Abstract
Objectives
The aim of this study was to determine the prevalence of Echinococcus multilocularis environmental contamination in an urban fringe—the Nopporo forest park of Sapporo city, Hokkaido, Japan. A secondary aim was to determine possible transmission risks areas by associating percentage occurrence of E. multilocularis-positive faeces with the different land-use classes.
Methods
Wild fox faeces collected from the environment were examined by intravital methods, such as the taeniid egg sucrose floatation technique, E. multilocularis coproantigen enzyme-linked immunosorbent analysis and DNA test of taeniid eggs by PCR. Geospatial maps produced by the Global Positioning System and Landsat data were analysed using geographic information system software to determine the association between percentage occurrences of E. multilocularis-positive fox faeces and land-use classes.
Results
Our findings showed high prevalence rates in both E. multilocularis egg and coproantigen-positive faeces (16 and 49%, respectively) in the investigated urban fringe forest park. Data revealed that percentage occurrence of E. multilocularis-positive fox faeces was associated with land-use classes, such as forest and open field (P < 0.05).
Conclusions
We conclude that Nopporo forest park in the urban fringe of Sapporo city, Hokkaido is a reservoir with a high prevalence of zoonotic infective agents for alveolar echinococcosis. Our findings suggest that interface habitats between forests or woodlands and open fields are indispensable for continued maintenance of the life-cycle of E. multilocularis and, as such, constitute high risk areas for echinococcosis transmission.
Keywords: Environmental contamination, Echinococcus eggs, Forest park, Fox faeces
Introduction
Alveolar echinococcosis, a fatal zoonotic disease caused by the parasite Echinococcus multilocularis, is one of the major threats to public health in Japan. To date, there has been a total of 424 reported cases that have been confirmed (surgical method) [1]. The status of most of these patients was considered to be severe and difficult to treat. Although the exact means of transmission is not clear, wild fox populations are widely recognised as the primary source of infective eggs, which are transmitted into the environment through wild fox faeces. Humans and intermediate host animals can become infected by ingesting E. multilocularis eggs present in food and/or water obtained from the contaminated environment.
A dramatic rise in the numbers of urban foxes during the past decades has been documented in Japan and Europe [2–5]. In fact, one study actually anticipated an urban cycle of E. multilocularis in Sapporo city [6]. This study recorded an increase in fox populations in urban and suburban areas of Hokkaido, especially protected forest parks and woodlands, and presumed that urban fringe forested areas to be suitable environments for maintaining the life-cycle of E. multilocularis. In Zurich, Switzerland, the probable causal factor behind the high prevalence of E. multilocularis in foxes in that city was the increased infection pressure among foxes in endemic surrounding areas [7, 8]. Deplazes et al. [3] used various factors to determine the degree of E. multilocularis contamination and found that the maximum infection risk areas were villages and urban fringes where rural and urban habitats intersect.
From a public health perspective, monitoring the infection status of wildlife definitive hosts is necessary as part of risk assessment programmes [9]. In the study reported here, we investigated the prevalence of environmental contamination by E. multilocularis in a forest park located in the urban fringe of Sapporo city by the intravital diagnosis of fox faeces. We predicted that forest parks and woodlands in urban fringes provide a pool of infective sources for alveolar echinococcosis that represents a significant threat to the highly populated urbanised neighbourhood.
Alternatively, land-use patterns are known to influence rodent population dynamics [10] and, therefore, have important roles in the distribution of E. multilocularis. For example, the land-use class grassland has been associated to high alveolar echinococcosis prevalence rates in humans [11, 12]; as such, grasslands are considered to be risk areas in endemic regions in Europe and China. In Hokkaido, however, the Bedford’s red-backed vole (Clethrionomys rufocanus bedfordiae) is the main prey of foxes [13] and the intermediate host of E. multilocularis rather than voles of the genus Microtus which is not present in Hokkaido Island. This difference is believed to affect the land-use pattern associated with transmission risk areas in northern Japan. Hence, it may be possible to determine risk areas of transmission to humans through the geospatial mapping of fox faecal locations.
The aim of this study was to determine the prevalence of E. multilocularis environmental contamination by an examination of fox faeces. We also determined possible transmission risk areas by associating the percentage occurrence of E. multilocularis-positive fox faeces with the different land-use classes.
