Abstract
Background
Deficiency of X-linked Inhibitor of Apoptosis (XIAP), caused by BIRC4 gene mutations, is the second known cause of X-linked Lymphoproliferative Disease (XLP), a rare primary immunodeficiency that often presents with life-threatening hemophagocytic lymphohistiocytosis (HLH). Rapid diagnosis of the known genetic causes of HLH, including XIAP deficiency, facilitates the initiation of life-saving treatment and preparation for allogeneic hematopoietic cell transplantation (HCT). Until now, a rapid screening test for XIAP deficiency has not been available.
Methods
In order to develop a flow cytometric screening test for XIAP deficiency, we first used lymphoblastic cell lines generated from controls and patients with BIRC4 mutations to identify 2 commercially available antibodies specific for native intracellular XIAP. Next, we used these antibodies to study control whole blood leukocyte XIAP expression. We then studied XIAP expression in leukocytes from patients with XLP due to BIRC4 mutations, maternal carriers, and patients following HCT.
Results
XIAP was expressed by the majority of all whole blood nucleated cells in normal controls. In contrast, XIAP was absent or decreased in all lymphocyte subsets, monocytes and granulocytes from 4 unrelated patients with XLP due to BIRC4 mutations. Bimodal distribution of XIAP expression was evident in two maternal carriers, with significant skewing towards cells expressing normal XIAP. Bimodal distribution was also observed in a patient following HCT.
Conclusions
Flow cytometric analysis of intracellular XIAP provides a rapid screening test for XLP due to XIAP deficiency. It also allows carrier detection and can be used to monitor donor versus recipient reconstitution following HCT.
Keywords: X-linked lymphoproliferative disease, XLP, X-linked inhibitor of apoptosis, XIAP, hemophagocytic lymphohistiocytosis, HLH, flow cytometry, BIRC4
INTRODUCTION
Deficiency of X-linked Inhibitor of Apoptosis (XIAP), caused by BIRC4 gene mutations, is the second known cause of X-linked Lymphoproliferative Disease (XLP), a rare primary immunodeficiency that often presents with life-threatening hemophagocytic lymphohistiocytosis (HLH) (1). The ability to rapidly diagnose the known genetic causes of HLH, including BIRC4 mutation, can hasten the initiation of life-saving treatment and preparation for allogeneic hematopoietic cell transplantation (HCT). Currently, rapid screening methods exist for 2 of the known genetic causes of HLH: SAP deficiency (2), the most common cause of XLP, and perforin deficiency (3), a common cause of Familial HLH. Until now, a rapid screening test has not been available for XLP due to XIAP deficiency, and sequencing of the BIRC4 gene has been the only means of diagnosis. The work that we present here describes our development of a rapid whole blood screening test for XIAP deficiency using flow cytometric analysis of intracellular XIAP expression.
METHODS
Patients and Normal Control Samples
Patients and relatives were evaluated at Cincinnati Children’s Hospital Medical Center. EDTA-anti-coagulated blood samples were taken after informed consent was obtained according to an Institutional Review Board-approved research protocol. Pediatric control samples were obtained from healthy pediatric patients and de-identified except for age and gender. Inclusion criteria for the normal pediatric samples included normal CBC indices and white blood cell differential. Adult control samples were obtained from healthy adult volunteers associated with the Cincinnati Children’s Hospital Diagnostic Immunology Laboratory. All control samples were obtained according to IRB-approved policies. Samples were held at room temperature prior to analysis.
Mutational Analysis of the BIRC4 Gene
Genomic DNA was extracted from peripheral blood according to standard protocols. The coding regions and the exon-intron boundaries of the BIRC4 gene were amplified by polymerase chain reaction (PCR) using primers flanking each of the 6 exons by standard methods. Direct bi-directional sequencing of PCR products was performed using the ABI 3730XL sequencer (PE Biosystems, Foster City, CA) with the same primers used for PCR amplification. Mutational data is reported based on the recommendation by the American College of Medical Genetics that nucleotide +1 is the A of the ATG-translation initiation codon. Primer sequences are available upon request.
Generation of EBV-immortalized Lymphoblastic B-cell lines (LCLs)
Peripheral blood mononuclear cells (PBMC) from patients or controls were separated from whole blood by Ficoll-Hypaque density gradient centrifugation. PBMC were incubated with EBV-containing supernatant in RPMI 1640 medium (Mediatech/Cellgro) with added 20% fetal bovine serum (FBS) (Gibco/Invitrogen), glutamine (Gibco/Invitrogen), penicillin and streptomycin (Gibco/Invitrogen) and cyclosporin (2mcg/ml) to generate LCLs (4).
