Abstract
Purpose
The aim of this study was to evaluate the feasibility and toxicity of concurrent chemoradiotherapy (CCRT) with S-1 in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN) in elderly cases and/or cases with comorbidity.
Methods
Fifty eligible patients with stage III (15 cases) or stage IV (35 cases) SCCHN were treated with CCRT. Thirteen cases had an advanced age of over 75 years and 37 cases had comorbidity. Definitive radiotherapy was delivered up to a total dose of 66–70.2 Gy. The patients received two courses of oral S-1 (40 or 50 mg twice a day [80 or 100 mg/day]) for 2 weeks followed by 1 week of rest while receiving CCRT.
Results
All the patients received the planned radiotherapy and at least one course of S-1. Grade 3 mucositis occurred in 20% of the patients (10/50). Grade 3 neutropenia occurred in 12% (6/50) and leukocytopenia occurred in 6% (3/50) of the cases. Pathologically, the complete response rates were 93% in stage III and 54% in stage IV.
Conclusion
Concurrent chemoradiotherapy with S-1 is a safe, well-tolerated and effective regimen for locally advanced SCCHN in elderly cases and/or cases with comorbidity.
Keywords: S-1, Chemoradiotherapy, Elderly cases, Cases with comorbidity
Introduction
In an attempt to improve therapeutic results (survival or function preservation), chemotherapy has been applied before (neoadjuvant), with (concurrent), or after (adjuvant or consolidation) conventional therapy (surgery and/or radiotherapy). The rationale for concurrent chemoradiotherapy (CCRT) is that chemotherapeutic agents may act as radiation sensitizers in addition to contributing their own anti-tumor effect [1]. Furthermore, effective chemotherapy may control micrometastasis outside of the lesions treated with radiotherapy. Some CCRT regimens have produced an improvement in overall survival, disease-free survival, locoregional control of the disease, or a decrease in distant metastasis when examined in randomized trials where they were compared with radiotherapy alone [2–4].
In terms of squamous cell carcinoma of the head and neck (SCCHN), chemotherapy mainly consists of a platinum analog (mainly cisplatin [CDDP]) and the continuous intravenous infusion of 5-fluorouracil (5-FU), especially in the neoajuvant chemotherapy studies. On the other hand, the bolus administration of CDDP has been the most well-known regimen in the CCRT studies [2–4]. Recently, docetaxel combined with radiotherapy has been applied as a radio-sensitizer for fresh HNSCC [5], and carboplatin with definitive radiotherapy has been used for patients receiving NAC [6].
However, regimens with strong impacts cannot be administered to patients of advanced age or with comorbidity like renal dysfunction or cardiovascular disease, since they entail severe toxicities like nephrotoxicity (occurring with CDDP) or mucositis/cardiotoxicity (occurring with the continuous intravenous infusion of 5-FU at high doses). Simultaneous combined therapy with 5-FU and radiotherapy in patients with SCCHN has been reported to improve the therapeutic results when compared with radiotherapy alone [7]. The radiosensitizing efficacy of 5-FU strongly depends on the continuous exposure of the tumor cells to 5-FU for 8 h or more following irradiation [8, 9]. Because of the short half-life of 5-FU, the drug must be administered continuously to achieve an effective level for prolonged tumor exposure.
S-1 is an oral anticancer agent comprising tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate at a molar ratio of 1:0.4:1 [10–12]. Tegafur is a prodrug of 5-FU, and 5-chloro-2,4-dihydroxypyridine enhances the serum 5-FU concentration through the competitive inhibition of DPD, an enzyme responsible for 5-FU catabolism. Potassium oxonate, a reversible competitive inhibitor of orotate phosphoribosyl transferase, inhibits the phosphorylation of 5-FU in gastrointestinal tissue, reducing the diarrhea associated with 5-FU. In a phase II trial of advanced and recurrent SCCHN (59 eligible cases), S-1 showed a high response rate of 28.8% with acceptable toxicities [13]. The daily oral administration of S-1 instead of intravenous 5-FU infusion may be useful for reducing toxicity and producing a radio-sensitizing effect, resulting in an anti-tumor effect. However, in concurrent chemoradiotherapy with S-1, it is impossible to avoid an increase in toxicities, compared with the single use of S-1. The success of concurrent chemoradiotherapy depends on a feasible and effective administration schedule and S-1 dose that can be combined with radiotherapy. A schedule of 2 weeks of administration followed by 1 week of rest is more feasible than a schedule of 4 weeks of administration followed by 2 weeks of rest over a 6-month period for the administration of S-1 as an adjuvant chemotherapy for locoregionally advanced SCCHN [14].
When performing chemoradiation with S-1, the daily administration of S-1 is essential; thus, a schedule of 2 weeks of S-1 administration followed by 1 week of rest was used in this study.
