Abstract
In the CNS, ATP induces the synthesis and release of neurotrophic factors, cell proliferation, and differentiation. The olfactory system is one site where multipotent progenitor cells continue to proliferate and differentiate into neurons throughout life. We tested the hypothesis that ATP initiates proliferation in olfactory epithelium by measuring 5-bromo-2-deoxyuridine incorporation. Adult mice were pre-treated intraperitoneally or intranasally with saline or purinergic receptor antagonists (pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate + suramin) 30 min prior to nasal instillation of ATP, UTP, ATPγS or saline (0 hr). Mice received three injections of 5-bromo-2-deoxyuridine between 42–46 hr, and were sacrificed at 2, 9 or 16 days post ATP instillation. ATP, UTP or ATPγS significantly increased 5-bromo-2-deoxyuridine incorporation compared to intranasal saline controls in groups pre-treated with saline. Saline, ATP, UTP or ATPγS instillation did not significantly increase 5-bromo-2-deoxyuridine incorporation in groups pre-treated with purinergic receptor antagonists. Similar results were observed in neonates and in a cultured slice preparation. Intranasal instillation of ATP also increased the protein levels of proliferating cell nuclear antigen in adults. Pre-treatment with purinergic receptor antagonists inhibited the ATP-induced increase in proliferating cell nuclear antigen. In adults, a subset of the cells that incorporated 5-bromo-2-deoxyuridine were immunoreactive to neuronal markers MASH 1, GAP43, and OMP at 2, 9, and 16 days, respectively. Collectively, these data indicate that purinergic receptor activation induces proliferation and neuronal differentiation in the mouse olfactory epithelium. We propose that extracellular ATP released upon injury could induce proliferation and promote the neuroregeneration of the olfactory epithelium.
Keywords: ATP, neuroregeneration, olfaction, progenitor cell, sustentacular cell, olfactory sensory neuron
The peripheral olfactory system is a good model to identify and study trophic factors involved in neuroregeneration. In the olfactory epithelium, during embryonic development and continuing through adulthood, neuronal progenitor cells called basal cells proliferate, differentiate into olfactory sensory neurons and eventually undergo apoptosis (Graziadei et al., 1978, Cowan and Roskams, 2002). The olfactory neuroepithelium is in direct contact with airborne pollutants, toxicants and microbes, and consequently, is often damaged. The neuroepithelium, however, is able to regenerate following injury (Schwob, 2002). Cells contain millimolar levels of ATP which could be released upon injury, activate purinergic receptors, and initiate an ATP signaling cascade that induces proliferation and regeneration of the olfactory epithelium.
Extracellular purine nucleotides act as trophic factors in the CNS (Neary et al., 1996) stimulating proliferation in vivo and in vitro (Rathbone et al., 1992a, Rathbone et al., 1999). Proliferation occurs after activation of purinergic receptors and is coupled to the production of inositol phosphates and other second messengers (Rathbone et al., 1992b). Recently, we have shown that G-protein coupled P2Y receptors are expressed on the glial-like sustentacular cells and the basal progenitor cells, and both P2Y and ionotropic P2X purinergic receptors are present on the olfactory sensory neurons (OSNs) (Hegg et al., 2003). The purinergic receptors are located on the dendrites, somas and axons of OSNs, on the cell somas and cytoplasmic extensions of sustentacular cells, and on the basal cell soma located in the basal layer.
We investigated whether ATP acts as a proliferative agent in the olfactory epithelium. To test the hypothesis that activation of purinergic receptors evokes increased proliferation in the olfactory epithelium, we measured proliferation in the presence of purinergic receptor agonists and antagonists. We observed that ATP, UTP and/or ATPγS, a non-hydrolyzable form of ATP, increases cell proliferation in vivo and in vitro. ATP-induced cell proliferation was blocked by purinergic receptor antagonists. Two weeks post-ATP instillation, the proliferating cells expressed markers for mature OSNs, indicating ATP promotes neurogenesis. Collectively, these data indicate that ATP acts as a neuroproliferative factor in the olfactory epithelium.
Experimental Procedures
Animals
All animal procedures were approved by Michigan State University’s Institutional Animal Care and Use Committee, and all applicable guidelines from NIH were followed. Adult male (6–8 weeks) and neonatal (post-natal day 1–5) Swiss Webster mice (Charles River, Portage, MI) were used.
Solutions
Ringer’s solution contained (mmol/L): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose; pH 7.4, 290–320 mOsms. Concentrated stocks of pharmacological agents were made in Ringer’s solution, stored at −20 °C and diluted on the day of the experiment.
In vivo proliferation studies
Adult mice were injected intraperitoneally (ip) with purinergic receptor antagonists (100 μmoles/kg suramin + PPADS) or an equivalent volume of phosphate buffered saline (PBS). In some experiments anesthetized adults (4% isoflurane) or unanesthetized neonates were intranasally instilled (in) with PPADS (40 nmoles/kg) and suramin (160 nmoles/kg). 30 min later, either 20 or 400 nmoles/kg ATPγS, a non-degradable form of ATP, 400 nmoles/kg ATP or UTP, or an equivalent volume of saline was instilled into the nares of anesthetized adult mice (4% isoflurane) or unanaesthetized neonates. Note that the concentration of ATP, equivalent to 200 μM, falls within the range of the reported EC50 values of purinergic receptors and is physiologically relevant as every cell has millimolar levels of ATP. Mice were injected 3 times with 5-bromo-2-deoxyuridine (BrdU) (180 mg/kg total) between 42–46 hr and tissue was collected at 2, 9 and 16 days. Adults were deeply anethesitized with 80 mg/kg ketamine/xylazine, and perfusion fixed with 4% paraformaldehyde. Heads were post-fixed overnight in 4% paraformaldehyde, and placed in RDO decalcifier (Apex Engineering Products Corporation, Aurora, IL) for 4 hr. Neonates were quickly decapitated and heads were post-fixed for 2 hours in 4% paraformaldehyde. Adult and neonate tissue was cryoprotected with 20% sucrose, oriented in Tissue Tek OCT (Sakura Finetek, Torrence, CA), and quickly frozen. Cryostat sections (20 μm) were collected onto Superfrost Plus slides (Electron Microscopy Sciences, Hatfield, PA) from level 3 of the mouse nasal cavity taken at the level of the second palatal ridge (Young, 1981).
