Abstract
Extensive peripheral nerve injuries can result in the effective paralysis of the entire limb or distal portions of the limb. The major determinant of functional recovery after lesions in the peripheral nervous system is the accurate regeneration of axons to their original target end-organs. We used the mouse femoral nerve as a model to study motor neuron regeneration accuracy in terms of regenerating motor neurons projecting to their original terminal pathway to quadriceps muscle vs. the inappropriate pathway to skin. Using a variety of surgical manipulations and the selective removal of Schwann cells in the distal nerve via molecular targeting, we have examined the respective roles of end-organ influence (muscle) vs. Schwann cells in this model system. We found evidence of a hierarchy of trophic support that regulates motor neuron regeneration accuracy with muscle contact being the most potent, followed by the number or density of Schwann cells in the distal nerve branches. Manipulating the relative levels of these sources of influence resulted in predictable projection patterns of motor neurons into the terminal pathway either to skin or to muscle.
Keywords: femoral nerve, PNS
Recovery of function following nerve lesions in the peripheral nervous system (PNS) depends on the accurate regeneration of axons to their original target end-organs. A recognized leader in the field of clinical nerve repair once stated that, “The core of the problem is not promoting axon regeneration, but in getting them back to where they belong” (Sunderland 1991). Regenerating motor axons are often misrouted to sensory targets, and sensory axons formerly innervating skin are often misrouted to muscle. Such misdirected regeneration is a major detriment to functional recovery and rehabilitation. Nevertheless, under certain conditions, axonal regeneration in the mammalian PNS can and does occur with a remarkable degree of accuracy and return of function. The cellular and molecular determinants of this limited, but nonetheless remarkable capacity for reestablishing connections remain largely unknown. An improved understanding of the mechanisms that influence motor neuron reinnervation of distal nerve pathways could have a direct bearing on improving functional nerve repair; since if accurate choices are not made at the terminal nerve branch level progressively finer discriminations (e.g., correct receptor or fiber reinnervation) might be rendered impossible by the lack of appropriate end-organ choices available at the termination of the tributary nerve.
A useful model to study terminal pathway reinnervation is the rodent femoral nerve. Proximally in the leg, motor axons are dispersed across the entire mixed nerve. Distally, the nerve divides into two approximately equal terminal branches; a motor branch to the quadriceps muscle and a purely sensory branch which continues as the saphenous nerve (Brushart 1988; Franz, Rutishauser et al. 2005). Much previous work from several laboratories has shown that following a proximal lesion regenerating motor neurons preferentially, albeit incompletely, reinnervate the terminal quadriceps branch (to muscle) versus the saphenous branch (to skin), (Madison, Archibald et al. 1996; Al-Majed, Neumann et al. 2000; Robinson and Madison 2005; Eberhardt, Irintchev et al. 2006; Uschold, Robinson et al. 2007). However, there is controversy concerning the degree of influence the terminal nerve pathways alone have on such preferential reinnervation, vs. the influence of the respective end-organs of muscle and skin (Brushart 1993; Hoke, Redett et al. 2006; Robinson and Madison 2006; Uschold, Robinson et al. 2007).
Given the vast amount of previous work regarding the general influence of Schwann cells on peripheral nerve regeneration (Hall 1989; Hall 2005; Chen, Yu et al. 2007) it is reasonable to suspect that Schwann cells may be a likely cellular candidate in the terminal nerve pathways to influence regeneration accuracy. Accordingly, it has been suggested that Schwann cells from the cutaneous and muscle pathways of the femoral nerve have distinct sensory and motor phenotypes that can impact the accuracy of axon regeneration of motor neurons (Hoke, Redett et al. 2006). Conversely, Madison and colleagues (Robinson and Madison 2005; Uschold, Robinson et al. 2007) have proposed that it is not inherent molecular differences between Schwann cells that determines the accuracy of motor neuron regeneration, rather motor neurons can be directed to reinnervate either the muscle or the cutaneous pathway depending on the relative balance of trophic influences from the terminal pathways and end-organs of muscle and skin. The current work was aimed at clarifying the role of Schwann cells in the denervated distal nerve branches in terms of their influence on the accuracy of motor neuron regeneration into their original terminal nerve pathway.
We took advantage of advances in molecular targeting technology to selectively remove Schwann cells in the two terminal nerve pathways distal to a femoral nerve lesion. Sofroniew and colleagues have developed a transgenic model system in which specific cellular removal can be targeted genetically in vivo (Sofroniew, Bush et al. 1999; Morshead, Garcia et al. 2003; Sofroniew 2005). The strategy was based on previous demonstrations that promoter sequences specific for particular cell-types can be linked to genes that encode toxic agents, and that such fusion gene constructs will be expressed and function in a cell-type-specific manner in transgenic animals (Sassone-Corsi and Borrelli 1986; Borrelli, Heyman et al. 1988). The promoter of glial fibrillary acidic protein (GFAP), an intermediate filament protein (Bignami, Eng et al. 1972; Eng and Ghirnikar 1994) was used to target astroglia and related cells. The thymidine kinase gene of the herpes simplex virus (HSV-TK) was used to achieve cellular removal.
One of the most encompassing classification schemes of Schwann cell types is the myelinating and non-myelinating phenotypes, both of which arise from a common pool of proliferating, immature, GFAP-expressing cells (Jessen and Mirsky 1992; Jessen and Mirsky 1999). Following development GFAP-expression is down-regulated. However, if adult Schwann cells loose contact with axons, such as distal to a nerve transection, they de-differentiate and re-enter the cell cycle to become immature, proliferating and GFAP-expressing cells, comparable to immature Schwann cells in neonatal nerve. Schwann cells that have de-differentiated after nerve injury and are proliferating and expressing GFAP can be selectively removed in these transgenic mice that express HSV-TK under the GFAP-promoter simply by the administration of GCV. Thus, the timing and location of Schwann cell removal can be regulated temporally by application of GCV following lesions of specific nerves.
The results of the current studies support the hypothesis of a hierarchy of trophic support from the terminal nerve pathways and muscle that regulate the accuracy of motor neuron regeneration. Within this hierarchy muscle contact is the most potent followed by the number/density of Schwann cells. There is no support for the idea that Schwann cells within the muscle pathway, by themselves, have a unique molecular identity that regulates regeneration accuracy of motor neurons.
