Abstract
Purpose
This study tested a prediction stemming from the hypothesis that anastrozole users experience heightened vitreo-retinal traction. This hypothesis was based on the knowledge that menopause increases the risk of intraocular tractional events such as posterior vitreous detachments (PVDs).
Methods
Retinal thickness was measured for 3 groups of amenorrheic women: (1) anastrozole users and (2) tamoxifen users undergoing adjuvant therapy for early-stage breast cancer, and (3) control subjects not using hormonal medication. Foveal shape indices were derived for subjects without PVDs.
Results
For anastrozole users, the distance to the temporal side of the fovea became less than the distance to the nasal side at a sufficient height above the foveal base. This effect did not exist for control subjects; the between-group difference was appreciable. Results concerning tamoxifen users were inconclusive.
Conclusions
The foveas of women using anastrozole appear to be subjected to more tractional force than are the foveas of women not using any hormonal medication.
Keywords: Aromatase inhibitor, Estrogen, Eye, Optical coherence tomography, Retina, Vitreous
INTRODUCTION
Adjuvant endocrine therapy for early-stage hormone-receptor-positive breast cancer has changed over the last several years for post-menopausal women [1], as aromatase inhibitors (AIs) supplant or succeed the longstanding gold-standard, the selective-estrogen-receptor-modulator (SERM) tamoxifen. Although breast cancer recurrence rates are reduced [2], side effects due to AI-induced estrogen depletion are common [3–5] since estrogen receptors are ubiquitous and have many physiologic roles [6].
The retina contains estrogen receptors [7], and there is evidence that naturally occurring estrogen reductions can increase vitreo-retinal traction. In particular, macular holes (which target the fovea but are rare) and posterior vitreous detachments (PVDs, which can be more diffuse and are common) each occur more often for women than for men [8, 9] and are menopause-related [10, 11]. Each condition involves the development or action of tractional forces at or within the retina [12, 13]. These considerations suggest that AI-induced estrogen depletion may lead to heightened vitreo-retinal traction.
Although tractional forces within the eye cannot be measured directly in humans, highly precise retinal thickness measurements can be obtained using commercially available intraocular imaging devices, and these thickness measurements then can be used for deriving indices of foveal shape that capture the action of tractional forces that distort the vitreo-retinal interface. This is the approach used in the present study to evaluate the hypothesis that anastrozole – the most commonly used AI – leads to heightened vitreo-retinal traction. To make these measurements, we employed optical coherence tomography (OCT), a technology that is revolutionizing the way in which the retina is examined [14].
For this study, we derived indices of foveal shape for three groups of amenorrheic women: anastrozole users, tamoxifen users, and age-matched (middle-age) control subjects not using hormone replacement and with no histories of breast cancer or medication for breast cancer. We tested the prediction that foveal shape distortions are present to a greater degree for anastrozole users than for control subjects, and we aimed to determine whether salient differences exist between anastrozole users and tamoxifen users. In addition, we used OCT to detect PVDs, which is necessary before evaluating potential effects of vitreo-retinal traction on foveal shape. We had shown previously that anastrozole users are more likely than tamoxifen users and control subjects to have small retinal hemorrhages [15], and it is important to understand in their own right the factors that might contribute to the development of these hemorrhages.
METHODS
Subjects
Data from three groups of women ages 47–69 years old are reported: (1) women using 1 mg anastrozole daily as adjuvant therapy, (2) women using 20 mg tamoxifen daily as adjuvant therapy, and (3) a control group of women not using any hormonally acting medications. All subjects were amenorrheic for at least 6 months and all anastrozole users were post-menopausal. There were 27 anastrozole users, 40 control subjects, and 25 tamoxifen users who met all the eligibility criteria and for whom OCT scans were of acceptable quality. The mean ages and durations of medication use are provided in Table 1 for each entire subject group and also for each group subdivided according to the presence or absence of PVDs. The tamoxifen users were the only subject group for whom a demographic variable was related to the presence of a PVD, with the mean duration of medication use being 1.5 years greater for tamoxifen users with PVDs than without PVDs. No tamoxifen or anastrozole user had taken her medication for > 5 years or < 4 months.
Table 1.