Immunohistochemistry
Cryostat sections (20 μm) of the olfactory epithelium were rehydrated with 0.1 M PBS for 20 min and permeabilized with 0.3% Triton X-100 for 20 min. Non-specific binding was blocked with 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in 0.1 M PBS for 1 hr. Tissue sections were incubated in 2 M HCl for 30 min at 65°C to denature DNA, and then incubated with rat anti-BrdU IgG (Abcam Inc., Cambridge, MA; 1:100) in 10% normal donkey serum overnight at 4° C. For double-labeling, either mouse anti-mammalian achaete-schute homolog 1 (MASH 1, 1:20, clone 24B72D11.1, BD Pharmingen, Franklin Lakes, NJ), mouse anti-growth associated protein 43 (GAP43, 1:100, clone gap7B10, Sigma-Aldrich, St. Louis, MO) or goat-anti-olfactory marker protein (OMP, 1:1000, Waco Chemicals, Richmond, VA) was included in the incubation. Immunoreactivity was detected using fluorescein isothiocyanate (FITC)-conjugated donkey anti-rat IgG ± tetramethyl rhodamine isothiocyanate (TRITC)-conjugated donkey anti-mouse or TRITC-conjugated donkey anti-goat (Jackson ImmunoResearch; 1:200 in 0.3% triton X 100 + 10% donkey serum in PBS for 2 hrs at 37° C). Sections were mounted with Vectashield mounting media for fluorescence (Vector Laboratories, Burlingame, CA). Antibody specificity was examined by omitting the primary antibody and no immunoreactivity was ever observed. Immunoreactivity was visualized on a Nikon Eclipse 2000-U microscope equipped with an xcite 120 fluorescence illumination system or an Olympus BX51-FL microscope equipped with a mercury arc lamp. FITC and TRITC dyes were excited at 488 and 543 nm and low pass filtered at 505–525 and 560–620 nm, respectively. In some instances, the brightness and contrast of the fluorescent images were altered post-hoc. In all cases, the same changes were applied to all images collected on that given day, and it was verified that immunoreactivity was not observed in the antibody specificity controls under the new settings.
Data Analysis
An experimenter blinded to the treatments manually counted the number of BrdU+ cells from 2–3 tissue sections from each mouse (3–8 mice/group) and data was averaged (n = number of sections). To determine if the intranasal instillation was effectively reaching the olfactory epithelium, trypan blue (0.4%) was instilled into the nares. The septal olfactory epithelium (septum) and ectoturbinate 2 and endoturbinate II (turbinates) were regions that were heavily stained with trypan blue (data not shown), indicating that compounds instilled into the nares permeates into the olfactory epithelium at these regions, although it is not known how long compounds remain in the nasal cavity before they are cleared. Thus, BrdU+ cells were tabulated in the septum and/or turbinates, and the combination of the septum and the turbinate data is designated entire olfactory epithelium. Data is expressed as a ratio of BrdU positive cells to linear length of olfactory epithelium, and is presented as entire olfactory epithelium unless specified otherwise. The length of the septal epithelium and the ectoturbinate 2 and endoturbinate II were measured using Metamorph software (Molecular Devices, Sunnyvale, CA). In some experiments, we quantitated the BrdU+ cells in the apical sustentacular cell layer, defined as the region where the apical-most cell nuclei reside, the middle neuronal layer, defined as the region where OMP immunoreactivity can be observed (e.g. Figure 5D), and the basal cell layer, defined as the region where MASH1 immunoreactivity can be observed (e.g. Figure 5A). The entire breadth of all three layers is designated “all layers”. This was performed to distinguish between basal cell and sustentacular cell proliferation, and to qualitatively examine differentiation. One and two way ANOVA statistics with the Dunnett’s and the Bonferroni multiple comparison post-hoc tests, respectively, were performed using Prism software (GraphPad Software, San Diego, CA).
Figure 5. ATP-induced proliferation of MASH1, GAP43 and OMP expressing cells.
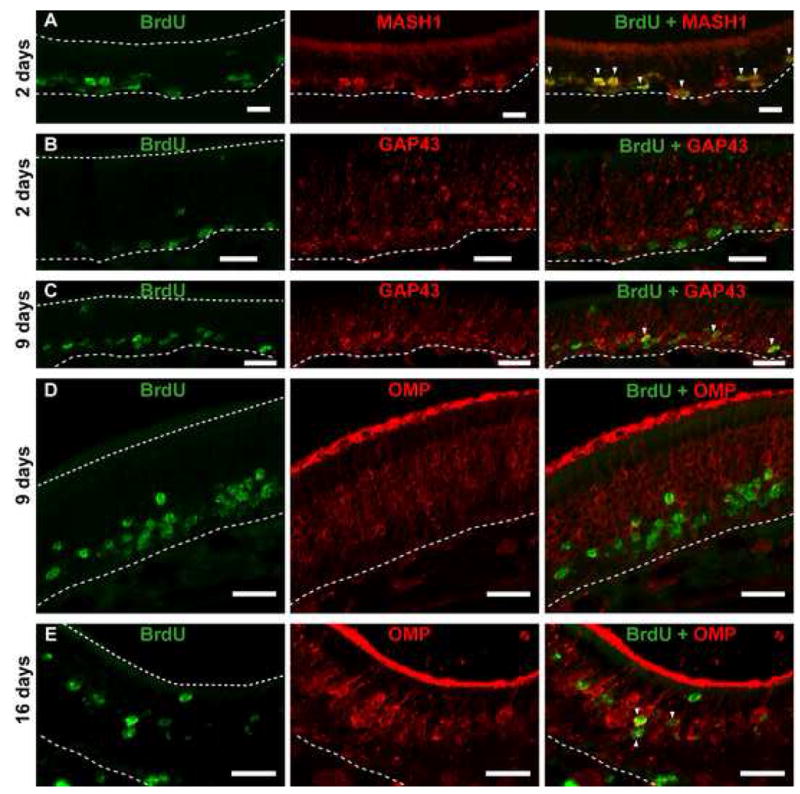
(A, B) ATP induces proliferation of cells (BrdU+; green) expressing MASH1 (red, white triangles △), a marker for neuronal progenitor cells, but not GAP43, a marker for immature neurons at 48 hrs post ATP instillation. Note: BrdU-labeled cells are located above the basement membrane (dotted white line) but below the GAP43-labeled immature neurons. (C, D) After 9 days ATP intranasal instillation, BrdU-labeled cells are now co-localized with GAP43-labeled immature neurons (white △), suggesting that the proliferating progenitor cells have differentiated into immature neurons and have migrated. BrdU-labeled cells do not co-localize with OMP (red), a marker for mature neurons. Note: BrdU-labeled cells are situated below the mature OSN layer (OMP). (E) After 16 days, BrdU-labeled cells (green) are co-localized with OMP-labeled mature neurons (red; white △), indicating that ATP can induce the proliferation and differentiation of neuronal progenitor cells into mature neurons. Scale bar = 5 μm. Dotted white lines indicate width of olfactory epithelium.
