Abstract
Polymer micelles with cross-linked ionic cores were prepared by using block ionomer complexes of poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) copolymer and divalent metal cations as templates. Doxorubicin (DOX), an anthracycline anticancer drug, was successfully incorporated into the ionic cores of such micelles via electrostatic interactions. A substantial drug loading level (up to 50 w/w %) was achieved and it was strongly dependent on the structure of the cross-linked micelles and pH. The drug-loaded micelles were stable in aqueous dispersions exhibiting no aggregation or precipitation for a prolonged period of time. The DOX-loaded polymer micelles exhibited noticeable pH-sensitive behavior with accelerated release of DOX in acidic environment due to the protonation of carboxylic groups in the cores of the micelles. The attempt to protect the DOX-loaded core with the polycationic substances resulted in the decrease of loading efficacy and had a slight effect on the release characteristics of the micelles. The DOX-loaded polymer micelles exhibited a potent cytotoxicity against human A2780 ovarian carcinoma cells. These results point to a potential of novel polymer micelles with cross-linked ionic cores to be attractive carriers for the delivery of DOX.
Keywords: Block copolymer micelles, doxorubicin, self-assembly, core-shell morphology
1. Introduction
Doxorubicin (DOX) is one of the most effective anthracycline antibiotics effective against a wide range of cancers. Despite DOX potency its clinical use is accompanied by severe side effects such as cardiotoxicity as well as tumors intrinsic and acquired drug resistance [1, 2]. Therefore, there is a need for development of novel delivery systems for DOX to improve its therapeutic efficacy while minimizing side effects and abrogating drug resistance.
Among the drug delivery systems, self-assembled polymer micelles based on amphiphilic block copolymers have attracted considerable attention as potential delivery vehicles for anti-cancer drugs [3–8]. The benefits of polymer micelles include the small size (10 to 100 nm) with a fairly narrow size distribution, core-shell architecture which leads to increased solubility and metabolic stability of incorporated chemotherapeutic agents. The micellar carriers with incorporated drugs were shown to have increased circulation time in the body and are believed accumulate in tumors due to the enhanced permeability and retention effect (EPR effect) [9]. In particular, DOX incorporated into mixed micelles of on poly(ethylene oxide) (PEO) and poly(propylene oxide) triblock copolymers (Pluronic) was shown to accumulate in tumor tissues more effectively than free DOX and cases showed remarkably improved DOX anti-tumor activity in drug resistant tumors [1]. One micellar formulation of DOX, SP1049C, was shown to be safe and effective in humans [10]. It was also reported that notable accumulation of micellar drugs in cancer can lead to complete tumor regression and substantial reduction of drug toxicity and other adverse effects [2]. These studies strongly suggest that incorporation of anticancer drugs in polymer micelles can increase the efficacy of cancer chemotherapy.
The core of the polymer micellar delivery system serves as a reservoir that accommodates drug molecules through a combination of hydrophobic, electrostatic interactions, hydrogen bonding or via chemical conjugation of the drug to the core-forming block of the copolymer. The choice of drug loading strategy strongly depends on the nature of the drug and chemical structure of the copolymer. Generally, physical entrapment of drugs in the micelle cores may be beneficial compared to micelle-forming polymer-drug conjugates in terms of simplicity of polymer preparation, micelle fabrication, and enhanced drug loading and bioavailability [3]. Nanofabrication of the polymer micelles has been recently advanced through the use of block copolymers with ionic and nonionic water-soluble segments (i.e.,“block ionomers”) [4, 8, 11, 12]. Such block copolymers react electrostatically with oppositely charged species resulting in block ionomer complexes (BIC). Neutralization of the polyion charges leads to formation of the hydrophobic domains, which tend to segregate in aqueous media. However, water-soluble nonionic segments, such as PEO, prevent aggregation and macroscopic phase separation of the complexes. As a result, the BIC self-assemble into particles of nanoscale size and form stable aqueous dispersions. The latter enable, uniquely, encapsulation of various charged therapeutic molecules, including large entities such as proteins and nucleic acids, into the micellar core [8, 13, 14]. This approach is now also used for incorporation of various small drug molecules in polymer micelles. In this work we explore this approach for delivery of DOX which represents weak base and is positively charged at physiological conditions. Direct interaction between DOX and poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) block copolymer results in spontaneous formation of BIC. In such complexes the ammonium group in the daunosamine part of DOX electrostatically binds to the carboxylic group of PMA segment of PEO-b-PMA copolymer [4]. The hydrophobic interactions between the anthracycline residues of DOX provide for additional stabilization of the complex. It was shown that the binding of DOX molecules to the polyanion segments of PEO-b-PMA proceeds as a highly cooperative process typical for the formation of regular interpolyelectrolyte or polyelectrolyte-surfactant complexes [4]. Similar approach has been recently explored for the loading of DOX into the complexes with Pluronic P85-b-poly(acrylic acid) (P85-b-PAA) and Pluronic F87-b-PAA block copolymers [5, 15]. Furthermore, DOX was directly entrapped into cross-linked Pluronic–PAA microgel particles via ion-exchange mechanism [16].
Recently, a novel type of polymer micelles with cross-linked ionic cores was introduced [6, 17–19]. Nanofabrication of these micelles was achieved by reacting PEO-b-PMA block copolymers with oppositely charged metal cations such as Ca2+. The metal-polyion complex cores of the resulting micelles were subsequently chemically cross-linked and the condensing ions removed. Resulting materials represented hydrophilic polyionic nanogels of core-shell morphology. The core comprised a network of cross-linked PMA polyanions surrounded with hydrophilic nonionic PEO shell. Such micelles displayed pH- and ionic strength-responsive hydrogel-like behavior. Furthermore, due to its polyionic character the core of such micelles could encapsulate oppositely charged therapeutic or diagnostic molecules. The cross-links increased stability of such micelles upon dilution, which can also be beneficial for drug delivery.