Western Blot Analysis of XIAP
PBMC from patients and controls were separated from whole blood by Ficoll-Hypaque density gradient centrifugation, followed by lysis of remaining red blood cells with incubation in ammonium chloride. Cells were then washed and pelleted. Alternatively, LCLs from patients and controls were washed and pelleted. Cell pellets were lysed in 1% NP40 Lysis Buffer with Complete Protease Inhibitors (Roche) and cleared by centrifugation. Protein concentration was determined by BCA assay (Pierce) and 5–90 µg total protein was separated by SDS-PAGE. After transfer to nitrocellulose, blots were probed with monoclonal anti-XIAP antibodies (clone 28, BD Biosciences, or clone 2F1, Abcam) followed by anti-β-actin antibody to serve as loading control (clone AC-15, Sigma). Bound antibodies were detected using appropriate HRP-conjugated secondary antibodies and SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Flow Cytometric Analysis of XIAP
Anticoagulated whole blood or LCLs from patients and controls were first fixed and permeabilized using a commercially available kit (Intraprep, Beckman Coulter). Cells were then incubated with one of several commercially available monoclonal anti-XIAP antibodies or equivalent microgram amounts of species-appropriate isotype control antibody for 15 minutes at room temperature. LCLs were studied using the mouse monoclonal antibodies: clone 28 (BD Biosciences), clone 48 (BD Biosciences), and clone 2F1 (Abcam), and the rabbit monoclonal anti-XIAP antibody: clone 3B6 (Cell Signaling). Whole blood samples were studied using either 1 mcg of clone 2F1 or 0.25 mcg of clone 48. After washing, cells were incubated with either PE-conjugated goat anti-mouse (BD Biosciences) or anti-rabbit (Jackson ImmunoResearch Laboratories) secondary antibodies as appropriate for 15 minutes at room temperature while protected from light. LCL samples were then washed and analyzed. Whole blood samples were washed followed by blocking of residual PE-conjugated anti-mouse antibody with mouse IgG1 antibody (BD Biosciences). Standard fluorochrome-conjugated surface marker antibody staining was then performed using mouse anti-CD3-PerCP, -CD4-Pacific Blue, -CD19-FITC, and -CD56-APC (BD Biosciences). Alternatively, to study XIAP expression within invariant natural killer T cells (iNKT cells), anticoagulated blood samples were first incubated with APC- or FITC-conjugated PBS-57 loaded CD1d tetramer (provided by the NIH Tetramer Core Facility, Atlanta, Georgia). Samples were analyzed on a BD FACSCanto Flow Cytometer using FACSDIVA software, with data analysis using FCS Express (De Novo Software). Lymphocytes were identified by forward and right angle light scatter. On average, list mode parameters of 30,000 events within the lymphocyte gate were acquired. The percentage positive staining for intracellular XIAP was determined with comparison to the background staining, measured using irrelevant species and isotype-specific control antibodies. Patient samples were analyzed concurrently with control samples. The performance of the flow cytometer was routinely verified by standard flow cytometric quality control protocols.
RESULTS
Detection of BIRC4 Mutations
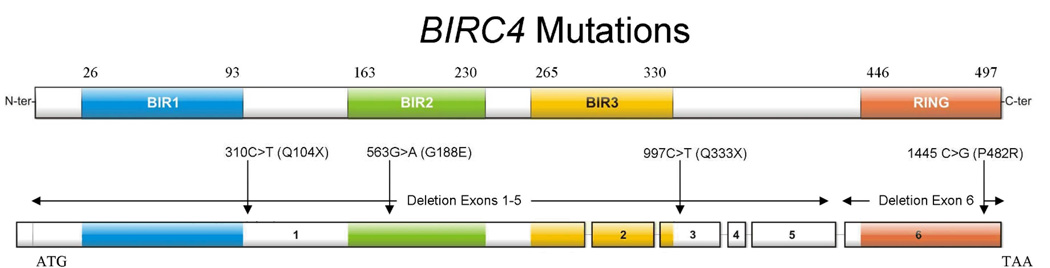
Mutations in the BIRC4 gene (which encodes XIAP) constitute the second most common (and second known) cause of XLP (1). Using standard amplification and sequencing methods performed by the clinical genetics laboratory at Cincinnati Children’s Hospital, we have identified 7 patients from 6 unrelated families who possess BIRC4 mutations. Patient 1 possesses an isolated deletion of exon 6. Patients 2 and 3 are maternally related and have large deletions spanning exons 1–5. Patient 4 possesses a nonsense mutation, 997C>T, resulting in an early stop codon, Q333X. Patient 5 was found to have a C-terminal missense mutation, 1445 C>G (P482R). Patient 6 possesses a nonsense mutation in exon 1, 310C>T, resulting in Q104X. Patient 7 possesses a missense mutation in exon 1, 563G>A (G188E). A schematic depicting the locations of the patient mutations within the BIRC4 gene and resultant XIAP protein is shown in Figure 1.
Figure 1.
A schematic of the 6 exons of the BIRC4 gene and resultant protein, XIAP, with patient mutations included.