Subject numbers (top), ages (middle), and durations of medication use (bottom) for each entire subject group and also for each subject group subdivided according to the presence or absence of PVDs For the ages and durations of use, non-parenthetical values are means and parenthetical values are standard deviations. Ages are given in years, and durations of use are given in months
| NUMBERS OF SUBJECTS | ||||
|---|---|---|---|---|
| All | no PVD | PVD | ||
| GROUP | Anastrozole | 27 | 13 | 14 |
| Control | 40 | 23 | 17 | |
| Tamoxifen | 25 | 19 | 6 | |
| AGE: years; mean (SD) | ||||
| All | no PVD | PVD | ||
| GROUP | Anastrozole | 57.8 (6.9) | 58.7 (6.4) | 56.5 (7.4) |
| Control | 58.5 (5.7) | 58.7 (6.8) | 58.2 (3.6) | |
| Tamoxifen | 55.6 (5.6) | 55.4 (6.1) | 55.6 (2.8) | |
| DURATION OF MEDICATION USE: months; mean (SD) | ||||
| All | no PVD | PVD | ||
| GROUP | Anastrozole | 18.1 (11.4) | 18.5 (12.5) | 17.8 (10.4) |
| Control | NA | NA | NA | |
| Tamoxifen | 26.2 (13.9) | 22.0 (10.6) | 39.5 (15.9) | |
All tamoxifen and anastrozole users had completed primary treatment for early-stage breast cancer, and all were able to perform their daily activities without restriction. None of the anastrozole or tamoxifen users had previously used a different adjuvant endocrine therapy, and none of the control subjects had previously used endocrine breast cancer medication, although two had had breast cancer. No subjects had ever used the SERM raloxifene.
All subjects met a rigorous set of eligibility criteria concerning ocular health, described previously [16–19]. The most salient criteria are: (i) best-corrected visual acuity of 20/20 or better in one eye and 20/25 or better in the other, (ii) no evidence or suspicion of eye disease, (iii) no diabetes, (iv) no myopia > 5 diopters, (v) no use of medication (other than anastrozole [17] or tamoxifen [20–22]) known to affect vision, and (vi) no ocular surgery.
Subject recruitment has been described previously [20, 23]. Recruitment information specified that “normal reading vision (corrective lenses OK)” was required. The study adhered to the tenets in the Declaration of Helsinki and was approved by the OHSU Institutional Review Board and the OHSU Cancer Institute. Written informed consent was obtained from all subjects before testing.
Procedures
Demographic and ophthalmologic data were collected using methods reported previously [15, 19]. Visual acuity and refractive error were measured for each eye using a Humphrey Automatic Refractor/Keratometer model 599 (Carl Zeiss Meditec Inc., Dublin, CA).
Retinal thickness along a 5-mm horizontal scan line through the fovea was measured using standard procedures with the Stratus® OCT (Carl Zeiss Meditec Inc., Dublin, CA) [24]. One eye per subject was tested. This test eye was chosen according to a 3-step procedure, applied in the following order: (i) the eye with the better acuity, (ii) the eye with less refractive error, (iii) subject preference. Only scans with a signal strength ≥ 7 (out of 10), as provided by the Stratus® software (version 4.0) were assessed for this paper. Scans with evident artifacts (e.g., misidentification of retinal boundary lines) were excluded.
Each 5000-μm horizontal line scan (the “B-scan”) was comprised of 512 “A-scans” corresponding to the retinal thickness at discrete intervals across the B-scan. These 512 retinal thickness values were exported to a statistics/graphics package (SYSTAT 11.0, San Jose, CA) for subsequent analysis for this paper. In addition, for each eligible subject, we visually examined the Stratus® OCT images themselves for the presence of PVDs, as indicated by the presence of vitreous strands or filaments connected to or situated above the retinal surface [12, 25]. The images were viewed with the graders (author EJT, author AE confirming) masked from identifying subject information. All determinations described next were likewise made with the investigators masked.
For each acceptable scan, we determined the locus of minimal retinal thickness relative to the center of the B-scan, which in the absence of artifacts corresponds to a subject’s point of fixation on the OCT target. This determination was made using the graphics capabilities of SYSTAT along with corresponding numerical spreadsheet data. In most cases, the locus of minimal thickness was defined as the center of the range of points (i.e., A-scans) with minimal thickness. Isolated points in the interval spanned by that range but with a thickness 1 or 2 μm different from the majority of points in the range were considered part of the range. When an isolated minimum value was separated by more than two points from a range of minimum values, the isolated point was excluded from the calculations. In the few cases when a range of minimal thicknesses did not exist, a single point with minimal thickness was validated as the correct choice by extrapolating from the nearby nasal and temporal foveal slopes.