Experimental Procedures
Conditional Removal of Schwann Cells
Transgenic animals were identified by genotyping as previously described (Bush, Savidge et al. 1998). Adult transgenic (TK) and non-transgenic wildtype (WT) mice were given a unilateral crush lesion of the sciatic nerve and continuous GCV delivery via a minipump (20 mg/kg/day) for the first seven days after surgery. After 14, 21 and 35 days post-injury animals were perfused with paraformaldehyde/gluteraldehyde, processed for plastic embedding, and stained with toluidine blue. The number of white blood cells and Schwann cells were quantified using analysis of 1µm plastic embedded sections. Counting frame fields (8564 µm2) were selected at random using a computer-driven microscope stage (CAST, Olympus); at least 15 frames were quantified per tissue sample. The number of positive cells per counting frame was determined, and final counts were expressed as number per mm2. At least 300 cells were counted for each tissue sample, and counts were carried out by blinded independent observers. Student t-tests were used to compare cell counts within groups. Differences were considered statistically significant when p < .05.
Surgical Procedures
All procedures were approved by the Veterans Affairs Medical Center animal use committee. General surgical procedures were carried out as previously described in detail (Robinson and Madison 2003). All of the retrograde labeling studies were carried out using TK transgenic mice. Briefly, TK mice (male and female, 20–22 g) were deeply anesthetized for all surgical procedures with a mixture of ketamine, xylazine and acepromazine (100, 6 and 1 mg/kg respectively) in normal (0.9%) saline.
In all groups the femoral nerve was exposed using an inguinal approach and the parent nerve was transected with microscissors ~5 mm proximal to the bifurcation of the nerve into its muscle and cutaneous branches. The nerve stumps were reapposed and repaired with fibrin sealant (Baxter Healthcare Products, Glendale, CA, USA). Two different repair groups were prepared that varied in terms of whether the distal nerve branches remained in continuity with their respective end-organs of muscle and skin.
In the End-Organ preparations (EO), the muscle branch and the cutaneous branch remained intact. In the No End-Organ groups (NEO), the muscle branch was transected at the quadriceps muscle, ligated and placed in a blind-ended silicone tube to prevent muscle contact by regenerating axons. The cutaneous branch was transected to be the same length as the muscle branch, and was also ligated and placed in a silicone tube. During the initial femoral nerve surgery, all animals also received implantation of osmotic minipumps subcutaneously on the back (Alzet, #1007) containing either ganciclovir (GCV, 20 mg/kg/day) to remove reactive and proliferating Schwann cells distal to the femoral nerve lesion, or saline (control); pumps were removed after the first week. Thus there were four different experimental groups; 1) End-Organ + Saline (N=6), 2) End-Organ + GCV (N=8), 3) No End-Organ + Saline (N=12), and 4) No End-Organ + GCV (N=7).
Retrograde Labeling and Counting of Motor Neurons
Eight weeks after nerve repair, the femoral nerve was re-exposed. The muscle and cutaneous branches were separated by silicone grease dams, trimmed to ~1 mm distal to the bifurcation, and randomly assigned to receive application of crystals of either fluoroscein dextran (FD, D-3306, Molecular Probes, Eugene, OR, USA) or tetramethylrhodamine dextran (TD, D-3308, Molecular Probes). After crystal application, each branch was sealed with silicone grease and separated from each other by plastic food wrap. Three days later the animal was perfused through the heart with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in PBS. The lumbar spinal cord was removed, post-fixed for several hours, and sucrose protected. The cord was frozen on dry ice and stored at −80 °C until being sectioned with a cryostat. Serial 25-µm frozen longitudinal sections were thawed in PBS, mounted onto glass slides, air dried and coverslipped with Prolong (P-7481, Molecular Probes) according to the manufacturer's instructions. All serial sections were examined, and all retrogradely labeled motor neurons containing a nucleus were identified using a composite filter set (#51006, Chroma Technology, Brattleboro, VT, USA) in a fluorescence-equipped Zeiss Axiophot microscope, at 250X magnification. Motor neuron counts were carried out by blinded independent observers and scored as either single-labeled (fluoroscein or tetramethylrhodamine only) or double-labeled (both fluoroscein and tetramethylrhodamine). Previously published work has documented the validity and reliability of these methods (e.g., lack of cross-contamination due to tracer leakage, and intra- and inter-counter reliability (see (Robinson and Madison 2004; Uschold, Robinson et al. 2007). Counting variation among the observers was ~2%. Motor neurons labeled from the muscle, the cutaneous, or both branches, were tabulated and their counts corrected for split cells (Abercrombie 1946). Control data from previously unlesioned quadriceps nerves (Robinson and Madison 2003) labeled 161 ± 4 motor neurons (N = 4, mean ± SE).
Statistical Analysis
Student t-tests for paired data were used within groups to compare the number of motor neurons labeled from the muscle and cutaneous branches. Differences were considered statistically significant when p < .05. Analysis of variance (using Student-Newman-Keuls post-hoc comparisons) was used to determine differences among groups. Differences were considered statistically significant when F < .05 and p < .05.