The present study for the first time examines the possible use of cross-linked PEO-b-PMA micelles as potential carriers for DOX. Towards this goal, the physicochemical properties of the micelles, the loading efficacy of DOX in the micelle cores and the release rate of DOX from the micelles were examined at different pH ranging from pH5.5 to pH7.4. Furthermore, the in vitro cytotoxicity studies were conducted to evaluate the biological activity of the DOX-loaded micelles. Altogether we demonstrate that remarkably high amount of DOX can be incorporated in the cross-linked micelles, and the drug loading and release can be effectively controlled by the changing the composition of the micelle core, pH and drug/micelles feeding ratio.
2. Experimental Section
2.1. Materials
PEO-b-PMA diblock copolymer (Mw/Mn = 1.45) was purchased from Polymer Source Inc., Canada. The block lengths were 170 and 180 repeating units for PEO and PMA, respectively. The concentration of carboxylate groups in the copolymer samples was quantified by potentiometric titration. Doxorubicin hydrochloride is a kind gift from Dong-A Pharmaceutical Company, South Korea. Calcium chloride, 1,2-ethylenediamine and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride were obtained from Sigma-Aldrich (St Louis, MO). All other chemicals were of reagent grade and used without further purification.
2.2. Synthesis of cross-linked polymer micelles
Polymer micelles with cross-linked ionic cores will be prepared by using BIC of PEO-b-PMA copolymer and divalent metal cations as templates by the previously described method with a slight modification [6]. In brief, PEO-b-PMA/Ca2+ complexes were prepared by mixing an aqueous solution of PEO-b-PMA with a solution of CaCl2 at a molar ratio of [Ca2+]/[COO−]=1.3. The 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1,2-ethylenediamine were then added to the dispersion of PEO-b-PMA/Ca2+ micelles. The reaction mixture was allowed to stir overnight at room temperature. The extent of degree of cross-linking was controlled by the ratio of amine functional groups to carboxylic acid groups. Byproducts of the cross-linking reaction and metal ions, which have cemented the ionic core, were removed by exhaustive dialysis of the reaction mixtures first, against 0.5% aqueous ammonia in the presence of ethylenediaminetetraacetic acid (EDTA), and then against distilled water.
2.3. Preparation of DOX-loaded micelles
The aqueous dispersions of cross-linked micelles were mixed with an aqueous solution of DOX (2 mg/ml) at various molar ratios of DOX to carboxylate groups of the micelles followed by incubation for 24 h at room temperature. Unbound DOX was removed by ultrafiltration using Amicon YM-30 centrifugal filter devices pretreated with DOX (MWCO 30,000Da, Millipore). Concentration of DOX in filtrates was determined by measuring the absorbance at 485 nm using Lambda 25 UV/VIS spectrophotometer. The extinction coefficient for DOX was determined to be 10,800 M−1cm−1 in water and 10, 400 M−1cm−1 in PBS buffer, respectively. The concentration of DOX present in the micelle was determined using a reversed-phase C18 column (Agilent Eclipse XDB, 150 × 4.6 mm i.d., 5 μm particle size) and Agilent 1200 HPLC system (G1353B UV detector, G1321A fluorescence detector, G1311A pump, G1329A Autosampler, G1316A column oven) at flow rate of 1 ml/min. The mobile phase was a linear gradient of an aqueous solution of 1.0 % acetic acid and acetonitrile solution (from 18% v/v to 82% v/v) [7]. Fluorescence detection of DOX was carried out at λex = 480 nm and λem = 590 nm.
2.4. Fluorescence studies
Fluorescence spectra of free-DOX and DOX-loaded micelles were recorded using a Cary Eclipse fluorescence spectrophotometer (Varian analytical instruments, CA) at λex = 480 nm with the bandwidth of 5 nm for excitation and emission. Solutions of free DOX and DOX-loaded micelles used for these studies were prepared at 50 μM equivalent concentrations of DOX.
2.5. Release studies of DOX-loaded cross-linked micelles
The release of DOX from the cross-linked micelles was evaluated in phosphate buffered saline (PBS, pH 7.4, 0.14 M NaCl), and acetate buffered saline (pH 5.5, 0.14 M NaCl) by dialysis method using a membrane tubing with 3,500 Da cut-off. DOX was sampled at selected time intervals. The concentrations of DOX present in the dialysate were determined spectrophotometrically by measuring absorbance at 485 nm as described above. The concentration of DOX released from the micelles was expressed as a percentage of the total DOX available and plotted as a function of time.
2.6. Electrophoretic mobility and size measurements
Electrophoretic mobility measurements were performed using a “ZetaPlus” analyzer (Brookhaven Instrument Co.) with a 30 mW solid-state laser operating at a wavelength of 635 nm. ζ-potential of the particles was calculated from the electrophoretic mobility values using Smoluchowski equation. Effective hydrodynamic diameters (Deff) of the particles were measured by photon correlation spectroscopy (DLS) in a thermostatic cell at a scattering angle of 90° using the same instrument equipped with a Multi Angle Sizing Option (BI-MAS). All measurements were performed at 25°C. Software provided by the manufacturer was used to calculate the size of the particles and polydispersity indices. The diameters mean values were calculated from the measurements performed at least in triplicate.
2.7. Atomic Force Microscopy (AFM)
Samples for AFM imaging were prepared by depositing 5 μL of an aqueous dispersion of micelles (ca. 1.0 mg/ml) onto positively charged 1-(3-aminopropyl)silatrane mica surface (APS-mica) for 2 minutes followed by surface drying under argon atmosphere. The AFM imaging was performed in air using a Multimode NanoScope IV system (Veeco, Santa Barbara, CA) operated in a tapping mode. The imaging was performed with regular etched silicon probes (TESP) with a spring constant of 42 N/m. The images were processed and the widths and heights of the particles were measured using Femtoscan software (Advanced Technologies Center, Moscow, Russia).