After the locus of minimal thickness was determined, we calculated the lateral distance from that locus to the foveal slopes in both the nasal and temporal directions. We did this at 10-μm increments up to 90 μm above the plane of minimal foveal thickness, and also at 3 μm (the lowest height for which these measurements could be made unambiguously for most eyes). We did not measure lateral distances at heights above 90 μm, since the maximal foveal height on the temporal side was less than 100 μm for more than 40% of all subjects. For heights of 10 μm and higher, the distances usually could be calculated as the intersection of the horizontal slice with the foveal slope, which was defined by linear interpolation between adjacent points when a measured point was not intersected directly. Occasionally, the horizontal slice intersected several adjacent points at the same height, in which case the average of these adjacent points was used. To quantify the nasal-temporal asymmetry at each height, we subtracted the temporal distance from the nasal distance.
Statistical Analyses
Simultaneous comparisons of central tendency across the three subject groups were made using parametric analyses of variance (ANOVAs) unless there were distant outliers, in which case non-parametric ANOVAs (Kruskal-Wallis tests) were used. Post-hoc comparisons between pairs of groups then were made using t-tests or Mann-Whitney U tests accordingly. In the text, none of the reported p-values for post-hoc comparisons have been adjusted for multiple comparisons. Significance is determined using a step-down Bonferroni procedure [26] in which the first (i.e., the most significant) comparison requires a significant ANOVA plus p ≤.05/3, the second most significant comparison requires a significant first comparison plus p ≤.05/2, and the third requires a significant second comparison plus p ≤.05. Additional statistics concerning within-group comparison of paired variables and linear regression are reported in the text. All analyses were conducted using SYSTAT 11.0 (San Jose, CA). All p-values are for 2-sided tests.
RESULTS
The Results section contains three subsections, which: (1) compare foveal shape indices across the three primary groups of subjects (anastrozole users, control subjects and tamoxifen users for whom PVDs were not detected and presumed absent), (2) assess relations between refractive error and the topography of the fovea at its base, and (3) extend analyses concerning refractive error to address the presence vs. absence of PVDs. Refractive error is important to assess because myopia is a major risk factor for PVDs [8].
Comparing the Foveal Shapes of the Different Groups
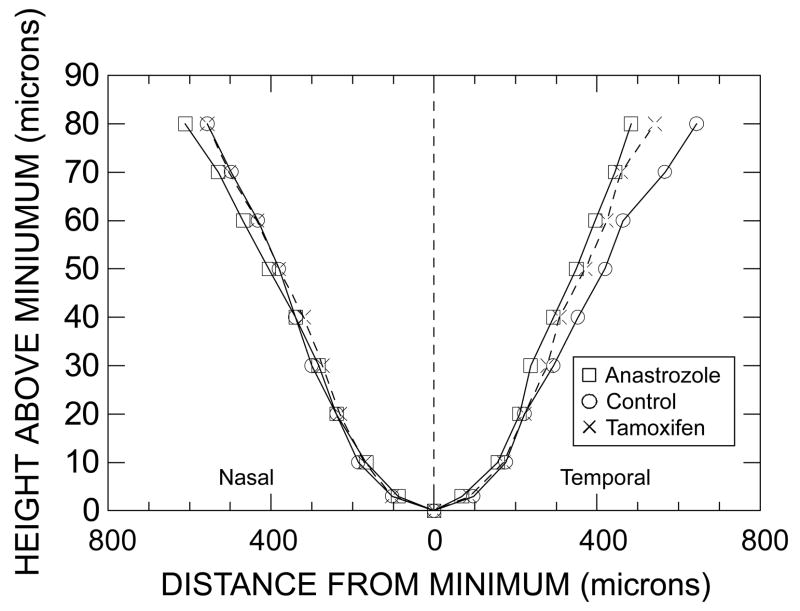
The foveal profiles for each of the three primary subject groups (median data) are shown in Fig. 1. The abscissa represents distances from the position of minimal thickness in the nasal (left) and temporal (right) directions, and the ordinate represents the degree by which the thickness of the fovea at any given locus exceeds the minimal thickness. Values along this ordinate are referred to as foveal heights. Normalization to the locus of minimal thickness reduces the influence of expected fixation disparities [27], and it provides a canonical way for extracting information about foveal shape.
Figure 1.
