Abstract
In addition to its role as a molecular chaperone, heat shock protein 72 (Hsp72) protects cells against a wide range of apoptosis inducing stresses. However, it is unclear if these two roles are functionally related or whether Hsp72 inhibits apoptosis by a mechanism independent of chaperone activity. The N-terminal adenosine triphosphatase domain, substrate-binding domain and the C-terminal EEVD regulatory motif of Hsp72 are all essential for chaperone activity. In this study, we show that Hsp72 mutants with a functional substrate-binding domain but lacking chaperone activity retain their ability to protect cells against apoptosis induced by heat and tumor necrosis factor alpha. In contrast, a deletion mutant lacking a functional substrate-binding domain has no protective capacity. The ability of the Hsp72 substrate-binding domain to inhibit apoptosis independent of the regulatory effects of the adenosine triphosphate-binding domain indicates that the inhibition of apoptosis may involve a stable binding interaction with a regulatory substrate rather than Hsp72 chaperone activity.
Keywords: Hsp72, Functional analysis, Apoptosis, Chaperone activity
Introduction
Heat shock protein 72 (Hsp72) protects mammalian cells from apoptosis induced by a variety of different stresses including heat, UVC radiation, ceramide, tumor necrosis factor alpha (TNFα), and some chemotoxic drugs (Ahn et al. 1999; Buzzard et al. 1998; Demidenko et al. 2006; Dressel et al. 2003; Gabai et al. 1997; Jaattela et al. 1992; Simon et al. 1995; Sliutz et al. 1996). As Hsp72 expression is induced in response to proteotoxic stresses such as heat, it may be important for preventing the excessive apoptosis of cells when organisms are exposed to extreme conditions. Hsp72 is expressed at high levels in many forms of cancer, where it is thought to enhance the viability of cancer cells and their resistance to apoptosis (Gabai et al. 2005; Nylandsted et al. 2000). The mechanisms by which Hsp72 regulates apoptosis remain controversial, but include reports of the protein suppressing stress-induced Jun N-terminal kinase (JNK) activation (Gabai et al. 1997; Mosser et al. 1997), inhibiting the translocation of the pro-apoptotic protein Bax to the surface of the mitochondria (Gotoh et al. 2004; Stankiewicz et al. 2005) and preventing the release of cathepsins from lysosomes (Nylandsted et al. 2004). While the multitude of different mechanisms have prompted the suggestion that Hsp72 regulates apoptosis simultaneously at multiple different points in the apoptotic pathway (Beere 2005), all studies to date have observed only single mechanisms operating in individual cell types and so have not confirmed this suggestion. The lack of reproducibility between the different cell models raises questions about the generality of these mechanisms and how they might all be integrated in a physiological context.
The question of integration is particularly important as Hsp72 belongs to a highly conserved family of molecular chaperones, collectively known as Hsp70, that participate in many different cellular functions (Agashe and Hartl 2000). As molecular chaperones, Hsp70 family members bind to exposed hydrophobic protein surfaces, inhibiting aggregation and promoting the refolding of denatured proteins (Kobayashi et al. 2000; Wang et al. 2005). This chaperone function is critical for the translation of proteins from ribosomes (Beckmann et al. 1990), the translocation of proteins through intracellular membranes (Kang et al. 1990) and is required for the remodeling of native protein structures such as clathrin-coated vesicles and steroid receptors (Chappell et al. 1986; Kosano et al. 1998). Hsp70 also has an important role in modulating protein homeostasis by targeting proteins for proteolytic degradation through the ubiquitin/proteasome and lysosomal systems (Dice 2007; Esser et al. 2004).