2.8. In vitro cytotoxicity studies
Cytotoxicity of DOX-loaded polymer micelles was assessed in human ovarian A2780 carcinoma cells by a standard MTT assay as described previously [6]. Briefly, cells were seeded in a 96-well microtiter plates with 10,000 cells per well and allowed to adhere for 24 h prior to the assay. Cells were exposed to various doses (0–200 μg/ml on doxorubicin basis) of DOX alone, polymer micelles alone, DOX-loaded micelles or liposomal doxorubicin (DOXIL®) for 6 h or 24 h at 37 °C, followed by washing with PBS, and maintaining in RPMI 1640 medium with 10% FBS for additional 72 h. 25 μl of MTT indicator dye (5 mg/ml) was added to each well and the cells were incubated for 2 h at 37 °C in the dark. 100 μl of 50% DMF-20% SDS solution was added to each well to dissolve the MTT formazan crystals and kept overnight at 37 °C. Absorption was measured at 570 nm in a microplate reader (SpectraMax M5, Molecular Devices Co., USA) using wells without cells as blanks. All measurements were taken eight times. The net absorbance was taken as index of cell viability. The reading taken from the wells with cells cultured with control medium was used as 100% viability value. The cell viability was calculated as Asample/Acontrol × 100%. Based on the results of the test, the IC50 values (the concentration which kill 50% of cells) were calculated by using GraphPad Prism Software (Version 5.0, GraphPad Software, San Diego California, USA).
3. Results and discussion
3.1 Preparation of cross-linked polymer micelles
PEG-b-PMA polymer micelles with cross-linked ionic core (clPEO-b-PMA) were synthesized via two step procedure that involves (1) condensation of PEO-b-PMA copolymers by Ca2+ ions into spherical micelles of core-shell morphology and (2) cross-linking of the cores of the micelles through the use of bifunctional agents [6, 17]. After completion of the corss-linking reaction the metal ions and the byproducts of the cross-linking reactions were removed by dialysis. The same stock solution of template PEO-b-PMA/Ca2+ micelles was used to synthesize cross-linked micelles with various targeted degree of cross-linking from 10% to 70%. It should be noted, that these numbers represented the targeted degrees of cross-linking, while the actual degrees of cross-linking were expected to be significantly less. Indeed, the efficiency of the carbodiimide-induced cross-linking reaction is affected strongly by a side reaction between water and the activated carboxylic groups that competes with the condensation reaction. In addition, the diamine could react either with two separate PMA chain segments to yield a cross-link or form a “loop” being bound to the same PMA chain as well as attach by only one amino group giving the free amine. It has been estimated that approximately two cross-links per block copolymer chain were formed in the micelles with targeted 20% degree of cross-linking [6]. This number corresponded to only ca. 10% yield of the cross-linking reaction. The resulting cross-linked micelles represented hydrophilic nanospheres with sizes ranging from ca. 100 to 200 nm. The core of such micelles comprised a swollen network of the cross-linked PMA chains and was surrounded by the shell of hydrophilic PEO chains [6, 17].
The clPEO-b-PMA micelles swelling was affected strongly by pH, ionic strength and degree of cross-linking [6, 17]. For example, the particle size and net negative charge of clPEO-b-PMA micelles studied in this work increased considerably as pH increased. The effective diameters and ζ-potential of such micelles with 20% targeted degree of cross-linking are shown in Figure 1A.
Figure 1.
Physicochemical characteristics of clPEO-b-PMA micelles (A) Effective diameter (Deff) and ζ-potential of clPEO-b-PMA micelles with 20% targeted degree of cross-linking as a function of pH. (B) Effective diameter (Deff) of clPEO-b-PMA micelles at various pH as a function of targeted degree of cross-linking.
Evidently, increased swelling at high pH was induced by ionization of the carboxylic groups of the PMA chains of the micelles. As the salt concentration of the external solution was increased from 10 mM to 150 mM NaCl at pH 7.4, the diameters of the swollen micelles decreased from ca. 240 nm to ca. 200 nm. The swelling the clPEO-b-PMA micelles was also strongly affected by the degree polymer cross-linking (Figure 1B). As expected the micelles with lower degrees of cross-linking exhibited significant increase in Deff upon increase of pH, whereas the micelles with higher degree of cross-linking (e.g. 40% and 60%) exhibited only modest swelling under the same conditions. The size and shape of cross-linked micelles at various pH values were further characterized by tapping-mode AFM. The particles were deposited at different pH and then dried on the mica. The typical images of clPEO-b-PMA micelles with different targeted degrees of cross-linking (20% and 70%) are presented in Figure 2.
Figure 2.
Tapping-mode AFM images of clPEO-b-PMA micelle deposited from aqueous solutions at different pH and dried on the mica. clPEO-b-PMA micelles with 20% targeted degree of cross-linking: (A) at pH 7.0; (B) at pH 5.3. clPEO-b-PMA micelles with 70% targeted degree of cross-linking: (C) at pH 7.0 and (D) at pH 5.3 Scan size in 3 μm.
As expected, the micelles formed flat circular structures upon adsorption on mica surface. The measured number-averaged heights and widths are presented in Table 1. The clPEO-b-PMA micelles with targeted degree of cross-linking of 20% deposited at pH 7.0 exhibited a high width versus height aspect ratio (ca. 32) suggesting substantial flattering of the particles. This observation was in agreement with the expected flexible, shape-adaptable character of the cross-linked micelles imparted by the PMA “soft” core material. Interestingly, under the same pH 7.0 the micelles with the higher degree of cross-linking (70%) were characterized by a significantly lower aspect ratio (ca. 9.1). These data suggest that increased number of cross-links within the PMA core provided substantial reinforcement of the cores of the micelles and resulted in the formation of relatively more stiff nanostructures. At pH 5.3 the clPEO-b-PMA micelles of any studied degree of cross-linking were characterized by significantly greater height and smaller widths values than the same micelles at pH 7.0. As a result the micelles had lower dimension aspect ratios of ca. 4.4 and 1.8 at 20% and 70% cross-linking respectively (Table 1). This is probably explained by contraction of the PMA cores upon acidification due to the protonation of the carboxylic groups. As a result, at lower pH the micelles become more robust upon deposition on the mica.
Table 1.