Graph of the height above the minimal foveal thickness (i.e., of the degree by which the thickness of the fovea at a given locus exceeds the minimal thickness) vs. the lateral distance from the locus of minimal thickness to the foveal slopes in the nasal (left) and temporal (right) directions. Squares (□) represent median data of anastrozole users, circles (○) represent median data of control subjects, and crosses (×) represent median data of tamoxifen users. Connecting lines are unbroken for the anastrozole users and control subjects, and are dashed for the tamoxifen users. Although heights vary along the ordinate and the distances vary along the abscissa, distance is the dependent variable. Data at a height of 90 μm are not included since the maximal height on the temporal side was < 90 μm for 10 of 56 subjects. All units are in microns (μm). Note that the scales on the two axes differ. The locus of minimal thickness itself defines a height of 0 μm. Non-PVD subjects only.
The foveal profiles of the three groups appeared to begin diverging at heights of ~30 μm temporally and higher nasally (if at all). By a height of 70 μm, a pronounced nasal-temporal asymmetry was evident for the anastrozole users, with the distance to the temporal side of the fovea being less than the distance to the nasal side (p =.045, Wilcoxon signed ranks test). Furthermore, the nasal-temporal asymmetry differed significantly among groups (p =.014, non-parametric ANOVA). The unadjusted p-values for comparisons involving pairs of groups were: (a) p =.006 for anastrozole users vs. control subjects, (b) p =.116 for anastrozole users vs. tamoxifen users, and (c) p =.100 for tamoxifen users vs. control subjects. The between-group comparisons for a height of 80 μm were qualitatively similar to those for 70 μm despite the loss of one anastrozole user and three control subjects whose maximal temporal heights did not reach 80 μm. At 60 μm, the corresponding ANOVA yielded p =.077.
Overall, the data show that the foveal profiles of anastrozole users and control subjects differ. For the anastrozole users, the distance to the temporal side of the fovea was less than the distance to the nasal side towards the upper margin of the fovea. The profiles of tamoxifen users seem to be intermediate in that they more closely resemble the profiles of the control subjects nasally and they are closer to the profiles of the anastrozole users temporally, but these observations are not conclusive. For the control subjects, the distance to the nasal side of the fovea was significantly less than the distance to the temporal side at heights of 70 μm (p =.018, paired t-test) and 80 μm (p =.018, paired t-test), but this direction of asymmetry is unextraordinary [27] since the nerve fiber layer exits the fovea along the horizontal meridian in the nasal direction to form the papillomacular bundle [28], thereby causing the nasal side of the fovea to become thicker than the temporal side. This routine observation shows how extraordinary the results are for the anastrozole users.
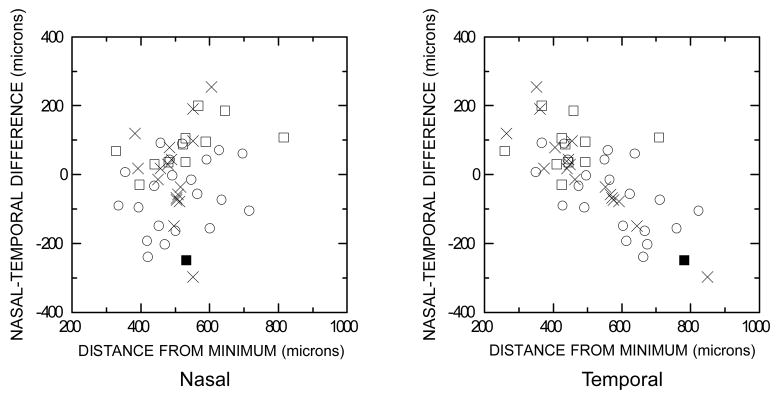
The data are consistent with the view that for anastrozole users without PVDs, the upper temporal aspect of the fovea tends to be pulled by the vitreous towards the optic nerve head (ONH), which is located nasal to the fovea and which provides an anchor for the vitreous [12, 25]. This view is supported by the data in Fig 2, which plots the individual nasal-temporal difference vs. the distances to the nasal (left) and temporal (right) sides of the fovea, all at a height of 70 μm. These graphs reveal that the nasal-temporal difference tended to vary systematically mainly with the distance to the temporal side. For subjects with PVDs, evaluation of shape distortions is complicated by the observation that PVDs usually were partial rather than complete, and bore a variety of positional relations to the fovea. The substantial heterogeneity precludes analyzing foveal topography data for PVD subjects at the present time.
Figure 2.