Results
Schwann Cell Removal
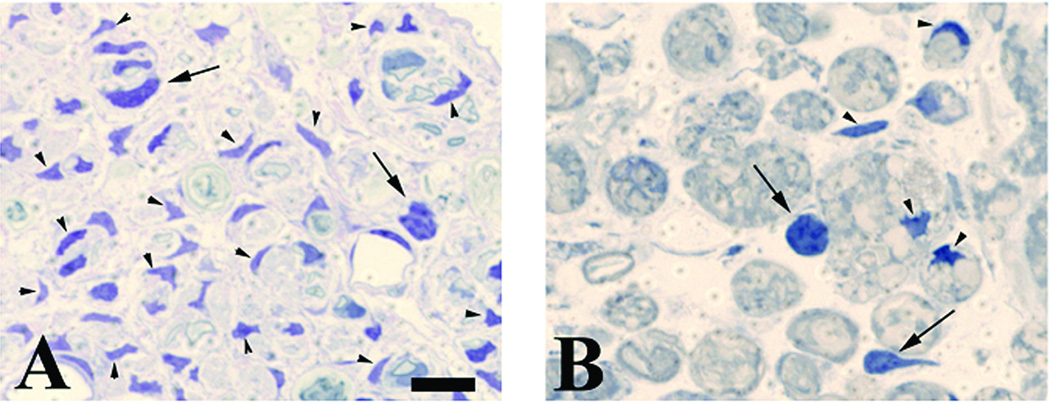
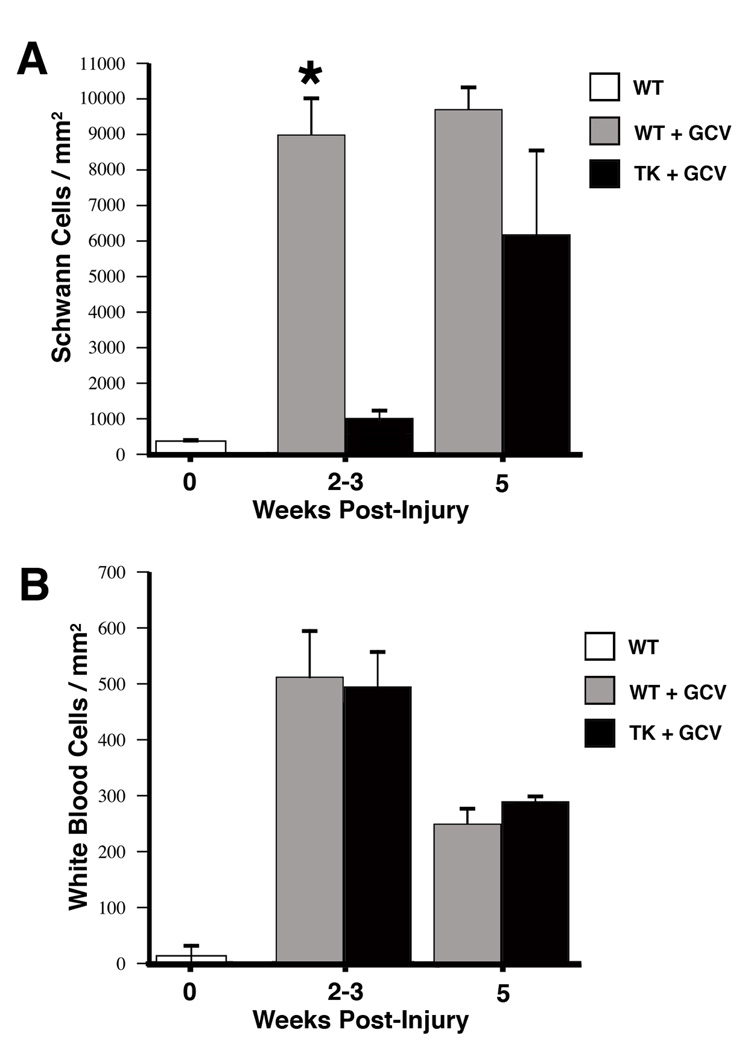
Schwann cell nuclei were identified by their characteristic dense staining and elongated or irregular morphology (Fig. 1a) and white blood cells were identified as being larger, moderately-dark cells, with rounded or oblong cell bodies and nuclei (Fig. 1b). In agreement with previous work (Sofroniew, Bush et al. 1999), after crush injury in wildtype mice there were significant increases (~18 fold) in the number of Schwann cell nuclei in the distal nerve as early as 14 days postinjury compared with uninjured nerve, even in the presence of GCV (Fig. 2A). These data show that administration of GCV to the wildtype animals did not change the expected increase in Schwann cell proliferation after nerve injury. Conversely, at all time points there were significantly fewer Schwann cells in the transgenic distal nerve compared to wildtype animals; expressed as number of Schwann cell nuclei per square mm (mean ± SEM). However, this difference decreased at the later time points. Due to the loss of axonal contact the original populations of Schwann cells in the transgenic animals distal to the nerve lesion de-differentiated and began to reexpress GFAP, and thus were selectively removed by application of GCV during the first 7 days postlesion. By 5 weeks postinjury, both TK and wildtype animals displayed an 18- and 28-fold increase respectively in Schwann cell nuclei compared to unlesioned nerve. The Schwann cell increase seen at 5 weeks postlesion in the TK animals given GCV during the first week is due to Schwann cell invasion (and subsequent proliferation) from the proximal nerve that accompanies regenerating axons, as has been seen in cases of freeze-thaw lesions (Fugleholm, Schmalbruch et al. 1994; Fugleholm, Sorensen et al. 1998).
Figure 1.
Representative high-power views of 1µm plastic-embedded sections stained with toluidine blue. WT and transgenic TK animals received a crush lesion of the sciatic nerve and implantation of a minipump to deliver ganciclovir (GCV; 20 mg/kg/day) for the first seven days after surgery. Nerve segments distal to the crush lesion were harvested 7, 14, or 35 days post-injury. Panels are from the 14-day time point. A) WT animal. Note the numerous Schwann cell nuclei (identified by their characteristic dense staining and elongated or irregular morphology; arrowheads). A few white blood cells are also present (identified as larger moderately dark cells with rounded or oblong cell bodies and nuclei; arrows). B) Transgenic animal (TK). Compared to the WT animal note the paucity of Schwann cells but similar density of white blood cells. Size bar in A represents 10 microns for both panels. The quantification of such sections is shown in figure 2.
Figure 2.
Counts of the number of Schwann cells (A) and white blood cells (B) in 1µm plastic-embedded sections in distal nerve at various times post-injury following continuous GCV administration during the first 7-days. Data expressed as mean ± SEM per mm2, N=4 TK animals and N=4 WT animals at each time point. A, top panel: There is a rapid and dramatic increase in the number of Schwann cells in WT animals compared to uninjured controls by 2–3 weeks, representing a greater than 15-fold. A similar increase (11-fold) in TK animals is not seen until 5 weeks post-injury and represents the ingrowth of Schwann cells from the proximal nerve that accompany regenerating axons. *=p<.05 between WT and TK. B, bottom panel: In contrast to the dramatic differences in the number of Schwann cells between WT and TG animals, there are no differences in the number of inflammatory cells (white blood cells). Compared to uninjured controls, both experimental groups show significant increases in the number of white blood cells typical of Wallerian degeneration.
