Abstract
Objective
A measure that identifies patients who are at high risk of mortality after prolonged ventilation will help physicians communicate prognosis to patients or surrogate decision-makers. Our objective was to develop and validate a prognostic model for 1-year mortality in patients ventilated for 21 days or more.
Design
Prospective cohort study.
Setting
University-based tertiary care hospital
Patients
300 consecutive medical, surgical, and trauma patients requiring mechanical ventilation for at least 21 days were prospectively enrolled.
Measurements and Main Results
Predictive variables were measured on day 21 of ventilation for the first 200 patients and entered into logistic regression models with 1-year and 3-month mortality as outcomes. Final models were validated using data from 100 subsequent patients. One-year mortality was 51% in the development set and 58% in the validation set. Independent predictors of mortality included requirement for vasopressors, hemodialysis, platelet count ≤150 ×109/L, and age ≥50. Areas under the ROC curve for the development model and validation model were 0.82 (se 0.03) and 0.82 (se 0.05) respectively. The model had sensitivity of 0.42 (se 0.12) and specificity of 0.99 (se 0.01) for identifying patients who had ≥90% risk of death at 1 year. Observed mortality was highly consistent with both 3- and 12-month predicted mortality. These four predictive variables can be used in a simple prognostic score that clearly identifies low risk patients (no risk factors, 15% mortality) and high risk patients (3 or 4 risk factors, 97% mortality).
Conclusions
Simple clinical variables measured on day 21 of mechanical ventilation can identify patients at highest and lowest risk of death from prolonged ventilation.
Keywords: Mechanical Ventilation, Illness severity scores, Outcomes, Statistical Model, Critical Illness, Prognosis
Introduction
As patient management strategies in the intensive care unit continue to advance, more patients are surviving the early acute phases of critical illness. However when multiorgan failure fails to resolve or leads to subsequent complications such as critical illness polyneuropathy, prolonged mechanical ventilation can result.(1, 2) The number of patients requiring prolonged mechanical ventilation (PMV) has been increasing over the last decade and promises to increase dramatically when members of the baby boom generation reach advanced age and become particularly susceptible to this complication.(3)
Patients requiring PMV consume a disproportionately high amount of healthcare resources, both in the ICU and after hospital discharge.(4, 5) Their short-term and long-term mortality is high (6), and they suffer a very heavy symptom burden for prolonged periods.(7, 8) Hospital survivors have a significant degree of functional and cognitive limitations, and a high readmission rate. (9) Some remain at high risk for death after hospital discharge, but not all. Prolonged hospitalization for PMV patients who are at high risk of death does not meet current standards of cost-effectiveness.(10) Considering the high symptom burden of this population and often poor outcomes, a mortality prediction model that identifies PMV patients with the highest and lowest risk for death would be useful to inform discussions of prognosis among clinicians and patients or their surrogate decision-makers. Such a model could also standardize illness severity in cohort studies examining outcomes and interventions in this resource intensive group of patients.
A consensus conference defined PMV as patients requiring invasive mechanical ventilation for at least 21 days after acute illness.(1) We conducted a prospective cohort study to develop and validate a mortality prediction model for adult patients meeting this definition. Our intention was to develop a model that would be practical for use in the clinical setting and have very high specificity in patients at highest risk of death.
Materials and Methods
Patients
A total of three hundred adult patients were prospectively enrolled from UNC Hospitals, a 640 bed university-based tertiary care medical center with 65 adult ICU beds that can accommodate mechanically ventilated patients. Two hundred patients were consecutively enrolled from November 2001 to January 2004 for the development set of the prognostic model. One hundred patients were consecutively enrolled from February 2004 to June 2005 to form the model's validation set. Enrollment criteria included requirement of mechanical ventilation after acute illness for at least 21 days after initial intubation. If patients were extubated within that initial 21 day period but needed reintubation, they were enrolled only if the period of spontaneous breathing was ≤72 hours. Exclusion criteria included age <18 years, severe burns, chronic neuromuscular diseases, chronic mechanical ventilation prior to admission, receipt of >7 days of mechanical ventilation before transfer from a referral center, prisoners, and refusal of consent.
Patients in adult medical and surgical ICUs were screened on a daily basis. All eligible patients were enrolled for review of existing medical records. We requested permission through primary physicians to approach patients, or as was usually necessary, their surrogates, to request consent for interviews and telephone follow-up. If patients or surrogates refused consent to participate, they were excluded from the study, including review of existing data. If a surrogate was not available, follow-up was achieved by review of medical records and the National Death Index. The research protocol was approved by the UNC Institutional Review Board.
Data Collection
On day 21 of mechanical ventilation, medical records were abstracted for demographic data, diagnoses, comorbidities, and premorbid functional status. Physiologic variables were recorded from the first day of ICU admission and from day 21 of mechanical ventilation. APACHE II scores were calculated using data from the first 24 hours of ICU admission.(11) Sequential Organ Failure Assessment (SOFA) scores were calculated using data from the first 24 hours of ICU admission and from data collected on day 21 of mechanical ventilation.(12) The Charlson Index score, a measure of medical comorbidities, was calculated from medical record data based on conditions present at day 21.(13) Premorbid functional status was assessed by the surrogate's perception of whether the patient needed assistance with any activity of daily living (ADL) during the 2 weeks prior to acute illness.
