Abstract
Objective
To determine the effect of antibiotic treatment for acute otitis media in children between 6 months and 2 years of age.
Design
Practice based, double blind, randomised, placebo controlled trial.
Setting
53 general practices in the Netherlands.
Subjects
240 children aged 6 months to 2 years with the diagnosis of acute otitis media.
Intervention
Amoxicillin 40 mg/kg/day in three doses.
Main outcome measures
Persistent symptoms at day four and duration of fever and pain or crying, or both. Otoscopy at days four and 11, tympanometry at six weeks, and use of analgesic.
Results
Persistent symptoms at day four were less common in the amoxicillin group (risk difference 13%; 95% confidence interval 1% to 25%). The median duration of fever was two days in the amoxicillin group versus three in the placebo group (P=0.004). No significant difference was observed in duration of pain or crying, but analgesic consumption was higher in the placebo group during the first 10 days (4.1 v 2.3 doses, P=0.004). In addition, no otoscopic differences were observed at days four and 11, and tympanometric findings at six weeks were similar in both groups.
Conclusions
Seven to eight children aged 6 to 24 months with acute otitis media needed to be treated with antibiotics to improve symptomatic outcome at day four in one child. This modest effect does not justify prescription of antibiotics at the first visit, provided close surveillance can be guaranteed.
Introduction
Antibiotics are currently the treatment of choice for acute otitis media in nearly all countries,1 which is rather surprising as their effectiveness seems limited in terms of clinical improvement.2–7 Although the worldwide crisis of multiple resistant strains of microbes underlines the importance of the prevention of overuse and misuse of antibiotics, the Netherlands is still the only country where only a minority of the episodes of acute otitis media are treated with antibiotics.1The outcome of acute otitis media in the Netherlands does not seem to be any worse than that in other countries.1
Several authors have advocated restriction of antibiotic treatment for acute otitis media to children at increased risk of poor outcome or complications,5,8 notably children under 2 years of age,4,9–14 although, surprisingly, there is virtually no empirical evidence as to the effectiveness of such treatment in these children.15 We therefore assessed outcome in a primary care based randomised trial of amoxicillin versus placebo.
Methods
Studypopulation
The study was conducted between February 1996 and May 1998 in the Netherlands, where all patients are treated initially by their own general practitioner. Children aged between 6 and 24 months were eligible if they presented with acute otitis media—defined as infection of the middle ear of acute onset and a characteristic ear drum picture (injection along the handle of the malleus and the annulus of the tympanic membrane or a diffusely red or bulging ear drum)—or acute otorrhoea. In addition, one or more symptoms of acute infection (fever, recent earache, general malaise, recent irritability) had to be present, in line with the Dutch guidelines.8
The following exclusion criteria were applied: antibiotic treatment in the preceding four weeks; proved allergy to amoxicillin; compromised immunity; craniofacial abnormalities; Down's syndrome; or being entered in this study before. The 53 participating general practitioners were trained to classify ear drums by using a standard set of slides depicting a range of common ear drum appearances with the emphasis on discriminating between acute otitis media and otitis media with effusion.16 The study protocol was approved by the ethical committee of the Children's Hospital of the University Medical Centre Utrecht; and all parents of the children gave written informed consent before enrolment.
Intervention
Patients received either amoxicillin suspension 40 mg/kg/day in three divided doses for 10 days or placebo suspension. Most patients in the Netherlands with acute otitis media receive decongestant nose drops, so all patients received one drop of oxymetazoline 0.025% in each nostril three times a day (Nasivin, Merck) for seven days. The use of paracetamol was allowed when the child was in pain, the amount being recorded in the diary. For each dose children under 1 year old received a 120 mg suppository and older children received 240 mg.
At the baseline visit the doctor recorded the history, the presence or absence of certain risk factors for acute otitis media, and the results of otoscopy. Parents were instructed to keep a 10 day diary showing occurrence of aural and gastrointestinal symptoms and administration of study medication, paracetamol, and nose drops. Follow up visits were scheduled on days four and 11 at the general practitioner's clinic, inquiry was made about remaining symptoms, and the ear drum was examined. At six weeks all children were visited at home by the first author (RD), and information was obtained about present and past symptoms, antibiotic use, and any referral to a paediatrician or otolaryngologist since day 11. Further otoscopy and tympanometry was also carried out.
Outcome measures
The primary outcome measure was persistent symptoms at day four, assessed by the doctor and defined as persistent earache, fever (⩾38oC), crying, or being irritable. In addition the prescription of another antibiotic because of clinical deterioration before the first follow up visit was to be considered a persistent symptom.