Analysis of the same nerve samples for white blood cells (as a gross marker of inflammation) showed no difference between the TK or wildtype animals at any of the time points (Fig. 2B). All lesioned nerves showed the expected increase in white blood cells compared to unlesioned control nerves. Theses results suggest that the conditional removal of Schwann cells did not add to the already extensive inflammation that is a hallmark of Wallerian degeneration and regenerating peripheral nerve.
Regeneration Accuracy of Motor Neurons
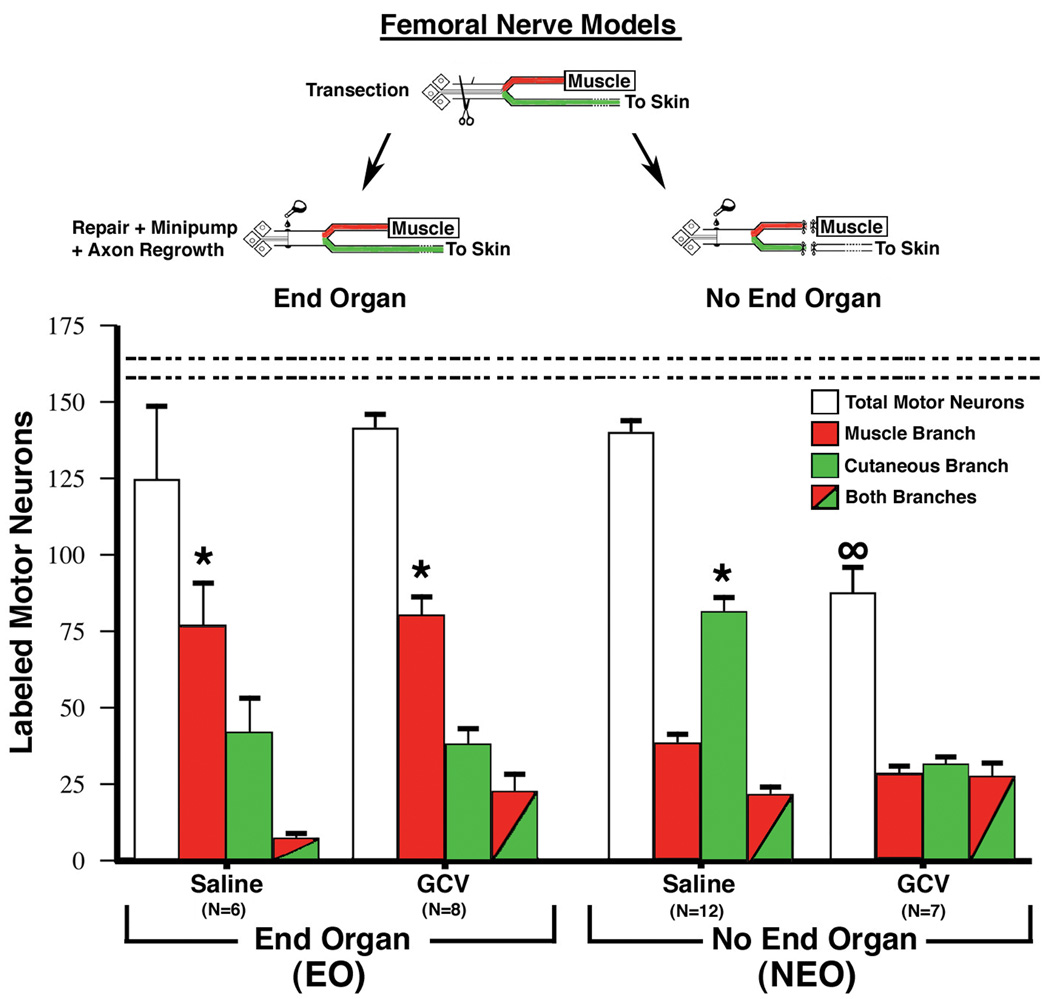
Eight weeks after the nerve repair the number of motor neurons projecting to each of the terminal branches was determined by retrograde transport of low molecular weight dextrans. In the normal femoral nerve, motor neurons project exclusively to the muscle pathway. Therefore, within the context of the femoral nerve choice model the measure of regeneration accuracy is the extent to which regenerating motor neurons return to their original pathway to muscle rather than the foreign pathway to skin. With end-organ contact (EO) significantly more motor neurons projected to the muscle branch. This result was seen whether the animals received saline or GCV minipumps during the first 7 days after nerve repair (Fig. 3, left hand side). The total number of motor neurons retrogradely labeled was not significantly different from control unoperated animals.
Figure 3.
Quantitative assessment of regeneration accuracy of motor neurons obtained 8 weeks after femoral nerve repair in adult transgenic TK mice. In the End-Organ model (EO) both terminal branches remained intact to their respective end-organs of muscle and skin. In the No End-Organ model (NEO) all end-organ contact was prevented by transecting, ligating, and capping both terminal branches equidistant from the parent nerve bifurcation. Eight weeks after femoral nerve repair application of labeled dextran tracers to the two terminal nerve branches just distal to the bifurcation of the parent nerve allowed quantification of the number of motor neurons projecting solely to one of the branches (red or green alone) or simultaneously to both branches (both colors). In the unlesioned femoral nerve motor neurons are only retrogradely labeled from the muscle branch. In the EO model significantly more motor neurons were retrogradely labeled from the muscle branch compared to the cutaneous branch when saline was delivered for the first seven days after surgery. The same result was found when ganciclovir (GCV) was delivered, which selectively removed the original population of Schwann cells distal to the nerve lesion (see text for details). Very different results were obtained in the NEO model. When saline was delivered significantly more motor neurons projected to the cutaneous branch. When GCV was delivered about 1/3 of motor neurons projected solely to the muscle branch, 1/3 solely to the cutaneous branch, and 1/3 simultaneously to both branches. In addition, the total number of motor neurons that regenerated was significantly less than the number of motor neurons retrogradely labeled from control nerves (dashed horizontal lines, 161±4). Data is expressed as the mean ± SEM, *= p<.05 between the two branches, ∞ = p<.05 total motor neurons compared to control.