Western Blot Analysis of XIAP Expression
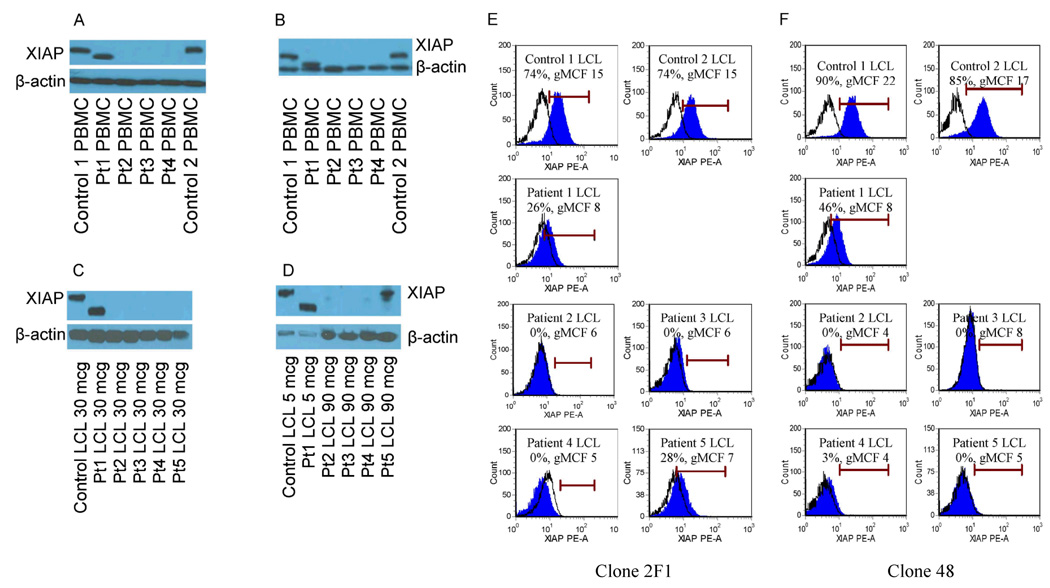
In order to anticipate the expected result of flow cytometric XIAP analysis of patient samples, XIAP expression was first studied by established western blotting techniques to determine the impact of patient BIRC4 mutation on protein expression. PBMC lysates were available for patients 1–4, and LCL lysates were available for patients 1–5. Using 2 different monoclonal antibodies against XIAP (clone 28, BD Biosciences, and clone 2F1, Abcam), western blot analysis of XIAP expression in PBMCs revealed that patient 1 possessed a shortened form of XIAP with lower than the normal predicted molecular weight, consistent with deletion of exon 6, indicating that this mutation allows for translation of truncated protein. Patients 2–4 showed a complete lack of protein (Figure 2A and 2B). Lysates generated from LCLs studied with the clone 28 antibody again revealed that patient 1 possessed a truncated protein, while patients 2–5 had undetectable protein when 20–30 mcg of protein was loaded into the gel (Figure 2C). However, a band corresponding to XIAP (appropriate molecular weight) was detectable from patient 5 with significant increase of protein loading (90 mcg) and extending the exposure time of the film for this patient (Figure 2D), indicating that this mutation likely allows for limited recognizable protein translation as detected by western blot analysis.
Figure 2.
Analysis of XIAP expression within PBMCs and LCLs generated from patients with XIAP deficiency and normal controls. Figure A and B show western blot analysis of PBMC lysates of patients 1–4, probed with clone 28 anti-XIAP antibody (A) or clone 2F1 (B). Gels were loaded with 30 mcg of protein. Figures C and D depict LCL lysates from patients 1–5 using the clone 28 antibody. Figure C shows 30 mcg of protein loaded into the gel. Figure D shows the same samples, but with 5 mcg of protein loaded for control and patient 1, and 90mcg of protein loaded for patients 2–5. β-actin serves as a loading control. Figures E and F illustrate flow cytometric detection of XIAP in LCLs generated from patients 1–5, with 2 controls shown for comparison, using the clone 2F1 (E) or clone 48 (F) anti-XIAP antibodies. Debris was excluded from analysis by creating a viable LCL gate based on the appearance of forward and right angle light scatter. Filled histograms represent XIAP staining, while open histograms represent isotype control antibody. Percentages of cells positive for XIAP and the geometric mean channel fluorescence (gMCF) are given within the histogram boxes.
Identification of Monoclonal Antibodies Specific for Native Intracellular XIAP
As described above, western blot analysis revealed that the BIRC4 mutations of patients 2–4 result in absence of detectable XIAP, while the mutations found in patients 1 and 5 result in expression of detectable mutated protein. Thus, we anticipated that flow cytometric analysis of XIAP should mirror these results when using an antibody specific for native intracellular XIAP. Accordingly, we used the LCLs generated from controls and patients 1–5 to identify commercially available antibodies that are specific for XIAP when used for flow cytometric analysis. We found that the clone 28 (BD Biosciences) and 3B6 (Cell Signaling) anti-XIAP antibodies were non-specific for detection of native intracellular XIAP by flow cytometry, as all patient LCLs appeared to have normal XIAP expression when these antibodies were used (data not shown). However, two mouse monoclonal antibodies, clone 2F1 (Abcam), which recognizes an epitope within the C-terminal region of XIAP spanning amino acids 352–449, and clone 48 (BD Biosciences), which recognizes an epitope within the region of XIAP spanning amino acids 268–426, showed clearly detectable intracellular XIAP in control LCLs and absence within LCLs generated from patients 2–4 (Figures 2E and 2F). Study of XIAP detection by flow cytometry in the LCL generated from Patient 1 (who has readily detectable truncated protein by western blot analysis) revealed XIAP detection less than the control LCLs. Additionally, study of the LCL generated from patient 5 revealed a low amount of protein staining using the clone 2F1 antibody and absence using the clone 48 antibody.