Patients were followed during the rest of their hospitalization for duration of mechanical ventilation, mortality, ICU and hospital disposition, and length of stay. Patients or surrogates who consented to telephone follow-up were contacted at 3 months, 6 months, and 12 months from the time of enrollment (day 21 of mechanical ventilation). They were interviewed regarding the patient's vital status, place of residence, number of hospital readmissions, requirement for mechanical ventilation, tracheostomy, feeding tubes, and the patient's functional status. Performance of 6 basic activities of daily living (ADLs) were assessed by questionnaires asking how much assistance patients needed with feeding, getting out of bed, walking, dressing, toileting, and bathing.(14) A written notification that a phone call was going to be made was mailed 2 weeks prior to the scheduled contact, and multiple telephone calls were attempted until the patient or surrogate was reached. Patients and surrogates were reminded that they had the option of not answering any or all of the questions. For hospital survivors that were enrolled for review of existing data only, and for patients who were lost to telephone follow-up, oneyear mortality was assessed by review of the National Death Index.
All data collection instruments were pretested using records from 10 patients who were not part of the study sample. Revisions were made after clarifications by all investigators. Data on the first 10 patients enrolled in the study were collected by both the primary data collector and the principle investigator to ensure concordance. Similar quality checks were conducted on a random sample of 10% of the first 100 patients enrolled. Subjective variables such as primary and secondary diagnoses and comorbidities were made by both the primary data collector and the principal investigator on all patients, and discrepancies were settled together. Interview instruments were prestested on a sample of 10 patients and surrogates, and revisions were made accordingly. Telephone interviewers received full instruction from the principal investigator, and mock interviews were conducted until performance was consistent and reproducible. Analysis on the development set model was not begun until follow-up on the validation set was completed and the database was closed.
Statistical Analysis
Summary analyses were performed on demographic and physiologic variables and expressed as mean ± standard deviation (SD) for normally distributed data and median, interquartile range (IQR) for non-normal data. Power analyses indicated that a logistic regression model with 200 patients and 130 expected deaths would have sufficient power to include 13 variables. These predictor variables were chosen a priori based upon clinical judgment and previous studies in different settings. (15-18) All were measured on day 21 of mechanical ventilation. The variables included age, premorbid independence in activities of daily living, PaO2/FIO2, inability to lift upper extremity from the bed, requirement for any dose of pressor (dopamine, norepinephrine, phenylephrine), platelet count, requirement for hemodialysis (any patient receiving hemodialysis between Day 19 and 22 of mechanical ventilation, or any patient with renal failure for whom hemodialysis had been indicated but withheld), and specific comorbidities (severe chronic pulmonary disease, peripheral vascular disease, diabetes mellitus with chronic complication, congestive heart failure). Bivariate analysis of associations between the primary outcome, death at one year, and the preselected predictor variables were performed for descriptive purposes. Potential collinearity was assessed by examining pairwise correlations and measuring variance inflation factors. Collinearity was not found to be an issue in our data and therefore did not affect our modeling strategy.
All variables identified a priori as potential predictors were included in a logistic regression model with death at one year as the primary outcome. The maximal model was reduced by the investigators by eliminating variables sequentially and comparing each new model by likelihood ratio tests and by comparing the area under the ROC curve for each new model. Calibration of the model was assessed using Pearson's chi-square goodness-of-fit test (GoF). Odds ratios and 95% confidence intervals associated with each variable in the final model are reported. A similar model was constructed using 3-month mortality (90 days after day 21 of mechanical ventilation) as the primary outcome.
For the validation phase of the study, values for predictive variables measured for the 100 patients in the validation set were entered into logistic regression models using the beta values from the reduced logistic regression model from the 200-patient development phase. Validation of the prediction model was established by comparing the area under the ROC curve, sensitivity, and specificity of the development and validation study models. Calibration of the validation study model was assessed using Pearson's GoF test. Similar analyses were performed using 3-month mortality as the primary outcome.
A clinical prediction rule was adapted from the final prediction model by assigning points to each predictive variable based on regression coefficients from the development model. Performance of this clinical prediction rule was assessed by comparing observed to predicted outcomes for one-year and 3-month mortality and by comparison of area under the ROC curve to that of the final model.
Data are presented as mean ± Standard Deviation or median (IQR). Area under ROC curves, sensitivity and specificity are presented as value (standard error). All analyses were performed using Stata 8.0 software (Stata Corporation, College Station, TX).
Results
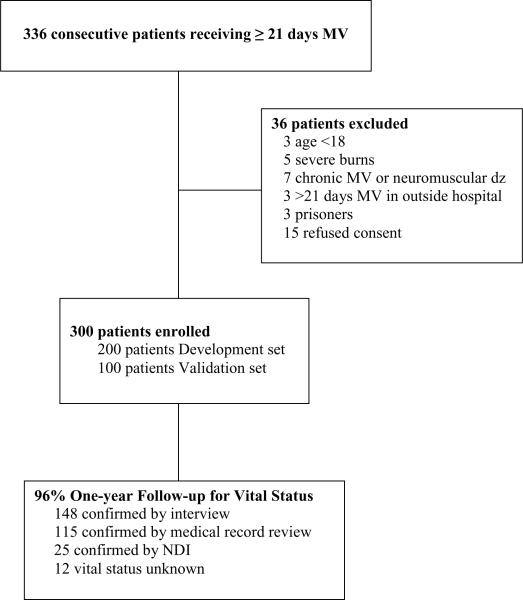
Of 336 consecutive patients who were eligible for the study, 36 were excluded (Figure 1). Vital status one year after enrollment was confirmed by telephone follow-up or medical record review in 263 patients and by NDI review in 25 patients. One-year mortality was unknown for 12 patients, for an overall follow up rate of 96%.
Figure 1.
Enrollment and follow-up data
Patient demographics and outcomes for the development and validation sets are presented in Tables 1 and 2. ICU admission diagnoses are shown in the table in the Appendix. The groups were mostly similar, but patients in the validation set had higher admission APACHE II scores and higher hospital and 3-month mortality. Thirty-seven of the patients who survived hospitalization were not confirmed to have died by review of medical records or telephone follow-up. NDI records were available for 25 of them. All were noted to have survived the year. The remaining 12 patients for whom NDI records were not yet available were counted as survivors based upon survival of the 25 patients lost to telephone follow-up who did have NDI data available.