Immunoblotting
Olfactory epithelium dissected from adult animals treated as previously described (above) were obtained from 6–9 mice and homogenized by sonication in Tris buffer [10 mM Tris-HCl (pH 7.6) containing 100 mM NaCl, 1 mM EDTA, 2 M activated Na3VO4, 50 mM NaF, and a protease inhibitor cocktail (1:1000, Sigma-Aldrich, St. Louis, MO)]. The concentration of protein in the homogenate was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Equivalent amounts of protein (20 μg/lane) for each sample were fractionated by polyacrylamide gel electrophoresis [0.75 mm thick TRIS-glycine denaturing reducing gels containing 0.1% SDS, 12.5% acrylamide/bisacrylamide (30:0.2), 4.6 M urea, and 375 mM Tris (pH 8.7)] and transferred to nitrocellulose membrane. After incubation with blocking buffer (0.38 M Na2HPO4, 0.17 M NaH2PO4 and 0.68 M NaCl) containing 0.05% Tween-20 and 5% bovine serum albumin (Sigma, St. Louis, MO), the membranes were probed with mouse anti-proliferating cell nuclear antigen (PCNA, 1:1000, Millipore, Billerica, MA) antibody overnight at 4°C. After washing with washing buffer (0.38 M Na2HPO4, 0.17 M NaH2PO4 and 0.68 M NaCl) containing 0.1% Tween-20, the membranes were incubated with HRP-labeled secondary antibody (Jackson Laboratory, West Grove, PA). Immunoreactive proteins were detected with a chemiluminescence reagent (ECL, Amersham Biosciences, Piscataway, NJ) and then exposed to Kodak X-ray film. For quantitative analyses, the membranes were probed again with mouse anti-actin antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA). Films were analyzed by Image J (NIH). Integrated optical densities (IOD)/μg protein were expressed as percentile changes from IOD/μg protein values of vehicle animals. The value of PCNA for each animal was then normalized to the value of actin. Each sample was measured on three independent gels.
In vitro BrdU incorporation in neonatal olfactory epithelium slices
To prepare olfactory epithelial slices, neonatal Swiss Webster mice (postnatal day 0–6) were quickly decapitated, and the skin and lower jaw were removed. Tissue was embedded in a carrot, mounted onto a vibratome-cutting block in ice cold Ringer’s solution and 300 μm slices were made. All slices from an individual mouse (4–5 slices) were cultured on a cell culture insert (Millicell-CM, Millipore, Billerica, MA) in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 1X B27 supplement (Invitrogen) and 100 U/ml penicillin G and 100 mg/ml streptomycin (Invitrogen). Slices were cultured in the following treatments: Vehicle (Ringer’s solution), ATP (100 μM), suramin (100 μM), or ATP + suramin at 37° and 5% CO2. In previous in vitro experiments, suramin alone and PPADS alone produced equal inhibition of the transient calcium increase induced by ATP in a slice preparation (Hegg et al., 2003). Thus, in the in vitro experiments, only suramin was used. Note that both suramin and PPADS are non-specific purinergic receptor antagonists, with similar potencies. Cultures were pre-incubated with purinergic receptor antagonists 30 min prior to stimulation with purinergics. Two hrs prior to the experimental endpoint a stock solution of 20 mM BrdU was added to the slice cultures for a final concentration of 200 μM. This 2 hr short duration of BrdU allows for identification of cell types that are in the S phase of the cell cycle. After 48 hrs, slices were fixed with 4% paraformaldehyde in PBS for 5 min, and cryoprotected in 30% sucrose in PBS. A 0 hr control group only received the BrdU treatment 2 hrs prior to fixation. Tissue was oriented in Tissue Tek OCT (Sakura Finetek, Torrence, CA), quickly frozen and cryostat sections (20μm) were collected onto Superfrost plus slides (Electron Microscopy Sciences, Hatfield, PA). BrdU immonhistochemistry was performed as described above. BrdU positive cells were counted in the olfactory epithelium by an experimenter blinded to the treatments and are expressed as a percentage of 0 hr cell counts. Cells were counted from 3 fields of view per slide and averaged (n= 6–9 slides from 3 animals in each group, i.e., 18–27 fields of view total). A one way ANOVA and Dunnett’s multiple comparison post-hoc test was performed using Prism software (GraphPad Software, San Diego, CA) unless specified.
Results
ATPγS induces proliferation in adult olfactory epithelium
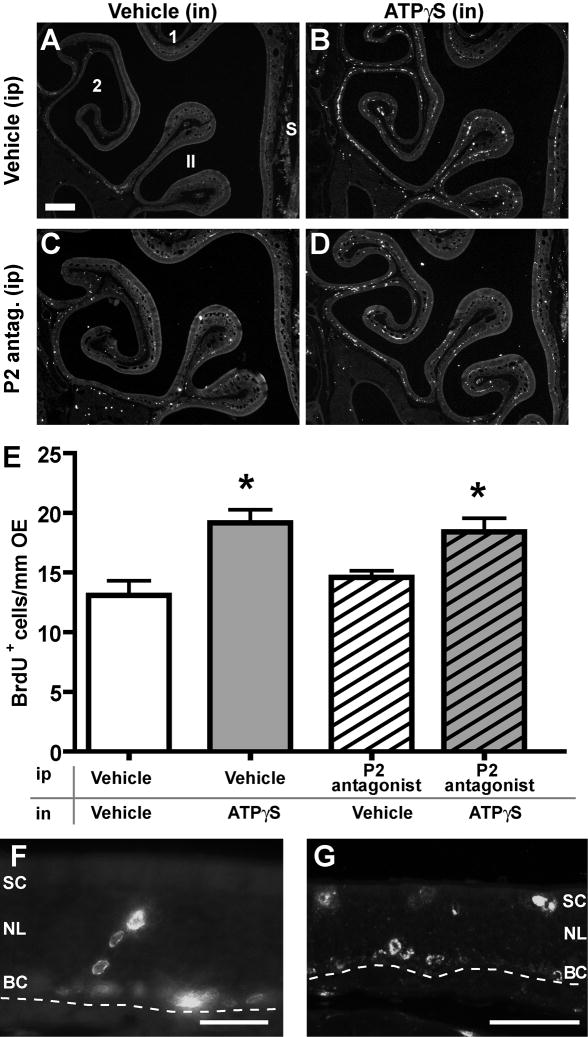
To determine if ATP induces proliferation in vivo, adult mice were intranasally instilled with ATPγS (200 μM) or vehicle and BrdU incorporation was monitored 48 hrs later in the septum and the ectoturbinate 2 and endoturbinate II (turbinates). ATPγS was utilized because it is a non-hydrolyzable analog of ATP and ectonucleotidases present in the olfactory epithelium (Braun and Zimmermann, 1998) could rapidly degrade ATP. Vehicle pretreatment followed by instillation of 200 μM ATPγS significantly increased the BrdU incorporation compared to vehicle instillation in the septum, turbinates and the entire olfactory epithelium (Table 1, Figure 1A, B, E; p <0.001, n = 23 sections each from 8 and 7 mice, respectively).