Dimensions of cross-linked PEO-b-PMA micelles measured by tapping mode AFM at various pHa
| Targeted degree of cross-linking (%) | pH | Havb, nm | Wavc, nm | Aspect ratio (Wav/Hav) |
|---|---|---|---|---|
| 20 | 7.0 | 3.45 ± 0.06 | 111.4 ± 0.4 | 32.3 |
| 5.3 | 18.9 ± 0.3 | 82.30 ± 0.14 | 4.4 | |
| 70 | 7.0 | 8.13 ± 0.22 | 74.30 ± 1.95 | 9.1 |
| 5.3 | 35.5 ± 0.5 | 62.3 ± 0.6 | 1.8 |
Cross-linked micelles were deposited from aqueous solutions with different pH onto a mica surface and allowed to dry in vacuo
Number-averaged heights (Hav) of the micelles
Number-averaged widths (Wav) of the micelles
3.2. DOX -loaded polymer micelles
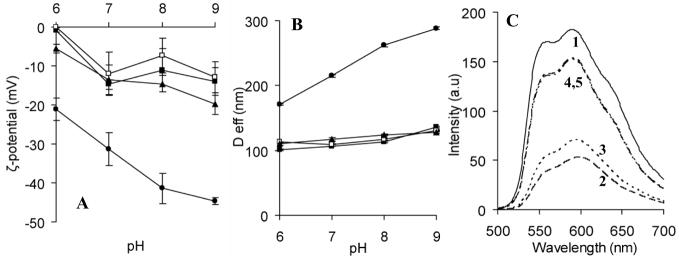
A weakly basic drug DOX (pKa= 8.2) was immobilized into the cores of clPEO-b-PMA micelles that contain PMA, a weak polyacid (apparent pKa is 5.5) [20], by simple mixing of this drug with the aqueous dispersion of the micelles. Briefly, the aqueous solutions of DOX (2mg/ml) and polymer micelles were mixed at various pH and incubated for 24 hours at room temperature. The composition of the mixtures expressed as the feeding molar ratio of DOX to carboxylate groups (R=[DOX]/[COO−]) was varied in the range from 0.25 to 1.5. Free unbound DOX was removed thoroughly by repeated filtrations through a 30 kDa molecular cutoff membrane to retain only the DOX-loaded micelles. Figure 3 presents the dependence of ζ-potential and effective diameter of DOX-loaded micelles as a function of pH. It is seen that upon loading with DOX, the net negative charge of the micelles was decreased in the entire range of the pH studied (Figure 3A). This provided evidence of the neutralization of the PMA segments in the micelle due to the electrostatic binding of DOX [4]. This was also accompanied by a decrease in the particle size. Notably, in contrast to the empty cross-linked micelles, the DOX-loaded micelles did not reveal pH-dependent swelling behavior (Figure 3B).
Figure 3.
(A) ζ-potential and (B) effective diameter (Deff) of DOX-loaded clPEO-b-PMA micelles as a function of pH. (▲) R=0.25, (■) R=0.5, (□) R=0.75, and (●) clPEO-b-PMA without DOX. (C) Fluorescence emission spectra of free DOX (1), DOX-loaded micelles at R = 0.25 (2), DOX-loaded micelles at R = 0.5 (3), DOX-loaded micelles in the presence of 0.15 M NaCl at R=0.25 (4) and R=0.5 (5). Targeted degree of cross-linking of clPEO-b-PMA micelles is 20%.
The binding of DOX with the micelles was further supported by quenching of DOX fluorescence (by ca. 70%) compared to the fluorescence of free DOX in an aqueous solution (Figure 3C). This fluorescence quenching is likely to due to π-π stacking interactions between DOX molecules bound to PMA chains in the micelle. Substantial reduction in DOX fluorescence intensity was previously attributed to the self-association of DOX molecules which is stabilized by stacking interactions of the adjacent aromatic chromophors [21–23]. However, the fluorescence intensity of DOX increased upon addition of 0.15 M sodium chloride to the DOX-loaded micelles. It appears that the DOX/PMA complex disintegrated in the presence of the salt and the free DOX was released from the micelles. Indeed, addition of low molecular weight salts to complexes of polyelectrolytes with oppositely charged species usually leads to destabilization of the system of the salt bonds. These data further reinforce the conclusion that DOX binding to the ionic cores of the clPEO-b-PMA micelles is governed by electrostatic interactions as well as stacking interactions between DOX molecules.
The size and ζ-potential of DOX-loaded micelles remained practically unchanged in the range of the feeding ratios 0.25 ≤ R ≤ 0.75 (Figure 3A,B). The DOX-loaded polymer micelles prepared under these conditions were stable in aqueous dispersions, exhibiting no aggregation or precipitation for a prolonged period of time (up to 3 months). Notably, in most cases (except pH equal 6.0) the DOX-loaded micelles prepared under these conditions exhibited negative values of ζ-potential, suggesting that the drug was in deficiency and PMA groups in excess. However, at higher feeding ratios, R ≥ 1, the size of the DOX-loaded micelles was significantly larger (ca. 150 nm) and their ζ-potential was weakly negative (ca. − 3.8 mV). Progressive aggregation was observed in the reaction mixtures over the time (data not shown). It appears that under the high DOX loadings the neutralization of the negative charge of PMA by DOX was practically complete and the resulting DOX/PMA complex was too hydrophobic so that the PEO segments in the corona of the micelles were unable to stabilize the particles in dispersion. The amount of DOX incorporated into cross-linked micelles was strongly affected by both the pH and feeding ratio of the components in the reaction mixtures.