The primary outcomes of interest in the present study were the feasibility and toxicity of CCRT with S-1, with tumor response being of secondary interest. The intent was to be able to recommend combination therapy for locally advanced SCCHN cases with advanced age and/or comorbidity.
Patients and methods
The eligibility criteria included histologically confirmed squamous cell carcinoma either of the larynx, oropharynx, hypopharynx, or oral cavity; measurable or clinically assessable disease; stage III or IV disease according to the 2002 staging system of the International Union Against Cancer (UICC); disease limited to the head and neck region (M0); an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better; no prior radiotherapy or chemotherapy; adequate bone marrow function, defined as a neutrophil count of >1,500 cells/mm3 and a platelet count of >100,000/mm3; and adequate liver function, defined as a total bilirubin level of <1.25 times the upper limit of normal (ULN), an aspartate or alanine aminotransferase (AST or ALT) level of <2.5 ULN, and an alkaline phosphatase level of <2.5 ULN. These criteria included patients with renal dysfunction (creatinine clearance rate between 40 and 60 mL/min), cardiovascular disease, cerebral disorders, and/or advanced age (>75 years). Patients with active peptic ulcers were not included in this study because S-1 induces mucositis, which in turn aggravates peptic ulcers.
The criteria for surgical resection excluded patients with invasion to the prevertebral muscle, invasion to the common or internal carotid artery (i.e., those showing positive results on the artery occlusion test), or bulky metastasis in the retro-pharyngeal lymph nodes. The pretreatment evaluation included a medical history, physical examination, electrocardiogram and chest X-ray in all patients. The laboratory evaluation consisted of a complete blood cell count, urinalysis, and serum chemistry values, including urea nitrogen, creatinine, calcium, albumin, AST, ALT, alkaline phosphatase, and bilirubin. Computed tomography (CT), magnetic resonance imaging (MRI) and neck ultrasonography analyses were performed in all the patients and were used as an adjunct to the clinical evaluation to define the tumor extent and the presence of nodal metastasis. To check for distant metastasis, positron emission tomography (PET), chest CT, abdominal ultrasonography, and bone scintigraphy examinations were performed.
Two courses of S-1, 50 or 40 mg twice a day, administration with radiotherapy were given according to a schedule of 2 weeks of administration followed by 1 week of rest (one course). The daily dose of S-1 was reduced by one dose level compared with the conventional adjuvant setting, i.e., from 120 mg/day (60 mg, twice a day) to 100 mg/day (50 mg, twice a day) and from 100 mg/day (50 mg, twice a day) to 80 mg/day (40 mg, twice a day). The minimum dose of S-1 studied was 80 mg/day (40 mg, twice a day). The S-1 was orally administered in the morning 2 h before the start of daily radiotherapy and again after the evening meal. To ascertain compliance with oral administration into the patient, the medication was checked daily by two or three nurses.
Radiotherapy was delivered 5 days a week using single daily fractions of 1.8 or 2.0 Gy. Megavoltage radiation was generated by a 6 MeV X-ray linear accelerator. The field size included the primary tumor and regional (cervical) metastasis. After approximately 40 Gy, the patients were reevaluated. The patients with an obvious clinical response completed the radiotherapy with a total dose of between 66.0 and 70.2 Gy. In non-responders with resectable tumors showing a progressive disease, surgery was recommended according to the physical condition of the patient.
The patients were monitored for toxicity (medical interview, physical examination, and complete blood cell counts) during the treatment. Blood and urine chemistries were performed twice a week. If the neutrophil count dropped to <1,000 cells/mm3 during CCRT, subcutaneous G-CSF (100 μg/body/day) was injected.
Toxicity was assessed during the treatment and for 4 weeks after treatment using the 2003 Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0). Unacceptable toxicity was defined as follows: any Grade 3 or higher non-hematological toxicity or Grade 4 hematological toxicity lasting more than 7 days or associated with fever, infection, or thrombocytopenia.
Although the major objective of this study was to evaluate the feasibility and toxicity, an attempt was made to evaluate the efficacy of this drug combined with radiotherapy in this patient population. In patients completing radiotherapy, the final response at the primary site and in the neck was evaluated 4–6 weeks later. A complete response (CR) was defined as the disappearance of all clinical evidence of disease and negative biopsy results for the primary site. The neck response was deemed complete with the disappearance of any adenopathy, as determined using ultrasonography. Fine-needle aspiration cytology was performed when residual nodal metastasis was suspected. A partial response (PR) was defined as a 50% or greater decrease in the product of two perpendicular diameters of the primary and regional tumors. The patients whose diseases did not fulfill the criteria for a PR were deemed to have no change (NC) or stable disease. The patients with less than a CR of the primary and neck tumors were considered for planned surgery 4–8 weeks after the completion of radiotherapy, and salvage surgery was recommended when appropriate for local and/or regional recurrence, if the patients could withstand surgical treatments.