Conversely, in the absence of end-organ contact (NEO) significantly more motor neurons reinnervated the cutaneous pathway as compared to the muscle pathway in those animals receiving saline-filled minipumps. Interestingly, this preference to reinnervate the cutaneous pathway in the absence of end-organ contact was not seen when the original population of Schwann cells in the distal nerve was removed by application of GCV. There was also a significant decrease in the total number of retrogradely labeled motor neurons compared to control unoperated animals in the NEO group that received GCV, indicating the difficulty faced by regenerating motor neurons into a distal nerve that both lacks end-organ contact and the original population of Schwann cells.
Discussion
There are three primary findings from these experiments: 1) it is possible to selectively remove the original population of Schwann cells distal to a peripheral nerve transection; 2) in the absence of end-organ contact there is a preferential projection of motor neurons to the cutaneous pathway, however if the original population of Schwann cells is removed this preference for the cutaneous pathway disappears, and 3) in the presence of end-organ contact there is a preference for motor neurons to project to the muscle pathway regardless of whether the original population of Schwann cells is left intact or removed. These findings support the idea that motor neurons will preferentially reinnervate either the muscle or cutaneous pathways depending upon the particular surgical model used, and argue against the possibility of original Schwann cells in the muscle pathway maintaining a specific molecular identity that can be recognized by regenerating motor axons (Brushart 1993; Brushart, Gerber et al. 1998; Hoke, Redett et al. 2006). It also suggests that the influence of the end-organ (i.e., muscle contact) is more important in shaping final projection patterns compared to the original population or density of Schwann cells in the two pathways.
Schwann Cell Removal
We confirmed and extended previous studies that had shown the selective removal of reactive Schwann cells distal to a nerve injury in this transgenic mouse line (Sofroniew, Bush et al. 1999). Extensive studies have shown that transgene-derived HSV-TK is regulated similarly to endogenous GFAP in CNS and PNS glial cells, and that other cell types do not express HSV-TK (Bush, Puvanachandra et al. 1999; Sofroniew, Bush et al. 1999). Previous work has also shown that GCV at the doses used in the present study has no detectable effects on non-transgene-expressing cells, that GCV kills only dividing transgene-expressing cells, and that the phosphorylated and lethal metabolites of GCV are not released in sufficient quantities by HSV-TK-expressing cells to affect neighboring, non-transgene expressing cells (ibid.).
We also investigated the possibility that the targeted removal of reactive Schwann cells distal to a nerve lesion might lead to an increased inflammatory response beyond that which is routinely present during Wallerian degeneration. Such increased inflammation might lead to reduced axonal regeneration. It is important to keep in mind however that inflammation is a normal and necessary occurrence during Wallerian degeneration, and that such inflammation is required for removal of myelin and axonal debris and subsequent axonal regeneration. Cell death in the distal nerve is a normal component of Wallerian degeneration, where 4–5% of invading macrophages as well as T-cells undergo apoptotic cell death (Kuhlmann, Bitsch et al. 2001). In fact, robust inflammation is essential for robust axonal regeneration in the PNS and if inflammation is experimentally reduced, by reducing macrophage infiltration, axonal regeneration is decreased (Dahlin 1995).
As expected during Wallerian degeneration, we found a marked increase (> 25 fold) in white blood cells compared to resting nerve. This increase in white blood cells was comparable for both WT and TK animals, even though there were dramatic differences (> 10 fold) in terms of increased numbers of Schwann cells. These findings suggest that the degree of inflammation (as grossly assessed by the number of white blood cells) is similar between WT nerve undergoing Wallerian degeneration and transgenic nerve undergoing Wallerian degeneration plus killing of resident Schwann cells. These results also suggest that the administration of GCV to the transgenic animals during the first week after nerve lesion effectively removed the vast majority of the original population of Schwann cells distal to the nerve transection. As expected from previous work with freeze-thaw nerve lesions, the number of Schwann cells in the distal nerve of TK animals did increase at the later time point of 5 weeks due to Schwann cell ingrowth (and subsequent proliferation) from the proximal nerve stump. Thus, under these conditions of delivering GCV only during the first seven days after surgery it is only the original population of Schwann cells in the distal stump that is targeted for removal due to their cell division and incorporation of GCV, while subsequently in-growing Schwann cells are not affected.
An additional aspect of inflammation in this animal model deserves mention. One might question whether central gliosis could be a confounding factor in these studies since the peripheral nerve transection may lead to increased glial activity in the spinal cord, and these cells would then be lesioned due to their expression of GFAP and HSV-TK. Such a result could influence motor neuron integrity and thus the robustness of axonal regeneration. We do not believe this would impact these studies since GCV does not normally cross the intact blood-brain-barrier, and thus would not affect central glia (e.g., see (Brewster, Raghavan et al. 1994).
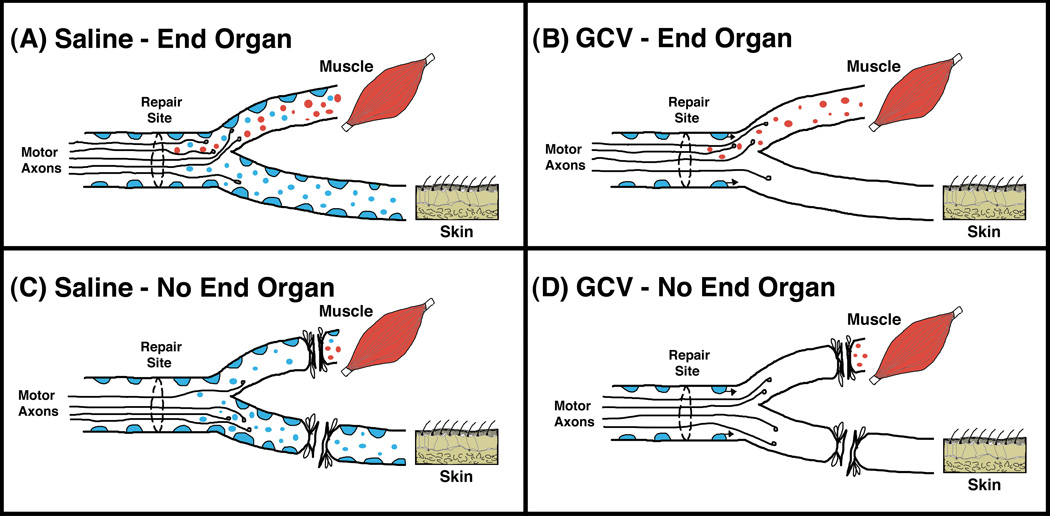
Muscle Contact and Trophic Hierarchy of Preferential Pathway Projections (Fig. 4)
Figure 4.