Graphs of the lateral distance to the nasal side of the fovea at a height of 70 μm minus the corresponding distance to the temporal side at the same height vs. the component distance to the nasal side (left) and to the temporal side (right). Same symbols as for Fig. 1 except that the filled square (■) signifies data from the one anastrozole user with outlying data. When this subject was retested ~2.5 years later (at age 54 years), her acuity had decreased from 20/20 to 20/25 and her nasal-temporal profile had become asymmetric in the opposite direction, more like that of most anastrozole users, for whom the distance to the temporal side of the fovea was less than the distance to the nasal side. All units are in microns (μm). The scales on the two axes are the same. Non-PVD subjects only.
Relations Pertaining to the Base of the Fovea
As stated in the preface, myopia is a major risk factor for PVDs [8]. Since myopic eyes tend to be longer than non-myopic eyes [29], the stretching forces exerted on the retina from its scleral (i.e., non-vitreal) side might distort the fovea at its base [30]. More specifically, since the nasally located ONH serves as breaker for these forces [31], we would expect the temporal side of the foveal base to be wider than the nasal side for myopic eyes. This possibility is evaluated next.
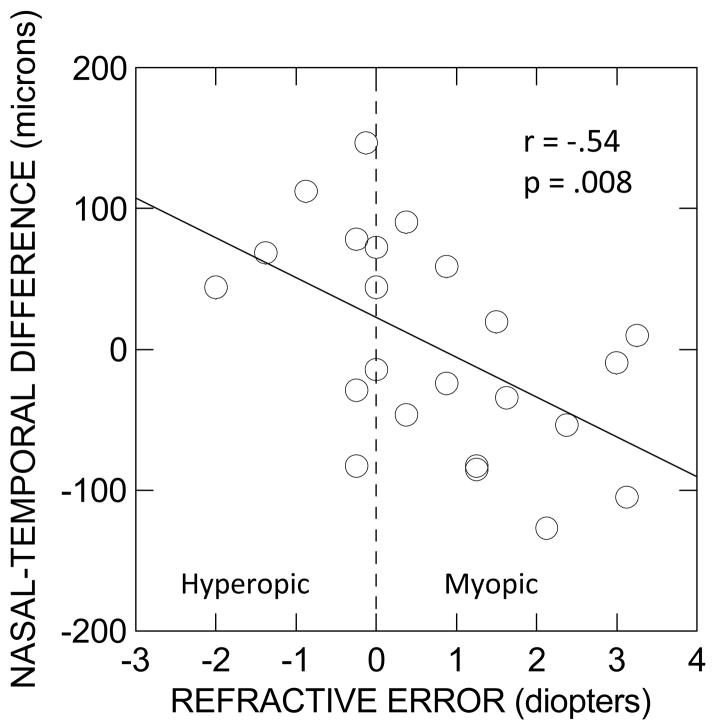
Figure 3 plots the nasal-temporal differences at a height of 10 μm vs. the spherical equivalent refractive error for control subjects without PVDs. The linear relation between these two variables is highly significant (r = −.54, p=.008) in the expected direction. This result itself constitutes evidence that tractional forces can distort the fovea, and together with the results from the previous subsection, it indicates that several tractional forces act on the fovea.
Figure 3.
Graph of the lateral distance to the nasal side of the fovea at a height of 10 μm minus the corresponding distance to the temporal side at the same height. vs. the spherical equivalent refractive error. Non-PVD control subjects only. The straight line is the least-squares linear regression line. The spherical equivalent refractive error is defined as the spherical refractive error plus one-half the cylindrical refractive error. The ordinate is in units of microns (μm), and the abscissa is in units of diopters.
Similar results were obtained at heights of 3 μm (r = −.64, p =.001) and at 20 μm (r = −.42, p =.048). At sufficiently great heights, there was no discernable relation between refractive error and nasal-temporal asymmetry.
It is uncertain whether comparable nasal-temporal asymmetries were related to the degree of refractive error for the tamoxifen users. At 10 μm, for example, r = −.31 (p =.21) after excluding an outlier flagged by SYSTAT. For the anastrozole users, there was little evidence for a relation between refractive error and nasal-temporal asymmetry (e.g., r = −.23 at 10 μm), but as shown later, non-PVD anastrozole users tended not to be myopic.
Because the foveal minimum often was displaced from the center of the OCT scan line, the location of this minimum could have differed systematically between groups. The average position of the foveal minimum (± 1 SEM) relative to the center of the scan was located at 64 ± 23 μm temporal, 10 ± 14 μm temporal, and 21 ± 22 μm temporal for the anastrozole users, control subjects, and tamoxifen users, respectively. These means did not differ significantly (p =.164) as assessed using an ANOVA. Nevertheless, the data suggest that the position of the foveal minimum might depart from normal for anastrozole users.