Materials and methods
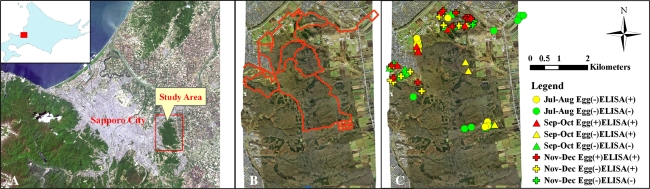
The study area was the Nopporo Forest Park (20.5 km2), which is located about 11–15 km from the centre of Sapporo, the capital city of Hokkaido, Japan (Fig. 1a). The presence of at least two fox families was confirmed in this park. Fox faeces were collected from July to December 2005 on a monthly basis (pooled bimonthly) on forest pathways, roads and agricultural fields located inside and at the peripheries of the park (Fig. 1b). A total of 131 fox faecal samples were collected during the 6-month study period. Prior to the analytical procedures, the faeces were frozen for >1 week at −80°C to render E. multilocularis eggs non-infective. Taeniid egg examination was conducted by the centrifugal flotation technique [14] using a sucrose solution with a specific gravity of 1.27. Sandwich enzyme-linked immunosorbent analysis (ELISA) using a monoclonal antibody (EmA9) was used for E. multilocularis coproantigen detection [15]. Morphologically, the Echinococcus egg is indistinguishable from those of other taeniid species [16]. Taeniid egg-positive faeces were subjected to copro-DNA examination. Briefly, 1 g of faeces was washed twice with 40 ml distilled water and the sediment resuspended with 30 ml sucrose solution (1.27 g). The supernatant was filtered through a 40-µm nylon mesh and the filtrate subjected to a second filtration through a 20-µm nylon mesh. The sediment was backwashed and centrifuged to obtain the taeniid eggs. DNA was extracted using the QIAamp DNA Mini kit tissue protocol (Qiagen, Valencia, CA). Specific amplification was carried out using the multiplex PCR procedure according to Trachsel et al. [17].
Fig. 1.
a Map of study area, Nopporo Forest Park, Hokkaido, Japan adjacent to Sapporo city [inset is Hokkaido map showing the location below (rectangle)]. bLines show the roads and pathways investigated. c Bi-monthly changes in the distribution of faecal contamination in the environment by foxes infected naturally by Echinococcus multilocularis
The exact location of fox faeces was recorded (± 50 cm) by a handheld Global Positioning System (GPS; Pathfinder Pro XR; Trimble, Sunnyvale, CA) using ArcPad software 6 (ESRI, Redlands, CA). Data were fed into a personal computer using the Microsoft Active Sync program. Bi-monthly distribution maps of fox faeces were created, and the locations of the faeces were determined using the ArcView 9 software package (ESRI, Redlands, CA). Spatial analysis of the location of fox faeces by land-use classes, such as ground, forest, building, open field, rice field and others, was performed using Landsat data and ArcMap 9 software.
Association between percentage occurrence of E. multilocularis-positive faeces and different land-use classes were analysed by the chi-square test. P < 0.05 was considered to be significant.
Results and discussion
The results of this study validate an earlier report that urban fringes offer suitable conditions for maintaining the life-cycle of E. multilocularis [6]. The prevalence rates of E. mutilocularis egg- and coproantigen-positive fox faeces significantly increased on a bi-monthly basis (Fig. 2), with means of 16 and 49%, respectively. Although no Echinococcus eggs were detected during the period July–August, a prevalence rate of 47% for coproantigen-positive faeces was registered during these months. Coproantigen can be detected during the pre-patent period of infection by this tapeworm before egg excretion is initiated. The prevalence rates of egg- and coproantigen-positive fox faeces in September and October were 14.54 and 45.45%, respectively; these increased remarkably in November and December, reaching 28.26 and 56.52%, respectively. The apparent low egg prevalence compared with the detection of the coproantigen can be accounted for by the intermittent egg excretion of this tapeworm even after maturation [18].
Fig. 2.