The loading capacity (expressed as mass of incorporated DOX per mass of polymer in DOX-loaded micelles) of cross-linked micelles loaded with DOX at various pH is presented in Figure 4. First, the changing the pH provided an opportunity to tune the electrostatic binding between the DOX and PMA chains of the micelles and to control the drug loading. At any given feeding ratio the maximal loading capacity of clPEO-b-PMA micelles was achieved at pH 7, when both the DOX molecule and the carboxylic group of the PMA were completely ionized. Much lower loadings were obtained at pH 6.0 or at pH 9.0, respectively. This is evidently due to either partial protonation of the carboxylic groups in the PMA cores of the micelles at acidic pH or deprotonation of positively charged amino group of DOX at alkaline pH. Second, there was an initial increase in the loading capacity of the cross-linked micelles as the feeding ratio of DOX increased from R=0.25 to R=0.5. However, an increase of the DOX concentration in the reaction mixture from R = 0.5 to R = 0.75 was accompanied with only slight increase in the DOX loading. It is likely that at R ≥ 0.75 a saturation limit of loading capacity was reached. Altogether, it appears that the loading capacity was mainly governed by the number of the carboxylate groups in the micelle available for DOX binding. In fact, the binding of the DOX with the PMA chains induced almost complete collapse of the micelles already at low concentration of the drug in the reaction mixture (R = 0.25, see Figure 3B). We suggest that the some steric restrictions may imposed on the cross-linked PMA chains that may limit the availability of the carboxylic groups in the micellar core for interaction DOX molecules and impede a further electrostatic binding of DOX. However, we cannot exclude a possibility that additional amounts of DOX can be physically trapped and retained in the micelles due to non-specific hydrophobic interactions of anthracycline groups of DOX with DOX/PMA complex regions. In this case similarly to previously reported micelles of DOX-conjugated PEO-b-poly(aspartic acid) [3], the relatively hydrophobic cores of the DOX-loaded clPEO-b-PMA micelles can serve as a carrier to physically entrapped DOX. We also speculate that as DOX molecules become incorporated, the number of contacts between the hydrophobic core of DOX-loaded micelles and the water will increase. This may be one reason for the neutralization of the net charge of the micelles at R ≥ 1 and destabilization of the micelle dispersion, discussed above.
Figure 4.
Loading capacity of DOX-loaded clPEO-b-PMA micelles (mg DOX/mg polymer) as a function of pH and feeding ratio: (▲) R=0.25, (■) R=0.5, and (□) R=0.75.
Interestingly, the micelles with higher degree of cross-linking exhibited significantly lower loading capacity compared to the micelles with lower degrees of core cross-linking. At R = 0.5, clPEO-b-PMA micelles with targeted degree of cross-linking of 70% contained only about 34% (w/w) of DOX vs 50% (w/w) in DOX-loaded micelles with targeted degree of cross-linking of 20%. A plausible explanation of this fact is probably the differences in the total content of carboxylate groups that decreases as the cross-linking degree increases. Additionally, the distribution of cross-linkers in the micelle core can also affect the loading capacity. Upon cross-linking reaction the carboxylate groups are buried in the polyion-metal complex core and their number and accessibility for carbodiimide activation may be restricted. In consequence, it is possible that the cross-linking reactions are limited to some exterior layer of the core. At higher degrees of cross-linking, the free volume of this layer should decrease, which may further hinder the accessibility of the micelle core to the drug molecules.
The topology of DOX-loaded micelles was further characterized by tapping-mode AFM imaging and typical images of DOX-loaded micelles are presented in Figure. 5. DOX-loaded micelles maintained their spherical morphology while exhibited significantly greater heights and smaller widths values, compared to the initial clPEO-b-PMA micelles. DOX-loaded micelles with 20% targeted degree of cross-linking prepared at pH 7.0 were characterized with an average height of 29.9 ± 0.69 nm and diameter of 86.6 ± 1.82 nm (Figure 5A). This corresponds to 11-fold decrease (from 32.3 to 2.9) in the dimension aspect ratio compared to the empty clPEO-b-PMA micelles and suggests that the cores of the micelles became more rigid due to the DOX binding. In contrast, the incorporation of DOX into the micelles with higher degree of cross-linking (70%) and consequently relatively rigid cores did not result in such pronounced changes in the dimensions compared to the initial clPEO-b-PMA micelles (the dimension aspect ratio changed from 9.1 to 2). Interestingly, the DOX-loaded micelles with 20% targeted degree of cross-linking appeared on the mica as oblong nanostructures (see height image in Figure 5A) whereas the DOX-loaded micelles with 70% targeted degree of cross-linking preserved their circular shapes (Figure 5B). To explain such behavior one should consider that the observed topographic images represented the DOX-loaded micelles absorbed onto a surface and, hence, also reflected the interaction of micelles with the substrate. Since the DOX-loaded micelles with 20% and 70% targeted cross-linking exhibited similar net charge values, the contribution of the electrostatic interactions of the micelles with the positively charged amino-modified mica surface (APS mica) into the observed topography of the DOX-loaded micelles may be comparable. The hydrophobic components arising from the DOX/PMA core-shell interface of the DOX-loaded micelles could also contribute to the particle - surface interactions and, thus, force an additional flattening of the micelle particles. It is likely, that DOX-loaded micelles with 20% targeted degree of cross-linking due to their greater flexibility may form greater number of contacts of the hydrophobic DOX/PMA exterior layer of the core with the mica surface and this can also account for additional flattering of the micelles on the surface.
Figure 5.
Tapping-mode AFM images of DOX-loaded clPEO-b-PMA micelles with various degrees of cross-linking in air. (A) 20%; (B) 70%. pH 7.0. Scan size in 3 μm. The inserts show 3D image of the same micelles. Bar equals 100 nm.
Overall, these data demonstrate that DOX-loaded micelles can be prepared by mixing of DOX with the aqueous dispersions of cross-linked micelles. The highest drug loading, 50% (w/w) was achieved at optimal pH 7.0. Such a remarkably high loading capacity of the cross-linked micelles might be valuable from a pharmaceutical point of view to decrease the total dose of formulation. It is likely that the composition of the core of the cross-linked micelles is the major factor affecting its loading capacity. Therefore, further studies toward elucidating the effect of nature of polyion block, cross-linking density and structure of cross-linker in the core on the structure, swelling properties, and DOX loading capacity are underway.
3.3. Release of DOX from cross-linked micelles
The release profiles of the DOX-loaded cross-linked micelles prepared at pH 7.0 and different feeding ratios, R, are depicted in Figure 6A. As is seen, DOX release from the clPEO-b-PMA micelles was a pH-dependent process. Indeed, DOX was liberated from the micelles significantly faster at pH 5.5 than at pH 7.4. At any given pH the release profiles were characterized by two distinct stages with a relatively rapid release of DOX over the first 8 h followed by the apparently slow release stage (up to 72 h). During the first hour at pH 5.5 up to 50% of DOX loaded into the micelles was released. However, there was no burst release of DOX at pH 7.4. The accelerated DOX release from micelles at the acidic pH was probably due to the protonation of carboxylic groups of PMA chains in the micelles. Interestingly, at pH 7.4 a significant retardation of the DOX release from the polymer micelles was observed in 5% dextrose solution compared to the solutions containing 0.14 M NaCl (dashed line in Figure 6A). Increase of low molecular mass electrolyte concentration is known to weaken DOX binding with carboxylic groups in the core, which evidently results in faster release of DOX [8]. Thus, the overall release of DOX from cross-linked polymer micelles was strongly affected by environmental pH and ionic strength.