Refractive Error Differences between Groups
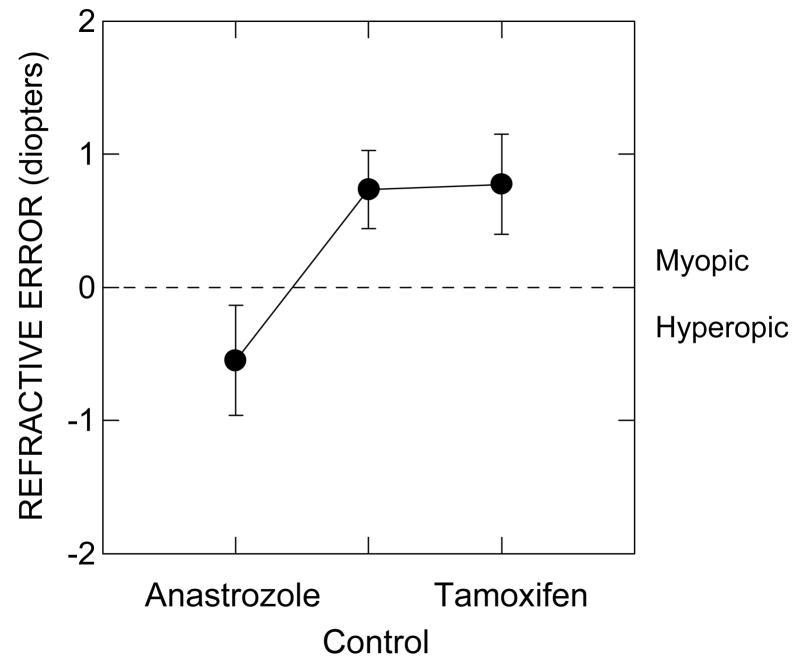
Anastrozole users without PVDs were less myopic than were each of the two other subject groups without PVDs. This is seen in Fig. 4, which plots the means and SEMs for the spherical equivalent refractive error for these groups. The overall significance level for the ANOVA was p =.032, with the comparison for pairs of groups being significant for anastrozole users vs. control subjects (unadjusted p =.014). For anastrozole users vs. tamoxifen users, the unadjusted significance level was p =.027. Only 3 out of 13 non-PVD anastrozole users had any degree of myopia, compared with 13 of 23 non-PVD control subjects, and 12 of 19 non-PVD tamoxifen users.
Figure 4.
The mean spherical refractive error ± the standard error of the mean (SEM) for the anastrozole users, control subjects, and tamoxifen users. Units are diopters. Data from non-PVD subjects only.
A plausible interpretation of these unforeseen results is that the ability of anastrozole to increase vitreo-retinal traction (see the first subsection of the Results) makes anastrozole users more susceptible to the development of myopia-induced PVDs. Furthermore, since the control subjects with PVDs were more myopic than the anastrozole users with PVDs, (p =.042, t-test), it seems that myopic anastrozole users were underrepresented generally. We used a t-test rather than a 3-level ANOVA because (a) only 6 of 25 tamoxifen users had PVDs, and (b) the tamoxifen users with PVDs used their medication significantly longer (p =.041, t-test) than did the tamoxifen users without PVDs (see Table 1). This result suggests that short-term tamoxifen users with PVDs may also have been underrepresented.
DISCUSSION
The results are consistent with the hypothesis that the foveas of women who use the AI anastrozole are subjected to more vitreo-retinal tractional force than are the foveas of women not using hormonal medication. In particular, the shape of the fovea appears to be altered, with the temporal side being displaced nasally near the foveal margin (~500 microns from the foveal center), and with the base probably shifted somewhat temporally. The displacement near the margin can exceed 100 microns, corresponding to ~0.35° or more of visual angle [32]. For reference, 0.35° is equivalent to the angle subtended by an object one centimeter wide viewed at a distance of one meter, or to approximately the angle spanned by the width of a pencil viewed at 25 inches. Overall, the fovea may be subjected to opposing tractional effects exerted from the vitreal and scleral sides of the retina, with the vitreous exerting a force containing a nasal-ward component vector, and with the scleral side exerting a force containing a temporal-ward component. The net result would be a shearing action.