Table 1.
Patient characteristics
| Variable | Development | Validation | P value |
|---|---|---|---|
| n=200 | n=100 | ||
| Age, mean ± SD | 55.7 ± 16.7 | 55.5 ± 16.6 | 0.92 |
| Age, median (IQR) | 58 (42-69) | 57 (44-66) | 0.82 |
| Male, n (%) | 120 (60) | 49 (49) | 0.07 |
| Race, n (%) | 0.31 | ||
| White | 124 (62) | 61 (61) | |
| African American | 61 (31) | 36 (36) | |
| Hispanic | 9 (5) | 1 (1) | |
| Asian | 4 (2) | 1 (1) | |
| Native American | 1 (0.5) | 1 (1) | |
| Premorbid Status | |||
| Residence, n (%) | n=181 | n=81 | 0.27 |
| Home | 171 (94) | 75 (93) | |
| Assisted Living Facility | 3 (2) | 4 (5) | |
| Skilled Nursing Facility | 7 (4) | 2 (2) | |
| Independent in ADLs, n (%) | n=175 | n=79 | 0.29 |
| 143 (82) | 60 (76) | ||
| APACHE II ICU Admit, mean ± SD | 20.6 ± 7.3 | 25.3 ± 6.8 | 0.0001 |
| SOFA Day 1 MV, mean ± SD | 9.9 ± 3.4 | 9.8 ± 2.9 | 0.78 |
| Day 21 Measurements | |||
| Advance Directives, n (%) | 0.94 | ||
| Do Not Resuscitate order | 12 (6) | 6 (6) | |
| Advanced Power of Attorney | 9 (5) | 6 (6) | |
| Living Will | 5 (3) | 2 (2) | |
| None | 168 (87) | 81 (85) | |
| Service, n (%) | 0.20 | ||
| Medicine | 79 (40) | 46 (46) | |
| General Surgery/Trauma | 56 (28) | 26 (26) | |
| Cardiac Surgery | 20 (10) | 5 (5) | |
| Thoracic Surgery | 18 (9) | 10 (10) | |
| Neurosurgery | 17 (9) | 4 (4) | |
| Transplant Surgery | 7 (4) | 5 (5) | |
| SOFA Day 21 MV, mean ± SD | 7.6 ± 3.9 | 7.7 ± 3.4 | 0.94 |
| PaO2/FIO2, mean ± SD | 219 ± 95.7 | 216 ± 110 | 0.82 |
| WBC, mean ± SD | 13.3 ± 8.6 | 11.7 ± 7.1 | 0.10 |
| Platelet count (x109/L), mean ± SD | 307 ± 216 | 243 ±159 | 0.009 |
| Pressors, n (%) | 33 (16) | 28 (28) | 0.02 |
| Hemodialysis, n (%) | 49 (25) | 32 (32) | 0.15 |
| Albumin, median (IQR) | 2.0 (1.7-2.4) | 2.0 (1.8-2.5) | 0.58 |
| BMI, mean ± SD | 30.2 ± 8.2 | 33.5 ± 11.9 | 0.009 |
| Charlson Index Score, mean ± SD | 2.7 ± 2.2 | 3.1 ± 2.3 | 0.16 |
| Specific Comorbidities, n (%) | |||
| Severe Chronic Pulmonary Disease | 22 (11) | 14 (14) | 0.45 |
| Chronic Vascular Disease | 16 (8) | 7 (7) | 0.78 |
| Diabetes with chronic complications | 23 (12) | 10 (10) | 0.70 |
| Congestive Heart Failure | 30 (15) | 15 (15) | 1.0 |
| Upper Extremity Strength, n (%) | 0.10 | ||
| Against gravity | 129 (66) | 59 (60) | |
| Withdraw to pain | 42 (21) | 17 (17) | |
| No movement | 25 (13) | 22 (22) | |
| Lower Extremity Strength, n (%) | 0.10 | ||
| Against gravity | 94 (48) | 42 (43) | |
| Withdraw to pain | 63 (32) | 26 (27) | |
| No movement | 38 (19) | 30 (31) | |
| Tracheostomy, n (%) | 167 (84) | 77 (77) | 0.38 |
| Days to Tracheostomy, n (%) | 17 (12-22) | 19 (12-26) | 0.11 |
ADLs: Activities of Daily Living. APACHE II: Acute Physiology and Chronic Health Evaluation System. SOFA: Sequential Organ Failure Assessment. WBC: White Blood Cell count. BMI: Body Mass Index
Table 2.