Prevalence rates of E. multilocularis egg- (circle) and EmA9 coproantigen (square)-positive faeces collected at Nopporo Forest Park at bimonthly intervals
The prevalence rates obtained in our study are comparable to the findings of other surveys carried out in endemic rural areas of Hokkaido [15, 19] and significantly higher than a reported survey performed in an urban setting [6]. Hofer et al. [8] reported that the percentage of E. multilocularis infection in the city of Zurich, Switzerland indicated a high prevalence in adjacent areas and that the observed decline in prevalence from recreational to urban areas was due to a lower predation on rodents by urban foxes. It has been reported that high environmental contamination with E. multilocularis eggs is a reflection of a high prevalence among definitive hosts, such as foxes [20].
The detection of E. multilocularis antigen levels in fox faecal samples collected in the field may provide a pragmatic methodology for the epidemiological surveillance of the infection status in wildlife hosts across large areas as well as provide an indication of the spatial distribution of infected faeces contaminating the environment [9]. The high prevalence rate determined in this study is quite alarming because the park is surrounded by public and residential buildings, schools, sports and camp site facilities. Moreover, it is frequently visited by local tourists, given its location adjacent to Sapporo city (population >1.8 million), the most populous city of Hokkaido.
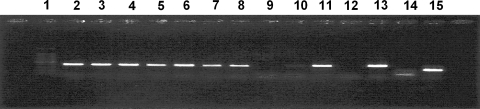
The copro-DNA test using multiplex PCR [17] identified 21 of 24 egg positive faeces as containing E. multilocularis eggs (Fig. 3). Three egg-positive faeces samples showed no reaction during the centrifugal flotation technique with the sucrose solution. None of the samples showed a reaction for Taenia taeniaeformis and E. granulosus; however, the PCR results suggested that an improved extraction technique for examining E. multilocularis copro-DNA should be explored. The absence of eggs despite high coproantigen prevalence rates in July–August may indicate a prepatent or early patent period of infection with a very low level of egg excretion among foxes in the study area. It has been reported that the coproantigen test has a higher likelihood of detecting infection in the late prepatent and early patent phases of infection [21], which is reflected in our results. It has also been reported that in a protected undisturbed population of foxes in Shiretoku National Park, Japan, infection with E. multilocularis ended in the summer, with a new infection being acquired in the early autumn [22]; consequently no eggs were detected during the summer period from the month of August onwards. Similarly, activities of foxes in Nopporo Forest Park were apparently less disturbed.
Fig. 3.
DNA amplification from ten taeniid-positive fox faeces using multiplex PCR. Lanes: 1 100-bp ladder, 2–11 samples, 12 negative control, 13E. multilocularis, 14E. granulosus, 15Taenia taeniaeformis
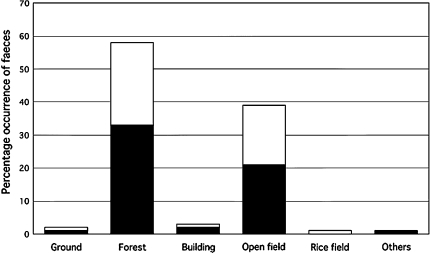
Geospatial mapping revealed that the locations of fox faeces varied bi-monthly (Fig. 1). Maps showed that fox faeces were mostly distributed at the northeastern part of the park, which happened to be adjacent to an agricultural university with animal and plant research stations. The distribution of fox faeces was also noted in the western area where vegetable gardens were cultivated by local residents. An analysis of land-use classes, however, revealed that the percentage occurrences of E. multilocularis-positive fox faeces were significantly higher in forests (33%) and open fields (21%), (P < 0.05; Fig. 4). Our data shows that fox faeces were detected more in forests and open fields than near animal stations, plant research plots, vegetable gardens and related agricultural fields. This finding is compatible to the biology of the red-backed voles in Hokkaido, the main prey of red foxes in this area [13], which generally favour both forests and open habitats [23, 24]. While forbs and grasses are the predominate food of Clethrionomys during the summer and bamboo grass during the winter, tree bark is also a major food item during the winter [25], thereby necessitating woodlands for their survival. Therefore, an interface between an open habitat and forest is required both in the home range of the red-backed vole and in the maintenance of the E. multilocularis life-cycle in Hokkaido. In contrast to Europe and China, the primary habitat of Microtus spp. is dependent mainly on grassland. Although infection of intermediate hosts does not require direct contact with definitive hosts, spatial interaction between fox home range and a landscape patch with susceptible small mammals is necessary [11]. Tsukada et al. [6] discovered that ten of the 11 fox den sites detected were located in parks and woodlands in the urban fringes of Sapporo city, a landscape also favourable to voles. In France, the completion of the life-cycle of E.multilocularis was found to require a spatial overlap between intermediate host species and definitive host faeces; this study also found that the densities of both Microtus sp. and fox faeces were higher in medium-height vegetation edges [26]. It has been suggested that at the regional scale, landscape affects human disease distribution through an interaction with small mammal communities and their population dynamics [12].