Peripheral Blood Leukocyte XIAP Expression in Normal Controls
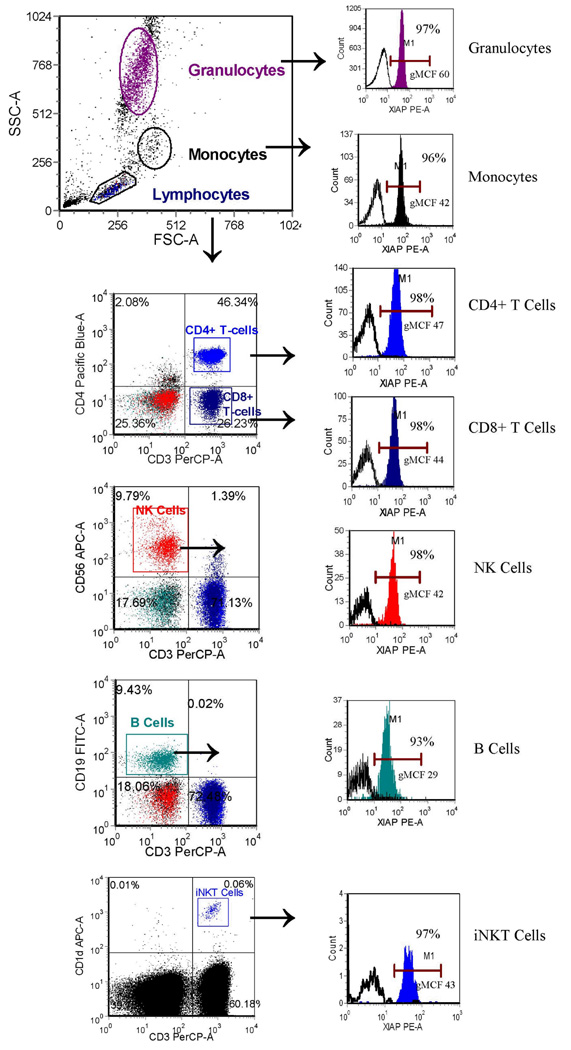
Rapid flow-cytometric screening tests should ideally be able to be performed using fresh whole blood samples, which avoids the time needed to generate cell lines. First, to determine our ability to detect normal XIAP in peripheral blood leukocytes, we used the clone 2F1 and clone 48 anti-XIAP antibodies to study 78 pediatric and 35 adult normal controls. EDTA-anti-coagulated blood samples were used based upon preliminary work showing optimal XIAP staining using EDTA versus blood collection tubes containing heparin or acid citrate dextrose (ACD) (data not shown). XIAP was detectable in the majority of all CD4+ and CD8+ T cells, NK cells, and B cells, as well as monocytes and granulocytes (Figure 3, Table 1). The clone 48 antibody provided consistently brighter staining compared to the clone 2F1 antibody, with higher mean channel fluorescence that translated into a higher percentage of each cell type having detectable XIAP compared to isotype control, keeping in mind that a PE-conjugated secondary reagent was used.
Figure 3.
Flow cytometric detection of XIAP in peripheral whole blood granulocytes, monocytes, CD4+ T cells, CD8+ T cells, NK cells, and B cells, as described in the methods section, using the clone 48 antibody. Gating strategy is as shown. Filled histograms represent XIAP staining, while open histograms represent control antibody. Percentages of cells positive for XIAP and the geometric mean channel fluorescence (gMCF) are given within the histogram boxes.
Table 1.
1A: Expression of XIAP (% of cells positive) in normal control adult and pediatric whole blood samples, using the clone 2F1 antibody. 1B: Expression of XIAP (% of cells positive and geometric mean channel fluorescence (gMCF)) in normal control adult and pediatric whole blood samples using the clone 48 antibody.
| Control Group | CD3+/CD4+ XIAP + |
CD3+/CD8+ XIAP+ |
CD3−/CD56+ XIAP+ |
CD3−/CD19+ XIAP+ |
|---|---|---|---|---|
| 1A. Clone 2F1 | ||||
| Pediatric: ages 7 months to 18 years, n=56, 45% male |
65–97%# | 61–96% | 59–95% | 45–90% |
| Adult: ages 19 to 60 years, n=11 , 36% male |
77–98% | 75–100% | 60–100% | 48–92% |
| 1B. Clone 48 | ||||
| Pediatric: ages 1year to 18 years; n=22, 45% male |
93–99% gMCF* 13–41 |
92–100% gMCF 13–39 |
93–100% gMCF 14–39 |
87–98% gMCF 10–27 |
| Adult: ages 22 to 60 years, n=24, 29% male |
94–100% gMCF 23–50 |
94–100% gMCF 21–52 |
94–100% gMCF 20–50 |
91–98% gMCF 14–35 |
percent of cells positive for XIAP, range calculated as mean +/− 2 standard deviations
geometric mean channel fluorescence, range calculated as mean +/− 2 standard deviations
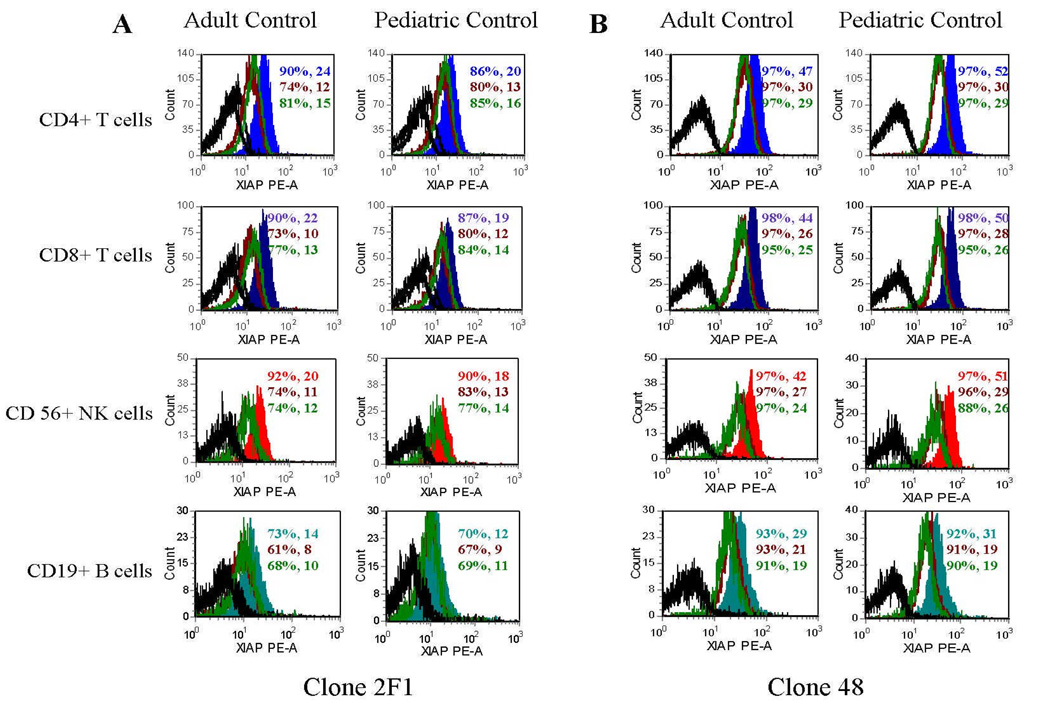
With regard to normal lymphocyte XIAP expression, XIAP expression was consistently lowest in B cells compared to CD4+ T cells, CD8+ T cells, and NK cells. Using student’s t-test, the differences between B cells and the other lymphocyte subpopulations were found to be statistically significant regardless of the antibody used or age group (for all comparisons p<0.02). Brighter XIAP staining was also noted in adult control versus pediatric control samples. To determine whether this could be due to timing differences in handling of adult and pediatric samples (adult control samples were generally analyzed within 6 hours of being drawn whereas the pediatric samples were analyzed 15–30 hours following blood draw), adult and pediatric samples were repeatedly studied over 36 hours. Adult and pediatric controls had comparable XIAP expression when analyzed immediately after sampling, and both groups showed an obvious decrease in detection of XIAP 12–36 hours after blood draw (Figure 4), indicating that there is likely no difference between adult and pediatric lymphocyte XIAP expression.