Table 1.
BrdU incorporation (BrdU+ cells/mm olfactory epithelium)
| Experimental conditions | Sustentacular cell layer | All layers of olfactory epithelium | |||||
|---|---|---|---|---|---|---|---|
| Ip injection | In instillation | Septum | Turbinates | Entire olfactory epithelium | Septum | Turbinates | Entire olfactory epithelium |
| Vehicle | Vehicle | 0.4±0.0 | 0.3±0.0 | 0.3±0.0 | 15.5±1.7 | 12.3±1.2 | 13.1±1.2 |
| Vehicle | ATPγS | 0.4±0.0 | 0.8±0.1 | 0.8±0.1 | 21.0±1.5* | 19.3±1.0* | 19.2±1.1* |
| P2 antagonist | Vehicle | 0.3±0.0 | 0.3±0.0 | 0.3±0.0 | 15.7±0.7 | 14.4±0.6 | 14.6±0.6 |
| P2 antagonist | ATPγS | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 | 23.6±1.1* | 17.5±1.2* | 18.4±1.2* |
, p<0.05 v. Vehicle (ip) Vehicle (in) Student’s t-test
Figure 1. ATPγS induces proliferation in the adult olfactory epithelium.
Adult mice were pre-treated with vehicle or purinergic receptor (P2) antagonists (100 μmoles/kg suramin + PPADS, ip) prior to intranasal (in) instillation of vehicle or 200 μM ATPγS. 48 hr later proliferation was measured as BrdU incorporation. (A–D) Representative images of BrdU-immunoreactivity in the septal (S), endoturbinate II (II) and ectoturbinate 2 (2) regions of the olfactory epithelium. Note the punctate BrdU-immunoreactivity near the basal lamina in the vehicle (ip) ATPγS (in) group (B). Scale bar represents 100 μm. (E) Quantitative analysis of BrdU+ incorporation. Data are expressed as mean (+ SEM) number of BrdU+ cells per linear length of total olfactory epithelium. n = 23, 23, 18, and 18 sections from 7, 8, 6, 6, mice, respectively. *, p < 0.01 v. all other groups, 2 way ANOVA with Bonferroni post-hoc tests. (F–G) Representative images of cell proliferation in the apical sustentacular cell layer (SC), the neuronal layer (NL), and the basal cell layer (BC). Dashed line, basement membrane, scale bars = 50 μm.
We examined the location of ATPγS-induced proliferation by analyzing BrdU incorporation in the septum and turbinates (Table 1). In the entire olfactory epithelium, there was no significant difference between BrdU+ cells in the septum v. turbinates (p = 0.09, 2 way ANOVA). In some instances we observed a few BrdU+ cells in the sustentacular cell layer of the olfactory epithelium, indicative of either sustentacular cell proliferation, or migration of proliferating basal cells to the apical regions (Figure 1F, G). To determine if ATPγS exposure evokes proliferation of sustentacular cells, BrdU+ cells were tabulated in the sustentacular cell layer of the olfactory epithelium (Table 1). Note that BrdU incorporation at 2 days post-ATPγS instillation is very low in the sustentacular cell layer such that BrdU incorporation in the basal cell layer would approximate BrdU incorporation in all the layers, and thus these data are not reported. In the septum, turbinates and entire olfactory epithelium, there was a very low level of BrdU incorporation in the sustentacular cell layer in the vehicle (ip, in) control group, with less than 1 BrdU+ cell/mm olfactory epithelium. Instillation of ATPγS did not significantly alter the number of BrdU+ cells 48 hrs later in the sustentacular cell layer of the septum, turbinates and entire olfactory epithelium (p>0.05, 2 way ANOVA, n=6). Overall, ATPγS-induced apical proliferation was not widespread, and if observed, was predominantly in the turbinates. Collectively, these data suggest that ATPγS does not preferentially induce proliferation in specific cell types or locations within the olfactory epithelium, but rather induces proliferation throughout the entire epithelium.
To investigate whether the ATPγS-induced increase in BrdU+ cells was mediated by purinergic receptors, we pre-treated adult mice with vehicle or purinergic receptor antagonists (100 μmoles/kg suramin + PPADS, ip) prior to intranasal (in) instillation of vehicle or 200 μM ATPγS. Pretreatment with 100 μmoles/kg purinergic receptor antagonists (ip) had no significant effect on BrdU incorporation in the vehicle (in) stimulated group (Table 1, Figure 1E; p>0.05 v. vehicle (ip and in), n = 18 sections from 6 mice). Pretreatment with 100 μmoles/kg of purinergic receptor antagonists (ip) also did not reduce the ATPγS-evoked increase in BrdU incorporation (Figure 1D, E; p<0.01 v. vehicle (ip and in), n = 18 sections from 6 mice).