The overall and disease-specific survival rates were calculated using the Kaplan–Meier method and were statistically analyzed using the generalized Wilcoxon test.
The study was approved by the local institutional review boards. The patients were informed of the investigational nature of this study and provided their written informed consent.
Results
Patient characteristics
Between December 2002 and July 2007, 50 patients received concurrent chemoradiotherapy as their primary therapy for SCCHN. The patient characteristics are shown in Tables 1 and 2. The median age was 68.0 years, while that of the 13 cases with advanced age was 78.6 years. According to the UICC staging system, 15 patients had stage III and 35 patients had stage IV tumors. Twenty-one cases had T4 tumors (42%), and 29 cases (58%) had N2 or N3 tumors (Table 3). There were 35 resectable cases (15 with stage III and 20 with stage IVA) and 15 unresectable ones (stage IVB). Thirty-seven cases had complications: 12 had a renal dysfunction (40 < Ccr < 60 mL/min), 8 had respiratory disorders, 14 had cardiovascular disorders, 6 had liver cirrhosis, 5 had cerebral infarction, and 4 had other comorbidity.
Table 1.
Patients’ characteristics (1)
| Characteristics | No. of patients (n = 50) |
|---|---|
| Age: range (median) | 38–88 (68) years |
| Sex | |
| Male | 47 |
| Female | 3 |
| Stage | |
| III | 15 |
| IV | 35 |
| Primary site | |
| Larynx | 9 |
| Oropharynx | 13 |
| Hypopharynx | 25 |
| Oral cavity | 3 |
| Differentiation | |
| Well | 8 |
| Moderately | 25 |
| Poorly | 17 |
| Resectability | |
| Resectable | 35 |
| Unresectable | 15 |
Table 2.
Patients’ characteristics (2)
| Cases with advanced age (75 years <): 13 cases | |
| Age: range (median) | 76–88 (78.6) years |
| Cases with comorbidity: 37 cases | |
| Renal dysfunction (40 < Ccr < 60 mL/min) | 12 cases |
| Respiratory disorder | 8 cases |
| Chronic obstructive pulmonary disease | |
| Cardiovascular disorder | 14 cases |
| Old myocardial infarction | |
| Angina | |
| Arrhythmia including atrial fibrillation | |
| Arteriosclerotic obliteration | |
| Liver cirrhosis | 6 cases |
| Old cerebral infarction | 5 cases |
| Others (alcoholism, malignant hyperpyrexia, epilepsy) | 4 cases |
Table 3.
TN classification
| T stage | |||||
|---|---|---|---|---|---|
| N stage | T1 | T2 | T3 | T4 | Total |
| N0 | 0 | 0 | 7 | 4 | 11 |
| N1a | 1 | 4 | 3 | 2 | 10 |
| N2a | 0 | 0 | 0 | 0 | 0 |
| N2b | 0 | 2 | 2 | 9 | 13 |
| N2c | 1 | 4 | 3 | 6 | 14 |
| N3 | 0 | 1 | 1 | 0 | 2 |
| Total | 2 | 11 | 16 | 21 | 50 |
Feasibility
Radiotherapy was completed to the planned dose of 66.0 or 70.2 Gy without early termination in all the patients (mean dose: 68.4 Gy). Nevertheless, the chemotherapy regimen was interrupted in 11 cases based on discussions with radiation oncologists considering physical conditions of patients: two cases with skin rash appearing 2 weeks after the start of S-1 administration, four cases with neutropenia, three cases with hepatic toxicity, and two cases with mucositis during the second course of S-1 administration. Three cases required a dose reduction of S-1 from 100 to 80 mg during the second course because of prolonged hematological toxicity. All the cases received at least one complete course of S-1 treatment (S-1 administration for 2 weeks) with radiotherapy. The completion rate of S-1 administration, as defined by the protocol, was 72%.
Adverse events
The adverse events observed during treatment are listed in Table 4. Grade 3 mucositis, neutropenia, leukocytopenia, and ALT and AST elevations were observed in 10 cases (20%), 6 cases (12%), 3 cases (6%) and 3 cases (6%), respectively. During CCRT, 18 of the 50 cases (36%) had difficulty eating with pain caused by mucositis and required nutritional support and the administration of S-1 using a gastric tube. With regard to the frequency and severity of adverse events, no significant difference was observed between patients with and those without a deteriorated Ccr. Fourteen patients had cardiovascular diseases, but the worsening of symptoms was not observed in any of the patients.
Table 4.