Model of motor neuron regeneration accuracy in the femoral nerve. We posit two main sources of trophic support for regenerating motor neurons and their axons; Schwann cells and muscle. Schwann cells in both terminal nerve branches are represented as blue, and trophic support from Schwann cells is represented as small blue circles. Trophic support originating from muscle is represented by small red circles. (A) When trophic support from both Schwann cells and muscle is present, more motor neurons project to the muscle branch and reinnervate muscle, indicating the potent influence of muscle on such regeneration. (B) When the original population of Schwann cells distal to the nerve lesion is removed via targeting with ganciclovir (GCV), the trophic support from muscle is still intact and once again more motor neurons project to the muscle branch and reinnervate muscle. Since GCV is only delivered during the first seven days, Schwann cells that grow into the distal nerve along with the regenerating axons after this time period will not be eliminated. (C) When trophic support from end-organs is prevented, but the original population of Schwann cells is left intact, more motor neurons project to the cutaneous branch because it has more Schwann cells and thus more trophic support from Schwann cells. This suggests that in the absence of the overriding support from muscle, it is the number and density of Schwann cells that determines the motor neuron projection choice. (D) When end-organ influences are prevented and Schwann cells distal to the nerve lesion are removed via targeting with ganciclovir (GCV), fewer motor neurons regenerate overall and those that do show no preference for either of the terminal nerve branches.
It was originally shown by Brushart in the rat femoral nerve that regenerating motor neurons initially project randomly into both distal terminal nerve branches, but that over time significantly more motor neurons are retrogradely labeled from the muscle branch (Brushart 1988; Brushart 1990). Pruning of inappropriate projections to the cutaneous branch was shown to be an important component of the development of such preferential projections to the muscle branch. On an individual neuron level such pruning could be an example of intraneuronal competition for trophic support, a concept termed trophomorphism by Crutcher and colleagues (Crutcher and Saffran 1990; Saffran and Crutcher 1990).
We have suggested that such trophic support could encompass anything that impacts neuronal survival such as growth factors, structural/metabolic precursors, and blood supply (Madison, Robinson et al. 2007; Uschold, Robinson et al. 2007). We have also posited that there are two main sources of trophic support for regenerating motor neurons: muscle, and the number/density of Schwann cells within the terminal nerve branches. The current studies were undertaken with the goal of manipulating the relative availability of these two sources of trophic support.
When muscle contact was maintained, significantly more motor neurons were retrogradely labeled from the muscle branch regardless of whether saline or GCV was administered (Fig. 3, end-organ). This suggests that contact with muscle is more important in determining preferential pathway projections than any influence of the original population of Schwann cells in the distal terminal nerve branches, consistent with muscle contact being at the top of the proposed hierarchy discussed above. The findings also show that axonal regeneration will take place even if distal Schwann cells are removed via molecular targeting, a finding that is similar to earlier regeneration studies using freeze-thawed nerve grafts that lacked any viable cells and yet supported axonal regeneration (Fugleholm, Schmalbruch et al. 1994; Fugleholm, Sorensen et al. 1998).
When muscle contact was prevented (Fig. 3, no end-organ) pathway projections depended upon whether the original Schwann cell population in the distal nerve branches was left intact (saline group) or removed (GCV group). When the original Schwann cell population was left intact, significantly more motor neurons projected to the cutaneous branch. We believe these findings are in keeping with the second level of the proposed trophic hierarchy, the absolute number/density of Schwann cells within the terminal nerve branches. As we and others have previously published, in the mouse there are significantly more myelinated axons (approximately double) within the cutaneous compared to the muscle distal branch, and thus concomitantly a greater number of Schwann cells in the cutaneous branch (Martini, Schmitz et al. 1992; Frei 1999; Sancho 1999; Robinson and Madison 2005). Not surprisingly, when we quantified the number of Schwann cells (identified by S-100 staining) within a set longitudinal area in each of the terminal nerve branches we found approximately 2–3 fold more Schwann cells in the cutaneous branch (Madison and Robinson, personal observations). Thus in the saline no-end-organ group there are significantly more Schwann cells in the cutaneous branch at the time of initial surgery, and significantly more motor neurons project to this branch by eight weeks. The disparity between the number of Schwann cells in each terminal nerve branch may also explain the preference that has been shown for motor neurons to project to the cutaneous branch when that branch is left intact and the muscle branch is ligated and capped just proximal to the quadriceps muscle (Robinson and Madison 2004; Robinson and Madison 2005) and most recently when the cutaneous branch is significantly enlarged via transgenic technology (Robinson and Madison 2009).
In the current no end-organ studies when the original population of Schwann cells was removed (Fig. 3; right most column), motor neurons projected equally to the muscle or cutaneous branch or to both branches simultaneously. We interpret this finding to support the notion that if both of the main sources of trophic support are removed from the distal nerve branches (muscle contact and Schwann cell number/density), the two pathways become as similar as possible and motor neurons project equally to both branches. In addition, when both of these sources of trophic support are removed, significantly fewer motor neurons overall regenerate into the distal nerve, thus highlighting the critical influence of such support. It is also interesting to note the increased number of double labeled motor neurons in the NEO groups and in the EO group given GCV, compared to the EO group which received saline. By this 8 week time point most axonal pruning will have already occurred, and thus the noted increase in double-labeled neurons could be due to either initial increased branching of the regenerating axons, or the continued maintenance of such collaterals, or both. Whatever the underlying mechanism is, this observation is consistent with the hypothesis that when Schwann cells are removed as a source of support for the regenerating axons it is more difficult for the motor neuron to make definitive projections to one branch or the other. In fact, increased axonal branching may be beneficial for a motor neuron when having to choose between a terminal nerve branch to muscle vs. one to skin since pruning of the inappropriate axon collateral to skin may take place. However, increased branching in other situations, e.g. as shown in the facial nerve paradigm, could be detrimental since both axon collaterals would contact muscle, and therefore it may be a more complicated choice that involves the “correct” muscle vs. the more simple case of muscle vs. skin (Guntinas-Lichius, Wewetzer et al. 2002).