ATP and UTP induce cell proliferation via a purinergic receptor mediated pathway
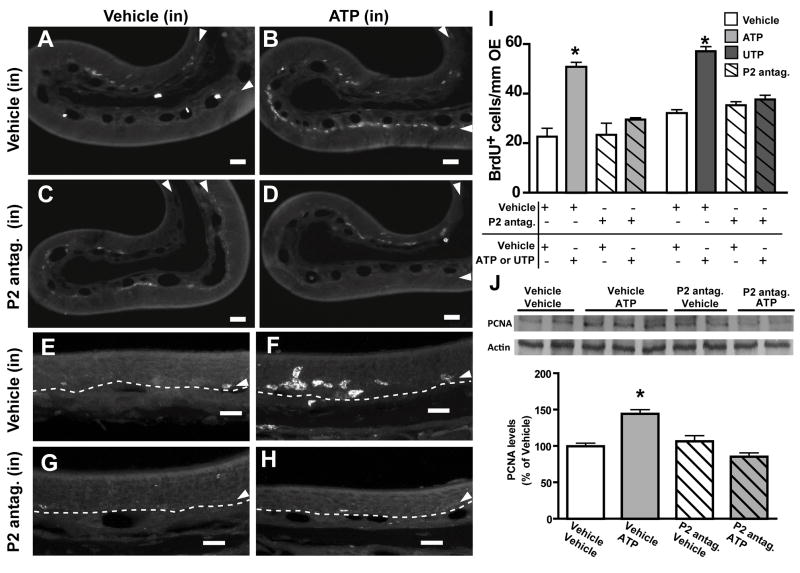
Based on these initial experiments, we could not conclude that ATPγS induces cell proliferation specifically via purinergic receptor activation. Review of our previous calcium imaging experiments showed that ATPγS activates less than 60% of the ATP-responsive olfactory sensory neurons, and 20% of the ATP-responsive sustentacular cells (Hegg et al., 2003). Therefore, even though ATPγS may have a longer signaling duration in the olfactory epithelium, we hypothesized that ATP may have a greater influence on cell proliferation due to activation of a greater number of cells. Furthermore, we postulated that intranasal instillation of antagonists may be more effective than ip injections due to the low level of vascularization in the olfactory epithelium. In addition, P2Y receptors are involved in the growth–promoting effects of ATP in the CNS (Rathbone et al., 1992b). Thus, we also tested the P2Y agonist UTP. Intranasal instillation of ATP or UTP (200 μM) significantly increased BrdU+ cells in the olfactory epithelium over vehicle control from 22.6 ± 3.4 to 50.7 ± 1.9 or 32.1 ± 1.4 to 57.1 ± 1.9 BrdU+ cells/mm OE, respectively (Figure 2A, B, E, F, I, p<0.001, n=9 sections from 3 mice, each group). Pre-intranasal treatment with purinergic receptor antagonists PPADS (40 nmoles/kg) and suramin (160 nmoles/kg) did not alter the levels of BrdU+ cells in the olfactory epithelium of control animals (23.4 ± 4.7 and 35.3 ± 1.4 BrdU+ cells/mm OE, for the ATP and UTP experiment, respectively, Figure 2C, G, I, n=9 sections from 3 mice, each group). However, pre-intranasal treatment with purinergic receptor antagonists significantly blocked ATP- and UTP-induced increases in BrdU+ cells (29.5 ± 0.8 and 37.6 ± 1.7 BrdU+ cells/mm OE, respectively, Figure 2D, H, I, p<0.01, n=9 sections from 3 mice, each group). These results indicate that ATP-induced cell proliferation is mediated via activation of P2X and/or P2Y receptors.
Figure 2. ATP-induced cell proliferation in adult mouse olfactory epithelium is mediated by purinergic receptor activation.
Representative BrdU immunoreactivity in endoturbinate 2 (A–D) and magnified view from ectoturbinate 2 (E–H) from each treatment group. Scale bars = 20 μm. (I) Quantitative data of BrdU+ cells/mm OE in each treatment group. * p<0.05 v. vehicle-vehicle group (Dunnett’s post-hoc test). (J) Representative immunoblot for PCNA protein levels is shown in the top panel, and the graphical representation of the data (expressed as percent control of vehicle) is shown in the bottom panel. *, p<0.05, v. vehicle-vehicle group (Dunnett’s post-hoc test).
To further investigate the proliferative effect of ATP, we performed immunoblot analysis to detect the presence of PCNA, a protein synthesized in early G1 and S phases of the cell cycle. Olfactory epithelium was dissected from mice treated with vehicle control or purinergic receptor antagonists in the absence or presence of ATP. ATP induced a significant upregulation of PCNA protein by 44 ± 6% compared with that in the control 48 hrs after treatment (Figure 2J, p<0.05, n = 9 mice). No statistically significant change in PCNA protein levels was observed following purinergic receptor antagonist treatment (Figure 2J, 106 ± 8 % control, p>0.05, n = 6 mice), however, in the presence of purinergic receptor antagonists, the ATP-evoked increase in PCNA protein was reduced to control levels (Figure 2J, 85 ± 6% of control, p>0.05, n = 6 mice). Collectively, these results confirm that activation of purinergic receptors induces cell proliferation in adult mouse olfactory epithelium.