Despite the many different roles performed by Hsp70 proteins, all members of the family possess only two key domains: a C-terminal peptide-binding domain that preferentially binds to exposed hydrophobic peptide sequences that characterize denatured proteins, and an N-terminal adenosine triphosphatase (ATPase) domain that regulates the binding affinity of the peptide-binding domain (Palleros et al. 1991). When adenosine triphosphate (ATP) is bound at the nucleotide-binding site, the peptide-binding domain possesses only a weak affinity for hydrophobic peptides but the hydrolysis of ATP to ADP results in a conformational change and an increase in peptide-binding affinity. This cycle of substrate-binding and release, regulated by ATP hydrolysis and ADP/ATP exchange is known as the chaperone cycle (Mayer and Bukau 2005). The chaperone cycle is further regulated by the presence of co-chaperones, such as Hsp40, that stimulate or inhibit ATP hydrolysis and nucleotide exchange, as well as targeting Hsp70 molecules to specific organelles and substrates (Mayer and Bukau 2005; Young et al. 2003). The C-terminal EEVD motif conserved in all cytosolic Hsp70 family members is known to interact with co-chaperones (Brinker et al. 2002) as well as with the N-terminal ATP-binding domain and is essential for Hsp70 function (Freeman et al. 1995).
While the search for the anti-apoptotic targets of Hsp72 continues, there has been less debate about how the Hsp72 protein recognizes and interacts with these targets. Is Hsp72 chaperone activity required for the remodeling of an apoptotic signaling complex in the same way it is required for remodeling the progesterone receptor (Kosano et al. 1998)? Mosser et al. (2000) reported that chaperone activity was required for the inhibition of apoptosis, while other authors have concluded that only the substrate-binding domain is required for protection (Li et al. 1992; Sun et al. 2006; Volloch et al. 1999). In this study, we expressed several different mutant forms of Hsp72 in cells and compared the ability of these proteins to inhibit apoptosis and promote the refolding of heat labile luciferase. We found that Hsp72 mutants with a functional substrate-binding domain but lacking ATPase activity were still capable of protecting cells from apoptosis induced by both proteotoxic and non-proteotoxic stresses, despite lacking chaperone function as measured by a luciferase re-activation assay.
Materials and methods
Construction of plasmids
Wild-type and mutant Hsp72 cell lines were generated using the retroviral plasmid MSCV-IRES-EGFP (kind gift from Dr. S. Jane, Royal Melbourne Hospital, Australia). This vector expresses the transgene and enhanced green fluorescent protein (EGFP) as a single transcript separated by an internal ribosomal entry site, upstream of the EGFP gene. Cells expressing equivalent levels of messenger RNA can be selected based on EGFP fluorescence. The coding region of human wild-type Hsp72 derived from pH2.3 (kind gift from Dr. C. Hunt, Washington University, MO, USA), the ATPase inactivating point mutant K71E (kind gift from Dr. L. Greene, NIH, USA; Rajapandi et al. 1998) and the C-terminal deletion mutant containing amino acids 1-611 (kind gift from Dr. R. Morimoto, North Western University, IL, USA; Freeman et al. 1995) were all cloned into the MSCV-IRES-EGFP plasmid. The ATPase domain deletion mutant containing amino acids 381-640, was amplified by PCR from human 293 cell cDNA using the following oligonucleotide primers: 5′-AACGGAATTCCTGACCATGGGGGACAAGTCCGAA-3′ and 5′-ACGGAATTCTACCTAATCTACCTCCTCAATGGTGG-3′. The amplified product was ligated into pGEM-T Easy plasmid (Promega, Madison, MI, USA) for sequence verification before being digested with EcoRI and ligated into the MSCV-IRES-EGFP plasmid. The boundary between the ATPase domain of Hsp72 and the C-terminal substrate-binding domain lies between amino acid residues 381 to 386, as deduced from structural studies (Morshauser et al. 1999; Sondermann et al. 2001; Zhu et al. 1996). The sequence for the 381-640 mutant was chosen to take advantage of a natural methionine for the initiation codon. All four proteins were expressed without affinity tags to avoid any possible fusion protein artefacts (Boice and Hightower 1997).
The MSCV-luc plasmid, used in the luciferase assay, was prepared by cloning the luciferase gene from the pGL3-Control vector (Promega) into MSCV vector.