Since every subject had 20/20 or better best-corrected visual acuity, the asymmetries of foveal shape must be compatible with the maintenance of excellent acuity. This probably means that the inferred distortion of foveal shape does not appreciably alter the spatial relations of the foveola (i.e., most densely packed) cone photoreceptors at their light gathering apertures, but instead mainly displaces the cone axons and other overlying neural processes that transmit and process visual information. Because this study was cross-sectional and required subjects to have excellent acuity in order to be eligible, the ability of any anastrozole-induced traction to cause acuity loss remains unknown. However, we observed in hindsight that the anastrozole users tended to have relatively little myopia. Because myopia is one of the strongest risk factors for PVDs [8], we may conjecture, therefore, that the combination of myopia with anastrozole use leads to a degree of traction that can cause vision change. Similarly, the low prevalence of myopia in our sample may have led us to underestimate the degree of traction among anastrozole users, and it is possible that disproportionately many anastrozole users opted not to volunteer for the study because they felt their vision no longer was normal, whether or not they ascribed their vision change to their anastrozole use. For the purpose of testing these conjectures and for most other reasons, it would be desirable to test anastrozole users longitudinally, before and after they start adjuvant therapy. It would also be desirable to determine whether corresponding effects occur for women using letrozole as initial adjuvant therapy, or as a result of switching from tamoxifen to an AI such as exemestane.
The foveal shape data are consistent with an ability of tamoxifen to increase vitreo-retinal traction, but the empirical results are neither conclusive nor definitive. However, tamoxifen has been reported to increase the prevalence of macular holes by about 5-fold [33], so it seems likely that tamoxifen can sometimes increase or exacerbate vitreo-retinal traction.
What are the implications for patients? Without baseline data, it is difficult to assign a cause to any relatively subtle visual changes that individual patients using anastrozole might experience. For women in their forties or fifties, a change in vision might result from or be attributed to the development of presbyopia, particularly if the corrected visual acuity remains excellent. For women in their sixties or older, a change in vision might result from or be attributed to the development of cataracts, particularly since tamoxifen can cause cataracts [22] (although perhaps by non-estrogenic means [34]). In addition, the prevalence of dry eye increases with age after menopause [35] and might result from hormonal imbalance [36]. The concern is that a medication-induced change in vision may sometimes be dismissed, either by the patient or her provider, incorrectly as an inevitable consequence of aging. There is evidence that reductions or changes in estrogen levels can affect temporal visual response properties [17], which might lead to subtle changes in visual perception. It remains unknown how intraocular changes due to anastrozole might affect the eyes or vision of women who have conditions such as diabetes or age-related maculopathy, or how long-term use of anastrozole may affect eyes generally. Prospective longitudinal studies with baseline data are needed.
Acknowledgments
The authors would like to thank the Oregon and Southwest Washington affiliate of Susan G. Komen for the Cure for assistance with subject recruitment. This research was funded by National Institutes of Health grant R01 EY014594 (to A. Eisner) and by an unrestricted grant to the Casey Eye Institute from Research to Prevent Blindness (New York, N.Y.)
References
- 1.Conte P, Frassoldati A. Aromatase inhibitors in the adjuvant treatment of postmenopausal women with early breast cancer: Putting safety issues into perspective. Breast J. 2007;13:28–35. doi: 10.1111/j.1524-4741.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 2.Buzdar AU, Coombes RC, Goss PE, et al. Summary of aromatase inhibitor clinical trials in postmenopausal women with early breast cancer. Cancer. 2008;112:700–709. doi: 10.1002/cncr.23193. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Garreau JR, Delamelena T, Walts D, et al. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192:496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Barkhem T, Nilsson S, Gustafsson JA. Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. Am J Pharmacogenomics. 2004;4:19–28. doi: 10.2165/00129785-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Munaut C, Lambert V, Noel A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001;85:877–882. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayreh SS, Jonas JB. Posterior vitreous detachment: clinical correlations. Ophthalmologica. 2004;218:333–343. doi: 10.1159/000079476. [DOI] [PubMed] [Google Scholar]