Activation of purinergic receptors promotes proliferation in neonatal olfactory epithelium
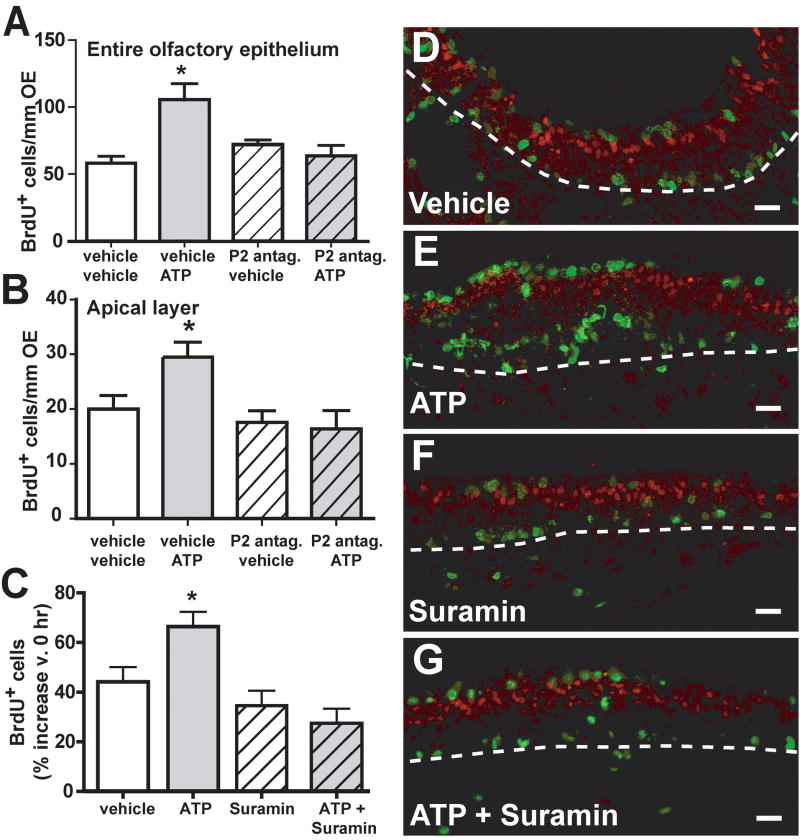
To determine if ATP acts as a proliferative factor throughout development, we performed the BrdU incorporation experiment in neonates. Intranasal instillation of 400 nmoles/kg ATP, equivalent to the adult ATP dose, significantly increased BrdU+ cells in the olfactory epithelium over vehicle control after 48 hrs from 58.3 ± 5.0 to 105.3 ± 12.2 BrdU+ cells/mm OE (Figure 3A, n=9 and 7 sections from 3 mice, p<0.001 v. vehicle control). Pre-intranasal treatment with purinergic receptor antagonists PPADS (40 nmoles/kg) and suramin (160 nmoles/kg) did not significantly alter the levels of BrdU+ cells in the olfactory epithelium of control animals (Figure 3A, 72.0 ± 3.4 BrdU+ cells/mm OE, n=8 sections from 3 mice, p > 0.05 v. vehicle control). However, pre-intranasal treatment with purinergic receptor antagonists significantly blocked ATP-induced increases of BrdU+ cells (Figure 3A, 63.6 ± 7.9 BrdU+ cells/mm OE, n=9 sections from 3 mice, p>0.05 v. vehicle control). Similar to the adult ATPγS experiment, there was no significant difference between BrdU incorporation between the septum and the turbinates (data not shown). These results indicate that in neonates, ATP induces cell proliferation via purinergic receptor activation throughout the entire olfactory epithelium.
Figure 3. ATP induces proliferation in neonates via purinergic receptor activation.
(A–B) Neonatal mice were instilled with PBS or 40 nmoles/kg PPADS + 160 nmoles/kg suramin (P2 antag.) 30 min prior to PBS or ATP (400 nmoles/kg). BrdU incorporation was measured 48 hr later in the entire olfactory epithelium (A) and in the apical layer (B). n = 7–9 sections from 3 mice, *, p<0.05 v. all groups, Dunnett’s post-hoc test. (C) In cultured neonatal olfactory epithelium slices proliferation was measured by BrdU incorporation and data are expressed as % BrdU+ cells compared to 0 hr vehicle (mean + SEM, n = 6). *, p<0.05 v. vehicle, Dunnett’s post-hoc test. (D–G) Representative olfactory marker protein (OMP; red) and 5-bromo-2-deoxyuridine (BrdU; green) immunoreactivity in neonatal olfactory epithelial slices cultured for 48 hrs in (C)Ringer’s solution vehicle, (D)ATP (100 μM), (E) suramin (100 μM) and (F) ATP + suramin (both 100 μM). Dotted white line depicts the basement membrane; scale bar = 20 μm.
There were more BrdU+ cells in the apical sustentacular cell layer in the neonates than in the adults in vehicle conditions (Figure 3B, 20.0±2.2, n=9 sections from 3 mice v. 0.3±0.0, adults e.g., Table 1). Interestingly, ATP significantly increased the number of BrdU+ cells in the apical layer compared to control (Figure 3B; 29.5±2.7 v. 20.0± 2.2, n=7 sections from 3 mice, p<0.05 v. vehicle control). Pre-intranasal treatment with purinergic receptor antagonists did not significantly alter the levels of BrdU+ cells in the olfactory epithelium of control animals (Figure 3B, 17.6±2.1 BrdU+ cells/mm OE, n=8 sections from 3 mice, p > 0.05 v. vehicle control). However, pre-intranasal treatment with purinergic receptor antagonists significantly blocked ATP-induced increases of BrdU+ cells (Figure 3B, 16.3±3.4 BrdU+ cells/mm OE, n=9 sections from 3 mice, p>0.05 v. vehicle control). In the neonates, purinergic receptor activation induced proliferation in putative sustentacular cells.
We also performed in vitro assays in which olfactory epithelial slices were cultured in the presence of vehicle (Ringer’s solution) or 100 μM ATP and/or 100 μM suramin for 48 hrs, and BrdU incorporation was evaluated (Figure 3C–G). After 48 hrs in vehicle, there was a 44±6% increase in BrdU incorporation compared to 0 hr control, indicating a level of inherent proliferation in the slice cultures (Figure 3C, D). After 48 hrs of ATP treatment a 66±6% increase in BrdU+ cells v. 0 hr control was observed, indicating that ATP significantly increased proliferation (Figure 3C, E, n = 9, p<0.05 v. vehicle, Dunnett’s post-hoc test). Similar to the neonatal in vivo study, we observed many BrdU immunoreactive cells in the apical layer, and ATP administration increased the number of apical BrdU+ cells (Figure 3D, E). Treatment of the slices with purinergic receptor antagonist suramin alone induced a level of BrdU incorporation similar to vehicle treatment, with a 35 ± 6% increase in BrdU+ cells v. 0 hr control (Figure 3C, F, n = 6, p > 0.05 v. vehicle, Dunnett’s post-hoc test). Co-treatment with ATP and suramin reduced the ATP-mediated increase in BrdU+ cells to 27 ± 6% BrdU+ cells compared to 0 hr control (Figure 3C, G, n = 7, p > 0.05 v. vehicle, Dunnett’s post-hoc test). A one way ANOVA indicated that there was a significant difference in BrdU incorporation among treatments (p = 0.0005), suggesting that ATP induces proliferation via purinergic receptor activation. Collectively, these results indicate that a neonatal cultured slice preparation may be a useful model for further proliferation studies.