Figure 6.
(A) Release profiles of DOX from clPEO-b-PMA mcelles at 37 °. (□) R = 0.25, pH 7.4, PBS buffer, (■) R = 0.25, acetate buffer (pH 5.5, 0.14 M NaCl), (◇) R = 0.5, pH 7.4, PBS buffer, (◆) R=0.5, pH 5.5, acetate buffer, (▲, dashed line) R = 0.5, pH 7.4, 5% dextrose solution. Insert represents DOX release plots at pH 7.4, PBS buffer as a function of (time)1/2. (B) Comparison of DOX release from (□) DOXIL®; (■) DOX-loaded clPEO-b-PMA, R = 0.5; (△) SP1049; and (▲) free DOX in RPMI 1640 cell culture media containing 10% FBS at 37°C. The loading capacity of DOX for each sample is 200 μg. The data represent averaged values and standard deviations calculated based on three independent experiments.
Of particular interest was the finding that the release rates at pH 7.4 were higher for the micelles loaded at feeding ratio R=0.5 than those loaded at R=0.25. For instance, during first 8 h hours the micelles with R=0.5 released 57.5 ± 9.7% of incorporated DOX, while the micelles with R=0.25 released only 32.9 ± 3.8%. This phenomenon may be rationalized by possible differences in DOX binding and localization in the core of the micelles as we assumed above. A relatively rapid release of the drug can occur when a significant amount of the drug resides at the core-shell interface because the drug does not have to cross large segments of the core to exit the micelle. The core dimensions were relatively the same in the both cases. However, the loading capacity of DOX-loaded micelles at R = 0.5 was higher. Consequently, a greater number of DOX molecules can be entrapped into the cores of DOX-loaded micelles prepared at R = 0.5 due to partition to the core-shell interface or through the hydrophobic interactions, and as a result, they will have a greater chance to be released quickly. This argument is also supported by the fact that the intrinsic fluorescence of DOX incorporated into the micelles with R = 0.5 was quenched to a lesser extent compared to the DOX-loaded micelles prepared at R = 0.25 (see Figure 3C). Alternatively, at lower loading there are more negative charged groups available in the micelles and DOX can be better retained by the electrostatic interactions. Furthermore, faster release of DOX was also observed for the DOX-loaded micelles with high density of cross-links (70%), which may have more peripheral drug localization and hence faster release (data not shown). Also it is worth mentioning that at the second slow stage of the release profile at pH 7.4 the overall release rates for all DOX-loaded micelles studied were virtually the same. Evidently the gradual decrease of drug release in time was related to some changes in the overall composition of the micelles, e.g. the rearrangement of the PMA chains in the cross-linked core. In addition, it needs to be pointed out that the release studies were performed not under the true “sink” conditions when the drug is constantly removed from the exterior solution. Therefore, in our static system an establishment of equilibrium between free DOX in solution and DOX entrapped into the micelles at the late stages of the release was possible. At lower pH the difference in the release behavior between the micelles prepared at various R was minimal since the substantial portion of the incorporated DOX was released during first two hours due to the fast dissociation of DOX/PMA complexes. These results provided additional indication that electrostatic interactions play an essential role in the binding and stabilization of DOX-loaded cross-linked micelles.
In order to understand the nature of the DOX release from clPEO-b-PMA micelles, the release data were analyzed in terms of Higuchi’s model that has been applied for the diffusion –controlled release of the drug from planar matrix [24]. According the model, the percentage of the drug released was plotted as a function of the square root of time. As expected, the fitting of all the time points on the release profile to the Higuchi model provided poor correlation coefficients due to the coexistence of two release stages. However, the plots for the initial rapid release stages showed good correlation with the Higuchi model for the both types of the DOX-loaded micelles studied. The representative plots of DOX release from the cross-linked micelles at pH 7.4 as a function of (time)1/2 are given as an insert in Figure 6A. Particularly, at pH 7.4 the correlation coefficients were 0.998 and 0.975 for the DOX-loaded clPEO-b-PMA micelles at R = 0.5 and R = 0.25, respectively. At pH 5.5, the fitting of the data provided lower correlation values (0.918 and 0.966, respectively), probably, due to the relatively quick release of the DOX from the micelles at these conditions. In addition, we analyzed the release data using Baker-Lonsdale model [25], which models the diffusion controlled release from matrices of the spherical shape. The release data at pH 7.4 fitted the Baker-Lonsdale model best: the plots showed very good correlation coefficients of 0.994 and 0.984 for the DOX-loaded clPEO-b-PMA micelles at R = 0.5 and R = 0.25, respectively. Thus, in general, the release of DOX from the cross-linked micelles appeared to be diffusion controlled. Similar to the regular block copolymer micelles [26], the structure of the cross-linked core of the micelles and the location of the drug had a significant influence on the rate of drug release from the clPEO-b-PMA micelles.
Since DOX-loaded cross-linked micelles retained the net negative charge, polycationic compounds such as protamine sulfate (PA) or double-chain surfactants (didodecyldimethylammonium bromide), were used to create an additional “protective” layer on the core-shell interface in the attempt to control the drug release. In fact, consecutive cooperative electrostatic sorption of different species by polyelectrolyte hydrogels has been proposed by V. Kabanov and A. Zezin as a general strategy to construct multilayer materials [27]. For example, addition of PA to the DOX-loaded clPEO-b-PMA micelles polymer was accompanied by a slight decrease of the particle sizes and change in the ζ-potential to weakly positive values (data not shown). The binding of PA to the DOX-loaded cross-linked micelles also resulted in the substantial decrease of the loading capacity of the micelles, which suggested that polycations competed with DOX for the binding with carboxylic group of PMA chains in the cross-linked cores and displaced DOX molecules from the micelles. However, no significant retardation of DOX release from such “mixed” clPEO-b-PMA micelles was observed.