It has been known for some time that a regenerating neuron will generate multiple collateral axons that enter the distal nerve (Cajal 1968 translation of 1928 publication). With the more recent advent of transgenic technology it has been possible to directly visualize and quantify this collateral sprouting process and it has been estimated that a regenerating axon has access to more than 100 distal stump Schwann cell tubes (Witzel, Rohde et al. 2005). Related studies have found that in the mouse femoral nerve model motor neurons sprout and maintain more collateral axons in a no-end-organ surgical preparation compared to one allowing interaction with the end-organs of muscle and skin (Redett, Jari et al. 2005). An interesting corollary to the idea of trophomorphism is that when a motor neuron is able to more clearly define a difference in the level of trophic support it will be more likely to withdraw axon collaterals that project to pathways with lower levels of trophic support, thus relieving the energy burden of maintaining multiple axon collaterals. Although not directly examining the number of axon collaterals, we have recently examined the influence of muscle contact on motor neuron projections to one or the other of the two terminal nerve pathways. We found that when the influence of muscle was maximized, the preference of motor neurons to project axons exclusively to the muscle pathway was significantly accelerated temporally, and the number of motor neurons projecting simultaneously to both pathways was significantly reduced (Uschold, Robinson et al. 2007). The collateral sprouting studies mentioned above (Redett, Jari et al. 2005) show this to also be the case at the axonal level; when contact with the end-organs of muscle and skin is allowed (resulting in a large differential of trophic support) fewer collaterals are maintained overall compared to when no end-organ contact is allowed (least differential of trophic support).
Towards a Molecular Understanding of Preferential Pathway Projections
The molecular mechanisms underlying preferential pathway projections in the rodent femoral nerve model remain largely unknown. Two schools of thought have emerged. One is that the Schwann cells of the respective pathways maintain a specific molecular identity that can be recognized by regenerating motor axons, and the other (favored by us) is that regenerating motor axons assess the relative levels of trophic support in each pathway and preferentially remain in the one that provides the greater amount of support; a general process that has previously been termed trophomorphism (Crutcher and Saffran 1990; Saffran and Crutcher 1990).
There have been some well-characterized molecular differences between the two terminal pathways that have been suggested as causative factors of preferential projections to the muscle pathway (e.g., preferential L2/HNK-1 expression by motor branch Schwann cells (Martini, Schmitz et al. 1992; Löw, Orberger et al. 1994; Martini, Schachner et al. 1994); enhanced BDNF/TrkB signaling (Eberhardt, Irintchev et al. 2006); differing levels of neurotrophins (Hoke, Redett et al. 2006)). Although such inherent molecular differences between the two pathways may modify preferential pathway projections, it seems unlikely that they are essential causative factors. The observed molecular differences between the branches remain whether or not end-organ contact is allowed (e.g., (Martini, Schachner et al. 1994), and yet the final preferred pathway can be either the muscle or cutaneous branch depending upon the specific surgical model (Robinson and Madison 2004; Robinson and Madison 2005; Robinson and Madison 2009).
The role of the polysialylated neural cell adhesion molecule (PSA) in terms of preferential pathway projections in the mouse femoral nerve model has recently been investigated. This elegant work has suggested an important role for PSA in enabling regenerating motor neurons to enhance their axonal arborization, and thus the ability to sample more basal lamina tubes in the distal nerve stump (Franz, Rutishauser et al. 2005). Preferential pathway projections were not present in NCAM −/− mice, or when PSA was removed enzymatically from wild-type mice. The authors conclude that the primary function of PSA may be to promote axon-axon de-adhesion, which then allows individual axons to respond to instructive guidance molecules expressed in the distal nerve pathways or end-organ. PSA may thus be necessary for regenerating motor axons to effectively compare levels of trophic support and allow motor neurons to respond to a hierarchy of such levels with preferential pathway projections.
In conclusion, although axonal regeneration in the PNS can be robust, extensive peripheral nerve injuries can result in the effective paralysis of the entire limb or distal portions of the limb due to the misrouting of regrowing axons. Refinement of microsurgical techniques involving the introduction of the surgical microscope and microsutures has increased the accuracy of the mechanical nerve repair process, however even using state-of-the-art current techniques there is still permanent functional comprise in up to 90% of adult human nerve repairs (Sunderland 1991; Madison, Archibald et al. 1992; Brushart 1998). It appears that the limits of mechanical repair techniques have been reached; this is not surprising given that the size of the finest suture material and needles (18–22 and 50–75 microns respectively) is still quite a bit larger than the size of the smallest axons that need to be repaired (sub-micron size). We are clearly in need of a greater understanding of the cellular and molecular events of nerve regeneration in order to develop fundamental improvements to the field of nerve repair. The femoral nerve model continues to be useful in studying the development of preferential pathway projections that occur under certain conditions, however there is much room for improvement in understanding the causation of such specificity and harnessing its effects for functional nerve repair. Once such an understanding is in hand, we may be able to make progress in getting axons “back to where they belong”(Sunderland 1991).