Fig. 4.
Percentage occurrence of fox faeces according to land-use classes as analysed using Landsat data. Filled barE. multilocularis-positive faeces, open barE. multilocularis-negative
Our data also confirmed that the distribution of fox faeces was associated with the foraging behaviour of the fox and may depend on the availability of food resources in an area [27]. A close relationship between voles and foxes through the parasite E. multilocularis in the field has been demonstrated [13].
The deposition of faeces by foxes in open field areas during the summer and fall, which are peak periods associated with soil-linked activities of humans in Hokkaido, increases the transmission risk of E. multilocularis eggs. Likewise, agricultural areas were cited as one of the highest factors influencing the urban cycle and infection pressure with E. multilocularis eggs [3]. In Europe, the prevalence rates of foxes have risen in many agriculture-dominated areas, particularly in France, The Netherlands, Germany, Austria, Slovakia and Poland; however, the life-cycle of E. multilocularis has also been established in many urban areas in which foxes are present at high population densities, thereby presenting an increased infection risk for large human populations [28, 29].
The results of our study confirm that the Nopporo forest park located in the urban fringe of Sapporo city functions as a reservoir, with a high prevalence of zoonotic infective agents for alveolar echinococcosis. We suggest that this interface habitat between forests or woodlands and open fields is indispensable for the intermediate host voles and for a continued maintenance of the life-cycle of E. multilocularis. These environments are contaminated with Echinococcus eggs and constitute high transmission risk areas for this fatal infection. Thus, the implementation of a control strategy would be a highly prudent step in these identified high-risk forest parks and woodlands in the urban fringes of Sapporo. A delay in implementing intervention programmes in combination with the increasing number of infected foxes foraging in close vicinity to humans in suburban and urban areas may possibly result in a serious public health hazard.
Contributor Information
Jose Trinipil G. Lagapa, Email: jtgl@rakuno.ac.jp
Masao Kamiya, Phone: +81-11-3884909, FAX: +81-11-3884909, Email: mkamiya@rakuno.ac.jp.
References
- 1.Oku Y, Kamiya M. Biology of echinococcus. In: Otsura M, Kamegai S, Hayashi S, editors. Progress of medical parasitology in Japan, vol. 8. Tokyo: Meguro Parasitological Museum; 2003. p. 293–318.
- 2.Hegglin D, Ward PI, Deplazes P. Anthelmintic baiting of foxes against urban contamination with Echinococcusmultilocularis. Emerg Infect Dis. 2003;9:1266–72. [DOI] [PMC free article] [PubMed]
- 3.Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanisation of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. [DOI] [PubMed]
- 4.Nonaka N, Kamiya M, Oku Y. Towards the control of Echinococcus multilocularis in the definitive host in Japan. Parasitol Int. 2006;55:S263–6. [DOI] [PubMed]
- 5.Kamiya M, Lagapa JT, Oku Y. Research on targeting sources of alveolar echinococcosis in Japan. Comp Immunol Microbiol Infect Dis. 2007;30:427–48. [DOI] [PubMed]
- 6.Tsukada H, Morishima Y, Nonaka N, Oku Y, Kamiya M. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology. 2000;120:423–8. [DOI] [PubMed]
- 7.Ewald D, Eckert J, Gottstein B, Straub M, Nigg H. Parasitological and serological studies on the prevalence of Echinococcus multilocularis Leuckart, 1863 in red foxes (Vulpes vulpes Linnaeus, 1758) in Switzerland. Rev Sci Tech. 1992;11:1057–61. [DOI] [PubMed]