Cell lines and stable transfections
The tumorigenic mouse fibroblast L929 cell line and the mouse mammary carcinoma 4T1.2 cell line (Lelekakis et al. 1999) were maintained in Dulbecco’s Modified Eagle’s Medium and alpha minimal essential medium, respectively, supplemented with 10% fetal calf serum and 1% penicillin–streptomycin. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. A single cell clone, L929.3, with growth properties similar to the parental line, was generated prior to introduction of modified Hsp72 genes. L929.3 cells constitutively expressing wild-type or mutant Hsp72 were generated by infection with the relevant retrovirus. Populations of cells expressing the gene of interest were generated by florescence-activated cell sorting (FACS; Becton Dickinson FACS Star, San Jose, CA, USA) based on EGFP expression. To minimize fluorescence fluctuations, FITC-conjugated beads were used as a reference for standardizing the fluorescence level when sorting the base vector (BV) and Hsp72 expressing cells. Expression of wild-type and mutant Hsp72 was confirmed by western blotting.
Treatment of cells
All experiments were performed on ∼50–70% confluent cell cultures. Immediately prior to each experiment, the medium was replaced with fresh, pH- and temperature-equilibrated medium. Cells were heated by immersion in a circulating water bath calibrated to the required temperature (42–44°C) ± 0.1°C for 15–60 min or treated with mouse TNFα (Sigma, St Louis, MO, USA) and 100 ng/ml actinomycin D (Sigma). Thermotolerance was induced by exposure of cells to a heat treatment of 43°C for 15 min followed by 6 h at 37°C.
Apoptosis assays
Cells were permeabilized with 0.1% Triton X-100 prior to being stained with propidium iodide (PI; Sigma) to reveal nuclear structure. Nuclear condensation was scored by fluorescence microscopy, as described previously (Buzzard et al. 1998). At least 200 cells were counted in each experiment, and the data were expressed as the mean ± SE of at least three independent experiments.
Long-term survival assay
Long-term survival of L929.3 cells was assessed by clonogenic assay. Briefly, dilutions containing known numbers of cells (1 to 10,000) were placed in Petri dishes at 37°C for 10–12 days. Colonies (>50 cells) derived from cells that survived the stress were stained with 0.1% crystal violet in 50% methanol and counted. The fraction of surviving cells was calculated as a ratio of the surviving fraction in untreated control samples.
Sulphorhodamine B proliferation assay
Two hundred to 500 cells were plated in 96-well plates, allowed to grow at 37°C, and harvested at specified times by fixing with 10% trichloroacetic acid (TCA). The wells were then stained with 0.4% sulphorhodamine B (Sigma) dissolved in 1% acetic acid. After extensive washing, the immobilized dye was dissolved in 10 mM Tris and the absorbance measured at 550 nm. The absorbance reflects the quantity of cellular proteins fixed by TCA and is therefore an indirect measure of cell number.
Western analysis
Samples containing equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. The following antibodies were used: Hsp27 mouse monoclonal antibody (clone G3.1, SPA-800, Stressgen Biotechnologies, Victoria, BC, Canada); Hsp60 rabbit polyclonal antibody (kind gift from Prof. N. Hoogenraad, La Trobe University, Australia); Hsp72 mouse monoclonal antibody specific for human Hsp72 (clone N15, kind gift from Dr. W. Welch, UCSF, USA); Hsp72 mouse monoclonal antibody that recognizes both human and mouse Hsp72 (clone C92, SPA-810, Stressgen); Hsp72/Hsp73 goat polyclonal antibody that recognizes both human and mouse Hsp72 and Hsp73 (clone K20, sc-1060, Santa Cruz Biotechnology, CA); Hsp90 mouse monoclonal antibody (clone AC88, SPA-830, Stressgen); EGFP rabbit polyclonal antibody (915-059, Stressgen); and β-tubulin antibody (sc-9104, Santa Cruz). HRP-conjugated secondary antibodies were detected by enhanced chemiluminescence (Lumilight, Roche Diagnostics, Sydney, NSW, Australia).
Luciferase assays
L929.3 or 4T1.2 cells were transiently transfected with the MSCV-luc plasmid by electroporation. After 24 h incubation, the cells were placed in fresh medium before heat treatment. Cycloheximide (CHX; Sigma) at 10 μg/ml was added before and/or after heat treatment to prevent de novo synthesis of luciferase and to ensure that only refolding of denatured pre-existing luciferase was observed. Cells were harvested and luciferase activity was measured using the Luciferase Assay System (Promega) according to the manufacturer’s instructions on a POLARstar luminometer (BMG Labtechnologies, Offenburg, Germany).