Outcomes
| Variable | Development | Validation | P value |
|---|---|---|---|
| N=200 | N=100 | ||
| Hospital Disposition, n (%) | 0.56 | ||
| Died | 82 (41) | 50 (51) | |
| Long Term Acute Care | 21 (11) | 13 (13) | |
| Rehabilitation | 59 (30) | 24 (24) | |
| Skilled Nursing Facility | 15 (8) | 5 (5) | |
| Home with Assistance | 19 (10) | 5 (5) | |
| Home Independent | 2 (1) | 1 (1) | |
| If Died | |||
| Received CPR at time of death, n (%) | 10 (12) | 3 (6) | 0.02 |
| Days DNR to Death, median (IQR) | 1 (0-3) | 2.5 (1-7) | 0.27 |
| Liberated from MV in hosp, n (%) | 106 (53) | 43 (43) | 0.23 |
| Liberated, hosp survivors | n=118 | n=50 | 0.88 |
| 95 (81) | 39 (78) | ||
| Reintubated, n (%) | 14 (8) | 14 (14) | 0.10 |
| Liberated from MV in one year, n (%) | 114 (58) | 47 (49) | 0.14 |
| Ventilator Days, median (IQR) | 35 (26-51) | 35 (27-54) | 0.71 |
| Ventilator Days, survivors | 39 (29-58) | 38 (29-52) | 0.72 |
| Ventilator Days, nonsurvivors | 32 (25-45) | 35 (25-59) | 0.29 |
| ICU length of stay, median (IQR) | 37 (28-52) | 36 (30-54) | 0.46 |
| Hospital length of stay, median (IQR) | 51 (36-72) | 50 (37-74) | 0.91 |
| Mortality, n (%) | |||
| Three Months | 83 (42) | 52 (52) | 0.08 |
| One Year: known follow up | n=175 | n=84 | 0.11 |
| 103 (59) | 58 (69) | ||
| One Year: includes NDI dataa | n=200 | n=100 | 0.18 |
| 103 (52) | 58 (58) |
All hospital survivors in the Development set who did not have a record of subsequent death at UNC survived the year based upon National Death Index (NDI) records. This assumption was made for 12 similar patients in the Validation set
Results of bivariate analyses are presented for descriptive purposes in Table 3. All predetermined predictor variables were included in the initial maximal logistic regression model. Requirement of vasopressors, platelets ≤150 x109/L, age ≥50, requirement of hemodialysis, and upper extremity weakness were independent predictors of death at one year in a reduced model. Clinically, upper extremity weakness was considered difficult to reproduce since the use of sedatives, which strongly affected this measurement, varied significantly between patients. This issue has affected the reliability of other illness severity models. (19) Therefore models with and without this variable were compared. The area under the ROC curve for the final reduced model shown in Table 4 (vasopressors, platelets ≤150 x109/L, age ≥50, requirement of hemodialysis) was 0.82 (se 0.03). This compares to 0.84 (se 0.02) for the model with the final four variables plus upper extremity weakness and 0.85 (se 0.03) for the maximal model, (p=0.46 for comparison of all three). For the final reduced model, sensitivity for identifying patients at ≥50% risk of death was 0.58 (se 0.16), and specificity was 0.91 (se 0.16). Sensitivity for identifying patients at ≥90% risk of death was 0.42 (se 0.12), and specificity was 0.99 (se 0.01). The model had good fit based on its non-significant GoF test (χ210df = 6.72, p = 0.75).
Table 3.
Bivariate analysis of associations between predetermined predictive variables and one-year mortality in development set
| Variable | n | Survived | Died | RR (95% CI) | p value |
|---|---|---|---|---|---|
| Age | |||||
| ≥50 years | 128 | 51 (40) | 77 (60) | 1.66 (1.19, 2.34) | 0.001 |
| <50 years | 72 | 46 (64) | 26 (36) | ||
| Activities of Daily Living | |||||
| Needs assistance with 1 ADL | 32 | 11 (34) | 21 (66) | 1.47 (1.07, 2.0) | 0.03 |
| No assistance needed | 143 | 79 (55) | 64 (45) | ||
| PaO2/FIO2, mean ± SD | 181 | 229 ± 96 | 208 ± 103 | -- | 0.21 |
| Upper Extremity Strength | |||||
| Cannot lift against gravity | 61 | 15 (25) | 46 (75) | 1.84 (1.44, 2.36) | 0.0001 |
| Can lift against gravity | 137 | 81 (59) | 56 (41) | ||
| Vasopressors | |||||
| Required | 33 | 2 (6) | 31 (94) | 2.18 (1.79, 2.66) | 0.0001 |
| Not Required | 165 | 94 (57) | 71 (43) | ||
| Platelets | |||||
| ≤150 x 109/L | 48 | 4 (8) | 44 (92) | 2.41 (1.93, 3.01) | 0.0001 |
| >150 x 109/L | 150 | 93 (62) | 77 (38) | ||
| Hemodialysis | |||||
| Required | 49 | 10 (20) | 39 (80) | 1.88 (1.49,2.37) | 0.0001 |
| Not Required | 151 | 87 (58) | 64 (42) | ||
| Chronic Pulmonary Disease | |||||
| Present | 22 | 10 (45) | 12 (55) | 1.07 (0.71, 1.60) | 0.76 |
| Absent | 178 | 87 (49) | 91 (51) | ||
| Peripheral Vascular Disease Present | 16 | 8 (50) | 8 (50) | 0.97 (0.58, 1.61) | 0.90 |
| Absent | 184 | 89 (48) | 95 (52) | ||
| Diabetes with chronic complication | |||||
| Present | 23 | 7 (30) | 16 (70) | 1.41 (1.03, 1.93) | 0.06 |
| Absent | 177 | 90 (51) | 87 (49) | ||
| Congestive Heart Failure | |||||
| Present | 30 | 12 (40) | 18 (60) | 1.20 (0.86, 1.67) | 0.31 |
| Absent | 170 | 85 (50) | 85 (50) |
Table 4.