Adverse events (n = 50)
| Grade 1/2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Hematological events | |||
| Leukocytopenia | 15 (30%) | 3 (6%) | 0 |
| Neutropenia | 15 (30%) | 6 (12%) | 0 |
| Anemia | 13 (26%) | 0 | 0 |
| Thrombocytopenia | 3 (6%) | 0 | 0 |
| Non-hematological events | |||
| Mucositis | 24 (48%) | 10 (20%) | 0 |
| Elevation of ALT, AST | 4 (8%) | 3 (6%) | 0 |
| Dermatitis | 15 (30%) | 1 (2%) | 0 |
| Skin rash | 2 (4%) | 0 | 0 |
Response
In terms of CR rate according to T and N stagings, the rate decreased as the T or N staging advanced (Table 5). The CR rate in the primary site (42/50, 84%) was higher than that of metastatic lymph nodes in the neck (29/39, 74%).
Table 5.
Response and CR rate according to T and N stagings
| Response | ||||
|---|---|---|---|---|
| T stage | CR | PR | NC | CR rate (%) |
| T1 (n = 2) | 2 | 0 | 0 | 100 |
| T2 (n = 11) | 10 | 1 | 0 | 91 |
| T3 (n = 16) | 14 | 1 | 1 | 88 |
| T4 (n = 21) | 16 | 3 | 2 | 76 |
| Total (n = 50) | 42 | 5 | 2 | 84 |
| N stage | CR | PR | NC | CR rate (%) |
|---|---|---|---|---|
| N1 (n = 10) | 10 | 0 | 0 | 100 |
| N2b (n = 13) | 9 | 2 | 2 | 69 |
| N2c (n = 14) | 9 | 5 | 0 | 64 |
| N3 (n = 2) | 1 | 1 | 0 | 50 |
| Total (n = 39) | 29 | 8 | 2 | 74 |
Of the 15 patients with stage III tumors, a CR was seen in 14 (93%), while the CR rate in the stage IV patients (35 cases) was 54% (16/35) (Table 6). None of the patients experienced disease progression while receiving CCRT. After the completion of the chemoradiotherapy, 16 patients had PR and 4 had NC. Four patients underwent operations after receiving CCRT. Of the remaining patients, 5 had unresectable tumors and 11 had resectable tumors but could not undergo surgery because of their physical condition.
Table 6.
Response according to staging and recurrent cases in CR cases
| Stage | Response | Recurrent cases in CR cases | |||
|---|---|---|---|---|---|
| CR | PR | NC | CR rate (%) | ||
| III (n = 15) | 14 | 0 | 1 | 93 | 3/14 (21%) |
| IV (n = 35) | 16 | 16 | 3 | 54 | 9/16 (56%) |
| Total (n = 50) | 30 | 16 | 4 | 60 | 12/30 (40%) |
Of the 14 CR cases with stage III tumors, 3 had recurrences (21%); the recurrence rate was 56% (9 cases) among the 16 CR cases with stage IV tumors. Among these cases, six had unresectable recurrences including two cases showing lung metastases, three cases with resectable recurrences underwent salvage surgery, and the remaining three cases with resectable recurrences could not undergo surgery because of their physical condition.
Survival
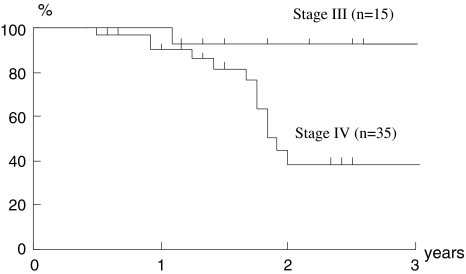
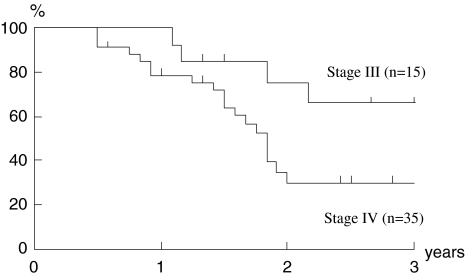
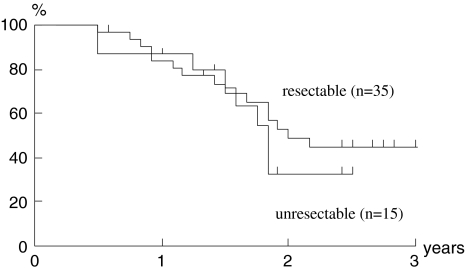
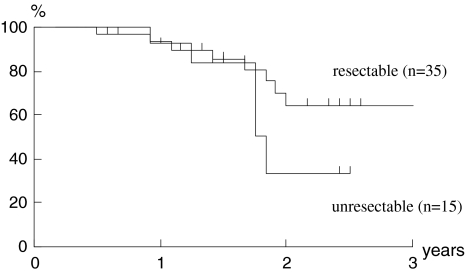
Although the number of cases was small and the follow-up period was short, a preliminary attempt to evaluate the outcome of the present study was made. The median follow-up period for the surviving patients was 26 months (range, 12–61 months). In Figs. 1 and 2, the 2-year disease-specific survival rate and the 2-year overall survival rate were 92 and 75% in the stage III group and 38 and 29% in the stage IV group, respectively. The two groups had significantly different 2-year disease-specific and overall survival rates (P < 0.05). In terms of resectability, the 2-year disease-specific survival rate and the 2-year overall survival rates of unresectable cases, i.e., cases showing stage IVB, were 33 and 32%, respectively. There were no significant differences compared to each survival rate of resectable ones (64 and 48%; Figs. 3, 4).