ATP promotes proliferation of neuronal and sustentacular progenitor cells in adults
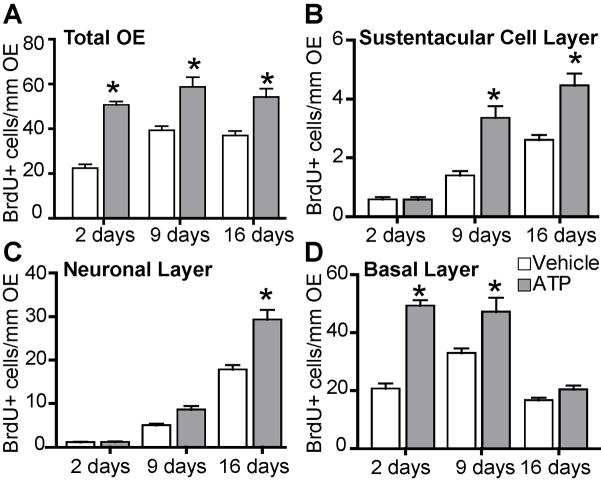
To determine if ATP induces the proliferation of neuronal and sustentacular progenitor cells we performed a new BrdU incorporation assay, extending the end point to 9 and 16 days following ATP application. This extended period allows for the basal cells to proliferate and differentiate into mature OSNs, a process that takes a minimum of 10 days (Schwob et al., 1995). Intranasal instillation of ATP significantly increased BrdU+ cells in the olfactory epithelium compared to vehicle controls by 125%, 49%, and 46% at 2, 9 and 16 days (Figure 4A, p<0.01 v. respective time control, Bonferroni’s post-hoc test; n=9 sections from 3 mice each group). The number of ATP-induced BrdU+ cells was not significantly different between 2, 9 and 16 days (50.7 ±1.6, 58.7±4.4, 54.1±3.8 BrdU+cells/mm OE, respectively), indicating that the BrdU+ cells survived through 16 days. To analyze the fate of ATP-induced cell proliferation, we counted BrdU+ cells in the apical sustentacular cell layer, the middle OSN layer and the basal progenitor cell layer. At 2 days, ATP-induced BrdU+ cells were primarily in the basal layer (Figure 4B v. 5C, D; 97% of the total BrdU+ cells). Immunohistochemical analysis showed colocalization of BrdU+ cells and the neuronal progenitor cell marker MASH 1, but not immature neuron marker GAP43 (Figure 5A, B). At 9 days, ATP significantly increased BrdU+ cells in the basal layer (Figure 4B; 32.9 to 47.2 BrdU+ cells/mm OE, p<0.001) and the sustentacular cell layer (Figure 4D; 1.4 to 3.4 BrdU+ cells/mm OE, p<0.001) suggesting proliferation of a sustentacular cell progenitor. ATP did not, however, increase BrdU incorporation in the OSN layer at 9 days (Figure 4C; 5.0 to 8.6 BrdU+ cells/mm OE, p>0.05). BrdU+ cells colocalized with GAP43 but not mature OSN marker OMP (Figure 5C, D). At 16 days, ATP-induced BrdU+ cells were significantly elevated in the sustentacular cell layer (Figure 4D; 2.6 to 4.5 BrdU+ cells/mm OE, p<0.001) and the OSN layer (Figure 4C; 17.8 to 29.3 BrdU+ cells/mm OE, p<0.001) but not in the basal layer (Figure 4B; 16.7 to 20.4 BrdU+ cells/mm OE, p>0.05). BrdU+ cells were primarily co-localized with OMP (Figure 5E). These data indicate that ATP induces proliferation of both OSN and sustentacular progenitor cells in adult mouse olfactory epithelium.
Figure 4. ATP promotes proliferation of OSN and sustentacular progenitor cells.
Quantitative analysis of BrdU incorporation in the entire olfactory epithelium (A), basal cell layer (B), neuronal layer (C), or sustentacular cell layer (D) 2, 9 or 16 days following ATP instillation. Data are expressed as mean (+ SEM) number of BrdU+ cells per linear length of the entire olfactory epithelium. *, p < 0.01 v. all other groups, 2 way ANOVA with Bonferroni post-hoc tests.
Discussion
This study was based on the hypothesis that ATP released following injury could promote regeneration in the adult olfactory epithelium. We examined whether ATP had a proliferative effect in the olfactory epithelium. We observed that both ATP and UTP increase the proliferation of cells through a purinergic receptor mediated pathway. Similar results with ATP were obtained from a neonatal in vivo experiment, and an in vitro study using a neonatal slice preparation. These results validate the use of a neonatal slice preparation in studying ATP-induced regenerative mechanisms in adult. Not surprisingly, in the neonates there was an inherently greater amount of cells undergoing proliferation in the vehicle treatment groups (compare Figure 2I v. Figure 3A). Nevertheless, ATP instillation significantly increased BrdU incorporation 1.8 fold in neonates. These data suggest that ATP may be a proliferative factor throughout development and into adulthood.
In the CNS, ATP’s proliferative effects appear to be mediated via activation of G-protein coupled P2Y purinergic receptors and are possibly coupled to the production of inositol phosphates and other second messengers (Rathbone et al., 1992b). When we designed the in vivo study, we initially elected to use the non-hydrolyzable ATP analog ATPγS, in hopes of prolonging the ATP signal duration. However, ATPγS, a non-selective P2 receptor agonist, activates less than 60% of the ATP-responsive olfactory sensory neurons, and 20% of the ATP-responsive sustentacular cells (Hegg et al., 2003). Subsequent experiments revealed that instillation of ATP induced a greater increase in cell proliferation v. vehicle than ATPγS (2.2 fold v. 1.5 fold increase, e.g., Figure 2I v. 1E). Moreover, we found that UTP, a P2Y receptor agonist, also increased proliferation (1.8 fold v. vehicle). Collectively, these data suggest that increased proliferation is mediated by activation of P2X and/or P2Y receptors.
The amount of inherent proliferation that occurred in our vehicle treated groups varied from ~13 BrdU+ cells (Figure 1) with an ip injection and an intranasal instillation of vehicle, to 23–32 BrdU+ cells (Figure 2) with two intranasal instillation of vehicle. This could be due to the additional intranasal instillation of saline in the second set of experiments, which may cause increased mechanical injury and a subsequent increase in proliferation. An alternative explanation is that is that we used the Swiss Webster strain, which is an outbred strain, and thus has substantial genetic variability from mouse to mouse. The variability with outbred strains, coupled with the fact that we obtained robust results, suggests that the ATP effect on proliferation is quite strong. In our experience, as well as in other labs, the C57BL/6 inbred strain has a significantly higher level of proliferation under control conditions than the Swiss Webster, indicating that there may be both strain differences as well as individual differences in basal cell proliferation. To be noted, however, is that the relative increase in proliferation following ATP instillation is roughly the same within each experiment, regardless of the individual performing the analysis or the mode of administration.