Model performance
| Three-Month Mortality | One-Year Mortality | |
|---|---|---|
| Variable | Development OR (95% CI) | Development OR (95% CI) |
| Vasopressor | 4.2 (1.2, 14.2) | 8.8 (1.6, 48.4) |
| Platelets ≤150 x 109/L | 7.1 (2.7, 18.6) | 14.5 (4.1, 50.8) |
| Age ≥50 years old | 3.5 (1.6, 7.8) | 5.6 (2.4, 12.9) |
| Requiring Hemodialysis | 3.1 (1.3, 7.5) | 2.9 (1.1, 7.7) |
| Three-Month Mortality | One-Year Mortality | |||
|---|---|---|---|---|
| Model | Development | Validation | Development | Validation |
| Area under ROC (se) | 0.81 (0.03) | 0.79 (0.05)a | 0.82 (0.03) | 0.82 (0.05)b |
| Sensitivityc (se) | 0.31 (.10) | 0.32 (.13) | 0.42 (0.12) | 0.44 (0.20) |
| Specificityc (se) | 0.97 (0.01) | 0.95 (0.02) | 0.99 (0.01) | 0.95 (0.02) |
p = 0.75 for comparison with Development set
p = 0.93 for comparison with Development set
Sensitivity and Specificity determined for 90% risk of death. Presented as value (standard error)
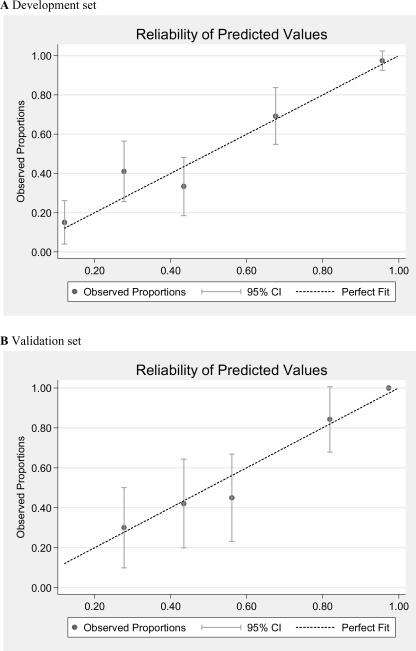
Using values measured in patients from the validation set, the same model had an area under the ROC curve of 0.82 (se 0.05), (p=0.93 compared to development set), and again demonstrated good fit (GoF χ216df = 18.31, p = 0.31). Comparisons of observed to predicted values for development and validation sets are shown in Figure 2. Reliability of the model was very consistent in the validation set. These four variables were also independent predictors of 3-month mortality in a separate model (GoF χ210df = 11.39, p = 0.33), and showed consistent performance in the validation set (GoF χ216df = 23.99, p = 0.09). (Table 4)
Figure 2.
Comparison of observed and predicted one-year mortality for patients divided into 5 equal sized groups from the Development set (2A) and Validation set (2B).
As a sensitivity analysis, patients in the validation set who were lost to follow up were assumed to have all died (rather survive, as was the case in the development set, confirmed by the NDI). The area under the ROC curve for that model was 0.76 (se 0.05). Sensitivity was 0.37 (se 0.18) and specificity remained high at 0.93 (se 0.02) for ≥90% likelihood of death.
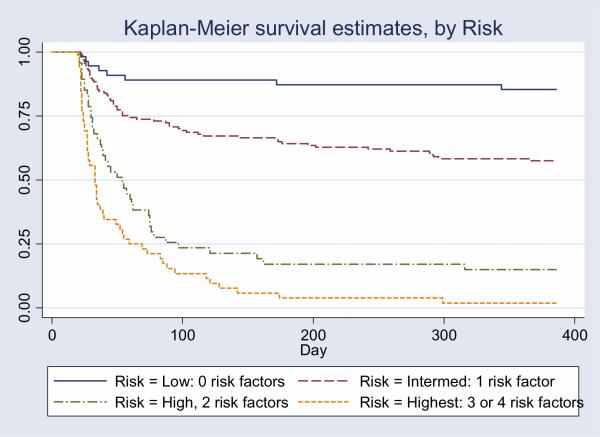
In order to create a prognostic scoring system that could ultimately be used by clinicians in daily practice, we assigned points to each of the four predictive variables in proportion to the regression coefficients from the development model. The regression coefficients were of similar magnitude, so we assigned one point for each risk factor resulting in a range of scores from 0 to 4. Performance of the 4-point prognostic scoring system (Prognosis for Prolonged Ventilation (ProVent) score) is shown in Table 5. Predicted and observed mortality for patients in the development set and observed mortality for patients in the validation set are included. In the development set, patients with the ProVent score of 0, representing no risk factors (n=41, 21%) had a 1-year mortality of only 15%. Patients with the score of 1, representing 1 risk factor (n=98, 50%) had a 1-year mortality of 42%. Patients with the score of 2, representing 2 risk factors (n=26, 13%), had mortality of 77% at 3 months and 88% at 1 year. Patients with the 3 or 4 risk factors had 3-month mortality of 90% and 1-year mortality of 97%. This highest risk group (scores 3 or 4) represents 16% of the development set and 24% of the validation set. The area under the ROC curve for ProVent score for the combined cohort is 0.82 (se 0.03; 95% C.I. 0.75, 0.88). For patients with ≥50% risk of death, sensitivity is 0.58 (se 0.12) and specificity is 0.95 (se 0.07). For patients with ≥ 90% risk of death, sensitivity is 0.32 (se 0.20) and specificity is 0.99 (se 0.01). Survival according to ProVent score risk group is shown in Figure 3.
Table 5.
Prognosis for Prolonged Ventilation (ProVent) Score
| Provent Score | Development Set | Validation Set | |||
|---|---|---|---|---|---|
| n (%) | Predicted 1-year Mortality | Observed 1-year Mortality | n (%) | Observed 1-year Mortality | |
| 95% (CI) | |||||
| 0 | 41 (21) | 0.12 (0.06, 0.21) | 0.15 | 14 (14) | 0.14 |
| 1 | 98 (50) | 0.44 (0.36, 0.53) | 0.42 | 42 (42) | 0.43 |
| 2 | 26 (13) | 0.83 (0.71, 0.90) | 0.88 | 21 (21) | 0.86 |
| 3 | 22 (11) | 0.97 (0.90, 0.99) | 0.95 | 13 (13) | 1.0 |
| 4 | 9 (5) | 0.99 (0.97, 1.0) | 1.0 | 8 (8) | 1.0 |
| Development Set | Validation Set | ||||
|---|---|---|---|---|---|
| Predicted 3-month Mortality | Observed 3-month Mortality | Observed 3-month Mortality | |||
| 95% (CI) | |||||
| 0 | 41 (21) | 0.10 (0.05, 0.17) | 0.12 | 14 (14) | 0.07 |
| 1 | 98 (50) | 0.32 (0.25, 0.40) | 0.29 | 42 (42) | 0.38 |
| 2 | 26 (13) | 0.67 (0.55, 0.77) | 0.77 | 21 (22) | 0.80 |
| 3 | 22 (11) | 0.90 (0.78, 0.95) | 0.91 | 13 (14) | 0.85 |
| 4 | 9 (5) | 0.97 (0.91, 0.99) | 0.89 | 8 (8) | 1.0 |
Variables measured on Day 21 of mechanical ventilation:
Age ≥50 = 1 point
Vasopressor = 1 point
Platelets ≤150 x 109/L = 1 point
Requires Hemodialysis = 1 point
Figure 3.