Fig. 1.
Disease-specific survival. Kaplan–Meier estimates of disease-specific survival in stage III and IV. Two-year disease-specific survival rates were 92% in stage III and 38% in stage IV. There was a significant difference between the two groups (P = 0.039)
Fig. 2.
Overall survival. Kaplan–Meier estimate of overall survival in stage III and IV. Two-year overall survival rates were 75% in stage III and 29% in stage IV. There was a significant difference between the two groups (P = 0.039)
Fig. 3.
Disease-specific survival. Kaplan–Meier estimates of disease-specific survival in resectable and unresectable cases. Two-year disease-specific survival rates were 48% in resectable and 32% in unresectable cases. There was no significant difference between the two groups (P = 0.598)
Fig. 4.
Disease-specific survival. Kaplan–Meier estimates of disease-specific survival in resectable and unresectable cases. Two-year disease-specific survival rates were 64% in resectable and 33% in unresectable cases. There was no significant difference between the two groups (P = 0.458)
Discussion
Recently, CCRT has been demonstrated to be highly effective in increasing survival in patients with locally advanced, especially unresectable, SCCHN in several studies [15–17]; however, few recent studies have examined CCRT or neoadjuvant chemotherapy for locally advanced SCCHN in patients with advanced age (over 75 years) or comorbidity [18, 19].
In terms of studies on CCRT for advanced SCCHN, a bolus administration of CDDP alone with or without the continuous infusion of 5-FU has been generally applied [20]; however, CDDP administration requires hydration, affecting heart function and inducing nephrotoxicity. Additionally, the continuous infusion of 5-FU at a high dose (800 or 1,000 mg/m2/day for 4 or 5 days) sometimes induces cardiovascular disorders [21]. For these reasons, the effective bolus administration of CDDP and/or the continuous infusion of 5-FU combined with radiotherapy is limited or prohibited in elderly patients with deteriorated renal function and in cases with comorbidity like cardiovascular dysfunction. In fact, a decrease in creatinine clearance with aging has been reported in patients with head and neck cancer in Japan [22].
With regard to the roles of chemotherapeutic agents combined with radiotherapy, a direct tumor-killing activity in addition to a radio-sensitizing activity is superior to a radio-sensitizing activity alone. Based on this concept, a multi-agent chemotherapy consisting of CDDP, 5-FU, methotrexate, and leucovorin or CDDP, 5-FU and docetaxel for advanced SCCHN cases with normal Ccr (>65 mL/min) and without comorbidity has been studied in our institutes [23–25].
S-1 (Taiho Pharmaceutical Co., Ltd, Tokyo, Japan) is a dihydropyrimidine dehydrogenase (DPD)-inhibitory fluoropyrimidine (DIF) that has produced the highest response rate among many oral anticancer agents used against unresectable advanced carcinomas in phase II studies [10]. S-1 is an oral anticancer agent comprising tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate, in a molar ratio of 1:0.4:1 [10–12]. The 5-chloro-2,4-dihydroxypyridine enhances the serum 5-FU concentration by the competitive inhibition of DPD, an enzyme responsible for 5-FU catabolism. In a phase II trial of advanced and recurrent SCCHN (59 eligible cases), S-1 showed a high response rate of 28.8%, with acceptable toxicities [13].