In the olfactory system there is a well documented phenomenon of zonal expression of phenotypic markers. Odorant receptors are expressed in distinct anatomical regions that are bilateral and symmetric in nature (Ressler et al., 1993, Vassar et al., 1993). Importantly, there is a zonal distribution of antigenically distinct progenitor cells (Murdoch and Roskams, 2008). Therefore, we characterized the ATP-induced cell proliferation in the septum, corresponding to zone 1, and turbinates, corresponding to zones 2–3 in the total olfactory epithelium (Ressler et al., 1993). There was no statistical difference between ATP-induction of cell proliferation between the septum and the turbinate locations, suggesting that ATP may have a universal, broad effect in the olfactory epithelium.
In adults we observed a very small population of proliferating cells located in the apical region of the olfactory epithelium, where sustentacular cell somas are located two days following ATP administration. This could indicate a more rapid migration of proliferating neuronal progenitor cells towards the apical regions. Indeed, after 48 hrs we could observe BrdU+ cells in the middle neuronal layer, e.g., Figure 1F and 2F, indicating that a subpopulation of proliferating cells were advancing through the olfactory epithelium. Or it may represent the “self” proliferation of sustentacular cells. The number of proliferating cells in the apical region was not significantly increased 48 hrs after in vivo instillation of ATPγS in adults in the turbinates (zone 2–3) or the septum (zone 1). However, at 9 and 16 days post ATP instillation, the number of BrdU immunoreactive cells in the apical layer increased significantly, indicating that ATP may induce the proliferation of sustentacular progenitor cells that reside in the basal layer. We also saw an ATP-induced time-dependent increase in co-expression of BrdU and markers for neuronal progenitor cells, immature neurons and mature olfactory sensory neurons. Thus, these results support the notion that sustentacular cells and OSNs arise from a common stem cell (Beites et al., 2005), and that ATP can induce the differentiation of both sustentacular and neuronal progenitor cells.
In neonates, we observed an increase in inherent proliferation in the apical region compared to adults. Our results support the previous observation that sustentacular cells undergo proliferation primarily during embryonic and post-natal stages to promote growth in surface area (Weiler and Farbman, 1998). Moreover, unlike in the adults, we observed an ATP-evoked increase in BrdU immunoreactive cells in the apical layer that is inhibited by purinergic receptor antagonism both in vitro and in vivo. Our results suggest that ATP may be a signal to induce sustentacular cell “self” proliferation.
In these studies, purinergic receptor antagonists administered alone did not affect the proliferation rate, suggesting that there may be other additional signals also involved in regulating cell turnover. Indeed, the level of neurogenesis is tightly regulated by a multitude of chemical signals produced by olfactory sensory neurons or sustentacular cells that influence the proliferation and differentiation of endogenous neuronal progenitor basal cells. Negative autoregulators of neurogenesis such as GDF11 (Wu et al., 2003) are released from neurons and inhibit proliferation and generation of new neurons in the olfactory epithelium. Thus, destruction of neurons removes the source of the inhibitory anti-proliferative signal, and promotes neuronal regeneration. Interestingly, ATP was able to override neuronal negative feedback signals to promote proliferation in the absence of injury.
The signals that lead to increased cell proliferation and neurogenesis after injury are poorly understood. The idea that cell death may be necessary to induce new cell production is supported by studies that have found increased neurogenesis after targeted apoptosis (Calof et al., 1996). A number of specific local molecular cues or cell-autonomous factors are candidates for mediating injury-induced increases in proliferating basal cells. This study demonstrates that ATP is sufficient to promote proliferation in vivo, in non-injured olfactory epithelium. The potential role of ATP in concert with other various signaling molecules, in normal and injury-induced neurogenesis remains to be determined in the olfactory epithelium. We have identified purinergic nucleotides as important local environmental signals in neuronal proliferation. Identification of factors that control and regulate proliferation and regeneration will have important implications on injury and repair therapeutics in both olfactory and neuronal tissue.
Acknowledgments
Research was supported by NIH NIDCD 006897.
Abbreviations
- BrdU
5-bromo-2-deoxyuridine
- FITC
fluorescein isothiocyanate
- GAP43
growth associated protein 43
- ip
intraperitoneal
- in
intranasal
- MASH 1
mammalian achaete-schute homolog 1
- OE
olfactory epithelium
- OMP
olfactory marker protein
- OSN
olfactory sensory neuron
- PBS
phosphate buffered saline
- PCNA
proliferating cell nuclear antigen
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
- TRITC
tetramethyl rhodamine isothiocyanate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Braun N, Zimmermann H. Association of ecto-5′-nucleotidase with specific cell types in the adult and developing rat olfactory organ. J Comp Neurol. 1998;393:528–537. doi: 10.1002/(sici)1096-9861(19980420)393:4<528::aid-cne10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Calof AL, Hagiwara N, Holcomb JD, Mumm JS, Shou J. Neurogenesis and cell death in olfactory epithelium. J Neurobiol. 1996;30:67–81. doi: 10.1002/(SICI)1097-4695(199605)30:1<67::AID-NEU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Roskams AJ. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc Res Tech. 2002;58:204–215. doi: 10.1002/jemt.10150. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA, Jacobson M. Handbook of Sensory Physiology. Vol. 9. New York: Springer; 1978. Continuous nerve cell renewal in the olfactory system; pp. 55–83. [Google Scholar]
- Hegg CC, Greenwood D, Huang W, Han P, Lucero MT. Activation of purinergic receptor subtypes modulates odor sensitivity. J Neurosci. 2003;23:8291–8301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. A novel embryonic nestin-expressing radial glia-like progenitor gives rise to zonally restricted olfactory and vomeronasal neurons. J Neurosci. 2008;28:4271–4282. doi: 10.1523/JNEUROSCI.5566-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Christjanson L, Deforge S, Deluca B, Gysbers JW, Hindley S, Jovetich M, Middlemiss P, Takhal S. Extracellular purine nucleosides stimulate cell division and morphogenesis: pathological and physiological implications. Med Hypotheses. 1992a;37:232–240. doi: 10.1016/0306-9877(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Deforge S, Deluca B, Gabel B, Laurenssen C, Middlemiss P, Parkinson S. Purinergic stimulation of cell division and differentiation: mechanisms and pharmacological implications. Med Hypotheses. 1992b;37:213–219. doi: 10.1016/0306-9877(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Supporting cell proliferation in the olfactory epithelium decreases postnatally. Glia. 1998;22:315–328. doi: 10.1002/(sici)1098-1136(199804)22:4<315::aid-glia1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]