Secondary outcome measures were clinical treatment failure at day 11, defined as persistent fever, earache, crying, being irritable, or no improvement in the appearance of the tympanic membrane, defined as persistent redness, bulging, or perforation of one or both tympanic membranes; the duration of fever (⩾38oC), pain, or crying, defined as the number of days until the first day on which these signs were considered absent and remained absent as recorded in the diary by the parents; the mean number of doses of analgesics given, based on the diaries; adverse effects mentioned in the diaries; and the percentage of children with middle ear effusion at six weeks. The diagnosis of effusion was based on combined otoscopy and tympanometry. Type B and C2 tympanograms (modified Jerger's classification) were regarded as indicative of the presence of fluid in the middle ear.17,18
Sample size and data analysis
Calculation of the sample size was based on the assumption of a minimum difference of 20% in primary outcome between the groups, with an α of 5%, a discriminating power of 80%, and an estimated 60% persistent symptoms in the placebo group.4 The total number of children required in each treatment arm was 79.
All analyses were carried out with SPSS on an intention to treat basis. We performed best and worst case analyses when necessary because of loss to follow up.
The prevalence of persistent symptoms at day four, clinical treatment failure at day 11, occurrence of middle ear effusion at six weeks, and side effects in the two groups were compared by calculating differences in risk with 95% confidence intervals. Durations of fever and of pain or crying, or both, were plotted by means of Kaplan-Meier curves, and differences between the treatment groups were tested by the log rank test. When diary data were incomplete and the last entry recorded fever or pain, the child was censored in the survival analysis. The difference in the mean analgesic consumption in the two groups was tested with the Mann-Whitney U test. All reported P values are two sided.
To adjust for possible confounding due to unequal distribution of baseline characteristics we used logistic regression analysis with the primary outcome measure as the dependent variable.
Assignment and blinding
After we obtained consent the children were randomly assigned to treatment with amoxicillin or with a placebo suspension with the same colour and taste. The suspensions were supplied to the participating doctors in a double blind fashion with computerised two block randomisation; doctors, parents, and investigators remained blinded throughout the study. During the trial the code of the allocation schedule was kept in the pharmacy of the University Medical Centre, Utrecht, and was broken only if severe complications or side effects occurred.
Results
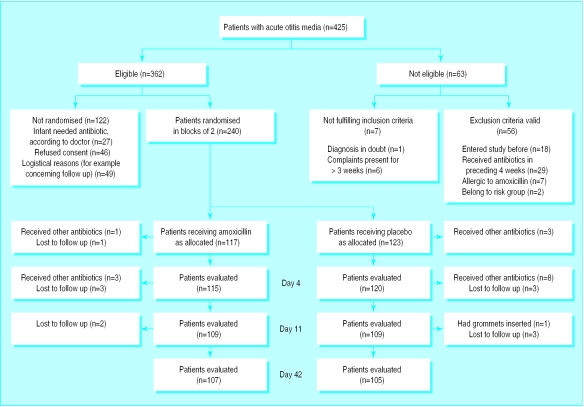
Participant flow and follow up—Of the 425 children with acute otitis media registered, 362 were eligible, and from these 240 were randomly assigned to one of the treatment groups (figure). Children in the antibiotic group and the placebo group differed in the prevalence of recurrent acute otitis media, regular attendance at a day care centre, and parental smoking habits (table 1).
Table 1.
Baseline characteristics of 240 children randomised in trial of antibiotic use for treatment of acute otitis media. Figures are numbers of children except for mean age
| Characteristic | Amoxicillin (n=117) | Placebo (n=123) |
|---|---|---|
| Mean age (months) | 13.3 | 13.3 |
| Male | 64 | 66 |
| Breastfed for >6 months | 21 | 22 |
| >2 children in family | 30 | 25 |
| Season of inclusion (Oct-March) | 76 | 79 |
| Smoking in household | 46 | 39 |
| Attendance at day care centre | 28 | 19 |
| Medical history: | ||
| Recurrent URTI | 37 | 33 |
| Recurrent AOM in family | 26 | 33 |
| Allergy | 14 | 9 |
| Recurrent AOM | 33 | 50 |
| Clinical presentation: | ||
| >3 days complaints | 57 | 54 |
| Earache | 82 | 82 |
| Fever | 79 | 80 |
| Perforation | 18 | 21 |
| Bilateral AOM | 75 | 76 |
| Bulging ear drum | 26 | 29 |
URTI=upper respiratory tract infection.
AOM=acute otitis media.