Generally, the dose of S-1 is determined according to the body surface area (BSA) as follows: in a patient with a BSA < 1.25 m2, 40 mg is administered twice a day (80 mg/day); in a patient with a BSA of >1.25 m2 but <1.5 m2, 50 mg is administered twice a day (100 mg/day); and in a patient with a BSA > 1.5 m2, 60 mg is administered twice a day (120 mg/day). In the present study, the dose of oral S-1 was reduced by one dose level (60 mg × 2/day to 50 mg × 2/day or 50 mg × 2/day to 40 mg × 2/day) because concurrent radiotherapy with S-1 at the doses used in the single agent treatment was thought to induce severe mucositis in the head and neck. The average single dose of S-1 per BSA was 29.5 mg/m2 (25.3–33.3 mg/m2) in the present study. The results of a pharmacokinetic study on S-1 showed that the plasma 5-FU concentration after S-1 administration at an average single dose of 35.9 mg/m2 twice a day was similar to that during the continuous intravenous infusion (CVI) of 5-FU at 300 mg/m2/day, and the range of the plasma 5-FU level after the administration of oral S-1 was narrower than that during the CVI of 5-FU, since the inclusion of a DPD inhibitor dampens the variation in the plasma 5-FU level [26]. The time-to-peak plasma concentration of 5-FU has been shown to reach a steady state 2–4 h after the administration of S-1. Based on this previous pharmacokinetic study, oral S-1 was administered 2 h before daily radiotherapy in the present study; theoretically, this timing should enable the plasma 5-FU concentration to peak and sensitize the tumor cells to radiation. Oral S-1 administration at a reduced dose was thought to allow a high Cmax and a greater area under the curve (AUC) of plasma 5-FU than the venous infusion of 5-FU at a dose of 250 mg/m2 during one course of chemotherapy with S-1 for 2 weeks [27]. Furthermore, there have been several reports regarding the sensitizing effect of S-1 on radiotherapy [28, 29]. Harada et al. reported that S-1 increases the in vivo radio-response of tumor xenografts derived from oral cancer cells, and that 5-FU has an ability to sensitize the in vitro radio-response of these cells by suppressing the activation of Akt/PKB, an important survival signal. Nakata et al. have shown that S-1 treatment enhances the response of 5-FU-resistant human colon carcinoma cells to radiotherapy through the down-regulation of thymidine synthase, an enzyme involved in tumor resistance to 5-FU and radiation.
In terms of the optimal schedule of CCRT with S-1 for oral squamous cell carcinoma, a schedule of 2 weeks of administration followed by 1 week of rest has been shown to cause fewer toxicities than a schedule of 4 weeks of administration followed by 2 weeks of rest, similar to our results in an adjuvant setting examining the use of S-1 for SCCHN [14, 30].
With regard to CCRT with S-1, Tsuji et al. reported that the recommended dose of S-1 was 80 mg/body/day (40 mg, twice a day) for cases with early glottic cancer (T2N0) [31]. The completion rate in the present study, as defined by the protocol, was 72% (36/50). Thirty-nine of the 50 cases (78%) received CCRT with two courses of S-1 administration, although dose reduction was needed in three cases. Considering the patients’ characteristics, i.e., cases with an advanced age and/or complications, CCRT with S-1 at the planned dose seems to be feasible. Since most cases with SCCHN have advanced diseases, 100 mg/body/day might be indispensable for advanced SCCHN cases with a high BSA.
Based on the above-mentioned pharmacokinetic results, oral S-1 administered twice a day at a reduced dose was thought to be capable of both direct tumor cell killing and radio-sensitization, similar to the CVI of 5-FU.
In the present study, mucositis was the most common adverse effect, i.e., Grade 3 mucositis occurred in 20% of the patients. This rate was higher than the results of a study on oral cancer in a younger patient group without comorbidity [30] and the phase I study on CCRT with S-1 for early glottic cancer [31]. Aging, comorbidity, and the radiation field might influence the severity of mucositis. The rate of Grade 3 mucositis in the present study seems lower compared to CCRT with the common bolus administration of CDDP [2, 4]. Some reasons are considered; first, S-1 is an oral agent resulting in a weaker radiosensitizing effect compared to CDDP. Second, in the present study nutrition support using a gastric tube was performed as soon as possible when the hyperemic mucosa was found during CCRT, because all the patients were elderly and/or had comorbidity. Similar to mucositis, neutropenia was also observed frequently, and Grade 3 neutropenia and leukopenia occurred in 12 and 6% of the patients, respectively. In an adjuvant setting study examining feasibility and safety, no patients with grade ≥3 neutropenia or mucositis were observed among cases receiving 2 weeks administration of S-1 followed by a 1-week rest [14]. The adverse events in the present study might have been induced by the combined treatment of radiotherapy with S-1. On the other hand, none of the patients in the present study experienced diarrhea, possibly because of the dose reduction.