Figure 4.
Flow cytometric detection of XIAP in pediatric and adult peripheral blood CD4+ T cells, CD8+ T cells, NK cells, and B cells at 0, 12, and 36 hours after obtaining fresh blood samples. Colored filled histograms represent XIAP expression at 0 hours after blood samples were obtained, maroon unfilled histograms represent XIAP expression at 12 hours after blood samples obtained, and green unfilled histograms represent XIAP expression at 36 hours after blood samples were obtained. Black unfilled histograms represent isotype control antibody staining. 3A was obtained using the 2F1 antibody. 3B was obtained using the clone 48 antibody. Percentages of cells positive for XIAP and the gMCF are given within the histogram boxes.
Peripheral Blood Leukocyte XIAP Expression in 4 Unrelated Patients with BIRC4 Mutations
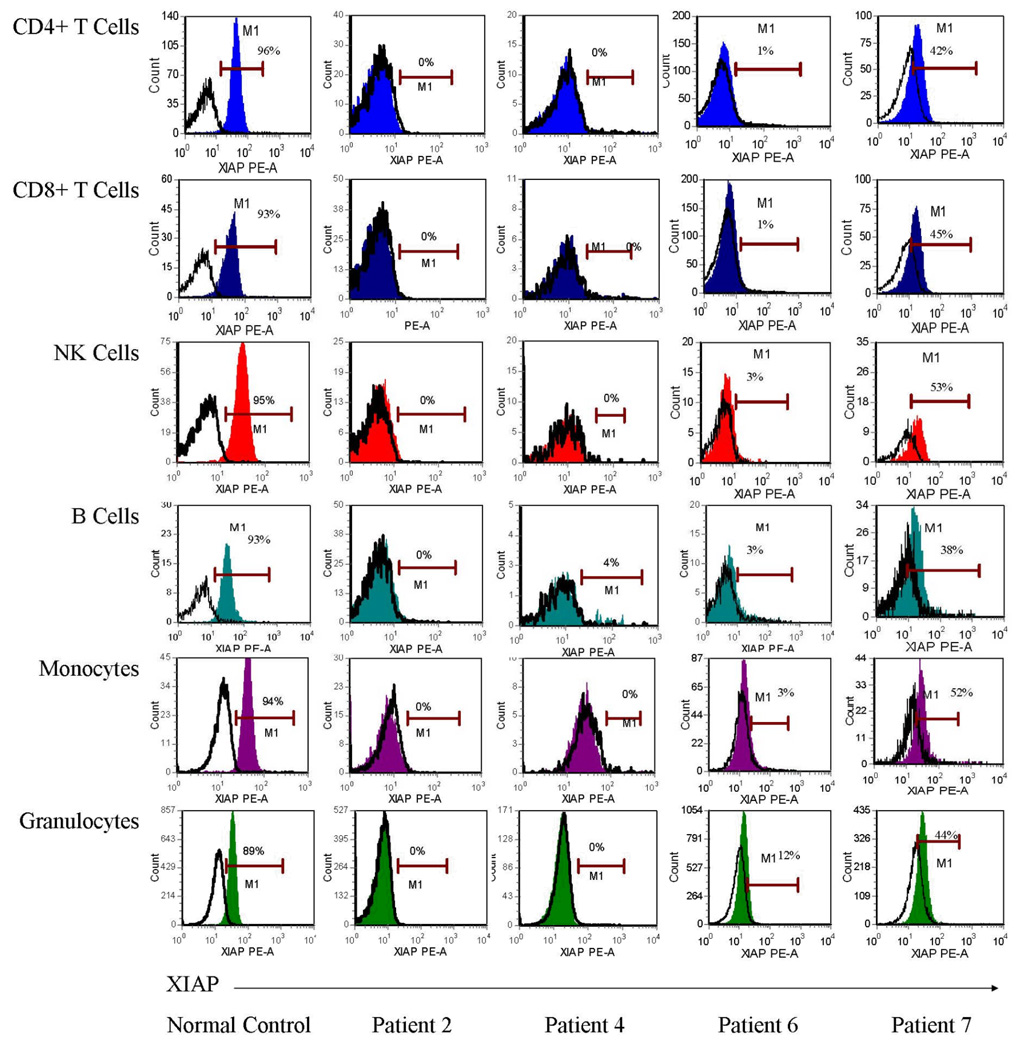
Next, to determine our ability to detect patient BIRC4 mutations with whole blood leukocyte XIAP analysis, fresh whole blood samples were obtained from patients 2, 4, 6, and 7. XIAP was absent in all CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and granulocytes of patients 2, 4, and 6, and decreased in patient 7 (Figure 5).