- 9.The Eye Disease Case-Control Study Group. Risk factors for idiopathic macular holes. Am J Ophthalmol. 1994;118:754–761. [PubMed] [Google Scholar]
- 10.Chuo JY, Lee TY, Hollands H, et al. Risk factors for posterior vitreous detachment: a case-control study. Am J Ophthalmol. 2006;142:931–937. doi: 10.1016/j.ajo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Evans JR, Schwartz SD, McHugh JD, et al. Systemic risk factors for idiopathic macular holes: a case-control study. Eye. 1998;12 (Pt 2):256–259. doi: 10.1038/eye.1998.60. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 13.Smiddy WE, Flynn HW., Jr Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137:525–537. doi: 10.1016/j.ajo.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 14.van Velthoven ME, Faber DJ, Verbraak FD, et al. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Eisner A, Falardeau J, Toomey MD, et al. Retinal hemorrhages in anastrozole users. Optom Vis Sci. 2008;85:301–308. doi: 10.1097/OPX.0b013e31816bea3b. [DOI] [PubMed] [Google Scholar]
- 16.Eisner A, Toomey MD, Incognito LJ, et al. Contrasting blue-on-yellow with white-on-white visual fields: roles of visual adaptation for healthy peri- or postmenopausal women younger than 70 years of age. Invest Ophthalmol Vis Sci. 2006;47:5605–5614. doi: 10.1167/iovs.05-1612. [DOI] [PubMed] [Google Scholar]
- 17.Eisner A, Toomey MD. The color appearance of stimuli detected via short-wavelength-sensitive cones: Comparisons with visual adaptation and visual field data for peri- or post-menopausal women under 70 years of age. Vision Res (March 14 epub preceding print version) 2008 doi: 10.1016/j.visres.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisner A, Toomey MD, Falardeau J, et al. Differential effects of tamoxifen and anastrozole on optic cup size in breast cancer survivors. Breast Cancer Res Treat. 2007;106:161–170. doi: 10.1007/s10549-006-9486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner A, Samples JR. High blood pressure and visual sensitivity. J Opt Soc Am A Opt Image Sci Vis. 2003;20:1681–1693. doi: 10.1364/josaa.20.001681. [DOI] [PubMed] [Google Scholar]
- 20.Eisner A, Incognito LJ. The color appearance of stimuli detected via short-wavelength-sensitive cones for breast cancer survivors using tamoxifen. Vision Res. 2006;46:1816–1822. doi: 10.1016/j.visres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Eisner A, Austin DF, Samples JR. Short wavelength automated perimetry and tamoxifen use. Br J Ophthalmol. 2004;88:125–130. doi: 10.1136/bjo.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorin MB, Day R, Costantino JP, et al. Long-term tamoxifen citrate use and potential ocular toxicity. Am J Ophthalmol. 1998;125:493–501. doi: 10.1016/s0002-9394(99)80190-1. [DOI] [PubMed] [Google Scholar]
- 23.Eisner A, O’Malley JP, Incognito LJ, et al. Small optic cup sizes among women using tamoxifen: assessment with scanning laser ophthalmoscopy. Curr Eye Res. 2006;31:367–379. doi: 10.1080/02713680600602547. [DOI] [PubMed] [Google Scholar]
- 24.Costa RA, Skaf M, Melo LA, Jr, et al. Retinal assessment using optical coherence tomography. Prog Retin Eye Res. 2006;25:325–353. doi: 10.1016/j.preteyeres.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–1479. doi: 10.1001/archopht.119.10.1475. [DOI] [PubMed] [Google Scholar]
- 26.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan A, Duker JS, Ko TH, et al. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields MB. Textbook of Glaucoma. Williams & Wilkins; Baltimore: 1998. Optic Nerve Head and Peripapillary Retina; pp. 72–107. [Google Scholar]
- 29.Gonzalez Blanco F, Sanz Fernandez JC, Munoz Sanz MA. Axial length, corneal radius, and age of myopia onset. Optom Vis Sci. 2008;85:89–96. doi: 10.1097/OPX.0b013e3181622602. [DOI] [PubMed] [Google Scholar]
- 30.Chui TY, Yap MK, Chan HH, et al. Retinal stretching limits peripheral visual acuity in myopia. Vision Res. 2005;45:593–605. doi: 10.1016/j.visres.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Enoch JM. Marked accommodation, retinal stretch, monocular space perception and retinal receptor orientation. Am J Optom Physiol Opt. 1975;52:376–392. doi: 10.1097/00006324-197506000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Drasdo N, Fowler CW. Non-linear projection of the retinal image in a wide-angle schematic eye. Br J Ophthalmol. 1974;58:709–714. doi: 10.1136/bjo.58.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronin BG, Lekich CK, Bourke RD. Tamoxifen therapy conveys increased risk of developing a macular hole. Int Ophthalmol. 2005;26:101–105. doi: 10.1007/s10792-005-5424-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JJ, Jacob TJ, Valverde MA, et al. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J Clin Invest. 1994;94:1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 36.Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. 2005;20:289–298. doi: 10.1080/09513590400027257. [DOI] [PubMed] [Google Scholar]