Outcome at day four—Persistent symptoms at day four occurred in 69 out of 117 children (59%) in the amoxicillin group and in 89 of 123 (72%) in the placebo group (difference in risk 13%; 95% confidence interval 1% to 25%) (table 2). Adjustment for recurrence, day care, and smoking as possible confounders in the logistic regression analyses showed an odds ratio of 1.79 (1.03 to 3.13). Among children with persistent symptoms four (one in the amoxicillin group and three in the placebo group) received other antibiotics. Three of these children were admitted to hospital (one in the amoxicillin group, two in the placebo group); one (placebo group) was admitted on the third day with meningitis but because of deterioration this child had already been started on another antibiotic on day two. The culture of cerebrospinal fluid yielded negative results, but the Gram stain suggested streptococcal meningitis. The two other children were admitted because of dyspnoea (amoxicillin group) and dehydration (placebo group). All four recovered without residual symptoms. Inclusion of the one child lost to follow up (amoxicillin group) in either outcome group did not materially change the findings.
Table 2.
Main outcome measures in infants with acute otitis media randomised to receive amoxicillin or placebo
| Measure | Amoxicillin | Placebo | Difference in % (95% CI) | P value |
|---|---|---|---|---|
| No (%) with: | ||||
| Persistent symptoms* at day 4 | 69/117 (59) | 89/123 (72) | 13 (1 to 25) | 0.03† |
| No improvement in eardrum at day 4 | 88/114 (77) | 99/120 (83) | 6 (−4 to 16) | 0.30† |
| Clinical treatment failure‡ at day 11 | 72/112 (64) | 84/120 (70) | 6 (−6 to 18) | 0.35† |
| Median duration of fever (days) | 2 | 3 | 1 | 0.004§ |
| Median duration of pain/crying (days) | 8 | 9 | 1 | 0.432§ |
| Mean consumption of analgesia in first 10 days (dose) | 2.3 | 4.1 | 1.8 | 0.004¶ |
Defined as still having earache or having fever, crying, being irritable, or having received other antibiotics.
χ2 test.
Defined as still having symptoms or no improvement, or both, in tympanic membrane.
Log rank test for Kaplan-Meier plot.
Mann-Witney U test.
Outcome at day 11—Clinical treatment failure at day 11 occurred in 72 out of 112 children (64%) in the amoxicillin group and in 84 of 120 (70%) in the placebo group (6%; −6% to 18%) (table 2). Eleven children received other antibiotics (three in the amoxicillin group, eight in the placebo group) and were recorded as treatment failures. One of these children (placebo group) needed admission to the hospital because of deterioration of symptoms of acute otitis media. Six children (three in each group) were lost to follow up between day four and day 11, and in one case (amoxicillin group) the evaluation of the ear drum was missing, although the symptoms were gone. A best case scenario (amoxicillin group analysed as “cured” and placebo group as “not cured”) did not show any significant difference in clinical treatment failure at day 11 (9%; −3% to 21%).
Duration of fever and pain or crying—The median time to cessation of fever was two days with amoxicillin and three days with placebo (P=0.004; log rank test). Median time to cessation of pain or crying was eight days with amoxicillin and nine days with placebo (P=0.432; log rank test).
Analgesic consumption—During the first three days, mean analgesic consumption in the amoxicillin group was 1.7 doses and in the placebo group 2.5 doses (P=0.018). Over the whole 10 days these figures were 2.3 and 4.1, respectively (P=0.004).
Outcome at six weeks—At six weeks 212 children were examined. Middle ear effusion was present in 69/107 (64%) in the amoxicillin group and in 70/105 (67%) in the placebo group (3%; −10% to 16%). The proportion of children with bilateral effusion was 48% in both groups. In addition, no clear differences were observed between the two groups as regards recurrent acute otitis media, use of antibiotics in this period, referrals to the otolaryngologist or paediatrician, or surgery.
Adverse effects—De novo diarrhoea was reported on day four in 17% (20/117) of the amoxicillin group and in 10% (12/123) of the placebo group (difference −7%; −16% to 2%). On day 10 these figures were 12% (14/117) and 8% (10/123), respectively (difference −4%; −12% to 4%). Of the children lost to follow up, five were withdrawn (all between day four and day 11) because of possible side effects, two because of diarrhoea (both in the amoxicillin group) and three because of skin rashes (all in the placebo group).
Compliance—According to the diaries the mean number of doses of study medication taken was 24.6 (82% of possible total) in the amoxicillin group and 23.2 (76%) in the placebo group (P=0.9). According to the suspension remaining in the returned bottles, 80% of the children in both groups had received the full amount, and 95% received at least 80% of the amount prescribed.