Figure 5.
Flow cytometric detection of XIAP in peripheral blood CD4+ T cells, CD8+ T cells, NK cells, B cells, monocytes and granulocytes from a representative normal control and XIAP deficient patients 2, 4, 6, and 7, using the clone 48 anti-XIAP antibody. Filled histograms represent XIAP staining, while open histograms represent control antibody.
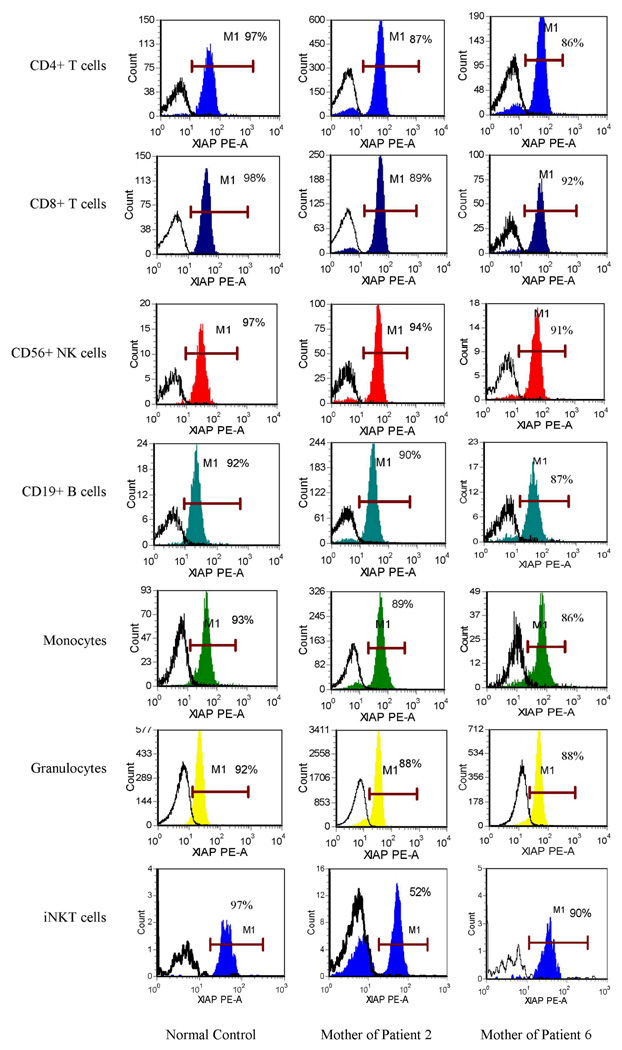
Detection of XIAP in Fresh Blood Samples from Maternal Carriers
Diagnosis of carrier status using flow cytometric analysis of protein expression is possible in several X-linked primary immune deficiencies through recognition of bimodal protein expression. However, significant skewing favoring cells expressing the normal gene has been reported in mothers of patients with XLP due to BIRC4 mutations, possibly due to a survival or selection advantage for the cells expressing normal XIAP (1). To determine whether we could detect carrier status with our method, and in order to study the effects of BIRC4 mutation on maternal leukocyte populations, fresh blood samples were obtained from the mothers of patients 1, 2, and 6. The mother of patient 1 did not show any abnormality of XIAP expression (data not shown), but this may be due to the fact that the deletion of exon 6 mutation that she carries results in expression of a detectable, truncated protein. The mother of patient 2, however, carries a deletion of exons 1–5, which results in absence of protein expression in cells harboring the mutated gene (as illustrated by her affected son). Multiple peripheral blood samples were obtained from this carrier. All leukocyte and lymphocyte subsets possessed significant skewing towards populations expressing normal XIAP, though bimodal distribution was typically evident (results obtained with the clone 48 antibody are shown in Figure 6). Interestingly, however, this carrier’s invariant Natural Killer T cells (iNKT cells) consistently displayed easily discernable bimodal distribution, with the two populations being nearly equivalent on four separate occasions. Bimodal distribution was more readily noted using the clone 48 antibody (though evident with either antibody) because of higher mean channel fluorescence of XIAP stained cells which enables wider separation from the isotype control histogram. The mother of patient 6 was also studied. She carries an early stop codon mutation in exon 1, resulting in absence of protein. This carrier also demonstrated marked skewing towards normal XIAP expression in all lymphocyte subsets, including the iNKT cell population.
Figure 6.
Flow cytometric detection of XIAP in peripheral blood CD4+ T cells, CD8+ T cells, NK cells, B cells, monocytes, granulocytes and invariant NKT cells from a representative control and the mothers of patients 2 and 6 using the clone 48 anti-XIAP antibody. Filled histograms represent XIAP staining, while open histograms represent control antibody.