Acknowledgements
We thank Baxter Healthcare for supplying the Tisseel fibrin sealant. Supported by the Office of Research and Development, Biological Laboratory Research and Development (BLRD) Service, Department of Veterans Affairs (to RDM). RDM is a Research Career Scientist for the BLRD Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;4:239–246. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, et al. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Eng LF, et al. Localization of the glial acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Heyman R, et al. Targetting of an inducible toxic phenotype in animal cells. Proc. Natl. Acad. Sci. USA. 1988;85:7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster ME, Raghavan K, et al. Enhanced delivery of ganciclovir to the brain through the use of redox targeting. Antimicrob. Agents Chemother. 1994;38:817–823. doi: 10.1128/aac.38.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Preferential motor reinnervation: a sequential double-labeling study. Restor. Neurol. Neurosci. 1990;1:281–287. doi: 10.3233/RNN-1990-13416. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair and Grafting. In: Green D, Hotchkiss R, Pederson R, editors. Green's Operative Hand Surgery. Vol. 4. New York: Churchill Livingston; 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Gerber J, et al. Contributions of pathway and neuron to preferential motor reinnervation. J. Neurosci. 1998;18(21):8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TME. Preferential reinnervation of motor nerves by regenerating motor axons. J. Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TME. Motor axons preferentially reinnervate motor pathways. J. Neurosci. 1993;13(6):2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, et al. Fulminant jujuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Cajal RS. Degeneration and Regeneration of the Nervous System. London and New York: Hafner Publishing Co.; Innervation of the peripheral stump; pp. 223–264. 1968 translation of 1928 publication. [Google Scholar]
- Chen ZL, Yu WM, et al. Peripheral regeneration. Ann. Rev. Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Saffran BN. Developmental remodeling of neuronal projections: Evidence for trophomorphism? Comments Developmental Neurobiology. 1990;1(2):119–141. [Google Scholar]
- Dahlin LB. Prevention of macrophage invasion impairs regeneration in nerve grafts. Brain Res. 1995;679:274–280. doi: 10.1016/0006-8993(95)00249-p. [DOI] [PubMed] [Google Scholar]
- Eberhardt KA, Irintchev A, et al. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp. Neurol. 2006;198(2):500–510. doi: 10.1016/j.expneurol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, et al. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J. Neurosci. 2005;25(8):2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei R, Mötzing S, Kinkelin I, Schachner M, Koltzenburg M, Martini R. Loss of distal axons and sensory Merkel cells and features indicative of muscle denervation in hindlimbs of P0-deficient mice. J. Neurosci. 1999;19:6058–6067. doi: 10.1523/JNEUROSCI.19-14-06058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugleholm K, Schmalbruch H, et al. Early peripheral nerve regeneration after crushing, sectioning, and freeze studied by implanted electrodes in the cat. J. Neurosci. 1994;14:2659–2673. doi: 10.1523/JNEUROSCI.14-05-02659.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugleholm K, Sorensen J, et al. Axonal elongation through acellular nerve segments of the cat tibial nerve: importance of the near-nerve environment. Brain Res. 1998;792:309–318. doi: 10.1016/s0006-8993(98)00160-7. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Wewetzer K, et al. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J. Neurosci. 2002;22(16):7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. The response to injury in the peripheral nervous system. J. Bone Joint Surg. Br. 2005;87(10):1309–1319. doi: 10.1302/0301-620X.87B10.16700. [DOI] [PubMed] [Google Scholar]
- Hall SM. Regeneration in the peripheral nervous system. Neuropathol. Appl. Neurobiol. 1989;15:513–529. doi: 10.1111/j.1365-2990.1989.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Schwann cells: Early lineage, regulation of proliferation and control of myelin formation. Curr. Opin. Neurobiol. 1992;2:575–581. doi: 10.1016/0959-4388(92)90021-c. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neuroscience. 1999;22:402–410. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Bitsch A, et al. Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. J. Neurosci. 2001;21(10):3401–3408. doi: 10.1523/JNEUROSCI.21-10-03401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Orberger G, et al. The L2/HNK-1 carbohydrate is carried by the myelin associated glycoprotein and sulphated glucuronyl glycolipids in muscle but not cutaneous nerves of adult mice. Eur. J. Neurosci. 1994;6:1773–1781. doi: 10.1111/j.1460-9568.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, et al. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J. Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, et al. Peripheral nerve injury. In: Cohen IK, Diegelman F, Lindblad WJ, editors. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: W.B. Saunders Co.; 1992. pp. 450–480. [Google Scholar]
- Madison RD, Robinson GA, et al. The specificity of motor neurone regeneration (preferential reinnervation) Acta Physiol. (Oxf) 2007;189(2):201–206. doi: 10.1111/j.1748-1716.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M, et al. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J. Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schmitz B, et al. The L2/HNK-1 carbohydrate epitope is involved in the preferential outgrowth of motor neurons on ventral roots and motor nerves. Eur. J. Neurosci. 1992;4:628–639. doi: 10.1111/j.1460-9568.1992.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, et al. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur. J. Neurosci. 2003;18(1):76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Redett R, Jari R, et al. Peripheral pathways regulate motoneuron collateral dynamics. J. Neurosci. 2005;25(41):9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Preferential motor reinnervation in the mouse: comparison of femoral nerve repair using a fibrin sealant or suture. Muscle Nerve. 2003;28(2):227–231. doi: 10.1002/mus.10422. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Motor neurons can preferentially reinnervate cutaneous pathways. Exp. Neurol. 2004;190(2):407–413. doi: 10.1016/j.expneurol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Manipulations of the mouse femoral nerve influence the accuracy of pathway reinnervation by motor neurons. Exp. Neurol. 2005;192(1):39–45. doi: 10.1016/j.expneurol.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Developmentally regulated changes in femoral nerve regeneration in the mouse and rat. Exp. Neurol. 2006;197(2):341–346. doi: 10.1016/j.expneurol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Influence of terminal nerve branch size on motor neuron regeneration accuracy. Exp. Neurol. 2009;215(2):228–235. doi: 10.1016/j.expneurol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Saffran BN, Crutcher KA. NGF-induced remodeling of mature uninjured axon collaterals. Brain Res. 1990;525(1):11–20. doi: 10.1016/0006-8993(90)91315-8. [DOI] [PubMed] [Google Scholar]
- Sancho S, Magyar JP, Aguzzi A, Suter U. Distal axonopathy in peripheral nerves of PMP22-mutant mice. Brain. 1999;122:1563–1577. doi: 10.1093/brain/122.8.1563. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Borrelli E. Transcriptional regulation by trans-acting factors. Trends Genet. 1986;2:215–219. [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Bush TG, et al. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res. 1999;835:91–95. doi: 10.1016/s0006-8993(99)01639-x. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerve injuries and their repair: A critical Appraisal. New York: Churchill Livingstone; 1991. [Google Scholar]
- Uschold T, Robinson GA, et al. Motor neuron regeneration accuracy: balancing trophic influences between pathways and end-organs. Exp. Neurol. 2007;205(1):250–256. doi: 10.1016/j.expneurol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, et al. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005;485(3):183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]