Discussion
In this study resolution of symptoms on day four was more common in those treated with amoxicillin than in those taking placebo. At day 11, no significant differences in symptoms and otoscopy results were observed. Amoxicillin shortened the duration of fever by one day, and analgesics were used more often in the placebo group.
What is already known about this subject
Several meta-analyses have shown that the effectiveness of antibiotics for acute otitis media is limited in terms of clinical improvement
For children under 2 years of age—a risk group with regard to poor outcome—the evidence of the effectiveness of antibiotics for this common condition is not conclusive
What this paper adds
This randomised study shows that seven to eight children, aged 6 to 24 months, with acute otitis media need to be treated with amoxicillin to improve symptomatic outcome at day four in one child
This is not sufficiently important clinically to prescribe antibiotics for every child with acute otitis media in this age group
Watchful waiting at the first visit is therefore justified for these children
The significant reduction of the duration of fever observed in the amoxicillin group is in accord with the results of Burke et al in children aged 3 to 10 years.2 We observed no difference between the two groups in pain or crying. This was also reported by Burke et al, and the amounts of analgesics taken in their study were comparable with those in ours.2 The fact that more analgesics were used in the placebo group could explain the lack of difference in duration of pain or crying.
The number of children with persistent symptoms in our study was high compared with other studies.2,19 Burke et al, however, included only older children and in young children symptoms are often prolonged.4,9 Contrary to our results complete resolution of symptoms was not mentioned by Kaleida et al.19
Our diagnoses were based on acute signs of infection and abnormality of the ear drum; this has shown to be adequate in other studies3,4 and is in accord with day to day practice in the Netherlands. An abnormal ear drum had to be seen because diagnosis based only on symptoms is not specific.20 According to the baseline characteristics the results in our sample are generalisable to the population seen in primary care in the Netherlands.1,21
The treatment regimen we used (amoxicillin 40 mg/kg/daily) is still the treatment of first choice.22 The dosage was deemed sufficient because incidences of resistant Streptococcus pneumonia and Haemophilus influenzae in the Netherlands remain low at <1 %23 and 6% (data on file 1998, Dutch National Institute of Public Health and Environmental Protection), respectively, and compliance in this study was good.
As primary outcome measure we combined earache, crying, and irritability because in these little children it is difficult to establish earache as such. We have shown that seven to eight children aged 6 to 24 months with acute otitis media needed to be treated to improve symptomatic outcome at day four in one child. This is not sufficiently important clinically to prescribe antibiotics for every affected child within this age group. Routine prescription of antibiotics would not prevent all cases of meningitis.24 Our conclusion is that watchful waiting at the first visit is justified for these children. Instead of antibiotics analgesics could be given for proper resolution of symptoms but more research is needed as to whether this is a good alternative.
Figure.
Trial profile and participant flow
Acknowledgments
We thank all the general practitioners who included patients for this trial.
Footnotes
Funding: Netherlands Organisation for Scientific Research (grant no 904-58-074).
Competing interests: Nasivinnose drops for this study were donated by E Merck Nederland BV.
References
- 1.Froom J, Culpepper L, Grob P, Barteld A, Bowers P, Bridges-Webb C, et al. Diagnosis and antibiotic treatment of acute otitis media: report from International Primary Care Network. BMJ. 1990;300:582–586. doi: 10.1136/bmj.300.6724.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke P, Bain J, Robinson D, Dunleavy J. Acute red ear in children: controlled trial of non-antibiotic treatment in general practice. BMJ. 1991;303:558–562. doi: 10.1136/bmj.303.6802.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Buchem FL, Dunk JHM, van't Hof MA. Therapy of acute otitis media: myringotomy, antibiotics, or neither? A double blind study in children. Lancet. 1981;ii:883–887. doi: 10.1016/s0140-6736(81)91388-x. [DOI] [PubMed] [Google Scholar]