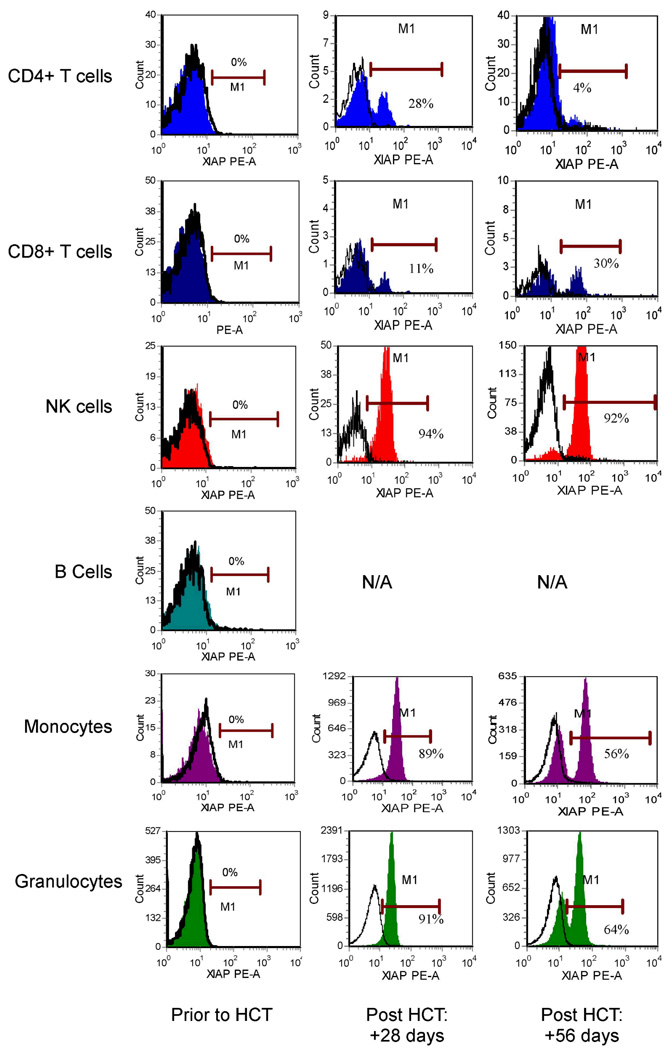
XIAP Expression in Fresh Blood Samples from Patients Following Allogeneic Hematopoietic Cell Transplant
We next sought to determine our ability to differentiate donor versus recipient reconstitution of hematopoietic cells in patients following allogeneic hematopoietic cell transplantation (HCT). Since their initial evaluations, patients 1 and 2 have undergone HCT, and both have developed mixed chimerism as determined by routine clinical genetic techniques using PCR-based amplification of informative chromosomal loci containing short tandem repeated sequences. Despite having between 9–18% recipient nucleated peripheral blood cells, flow cytometric study of patient 1 has failed to identify 2 populations of lymphocytes with differential XIAP staining (which is not surprising given the detectable XIAP noted on staining of the LCL generated from this patient) (data not shown). Patient 2, however, whose mutation leads to absence of protein expression, readily displays evidence of mixed chimerism by flow cytometric analysis (Figure 7). At 28 days following HCT, this patient displayed a bimodal pattern of XIAP expression limited to the T-cell compartment, while the other cell subsets displayed a pattern of XIAP expression consistent with normal donor expression. At this time point, approximately 0.05% of circulating nucleated white blood cells represented host T cells (in approximate numbers: 12,500 white blood cells/mcL of whole blood; 130 total lymphocytes/mcL of whole blood [based on clinical complete blood count] with 6% of lymphocytes being T cells; and 20% of T cells being XIAP positive [hypothesized to be of donor origin]). These results correlated with the 1% or less (limit of detection) of peripheral blood nucleated cells found to be of recipient origin by standard clinical genetic analysis of unsorted white blood cells, obtained in parallel. At 56 days following HCT, expression of XIAP appeared bimodal in all lineages, suggesting both donor and host cell populations within all hematopoietic cell types (mixed chimerism).
Figure 7.
Flow cytometric detection of XIAP in peripheral blood CD4+ T cells, CD8+ T cells, NK cells, B cells, monocytes and granulocytes from XIAP deficient patient 2, prior to and at +28 and +56 days following allogeneic hematopoietic cell transplant (HCT) using the clone 48 anti-XIAP antibody. Filled histograms represent XIAP staining, while open histograms represent control antibody. N/A=not applicable, as there was no significant B-cell reconstitution at these time points.
DISCUSSION
XLP is a rare but severe, life-threatening inherited immune deficiency which often acutely presents with HLH. Because of the gravity of the clinical situation of patients with HLH, the ability to rapidly screen patients for the known genetic causes of HLH can facilitate the initiation of life-saving treatment and preparation for HCT. The work we present here demonstrates that flow cytometric analysis of intracellular XIAP expression by peripheral blood lymphocytes provides a reliable rapid screening test to detect patients with XLP due to BIRC4 mutations. Importantly, our method was accurate in identifying all patients with absence of protein. Given that the majority of patients to date (described here and by Rigaud et al (1)) have mutations which result in absence of detectable protein expression, the majority of mutations are likely amenable to rapid detection with this method. Further experience is needed to more accurately assess the ability of this method to identify patients with mutations that do not result in absence of XIAP, such as in patients 1, 5, and 7 of this study. Patient 7 was the only patient with a missense mutation whose peripheral blood was available for study. Notably, we easily observed decreased detectable protein expression. We were also able to easily observe a significant abnormality of XIAP expression in the LCL derived from patient 5 (who also possesses a missense mutation). However, we were not able to study a fresh blood sample from this patient to confirm that a significant abnormality of protein expression would have been evident by peripheral blood analysis. We were additionally unable to study a peripheral blood sample from patient 1, whose deletion of exon 6 also allows (truncated) protein expression.