Results
Stable expression of equivalent levels of wild-type and mutant Hsp72 proteins
L929.3 cells were infected with MSCV-IRES-EGFP vector carrying either the wild-type or mutant Hsp72 genes (Fig. 1a). Bulk populations of cells expressing Hsp72 were selected according to their EGFP fluorescence (Fig. 1b). Base vector control cells were established by infecting L929.3 cells with the MSCV-IRES-EGFP base vector. Sorted cells were expanded and maintained as stable populations expressing a specific level of EGFP that was directly related to the expression of the co-expressed Hsp72 (data not shown). Expression of all Hsp72 variants was confirmed by western blotting (Fig. 1c). N15, an antibody that is specific for human Hsp72, detected the full length Hsp72, 381-640, and K71E but failed to detect the 1-611 deletion mutant lacking the extreme C-terminus. The lower staining intensity of the K71E mutant compared to full length Hsp72 and the 381-640 mutant suggests that K71E was present at lower levels than the other two proteins despite the cells expressing equivalent levels of EGFP (Fig. 1b). This may be due to a higher rate of protein turnover. To confirm that only human Hsp72 was being expressed in these mouse cells, we immunostained a matching membrane with C92, which detects both human and mouse Hsp72. The C92 antibody confirmed that L929.3 cells do not express Hsp72 in their resting state (lane marked BV) and that the expression of the human Hsp72 constructs, the full length Hsp72, 381-640, and K71E were consistent to what was detected using the N15 antibody. The C92 antibody also failed to detect the 1-611 mutant. Truncation at the C-terminal of 1-611 might have prevented the presentation of its C-terminal epitope to N15 and C92. Nevertheless, expression of 1-611 was confirmed with the polyclonal antibody K20 that recognizes both Hsp72 and Hsp73 (Fig. 1d).
Fig. 1.
Generation of wild-type and mutant Hsp72 expressing L929.3 cells with equivalent protein levels. a Schematic representation of Hsp72 and the mutant constructs. b Base vector MSCV-IRES-EGFP (BV), wild-type (WT), and mutant Hsp72 expressing cells were sorted with a set gate for similar EGFP profiles reflecting similar copy numbers of the mutant proteins. c, d EGFP sorted L929.3 cells were immunoblotted with the antibodies, C92, N15 and K20. β-Tubulin was used as a loading control for all western blots. A representative sample blot is shown. Antibody specificity: N15: human Hsp72, C92: human/mouse Hsp72, K20: human/mouse Hsp72/73
Expression of mutant Hsp72 proteins lacking ATPase activity reduces cell proliferation but does not induce a stress response
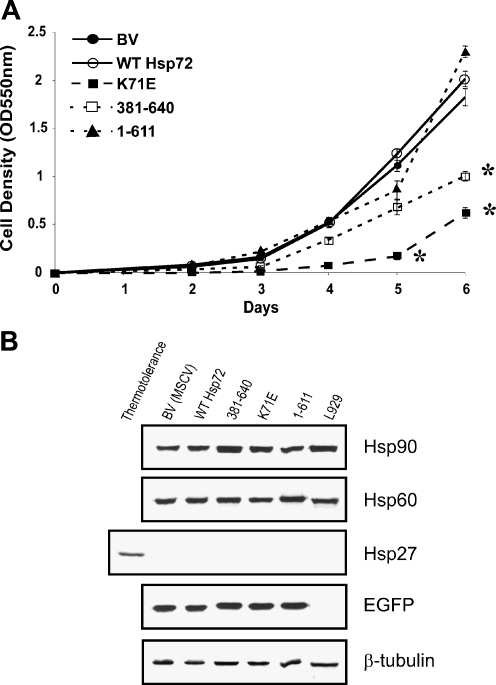
The rate of proliferation of BV, wild-type Hsp72, and 1-611 expressing cells was similar, while the 381-640 and K71E expressing cells grew more slowly over 6 days (50% and 37% of control rate, respectively; Fig. 2a). Since the 381-640 construct has no ATPase domain and K71E has a defective ATPase domain, the slower growth rate is consistent with a lack of ATPase activity.
Fig. 2.