The release behavior of DOX incorporated into the clPEO-b-PMA micelles (R = 0.5) was further compared to the clinically relevant DOX-formulations, Doxil® (DOX-loaded PEO-coated liposomes) and SP1049C (DOX-encapsulated micelles assembled from two different Pluronic copolymers, L61 and F127 in 1:8 w/w ratio). In vitro DOX release from various formulations in RPMI 1640 cell culture media containing 10% FBS is presented in Figure 6B. Doxil® formulation was characterized by a very slow DOX release from a carrier whereas Pluronic micelles (SP1049C formulation) exhibited burst release behavior similar to a free DOX. The DOX-loaded clPEO-b-PMA micelles showed intermediate release rates. Comparison among these various formulations revealed that the structure of polymer nanocarrier and the method of drug encapsulation are essential determinants of the carrier properties and have a dramatic effect on the drug release characteristics. The observed differences in the release characteristics between SP1049 and Doxil® formulations are consistent with the clinical pharmacokinetic data [28, 29]. Specifically, the liposomal DOX exhibited very low clearance rate (0.02 ml/(min kg) [28]. As a result, the DOX-associated toxicities such as cardiomyopathy and myelosuppression were significantly decreased [30]. However, the high residence time of DOX incorporated in liposomes was recently linked to the adverse mucocutaneous reactions such as palmar–plantar erythrodysesthesia (hand-foot syndrome) and mucositis/stomatitis [31, 32]. In contrast, Pluronic micelles did not change significantly the clearance rate of DOX due to the relatively low stability of the micelles against dilution. However, the appearance of Pluronic unimers (single block copolymer chains) in circulation was attributed to the improved tumor cell sensitivity to DOX chemotherapy by inhibiting the drug efflux that leads to tumor cell resistance against this drug [33, 34]. Therefore, the issues related to the appropriate rate of drug release from the carrier are of great importance. Drug incorporated into the carrier needs to be released to be bioavailable and exert its biological activity. Concomitantly, it needs to stay in the carrier long enough to achieve substantial levels of tumor drug accumulation. We believe that the rational design of the overall structure of the cross-linked polymer micelles and, in particularly, of their ionic cores might be an effective tool to tune the DOX release rates, to affect tumor/host cell interactions, and to achieve desirable therapeutic effect. The pH-sensitive drug release from the cross-linked polymer micelles is also advantageous for tumor targeting due to a lower pH in the interstitial space of solid tumors relative to the normal tissues [35]. In addition, since endocytosis is a main mechanism for the internalization of cross-linked micelles into the cancer cells [6], pH-sensitivity becomes a further benefit. Since intracellular lysosomes and endosomes compartments are acidic, the transport of the micelles in these compartments can potentially trigger a release of a bolus of drug from the carrier. Future studies will determine pharmacokinetics and biodistribution of the DOX-loaded clPEO-b-PMA micelles to underline the relationship between the composition of the micelles, drug rate release, and extent of bioavailability at the site of action.
3.5. In vitro cytotoxicity
Cytotoxic activity of DOX-loaded clPEO-b-PMA micelles (R = 0.5) was assessed against human A2780 ovarian carcinoma cells and was compared to the Doxil® and SP1049 formulations. Calculated IC50 values for all formulations are summarized in Table 2. Up to 100-fold difference in cytotoxicity of the DOX formulations studied was observed and it was incubation time-dependent. As expected, DOX-loaded Pluronic formulation reduced cell viability at the doses comparable to those for free DOX. DOX incorporated into the clPEO-b-PMA micelles displayed lower cytotoxic activity than free DOX. However, DOX-loaded clPEO-b-PMA micelles were characterized by much higher IC50 values compared to Doxil® formulation. Importantly, it was found that cross-linked polymer micelles alone were not toxic in the whole range of concentrations used for the treatment with DOX-polymer micelle formulation. Since the DOX released from the micelles plays an essential role in the cytotoxic activity, the reduction in cytotoxicity of DOX incorporated into polymer cross-linked micelles or liposomes was consistent with the corresponding sustained manner of DOX release from the nanocarriers.
Table 2.
In vitro cytotoxicity of various DOX formulations against A2780 cells
| Sample | IC50 (DOX equivalents in μg/mL); | |
|---|---|---|
| 6 h incubation | 24 h incubation | |
| Free DOX | 0.053 ± 0.016 | 0.024 ± 0.013 |
| DOX-loaded clPEO-b-PMAa | 3.26 ± 0.40 | 0.38 ± 0.04 |
| DOXIL | n.d.b | 27.69 ± 1.59 |
| SP1049C | 0.062 ± 0.022 | 0.015 ± 0.004 |
DOX-loaded clPEO-b-PMA micelles with targeted degree of cross-linking of 20% were prepared at R = 0.5 and pH 7.0
Cytotoxicity was not detected up to 200 μg/mL.
4. Conclusion
Novel hydrophilic polymer micelles with cross-linked ionic cores were synthesized and evaluated as potential drug delivery carriers of DOX. The ionic character of the cores allowed to achieve a very high level of DOX loading (50% w/w) into the cross-linked micelles. The DOX-loaded cross-linked micelles exhibited pH–sensitive drug release character: protonation of carboxylic groups in the cores of the micelles at the acidic conditions resulted in the accelerated DOX release. Importantly, the composition of the cross-linked micelles can be easily tuned to modulate drug loading efficiency and drug release properties. Therefore, we believe that polymer micelles with cross-linked ionic cores are promising supramolecular carriers that may allow to control the biodistribution and pharmacokinetics of the dug to improve the therapeutic outcomes.