Kaplan Meier Curves by Risk group for combined cohort: Low = ProVent score 0 (no risk factors), n = 55 (18% of cohort); Intermediate = ProVent score 1 (1 risk factor), n = 137 (47% of cohort); High = Provent score 2 (2 risk factors), n = 47 (16% of cohort); Highest = ProVent score 3 or 4 (3 or 4 risk factors), n = 52 (17% of cohort). Day 0 is the time of intubation.
Data on functional status were available for 57% of 1-year survivors. There were no differences between patients with and without available data for age (p=0.4), SOFA score at day 21 (p=0.30), Charlson Score (p=0.88) or premorbid independence in ADLs (p=0.68). Only 24% of survivors were independent in all ADLs after 1 year. Thirty-nine percent of survivors with ProVent scores of 0, and 18% of survivors with ProVent scores of 1 were independent in all ADLs. None of the patients with ProVent scores of 2 or greater were both alive and independent in all ADLs after 1 year.
Discussion
This prospective cohort study confirms that 4 easily measured variables recorded at day 21 of ventilation can identify patients who are both at high risk and low risk of mortality during prolonged mechanical ventilation. This prognostic model has very high specificity, limiting the possibility of inappropriately poor prognoses. The model performed well during validation in a cohort that was enrolled during a different time period than the development set and that had higher illness severity. Three of the four variables that are independent predictors of mortality-- requirement of pressors, requirement of hemodialyis, and platelet count ≤150 × 109/L, reflect ongoing systemic inflammation and multiorgan failure. The other prognostic variable, age 50 or older, likely reflects lower physiologic reserve independent of acute organ failure and specific comorbidities. It may also reflect less willingness on the part of older patients or surrogates to endure weeks and months of invasive care when progress does not seem apparent. (20)
Much has been written about how PMV patients require a unique approach to care due to differences in physiology.(1, 21-23) However few studies of interventions in this patient population have been published. The ability to standardize illness severity would facilitate the design of cohort studies evaluating interventions to improve process of care and survival. For example, due to issues of high costs and limited resources, hospitals are compelled to discharge PMV patients to various post-ICU settings including respiratory care units, long-term care hospitals (LTCH), or even skilled nursing facilities for continued weaning and management.(24-26) These facilities have been proliferating at a high pace in order to meet increasing demand.(27) While it is possible that these facilities decrease hospital costs, it is not clear whether outcomes are affected. This prognostic model was developed and validated in a population with relatively limited access to post-acute care weaning facilities. Therefore this model provides an acute care baseline against which outcomes from care in different settings can be compared. Variables for the model are measured before most LTCH transfers occur,(25) so illness severity can be standardized before transfer to alternative care settings.
Two prognostic models have been published for PMV patients managed in long-term acute care hospitals,(15, 16) but neither have been validated, and only one included long-term follow-up. In one study, existing illness severity scores demonstrated poor discrimination and calibration for hospital mortality in PMV patients at an LTCH. When measured on the day of admission to the LTCH, the area under the ROC curve was less than 0.70 for APACHE II, SAPS II, MPM II and LODS. (28)
The majority of patients with advanced illnesses wish not to be kept alive on life support when there is little hope for a meaningful recovery.(29) Focus group studies involving PMV patients and their families have revealed that they would benefit from more direct communication with healthcare providers, especially with regard to prognosis.(30) Another study of prognostication during physician-family discussions about limiting life support revealed that prognoses for long-term survival were given in only 12% of conferences.(31) In the SUPPORT study (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment), of the 1494 patients who spent more than 14 days in the ICU, fewer than 40% reported that their physicians had talked with them about their prognoses or preferences for life-sustaining treatment.(32) The simple prognostic model developed in this study could enhance communication of prognosis to these patients and their surrogates by providing objective estimates of short-term and long-term outcome.
A major strength of this study is the heterogeneous patient population including medical and surgical patients as well as patients with major trauma. The prognostic model does not require assignment of a specific diagnosis, which is usually difficult in critically ill patients with multiple active processes. Nor does it require assessments of neurological function which can be unreliable in patients receiving sedation.(19) Selection bias was limited by consecutive and prospective enrollment and a high follow-up rate for mortality.
This study has several important limitations. Differences in management at other centers or communities could result in worse performance of this model in those settings. External validation using multiple tertiary care centers in diverse regions is warranted before clinical or research application of this model is considered.(33) While the study was large enough to have sufficient power to assess the preselected variables in the study protocol, other potential predictors may not have been examined. However the variables that were studied produced a model that is simple, reproducible, and highly specific.