Effect of wild-type and mutant Hsp72 proteins on cell proliferation and on the expression of other stress proteins. a The growth rate of each line was measured using the sulphorhodamine B assay. The mean ± standard error from at least three independent experiments is shown. Statistical analysis by Student’s t test; *p < 0.05. b EGFP sorted L929.3 cells expressing various Hsp72 proteins were harvested. Samples containing equal protein were immunoblotted with a panel of antibodies to detect Hsp90, Hsp60, Hsp27, and EGFP. β-Tubulin was used as loading control. A lane containing protein from thermotolerant L929.3 cells was added to demonstrate that the Hsp27 antibody was functional
The identical staining patterns of N15 (human Hsp72) and C92 (human and mouse Hsp72) immunoblots demonstrated that no mouse Hsp72 was induced in L929.3 cells as a result of the expression of the various human Hsp72 wild-type and mutant proteins (Fig. 1c). Western blotting confirmed that the expression of other Hsps (Hsp90, Hsp60, and Hsp27) was not altered by the presence of the different constructs (Fig. 2b). Hsp27 could be detected only in thermotolerant cells. EGFP expression measured by western blotting analysis confirmed that cells with similar levels of EGFP fluorescence had been selected by FACS sorting (Fig. 2b). Thus, the expression of wild-type and mutant human Hsp72 proteins did not induce a stress response in the L929.3 cells.
Constitutive expression of wild-type Hsp72, mutants K71E, and 381-640, but not 1-611, inhibits both heat- and TNFα-induced apoptosis in L929.3 cells
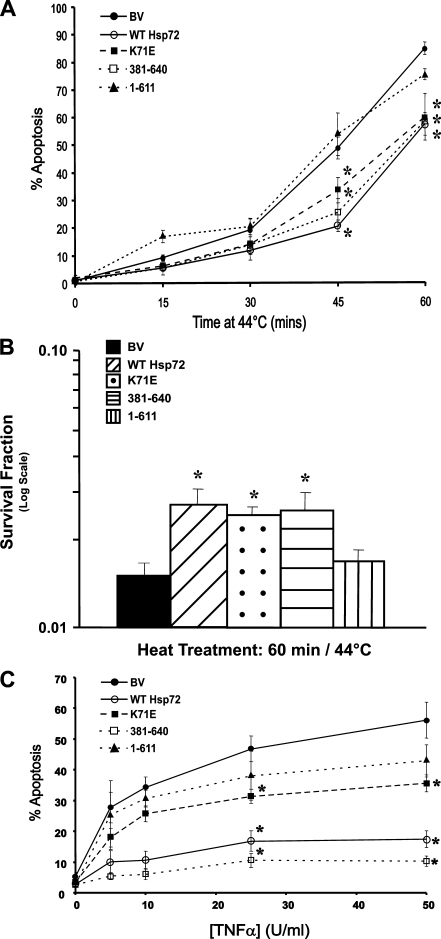
The expression of human Hsp72 provided L929.3 cells with substantial protection against heat-induced apoptosis (Fig. 3a). The K71E and 381-640 proteins, both lacking a functional ATPase domain, provided a similar degree of protection, while cells expressing the 1-611 protein displayed an equivalent rate of apoptosis to the BV cells. This pattern of protection was not restricted to the induction of apoptosis within the 22 h after heating but was consistent with the ability of wild-type, K71E, and 381-640 proteins to provide a long-term survival advantage to heated cells, as measured by a colony forming assay after heating at 44°C for 60 min (Fig. 3b). Thus, the ability of constitutively expressed Hsp72 to protect cells from heat-induced cell death appears to require the C-terminal substrate-binding domain but is not dependent on ATPase activity.
Fig. 3.