CCRT with S-1 produced a high compete response rate (60%) in advanced cases with advanced age and/or comorbidity. Compared with a previous study examining CCRT with carboplatin (CBDCA) and UFT for SCCHN cases with a poor PS (2 and 3), the CR rate of CCRT with S-1 was similar to that of CCRT with CBDCA plus UFT (61%) [18]; however, the study on CCRT with CBDCA plus UFT included cases with nasopharyngeal carcinoma (9 out of 62 cases), which is sensitive to CCRT, and a smaller numbers of cases with stage IV disease (35 out of 62 cases) and with unresectable lesions (15 out of 62 cases, data not included in the published manuscript). It is difficult to compare CCRT with S-1 to that with CDDP in terms of effectiveness, toxicity, survival and quality of life, because 37 of 50 cases had comorbidity including apparent renal dysfunction. Furthermore 12 of the 13 elderly patients showed low Ccr rate with less than 75 mL/min. These patients are not able to renal toxicity induced by CDDP and massive hydration before and after CDDP administration. Compared to the previous reports regarding CCRT with CDDP in locally advanced SCCHN in not elderly patients without comorbidity [2, 4], the effectiveness and survival rates of CCRT with S-1 seem to be slightly worse.
In conclusion, the present regimen of concurrent chemoradiotherapy with S-1 for advanced SCCHN cases with an advanced age or with comorbidity is feasible and well-tolerated, suggesting this regimen to be beneficial. These results warrant further investigation in terms of their effect on patient outcome and function, and a phase II randomized controlled study examining CCRT with S-1 or weekly docetaxel has been started in locally advanced SCCHN patients with the same baseline patient characteristics.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Douple EB, Richmond RC, O’Hara JA, Coughlin CT (1985) Carboplatin as a potentiator of radiation therapy. Cancer Treat Rev 12(Suppl A):111–124 [DOI] [PubMed]
- 2.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098 [DOI] [PubMed]
- 3.Cohen EEW, Lingen MW, Vokes EE (2004) The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 22:1743–1752 [DOI] [PubMed]
- 4.Urba SG, Moon J, Shankar Giri PG, Adelstein DJ, Hanna E, Yoo GH, LeBlanc M, Ensley JF, Schuller DE (2005) Organ preservation for advanced respectable cancer of the base of tongue and hypopharynx: a southwest oncology group trial. J Clin Oncol 23:88–95 [DOI] [PubMed]
- 5.Fujii M, Tsukuda M, Satake B, Kubota A, Kida A, Kohno N, Okami K, Inuyama Y (2004) Phase I/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol 9(2):107–112 [DOI] [PubMed]
- 6.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM Jr, Haddad RI (2007) TAX 324 Study Group. Cisplatin, fluorouracil alone or with decetaxel in head, neck cancer. N Engl J Med 357(17):1705–1715 [DOI] [PubMed]
- 7.Lo TC, Wiley AJ, Ansfield FJ, Brandenburg JH, Davis HL Jr, Gollin FF, Johnson RO, Ramirez G, Vermund H (1976) Combined radiation therapy and 5-fluorouracil for advanced squamous cell carcinoma of the oral cavity and oropharynx: a randomized study. Am J Roentgenol 126:229–235 [DOI] [PubMed]
- 8.Byfield JE, Calabro-Jones P, Klisak I, Kulhanian F (1982) Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-fluorouracil or ftorafur and X-rays. Int J Radiat Oncol Biol Phys 8:1923–1933 [DOI] [PubMed]
- 9.Smalley S, Kimler B, Evans R (1991) 5-Fluorouracil modulation of radiosensitivity in cultured human carcinoma cells. Int J Radiat Oncol Biol Phys 20:207–211 [DOI] [PubMed]
- 10.Shirasaka T, Shimamoto Y, Ohshima H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potenciation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557 [DOI] [PubMed]
- 11.Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M (1996) Antitumor activity of 1M tegafur-0.4 M 5-chloro-2, 4-dihydoxypyridine-1M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606 [PubMed]
- 12.Schoffski P (2004) The modulated oral fluropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 15:85–106 [DOI] [PubMed]
- 13.Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B (2001) Late phase II study of S-1 in patients with advanced head and neck cancer. Gan To Kagaku Ryoho 28:1381–1390 [PubMed]
- 14.Tsukuda M, Kida A, Fujii M, Kono N, Yoshihara T, Hasegawa Y, Sugita M, Chemotherapy Study Group of Head, Neck Cancer (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889 [DOI] [PMC free article] [PubMed]