- 8.Hofer S, Gloor S, Müller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicolaterrestris) in the city of Zürich, Switzerland. Parasitology. 2000;120:135–42. [DOI] [PubMed]
- 9.Pleydell DR, Raoul F, Tourneux F, Danson FM, Graham AJ, Craig PS, et al. Modelling the spatial distribution of Echinococcus multilocularis infection in foxes. Acta Trop. 2004;91:253–65. [DOI] [PubMed]
- 10.Delattre P, Pascal M, Damange JP. Towards a strategy for the epidemiological study of alveolar echinococcosis. Apropos of cases of infestation seen in Microtusarvalis P. in the Doubs (France) (in French). Ann Parasitol Hum Comp. 1985;60:389–405. [DOI] [PubMed]
- 11.Danson FM, Giraudoux P, Craig PS. Spatial modelling and ecology of Echinococcus multilocularis transmission in China. Parasitol Int. 2006;55[Suppl]:227–31. [DOI] [PubMed]
- 12.Giraudoux P, Craig PS, Delattre P, Bao G, Bartholomot B, Harraga S, et al. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology. 2003;127[Suppl]:S121–31. [PubMed]
- 13.Saitoh T, Takahashi K. The role of vole populations in prevalence of the parasite (Echinococcus multilocularis) in foxes. Res Popul Ecol. 1998;40:97–105. [DOI]
- 14.Ito S. Modified Wisconsin sugar centrifugal-flotation technique for nematode eggs in bovine faeces. J Jpn Vet Med Assoc. 1980;33:424–9.
- 15.Morishima Y, Tsukada H, Nonaka N, Oku Y, Kamiya M. Coproantigen survey for Echinococcus multilocularis prevalence of red foxes in Hokkaido, Japan. Parasitol Int. 1999;48:121–34. [DOI] [PubMed]
- 16.Thompson RCA. Biology and systematics of Echinococcus. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and hydatid disease. Wallingford: CAB International; 1995. p. 1–50.
- 17.Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–20. [DOI] [PubMed]
- 18.Kamiya M, Lagapa JT, Ganzorig S, Kobayashi F, Nonaka N, Oku Y. Echinococcus risk among domestic definitive hosts, Japan. Emerg Infect Dis. 2007;13:346–7. [DOI] [PMC free article] [PubMed]
- 19.Tsukada H, Hamazaki K, Ganzorig S, Iwaki T, Konno K, Lagapa JT, et al. Potential remedy against Echinococcus multilocularis in wild red foxes using baits with anthelmintic distributed around fox breeding dens in Hokkaido, Japan. Parasitology. 2002;125:119–29. [DOI] [PubMed]
- 20.Gottstein B, Saucy F, Deplazes P, Reichen J, Demierre G, Busato A, et al. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg Infect Dis. 2001;7:408–12. [DOI] [PMC free article] [PubMed]
- 21.Kapel CM, Torgerson PR, Thompson RC, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86. [DOI] [PubMed]
- 22.Nonaka N, Tsukada H, Abe N, Oku Y, Kamiya M. Monitoring of Echinococcus multilocularis infection in red foxes in Shiretoko, Japan, by coproantigen detection. Parasitology. 1998;117:193–200. [DOI] [PubMed]
- 23.Kaneko Y, Nakata K, Saitoh T, Stenseth NC, Bjornstad ON. The biology of the vole Clethrionomys rufocanus: a review. Res Popul Ecol. 1998;40:21–37. [DOI]
- 24.Ota K, Jameson EW. Ecological relationships and economic importance of Japanese Microtinae. Ecology. 1961;42:184–6. [DOI]
- 25.Ota K, editor. Study on wild murid rodents in Hokkaido (in Japanese). Sapporo: Hokkaido University Press; 1984.
- 26.Guislain MH, Raoul F, Poulle ML, Giraudoux P. Fox faeces and vole distribution on a local range: ecological data in a parasitological perspective for Echinococcusmultilocularis. Parasite. 2007;14:299–308. [DOI] [PubMed]
- 27.Macdonald D. The encyclopedia of mammals:1. London: George Allen and Unwin; 1984. p. 68–75.
- 28.Craig PS. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16:437–44. [DOI] [PubMed]
- 29.Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55:S187–91. [DOI] [PubMed]