneu.20304. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hilgenberg LG, Su H, Gu H, O’Dowd DK, Smith MA. alpha3Na(+)/K(+)-ATPase Is a Neuronal Receptor for Agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190:72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Johnson OL, Ouimet CC. A regulatory role for actin in dendritic spine proliferation. Brain Res. 2006;1113:1–9. doi: 10.1016/j.brainres.2006.06.116. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Cotman SL, Halfter W, Cole GJ. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J Neurobiol. 2003;55:261–277. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- Konur S, Yuste R. Imaging the motility of dendritic protrusions and axon terminals: roles in axon sampling and synaptic competition. Mol Cell Neurosci. 2004;27:427–440. doi: 10.1016/j.mcn.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Giger RJ. Probing microtubule +TIPs: regulation of axon branching. Curr Opin Neurobiol. 2005;15:58–66. doi: 10.1016/j.conb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, Bettler B, Ruegg MA. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci. 2007;27:7183–7195. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Li Z, Hilgenberg LG, O’Dowd DK, Smith MA. Formation of functional synaptic connections between cultured cortical neurons from agrin-deficient mice. J Neurobiol. 1999;39:547–557. doi: 10.1002/(sici)1097-4695(19990615)39:4<547::aid-neu8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Lin YL, Lei YT, Hong CJ, Hsueh YP. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Mantych KB, Ferreira A. Agrin differentially regulates the rates of axonal and dendritic elongation in cultured hippocampal neurons. J Neurosci. 2001;21:6802–6809. doi: 10.1523/JNEUROSCI.21-17-06802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Lüscher D, Hettwer S, Wölfel J, Ladner AP, Ster J, Gerber U, Rülicke T, Kunz B, Sonderegger P. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell. 2009;136:1161–71. doi: 10.1016/j.cell.2009.02.034. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol Cell Neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- McMahan UJ, Horton SE, Werle MJ, Honig LS, Kroger S, Ruegg MA, Escher G. Agrin isoforms and their role in synaptogenesis. Curr Opin Cell Biol. 1992;4:869–874. doi: 10.1016/0955-0674(92)90113-q. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Daniels MP. Targeting of recombinant agrin to axonal growth cones. Mol Cell Neurosci. 2003;24:1180–1196. doi: 10.1016/j.mcn.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neumann FR, Bittcher G, Annies M, Schumacher B, Kroger S, Ruegg MA. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci. 2001;17:208–225. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Pun S, Tsim KW. Antisense agrin cDNA transfection blocks neuroblastoma cell-induced acetylcholine receptor aggregation when co-cultured with myotubes. Mol Cell Neurosci. 1997;10:87–99. doi: 10.1006/mcne.1997.0637. [DOI] [PubMed] [Google Scholar]
- Ramseger R, White R, Kröger S. Transmembrane form agrin-induced formation requires lipid rafts and the activation of Fyn and MAPK. J Biol Chem. 2009;284:7697–7705. doi: 10.1074/jbc.M806719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wolfel J, Luscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, Sonderegger P. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007;21:3468–3478. doi: 10.1096/fj.07-8800com. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Serpinskaya AS, Feng G, Sanes JR, Craig AM. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev Biol. 1999;205:65–78. doi: 10.1006/dbio.1998.9112. [DOI] [PubMed] [Google Scholar]
- Smith MA, Hilgenberg LG. Agrin in the CNS: a protein in search of a function? Neuroreport. 2002;13:1485–1495. doi: 10.1097/00001756-200208270-00001. [DOI] [PubMed] [Google Scholar]
- Stone DM, Nikolics K. Tissue- and age-specific expression patterns of alternatively spliced agrin mRNA transcripts in embryonic rat suggest novel developmental roles. J Neurosci. 1995;15:6767–6778. doi: 10.1523/JNEUROSCI.15-10-06767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Callaway JL, Dent EW, Kalil K. Interstitial branches develop from active regions of the axon demarcated by the primary growth cone during pausing behaviors. J Neurosci. 1998;18:7930–7940. doi: 10.1523/JNEUROSCI.18-19-07930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournell CE, Bergstrom RA, Ferreira A. Progesterone-induced agrin expression in astrocytes modulates glia-neuron interactions leading to synapse formation. Neuroscience. 2006;141:1327–1338. doi: 10.1016/j.neuroscience.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Tsen G, Halfter W, Kroger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995;270:3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- Uhm CS, Neuhuber B, Lowe B, Crocker V, Daniels MP. Synapse-forming axons and recombinant agrin induce microprocess formation on myotubes. J Neurosci. 2001;21:9678–9689. doi: 10.1523/JNEUROSCI.21-24-09678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston C, Gordon C, Teressa G, Hod E, Ren XD, Prives J. Cooperative regulation by Rac and Rho of agrin-induced acetylcholine receptor clustering in muscle cells. J Biol Chem. 2003;278:6450–6455. doi: 10.1074/jbc.M210249200. [DOI] [PubMed] [Google Scholar]
- Weston C, Yee B, Hod E, Prives J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J Cell Biol. 2000;150:205–212. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzen U, Cole GJ, Halfter W. Agrin is a chimeric proteoglycan with the attachment sites for heparan sulfate/chondroitin sulfate located in two multiple serine-glycine clusters. J Biol Chem. 2003;278:30106–30114. doi: 10.1074/jbc.M212676200. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]