Acknowledgments
This work was supported by National Institutes of Health grants CA116590 (T.K.B.) and 2RO1 CA89225 (A.V.K.). The authors are grateful to Dr. Jean Grem for providing the DOXIL® sample, Dr. Luda Shlyakhtenko for assistance with AFM studies, and Dr. Maggie Rybak for assistance with in vitro cytotoxicity experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alakhov VY, Klinkski E, Li S, Pietrzynski G, Venne A, Batrakova E, Bronich TK, Kabanov AV. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids and Surfaces B: Biointerfaces. 1999;16:113–134. [Google Scholar]
- 2.Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, Okano T. Selective delivery of adriamycin to a solid tumor using a polymeric micelle carrier system. J Drug Target. 1999;7:171–186. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, Okano T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release. 1998;50(1–3):79–92. doi: 10.1016/s0168-3659(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 4.Bronich TK, Nehls A, Eisenberg A, Kabanov VA, Kabanov AV. Novel drug delivery systems based on the complexes of block ionomers and surfactants of opposite charge, Coll.Surfaces. B:Biointerfaces. 1999;16:243–251. [Google Scholar]
- 5.Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC. Complexation and release of doxorubicin from its complexes with pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release. 2007;121:137–145. doi: 10.1016/j.jconrel.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Bontha S, Kabanov AV, Bronich TK. Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release. 2006;114(2):163–174. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Kwon GS, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Block copolymer micelles for drug delivery: Loading and release of doxorubicin. J Control Release. 1997;48:195–201. [Google Scholar]
- 8.Kabanov AV, Bronich TK, Kabanov VA, Yu K, Eisenberg A. Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules. 1996;29:6797–6802. [Google Scholar]
- 9.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong A, Brewer J, Newman C, Alakhov V, Pietrzynski G, Campbell S, Corrie P, Ranson M, Valle JW. SP1049C as first-line therapy in advanced (inoperable or metastatic) adenocarcinoma of the oesophagus: A phase II window study. J Clin Oncology ASCO Annual Meeting Proceedings Part I. 2006;24:4080. [Google Scholar]
- 11.Bronich TK, Kabanov AV, Kabanov VA, Yu K, Eisenberg A. Soluble complexes from poly(ethylene oxide)-block-polymethacrylate anions and N-alkylpyridinium cations. Macromolecules. 1997;30(12):3519–3525. [Google Scholar]
- 12.Bronich TK, Popov AM, Eisenberg A, Kabanov VA, Kabanov AV. Effects of block length and structure of surfactant on self-assembly and solution behavior of block ionomer complexes. Langmuir. 2000;16:481–489. [Google Scholar]
- 13.Oh KT, Bromberg L, Hatton TA, Kabanov AV. Block ionomer complexes as prospective nanocontainers for drug delivery. J Control Release. 2006;115(1):9–17. doi: 10.1016/j.jconrel.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Solomatin SV, Bronich TK, Bargar TW, Eisenberg A, Kabanov VA, Kabanov AV. Environmentally responsive nanoparticles from block ionomer complexes: effects of pH and ionic strength. Langmuir. 2003;19:8069–8076. [Google Scholar]
- 15.Tian Y, Ravi P, Bromberg L, Hatton TA, Tam KC. Synthesis and aggregation behavior of pluronic F87/poly(acrylic acid) block copolymer in the presence of doxorubicin. Langmuir. 2007;23:2638–2646. doi: 10.1021/la060780a. [DOI] [PubMed] [Google Scholar]
- 16.Bromberg L, Hatton TA. Smart Microgel Studies. Polyelectrolyte and Drug-Absorbing Properties of Microgels from Polyether-Modified Poly(acrylic acid) Langmuir. 2003;19(21):8675–8684. [Google Scholar]
- 17.Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. Polymer micelle with cross-linked ionic core. J Am Chem Soc. 2005;127(23):8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 18.Bronich TK, Ouyang M, Kabanov VA, Eisenberg A, Szoka FC, Jr, Kabanov AV. Synthesis of vesicles on polymer template. J Am Chem Soc. 2002;124(40):11872–11873. doi: 10.1021/ja020509p. [DOI] [PubMed] [Google Scholar]
- 19.Bronich TK, Bontha S, Shlyakhtenko LS, Bromberg L, Hatton TA, Kabanov AV. Template-assisted synthesis of nanogels from Pluronic-modified poly(acrylic acid) J Drug Target. 2006;14(6):357–366. doi: 10.1080/10611860600833781. [DOI] [PubMed] [Google Scholar]
- 20.Grimshaw PE, Nussbaum JH, Grodzinsky AJ, Yarmush ML. Kinetics of electrically and chemically induced swelling in polyelectrolyte gels. J Chem Phys. 1990;93:4462–4472. [Google Scholar]
- 21.Husain N, Agbaria RA, Warner IM. Spectroscopic analysis of the binding of doxorubicin to human. alpha.-1 acid glycoprotein. J Phys Chem. 1993;97(41):10857–10861. [Google Scholar]
- 22.Menozzi M, Valentini L, Vannini E, Arcamone F. Self-association of doxorubicin and related compounds in aqueous solution. J Pharm Sci. 1984;73(6):766–770. doi: 10.1002/jps.2600730615. [DOI] [PubMed] [Google Scholar]
- 23.Kitaeva MV, Melik-Nubarov NS, Menger FM, Yaroslavov AA. Doxorubicin-poly(acrylic acid) complexes: interaction with liposomes. Langmuir. 2004;20(16):6575–6579. doi: 10.1021/la0497144. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 25.Radin S, Chen T, Ducheyne P. The controlled release of drugs from emulsified, sol gel processed silica microspheres. Biomaterials. 2009;30:850–858. doi: 10.1016/j.biomaterials.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 26.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Coll Surfaces, B:Biointerfaces. 1999;16:3–27. [Google Scholar]
- 27.Kabanov VA, Skobeleva VB, Rogacheva VB, Zezin AB. Sorption of Proteins by Slightly Cross-Linked Polyelectrolyte Hydrogels: Kinetics and Mechanism. J Phys Chem B. 2004;108(4):1485–1490. [Google Scholar]
- 28.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 29.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. British Journal of Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 31.Elbayoumi TV, Torchilin VP. Tumor-specific antibody-mediated targeted delivery of Doxil reduces the manifestation of auricular erythema side effect in mice. Int J Pharm. 2008;357(1–2):272–279. doi: 10.1016/j.ijpharm.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong RL, Tseng YL. Phase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in patients with solid tumors: the relation between pharmacokinetic property and toxicity. Cancer. 2001;91(9):1826–1833. [PubMed] [Google Scholar]
- 33.Batrakova EV, Kabanova AV. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers for overcoming drug resistance in cancer. Advanced Drug Delivery Reviews. 2002;54(5):759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 35.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]