It is possible that objective prognostic information will not change physician practice. In the SUPPORT trial, an intervention using a sophisticated prognostic model designed to facilitate discussion of prognosis and wishes for aggressive care in acutely ill patients had no significant impact on these outcomes. (34) There are several reasons why the prognostic model in this study could have a more significant impact than that of the SUPPORT trial. The prognostic score is simple to understand and can be assessed by the clinician at the bedside within seconds rather than relying on an intermediary with a complicated formula. The prognostic information comes later in the patients' clinical course when extensive efforts have been made on the patient's behalf, yet progress has stalled and reserve is limited. Both clinicians and surrogates may be more likely to accept a change in the course of care when poor outcomes are expected despite weeks of maximal treatment.
The majority of physicians find prognostication to be stressful and difficult, and they feel that they have inadequate training in this area. (35) They are particularly concerned about being wrong, especially when withholding or withdrawing life support is a possible outcome of decision-making. Variables were selected a priori for this model with an aim to identify the patients at highest risk of death. Consequently the model has very high specificity (0.99) for patients with a 90% mortality risk. There is minimal chance of misclassifying a patient as very high risk (false positive). Measuring specificity at a high mortality risk comes at the expense of lower sensitivity. A many as 58% of patients who ultimately died were not classified in the highest risk group (false negatives). When prognosticating however, most clinicians are worried more about giving negative prognoses for patients who would otherwise survive,(35) favoring a mortality model with high specificity.
Of course, objective prognostic information will not change physician practice in isolation. Other important factors are necessary to improve patient/family communication about end-of-life issues.(36, 37) Finally, such a scoring system should not be used to replace clinician judgment regarding likely outcomes, but rather to inform those judgments.(38)
Conclusions
Patients receiving PMV who are at the highest risk of death can be identified based upon the requirement of either vasopressors or hemodialysis, or the presence of platelet counts ≤150 × 109/L or age over 50. Following external validation, a prognostic scoring system using these risk factors could facilitate earlier and more definitive discussions between clinicians and patients or surrogates regarding appropriate goals of care.
Acknowledgments
This work was supported by National Institutes of Health grants K23 HL067068 (SSC) and K23 HL081048 (CEC)
Appendix
| ICU Admitting Diagnosis | Development Set n=200 | Validation Set n=100 |
|---|---|---|
| Pulmonary Fibrosis | 3 (2) | 0 |
| COPD | 4 (2) | 2 (2) |
| ARDS | 10 (5) | 5 (5) |
| Respiratory Arrest | 3 (2) | 5 (5) |
| Cystic Fibrosis | 5 (3) | 1 (1) |
| Pneumonia | 13 (7) | 14 (14) |
| Sepsis | 14 (7) | 8 (8) |
| Congestive Heart Failure | 2 (1) | 4 (4) |
| Myocardial Infarction | 3 (2) | 0 |
| Cardiac Arrest | 5 (3) | 2 (2) |
| Intracranial hemorrhage, | 3 (2) | 0 |
| nonoperative | ||
| Overdose | 1 (<1) | 2 (2) |
| Neuromuscular Weakness | 8 (4) | 0 |
| Hepatic Failure | 1 (<1) | 2 (2) |
| Gastrointestinal hemorrhage | 3 (2) | 2 (2) |
| Pancreatitis | 6 (3) | 1 (1) |
| Other GI condition | 1 (<1) | 2 (2) |
| Hematologic Malignancy | 2 (1) | 0 |
| Other Malignancy | 1 (<1) | 1 (1) |
| Other Medical | 4 (2) | 1 (1) |
| Multiple Trauma | 32 (16) | 10 (10) |
| Head Trauma | 4 (2) | 0 |
| C-spine Injury | 3 (2) | 1 (1) |
| Coronary Artery Bypass Graft | 5 (3) | 1 (1) |
| Heart Valve Surgery | 8 (4) | 2 (2) |
| Thoracic Surgery | 13 (7) | 6 (6) |
| GI perforation/Obstruction | 7 (4) | 6 (6) |
| Other GI surgery | 6 (3) | 7 (7) |
| Vascular Surgery | 7 (4) | 2 (2) |
| Surgery for intracranial hemorrhage | 8 (4) | 2 (2) |
| Heart Transplant | 3 (2) | 2 (2) |
| Lung Transplant | 1 (<1) | 1 (1) |
| Liver Transplant | 8 (4) | 6 (6) |
| Other Surgery | 3 (2) | 2 (2) |
Data presented as n (%). Percentages do not add to 100 due to rounding. Other Medical includes asthma, diabetic ketoacidosis, pulmonary embolus, meningitis, acute renal failure, 1 case each.
