Abstract
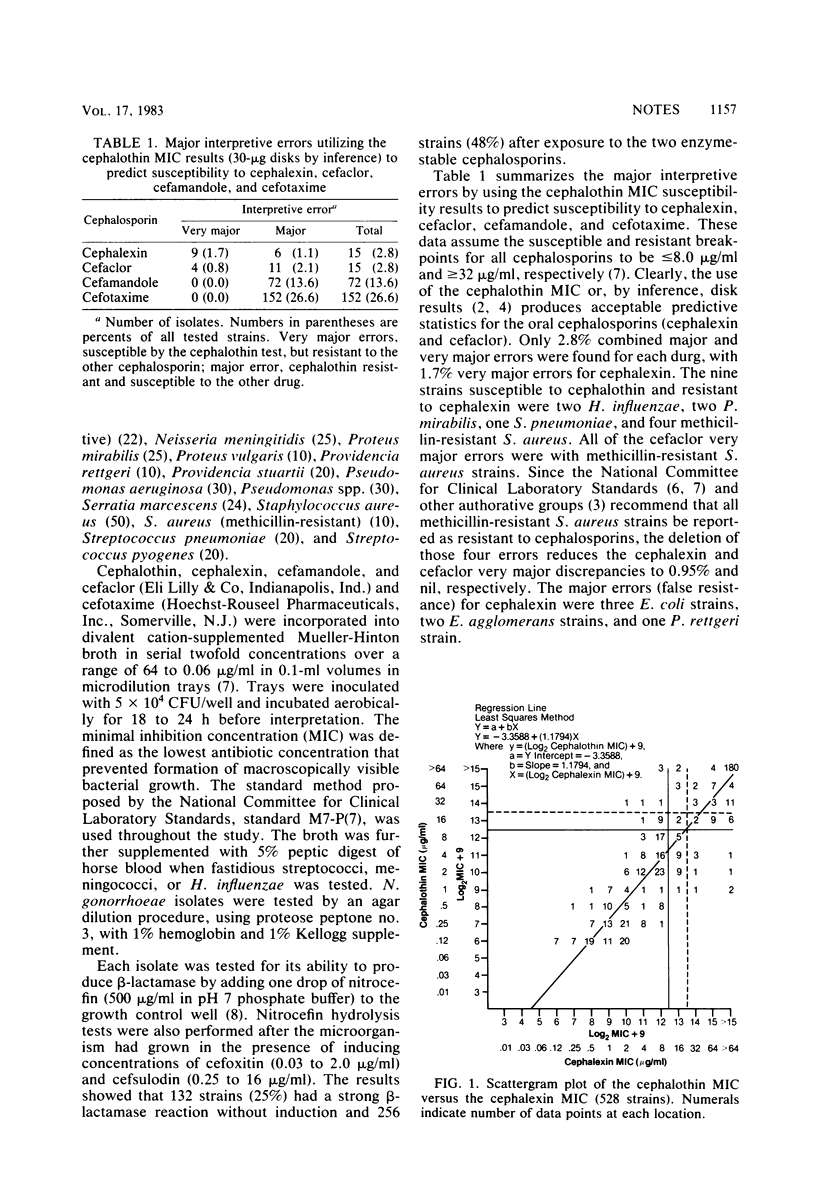
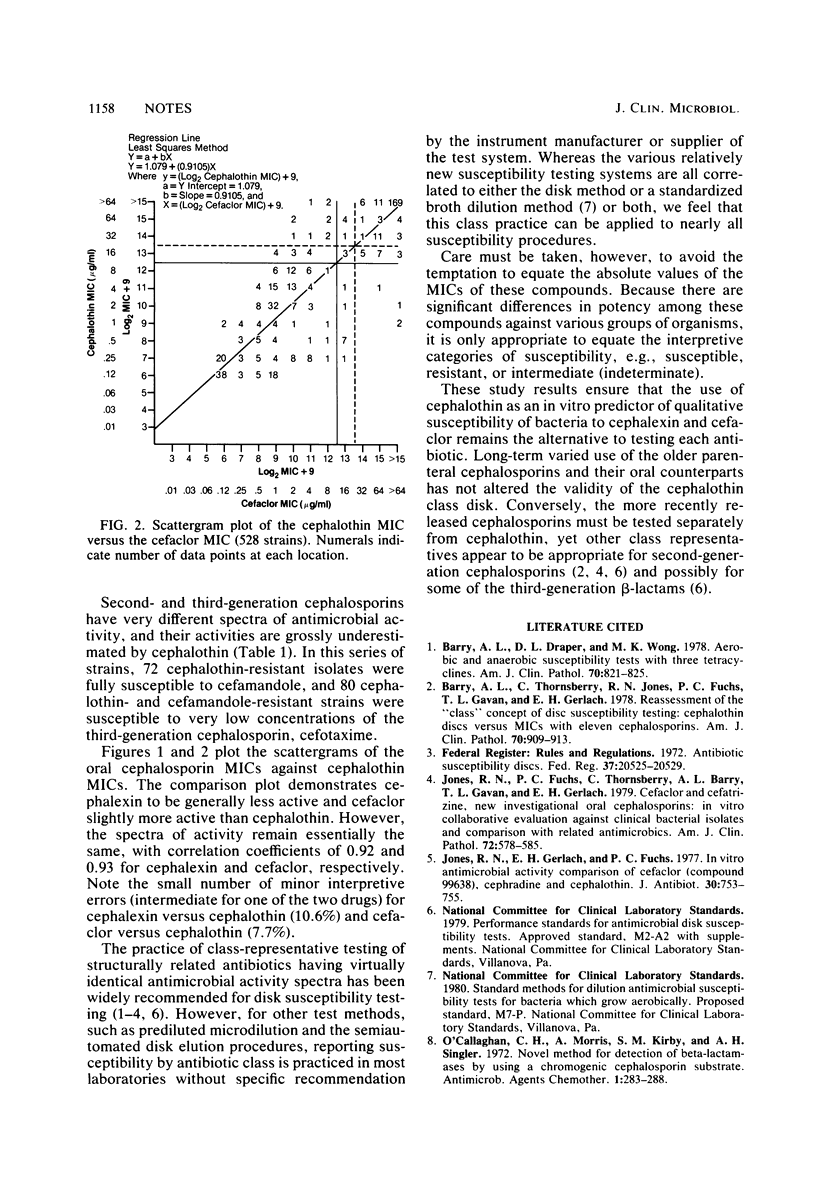
The validity of the class representative concept for in vitro susceptibility testing of older cephalosporins was reevaluated. Two oral cephalosporins, cephalexin and cefaclor, were compared with the established cephalosporin class representative, cephalothin, by using reference microdilution minimal inhibitory concentrations of 528 isolates of a wide variety of gram-positive and gram-negative bacterial pathogens. For each comparison, there were only 15 (2.8%) random major and very major interpretive discrepancies. Additional comparisons confirmed the need to test second-generation (cefamandole) and third-generation (cefotaxime) cephalosporins separately. These results provide reasonable assurance that the use of cephalothin as an in vitro predictor of qualitative bacterial susceptibility to these two oral cephalosporins remains an acceptable alternative to testing each antibiotic individually.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Draper D. L., Wong M. K. Aerobic and anaerobic susceptibility tests with three tetracyclines. Reassessment of the "class concept" of disk testing. Am J Clin Pathol. 1978 Nov;70(5):821–825. doi: 10.1093/ajcp/70.5.821. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H. Reassessment of the "class" concept of disk susceptibility testing. Cephalothin disks versus minimal inhibitory concentrations with eleven cephalosporins. Am J Clin Pathol. 1978 Dec;70(6):909–913. doi: 10.1093/ajcp/70.6.909. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Thornsberry C., Barry A. L., Gavan T. L., Gerlach E. H. Cefaclor and cefatrizine, new investigational orally administered cephalosporins. In-vitro collaborative evaluation against clinical bacterial isolates and comparison with related antimicrobics. Am J Clin Pathol. 1979 Oct;72(4):578–585. doi: 10.1093/ajcp/72.4.578. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Gerlach E. H., Fuchs P. C. In vitro antimicrobial activity comparison of cefaclor (compound 99638), cephradine, and cephalothin. J Antibiot (Tokyo) 1977 Sep;30(9):753–755. doi: 10.7164/antibiotics.30.753. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]