- 4.Appelman CLM, Claessen JQPJ, Touw-Otten FWMM, Hordijk GJ, de Melker RA. Co-amoxiclav in recurrent acute otitis media: placebo controlled study. BMJ. 1991;303:1450–1452. doi: 10.1136/bmj.303.6815.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froom J, Culpepper L, Jacobs M, de Melker RA, Green LA, van Buchem FL, et al. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ. 1997;315:98–102. doi: 10.1136/bmj.315.7100.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld RM, Vertrees JE, Carr J, Cipolle RJ, Uden DL, Giebink GS, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: meta-analysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994;124:355–367. doi: 10.1016/s0022-3476(94)70356-6. [DOI] [PubMed] [Google Scholar]
- 7.Del Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ. 1997;314:1526–1529. doi: 10.1136/bmj.314.7093.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appelman CLM, Bossen PC, Dunk JHM, Lisdonk EH, de Melker RA, van Weert HCPM. NHG standard otitis media acuta. (Guideline on acute otitis media of the Dutch College of General Practitioners.) Huisarts Wet. 1990;33:242–245. [Google Scholar]
- 9.Hoberman A, Paradise JL, Burch DJ, Valinski WA, Hedrick JA, Aronovitz GH, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463–470. doi: 10.1097/00006454-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Laxdal OE, Merida J, Jones RHT. Treatment of acute otitis media: a controlled study of 142 children. Can Med Assoc J. 1970;102:263–268. [PMC free article] [PubMed] [Google Scholar]
- 11.Mandel EM, Casselbrant ML, Rockette HE, Bluestone CD, Kurs-Lasky M. Efficacy of 20- versus 10-day antimicrobial treatment for acute otitis media. Pediatrics. 1995;96:5–13. [PubMed] [Google Scholar]
- 12.Alho OP, Laara E, Oja H. What is the natural course of recurrent acute otitis media in infancy? J Fam Pract. 1996;43:258–264. [PubMed] [Google Scholar]
- 13.Iino Y, Nakamura Y, Koizumi T, Toriyama M. Prognostic factors for persistent middle ear effusion after acute otitis media in children. Acta Otolaryngol (Stockh) 1993;113:761–765. doi: 10.3109/00016489309135897. [DOI] [PubMed] [Google Scholar]
- 14.Hathaway TJ, Katz HP, Dershewitz R, Marx TJ. Acute otitis media: who needs posttreatment follow-up? Pediatrics. 1994;94:143–147. [PubMed] [Google Scholar]
- 15.Damoiseaux RAMJ, van Balen FAM, Hoes AW, de Melker RA. Antibiotic treatment of acute otitis media in children under two years of age: evidence based? Br J Gen Pract. 1998;48:1861–1864. [PMC free article] [PubMed] [Google Scholar]
- 16.Wormald PJ, Browning GG, Robinson K. Is otoscopy reliable? A structured teaching method to improve otoscopic accuracy in trainees. Clin Otolaryngol. 1995;20:63–67. doi: 10.1111/j.1365-2273.1995.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 17.Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol. 1970;92:311–324. doi: 10.1001/archotol.1970.04310040005002. [DOI] [PubMed] [Google Scholar]
- 18.Zielhuis GA, Heuvelmans-Heinen EW, Rach GH, van den Broek P. Environmental risk factors for otitis media with effusion in preschool children. Scand J Prim Health Care. 1989;7:33–38. doi: 10.3109/02813438909103668. [DOI] [PubMed] [Google Scholar]
- 19.Kaleida PH, Casselbrant ML, Rockette HE, Paradise JL, Bluestone CD, Blatter MM, et al. Amoxicillin or myringotomy or both for acute otitis media: results of a randomized clinical trial. Pediatrics. 1991;87:466–474. [PubMed] [Google Scholar]
- 20.Kontiokari T, Koivunen P, Niemela M, Pokka T, Uhari M. Symptoms of acute otitis media. Pediatr Infect Dis J. 1998;17:676–679. doi: 10.1097/00006454-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bruijnzeels MA, van Suijlekom-Smit LWA, van der Velden J, van der Wouden JC. The child in general practice. Dutch national study of morbidity and interventions in general practice. Utrecht: NIVEL; 1993. [Google Scholar]
- 22.Berman S. Otitis media in children. N Engl J Med. 1995;332:1560–1565. doi: 10.1056/NEJM199506083322307. [DOI] [PubMed] [Google Scholar]
- 23.Hermans PWM, Sluijter M, Elzenaar K, van Veen A, Schonkeren JJM, Nooren FM, et al. Penicillin-resistant Streptococcus pneumoniae in the Netherlands: results of a 1-year molecular epidemiologic survey. J Infect Dis. 1997;175:1413–1422. doi: 10.1086/516474. [DOI] [PubMed] [Google Scholar]
- 24.Rothrock SG, Harper MB, Green SM, Clark MC, Bachur R, McIlmail DP, et al. Do oral antibiotics prevent meningitis and serious bacterial infections in children with streptococcus pneumoniae occult bacteremia? A meta-analysis. Pediatrics. 1997;99:438–444. doi: 10.1542/peds.99.3.438. [DOI] [PubMed] [Google Scholar]