Expression of wild-type Hsp72, K71E, or 381-640, but not 1-611, inhibits heat- and TNFα-induced apoptosis in L929.3 cells. a Induction of apoptosis as assessed by apoptotic morphology following PI staining 22 h after exposure to heat at 44°C. Data are expressed as mean ± standard error from at least three independent experiments. b Long-term survival after heat shock at 44°C for 60 min. c Apoptosis induction 22 h following addition of various doses of TNFα in the presence of 100 ng/ml of actinomycin D (Act D), as scored by nuclear morphology following PI staining. Data are expressed as mean ± standard error from at least three independent experiments. Statistical analysis by Student’s t test; *p < 0.05
Heat induces extensive protein denaturation in cells and stimulates the expression of HSPs required for protein repair. Thus, it is unclear if the ability of the substrate-binding domain of Hsp72 to protect cells from heat-induced cell death is due to its ability to directly inhibit apoptosis or whether it is related to the role of Hsp72 in protein repair. To address this, L929.3 cells were treated with 5 to 50 U/ml of TNFα and 100 ng/ml of actinomycin D (Act D) for 22 h prior to scoring for apoptotic nuclear morphology (Fig. 3c). TNFα acts on the extrinsic apoptotic pathway and does not cause any protein denaturation, therefore precluding any role for Hsp72 in protein repair. The 381-640 mutant was as effective as wild-type Hsp72 in protecting L929.3 cells from TNFα, with less, but still significant protection conferred by the K71E construct. The 1-611 construct resulted in a similar sensitivity to TNFα as the base vector. Long-term survival could not be assessed since the addition of Act D to block pro-survival pathways downstream of TNF-R1 ultimately kills the cells by blocking transcription. Hence, as for heat, the ATPase activity of Hsp72 is not required for protection of cells from TNFα.
Protection of luciferase activity during heat stress
L929.3 cells expressing the various Hsp72 constructs were exposed to heat at 42°C or 43°C for 20 min, 24 h after transfection with a luciferase expression vector. Residual luciferase activity was measured immediately after heat exposure. No protection of luciferase activity was observed in either the wild-type Hsp72 protein or any of the Hsp72 mutants (Fig. 4a). The level of expression of wild-type human Hsp72 in L929.3 cells is shown in Fig. 4b. To determine if a more effective protection of luciferase activity was possible, thermotolerance was induced in parental L929.3 cells 24 h after transfection of the luciferase vector. The 43°C 15 min heat treatment used to induce thermotolerance resulted in inhibition of more than 90% of the luciferase activity present, but new luciferase protein expressed during the 6-h recovery after heating resulted in a partial recovery of activity. This lower level of luciferase activity was used as the base level for the thermotolerant cells. In the thermotolerant cells, luciferase activity was significantly protected from denaturation after heating at both 42°C and 43°C (Fig. 4a). This improved protection compared to the transgenic cells may be due to the higher level of Hsp72 induced in thermotolerant cells compared to those expressing human Hsp72 (Fig. 4b) and due to the expression of co-chaperones. However, Nollen et al. (1999) reported that thermotolerant cells were more resistant to heat than cells expressing identical levels of Hsp72.
Fig. 4.
The effect of wild-type and mutant Hsp72 on the denaturation of luciferase after heat shock. a L929.3 base vector control (BV), wild-type and mutant (K71E, 1-611, 381-640) Hsp72 expressing cells and thermotolerant BV cells were transiently transfected with 5 μg of MSCV-IRES-DsRed luciferase plasmid. After 24 h, cells were treated with heat shock at 42°C or 43°C for 20 min, immediately harvested and assayed for luciferase activity. Luciferase activity as percent of that in non-stressed cells is shown. Data are expressed as mean ± standard error from at least three independent experiments. b Expression of mouse Hsp72 in thermotolerant L929.3 cells, compared to the constitutive expression of exogenous wild-type human Hsp72. c Luciferase activity in 4T1.2 BV and wild-type Hsp72 expressing clones. d Expression of human Hsp72 in two clones of 4T1.2 cell lines. Statistical analysis by Student’s t test; *p < 0.05
While Hsp72 failed to protect luciferase activity from heat denaturation in L929.3 cells, when human Hsp72 was expressed in 4T1.2 breast carcinoma cells, a modest protection of luciferase activity was observed after heating at 42°C but not at 43°C (Fig. 4c). The lack of protection at 43°C is possibly due to the increased damage to luciferase compared to that seen at 42°C (32% residual activity after 42°C compared to 13% after 43°C) and the inadequate level of protective chaperones present in the cells. The level of expression of human Hsp72 in the 4T1.2 cells is shown in Fig. 4d. This difference in the ability of Hsp72 to protect luciferase from heat denaturation in L929.3 cells, in 4T1.2 cells, and in thermotolerant L929.3 cells suggests that the chaperone function of Hsp72 is highly dependent on the internal cell environment and the presence of co-chaperone proteins. To explore this further, we compared the levels of co-chaperones (Hsp90, Hsp73, and Hsp40) by western analysis, but did not detect marked differences in the total levels of these proteins between the 4T1.2 and L929.3 cell lines (data not shown). Hence, the difference in the capacity of human Hsp72 to protect luciferase in 4T1.2 and L929.3 cells cannot be explained simply by increased expression of co-chaperones in the 4T1.2 cells.