This existence of mutations resulting in detectable XIAP raises the question of whether it would be prudent to routinely screen samples using both the clone 2F1 and clone 48 antibodies, reasoning that one may garner a higher sensitivity to detect BIRC4 mutations that do not result in absence of protein. However, the utility and practicality of this remains uncertain. XIAP, originally discovered in 1996 by Duckett et al after a nucleotide database search for a mammalian homologue to the Drosophila IAP-like protein (5), is widely expressed in many tissues including cells of hematopoietic origin (1,5,6). We were therefore surprised that we were not able to confirm the presence of XIAP in all normal control lymphocytes using the clone 2F1 antibody (see Table 1). However, we did observe a shift in the entire population relative to isotype control, indicating that XIAP is probably present in every cell. The “negative” cells are likely the result of a technical limitation of this antibody. This is supported by the observation that the clone 48 antibody allowed detection of XIAP in essentially all normal control cells. The clone 48 antibody consistently yielded brighter staining than the 2F1 clone, with higher mean channel fluorescence compared to isotype control staining which translates into higher percentages of cell populations being “positive” for XIAP compared to isotype control. In our experience thus far, this facilitates the recognition of bimodal/chimeric patterns, and the clone 48 antibody likely represents the best single antibody for routine use.
A significant benefit of whole blood flow cytometric protein analysis, as compared to western blot analysis of protein lysates, is the ability to easily study individual cells and defined leukocyte and lymphocyte subpopulations. Because of this, we were able to note that B-cell expression of XIAP is consistently lower than the other lymphocyte subsets. This suggests that there may be differential function of XIAP between B cells and T and NK cells. Given that HLH is a disorder of cytotoxic lymphocyte function and homeostasis, a prominent role of XIAP within cytotoxic T and NK cells would not be surprising. Flow cytometry also offers the possibility for carrier detection, detection of donor reconstitution or mixed chimerism following allogeneic HCT, and detection of revertant mosaicism. Female carriers of other X-linked immune deficiencies, such as XLP due to SAP deficiency and X-linked chronic granulomatous disease (CGD), can often be identified by flow cytometry on the basis of a bimodal distribution, corresponding to random X-chromosome inactivation (lyonization) (2,7,8). In contrast, however, we noted a striking skewing towards cells expressing normal XIAP in 2 maternal carriers, supporting the possibility of a survival or selection advantage for XIAP-expressing cells. Even more intriguing, however, was the consistent observation of evenly distributed bimodal iNKT cell populations in the mother of patient 2 on multiple occasions, suggesting that XIAP may not be a requirement for iNKT cell development and survival as has been previously postulated (9).
In summary, we have described a multi-parameter flow cytometric assay that allows rapid screening of patients for XIAP deficiency. We recommend that testing be considered during the evaluation of male patients with HLH or any of the clinical phenotypes of XLP, as rapid diagnosis can facilitate initiation of treatment and preparation for allogeneic HCT. Given that mutations exist which allow the translation of abnormal but detectable protein, genetic sequencing of the BIRC4 gene remains the gold standard for confirming diagnosis. Notably, however, this assay may aid in the interpretation of sequencing results, as it offers a tool to correlate BIRC4 genotypes with XIAP expression profiles. This information is especially important when new and/or unreported sequence variants are encountered. Following patient diagnosis, flow cytometric screening for maternal carrier status can be performed, but with the realization that carriers may demonstrate significant skewing favoring the normal cell populations, which may prevent the recognition of bi-modal distribution. This method also offers a simple means of lineage-specific monitoring of donor versus host reconstitution following patient treatment with allogeneic HCT.
Acknowledgements
The authors wish to thank the patients and their families, who have generously contributed samples for study. We thank the NIH Tetramer Core Facility for supplying the PBS-57 loaded CD1d tetramer. PBS-57 was originally developed by Dr. Paul Savage. We also thank Barb Lawall and Sue Vergamini for technical flow support, and the clinical genetics lab at CCHMC for the BIRC4 genetic analysis. We are grateful that this work was supported by the Histiocytosis Association of America and by NIH R03 1R03AI079797-01.
This work was supported by grants from the Histiocytosis Association of America and by NIH R03 1R03AI079797-01.
References
- 1.Rigaud S, Fondanèche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 2.Tabata Y, Villanueva J, Lee SM, Zhang K, Kanegane H, Miyawaki T, et al. Rapid detection of intracellular SH2D1A protein in cytotoxic lymphocytes from patients with X-linked lymphoproliferative disease and their family members. Blood. 2005;105:3066–3071. doi: 10.1182/blood-2004-09-3651. [DOI] [PubMed] [Google Scholar]
- 3.Kogawa K, Lee SM, Villanueva J, Marmer D, Sumegi J, Filipovich AH. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002;99:61–66. doi: 10.1182/blood.v99.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Tosato G, Cohen JI. Generation of Epstein-Barr Virus (EBV)–Immortalized B Cell Lines. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im0722s76. sup 76:Unit 7.22. [DOI] [PubMed] [Google Scholar]
- 5.Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, et al. A conserved family of cellular genes related to baculovirus iap gene and encoding apoptosis inhibitors. EMBO. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 6.Mufti AR, Burstein E, Duckett CS. XIAP: cell death regulation meets copper homeostasis. Arch Biochem Biophys. 2007;463:168–174. doi: 10.1016/j.abb.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 8.Crockard AD, Thompson JM, Boyd NA, Haughton DJ, McCluskey DR, Turner CP. Diagnosis and carrier detection of chronic granulomatous disease in five families by flow cytometry. Int Arch Allergy Immunol. 1997;114:144–152. doi: 10.1159/000237660. [DOI] [PubMed] [Google Scholar]
- 9.Latour S. Natural killer T cells and X-linked lymphoproliferative syndrome. Curr Opin Allergy Clin Immunol. 2007;7:510–514. doi: 10.1097/ACI.0b013e3282f1bad6. [DOI] [PubMed] [Google Scholar]