References
- 1.MacIntyre NR, Epstein SK, Carson S, Scheinhorn S, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 2.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18(3):461–76. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 3.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit Care Med. 2004;32(11):2219–26. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 4.Wagner DP. Economics of prolonged mechanical ventilation. Am Rev Respir Dis. 1989;140(2 Pt 2):S14–8. doi: 10.1164/ajrccm/140.2_Pt_2.S14. [DOI] [PubMed] [Google Scholar]
- 5.Douglas SL, Daly BJ, Gordon N, Brennan PF. Survival and quality of life: short-term versus long-term ventilator patients. Crit Care Med. 2002;30(12):2655–62. doi: 10.1097/00003246-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cox CE, Carson SS, Lindquist JAH, Olson MK, Govert JA, Chelluri L. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Critical Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RE. The symptom burden of chronic critical illness. Crit Care Med. 2004;32(7):1527–34. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction. Another burden for the chronically critically ill. Archives of Internal Medicine. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 9.Carson SS. Outcomes of prolonged mechanical ventilation. Curr Opin Crit Care. 2006;12(5):405–11. doi: 10.1097/01.ccx.0000244118.08753.dc. [DOI] [PubMed] [Google Scholar]
- 10.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35:1918–1927. doi: 10.1097/01.CCM.0000275391.35834.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Critical Care Medicine. 1986;311:818–829. [PubMed] [Google Scholar]
- 12.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Scott WK, Macera CA, Cornman CB, Sharpe A. Functional health status as a predictor of mortality in men and women over 65. J Clin Epidemiology. 1997;50:291–296. doi: 10.1016/s0895-4356(96)00365-4. [DOI] [PubMed] [Google Scholar]
- 15.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1568–73. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 16.Dematte D'Amico JE, Donnelly HK, Mutlu GM, Feinglass J, Jovanovic BD, Ndukwu IM. Risk assessment for inpatient survival in the long-term acute care setting after prolonged critical illness. Chest. 2003;124(3):1039–45. doi: 10.1378/chest.124.3.1039. [DOI] [PubMed] [Google Scholar]
- 17.Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Impact of renal dysfunction on weaning from prolonged mechanical ventilation. Critical Care. 1997;1(3):101–104. doi: 10.1186/cc112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook DJ, Rocker D, Marshall MD, Sjokvist P, Dodek P, Griffith L, Freitag A, Varon J, Bradley C, Levy M, et al. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. New Engl. J. Med. 2003;349:1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 19.Polderman KH, Jorna EM, Girbes AR. Inter-observer variability in APACHE II scoring: effect of strict guidelines and training. Intensive Care Med. 2001;27:1365–9. doi: 10.1007/s001340101012. [DOI] [PubMed] [Google Scholar]
- 20.Suchyta MR, Clemmer TP, Elliott CG, Orme JF, Morris AH, Jacobson J, Menlove R. Increased mortality of older patients with acute respiratory distress syndrome. Chest. 1997;111:1334–1339. doi: 10.1378/chest.111.5.1334. [DOI] [PubMed] [Google Scholar]
- 21.Scheinhorn DJ, Stearn-Hassenpflug M. Provision of long-term mechanical ventilation. Crit Care Clin. 1998;14(4):819–32. doi: 10.1016/s0749-0704(05)70032-4. [DOI] [PubMed] [Google Scholar]
- 22.Scheinhorn DJ, Chao DC, Hassenpflug MS, Gracey DR. Post-ICU weaning from mechanical ventilation: the role of long-term facilities. Chest. 2001;120(6 Suppl):482S–4S. doi: 10.1378/chest.120.6_suppl.482s. [DOI] [PubMed] [Google Scholar]
- 23.Nierman DM, Nelson JE. A structure of care for the chronically critically ill. Critical Care Clinics. 2002;18(3):477–492. doi: 10.1016/s0749-0704(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 24.Rudy EB, Daly BJ, Douglas S, Montenegro HD, Song R, Dyer MA. Patient outcomes for the chronically critically ill: special care unit versus intensive care unit. Nurs Res. 1995;44(6):324–31. [PubMed] [Google Scholar]
- 25.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, Knight EB, Petrak RA, Ventilator Outcomes Study Group Ventilator-dependent survivors of catastrophic illness transferred to 23 long-term care hospitals for weaning from prolonged mechanical ventilation. Chest. 2007;131(1):76–84. doi: 10.1378/chest.06-1079. [DOI] [PubMed] [Google Scholar]
- 26.Corrado A, Roussos C, Ambrosino N, Confalonieri M, Cuvelier A, Elliott M, Ferrer M, Gorini M, Gurkan O, Muir JF, Quareni L, Robert D, Rodenstein D, Rossi A, Schoenhofer B, Simonds AK, Strom K, Torres A, Zakynthinos S. Respiratory intermediate care units: a European survey. Eur Respir J. 2002;20(5):1343–50. doi: 10.1183/09031936.02.00058202. [DOI] [PubMed] [Google Scholar]
- 27.Medicare Payment Advisory Commission Chapter 5. Defining long-term care hospitals. June 2004 report). Available at: www.medpac.gov/publications. Accessed November, 2004.
- 28.Carson SS, Bach PB. Predicting mortality in patients suffering from prolonged critical illness. Chest. 2001;120:928–933. doi: 10.1378/chest.120.3.928. [DOI] [PubMed] [Google Scholar]
- 29.Heyland DK, Dodek P, Rocker G, Groll D, Gafni A, Pichora D, Shortt S, Tranmer J, Lazar N, Kutsogiannis J, Lam M, for the Canadian Researchers End-of-Life Network (CARENET) What matters most in end-of-life care: perceptions of seriously ill patients and their family members. CMAJ. 2006;174(5) doi: 10.1503/cmaj.050626. Online-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson JE, Kinjo K, Meier DE, Ahmad K, Morrison RS. When critical illness becomes chronic: informational needs of patients and families. J Crit Care. 2005;20(1):79–89. doi: 10.1016/j.jcrc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35:442–448. doi: 10.1097/01.CCM.0000254723.28270.14. [DOI] [PubMed] [Google Scholar]
- 32.Teno JM, Fisher E, Hamel MB, Wu AW, Murphy DJ, Wenger NS, Lynn J, Harrell FE. Decision-making and outcomes of prolonged ICU stays in seriously ill patients. J Am Geriatr Soc. 2000;48(5 Suppl):S70–74. doi: 10.1111/j.1532-5415.2000.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 33.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 34.A controlled trial to improve care for seriously ill hospitalized patients. The Study to Understand Prognoses for Outcomes and Risks of Treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1541–8. [PubMed] [Google Scholar]
- 35.Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389–2395. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 36.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–78. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 37.Curtis JR, Patrick DL, Shannon SE, et al. The family conference as a focus to improve communication about end-of-life care in the intensive care unit: opportunities for improvement. Crit Care Med. 2001;29(Suppl 2):N26–N33. doi: 10.1097/00003246-200102001-00006. [DOI] [PubMed] [Google Scholar]
- 38.Lynn J, Teno JM, Harrell FE. Accurate prognostications of death. Opportunities and challenges for clinicians. West J Med. 1995;163(3):250–7. [PMC free article] [PubMed] [Google Scholar]