- 15.Jeremic B, Shibamoto Y, Stanisavljevis B, Milojevic L, Milicic B, Nikolic N (1997) Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol 43:29–37 [DOI] [PubMed]
- 16.Adelstein DJ, Li Y, Adams GL, Wagner H, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98 [DOI] [PubMed]
- 17.Bensadoun RJ, Benezery K, Dassonville O, Magne N, Poissonnet G, Ramaiolia Lemanski C, Bouldin S, Tortochaux J, Peyrade F, Nrasy PY, Chamorey E, Vallicioni J, Seng H, Alzieu C, Gery B, Chauvel P, Schneider M, Santini J, Demard F, Calais G (2006) French multicenter phase III randomized study testing concurrent twice-a day radiotherapy and cisplatin/5-fluorouracil chemotherapy (BiRCF) in unresectable pharyngeal carcinoma: results at 2 years (FNCLCC-GORTEC). Int J Radiat Oncol Biol Phys 64:983–999 [DOI] [PubMed]
- 18.Katori H, Tsukuda M, Taguchi T (2007) Concurrent chemoradiotherapy with carboplatin and uracil-f tegafur (UFT) for patients with poor performance status with locally advanced squamous cell carcinoma of the head and neck (SCCHN). Acta Otolaryngol 127:1099–1104 [DOI] [PubMed]
- 19.Koussis H, Scola A, Bergamo F, Tonello S, Basso U, Karahontzitis P, Chiarion-Sileni V, Pasetto L, Ruol A, Loreggian L, Lora O, Bottin R, Marioni G, Donach M, Jirillo A (2008) Neoadjuvant carboplatin and vinorelbine followed by chemoradiotherapy in locally advanced head and neck or oesophageal squamous cell carcinoma: a phase II study in elderly patients with poor performance status. Anticancer Res 28:1383–1388 [PubMed]
- 20.Seiwert TY, Cohen EEW (2005) State-of-the-art management of locally advanced head and neck cancer. Br J Cancer 92:1341–1348 [DOI] [PMC free article] [PubMed]
- 21.Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, Karabelis A, Tsavaris N (2008) Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 134:75–82 [DOI] [PubMed]
- 22.Nishimura G, Tsukuda M, Horiuchi C, Satake K, Yoshida T, Taguchi T, Nagao J, Kawakami M, Kondo N, Matsuda H, Mikami Y (2007) Decrease of creatinine clearance rate with aging in patients with head and neck cancer in Japan. Int J Clin Oncol 12:120–124 [DOI] [PubMed]
- 23.Katori H, Tsukuda M, Mochimatu I, Ishitoya J, Kawai S, Mikami Y, Matsuda H, Tanigaki Y, Horiuchi C, Ikeda Y, Taguchi T, Ono M, Yoshida T, Hirose S, Sakuma Y, Yamamoto K (2004) Phase 1 trial of concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). Br J Cancer 90:348–352 [DOI] [PMC free article] [PubMed]
- 24.Taguchi T, Tsukuda M, Mikami Y, Matsuda H, Horiuchi C, Yoshida T, Nishimura G, Ishitoya J, Katori H (2006) Concurrent chemoradiotherapy with cisplatin, 5-fluorouracil, methotrexate, and leucovorin in patients with advanced resectable squamous cell carcinoma of the larynx and hypopharynx. Acta Otolaryngol 126:408–413 [DOI] [PubMed]
- 25.Katori H, Tsukuda M, Taguchi T (2007) Analysis of efficacy and toxicity of chemotherapy with cisplatin, 5-fluorouracil, methotrexate and leucovorin (PFML) and radiotherapy in the treatment of locally advanced squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 59:789–794 [DOI] [PubMed]
- 26.Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Phamacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005 [PubMed]
- 27.Yamada Y, Hamaguchi T, Goto M, Muro K, Matsumura Y, Shimada Y, Shirao K, Nagayama S (2003) Plasma concentrations of 5-fluorouracil and F-β-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenese inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil. Br J Cancer 89:816–820 [DOI] [PMC free article] [PubMed]
- 28.Harada K, Kawaguchi S, Supriatno Kawashima Y, Yoshida H, Sato M (2005) S-1, an oral fluoropyrimidine anti-cancer agent, enhanced radiosensitivity in a human oral cancer cell line in vivo and in vitro: involvement possibility of inhibition of survival signal, Akt/PKB. Cancer Lett 226:161–168 [DOI] [PubMed]
- 29.Nakata E, Fukushima M, Takai Y, Nemoto K, Ogawa Y, Nomiya T, Nakamura Y, Milas L, Yamada S (2006) S-1, an oral fluoropyrimidine, enhances radiation response of DLD-1/FU human colon cancer xenografts resistant to 5-FU. Oncol Rep 16:465–471 [PubMed]
- 30.Harada K, Ferdous T, Yoshida H (2007) Investigation of optimal schedule of concurrent radiotherapy with S-1 for oral squamous cell carcinoma. Oncol Rep 18:1077–1083 [PubMed]
- 31.Tsuji H, Kiba T, Nagata M, Inoue T, Yukawa H, Yamashita T, Shimode Y, Murata H, Nagata K, Tomoda K (2006) A phase I study of concurrent chemoradiotherapy with S-1 for T2N0 glottic carcinoma. Oncology 71:369–373 [DOI] [PubMed]