The expression of wild-type Hsp72, but not mutant constructs, restores luciferase activity after heat stress
Since minimal protection of luciferase activity from direct heat inactivation was observed, the luciferase assay was modified to investigate whether Hsp72 could assist in the recovery of luciferase activity after denaturation. Cells were transfected with the luciferase vector and heated as described previously, but after heat, were allowed to recover for varying times at 37°C in the presence of cycloheximide. CHX was added to ensure that the measured luciferase activity was derived from the reactivation of pre-existing protein rather than expression of new luciferase protein. Addition of 10 μg/ml of CHX before and/or after heat treatment was equally effective in blocking new protein synthesis, but treatment with CHX before heat strongly protected luciferase from heat damage (data not shown). This is due to the protein synthesis-independent thermotolerant state induced by CHX, as reported previously (Lee and Dewey 1987). Therefore, for these experiments, CHX was added immediately after heat exposure, resulting in inhibition of new protein synthesis (including heat-induced expression of endogenous HSP) without inducing thermotolerance.
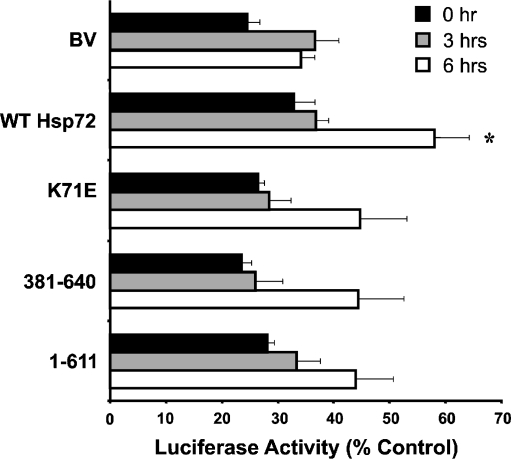
L929.3 cells expressing the various Hsp72 constructs and transfected with the luciferase vector were subjected to heat shock at 42°C for 20 min, treated with CHX, and allowed to recover for the specified time at 37°C. After 3 or 6 h, cells were harvested, lysed, and frozen at −70°C. These frozen extracts were later thawed and assayed together for luciferase activity. While there was no significant difference in luciferase activity 3 h after heating, the cells expressing wild-type Hsp72 showed a significantly improved recovery of activity after 6 h (Fig. 5). In comparison, the cells expressing mutant Hsp72 proteins possessed an increased level of luciferase activity compared to the BV control, but this difference was not statistically significant. Thus, the expression of wild-type Hsp72, but not mutant Hsp72 proteins, confers a significant increase in chaperone activity in L929.3 cells. It would have been interesting to assess luciferase refolding at longer time points, but the toxicity of CHX after 8–9 h did not allow us to assess these longer recovery times.
Fig. 5.
Expression of wild-type but not mutant Hsp72 enhances recovery of luciferase activity. L929.3 BV, wild-type Hsp72, and mutant (K71E, 381-640, or 1-611) cells were exposed to mild heat shock at 42°C for 20 min followed by recovery at 37°C for 0, 3, or 6 h in the presence of 10 μg/ml of cycloheximide. Samples were harvested and assayed for luciferase activity, which was expressed as percent of that in non-heated cells. Data are expressed as mean ± standard error from three independent experiments. Statistical analysis by Student’s t test; *p < 0.05
Discussion
The mechanism by which Hsp72 inhibits stress-induced apoptosis remains clouded by controversy. One study reported that the anti-apoptotic function of Hsp72 is dependent on chaperone activity, as both the N-terminal ATP-binding domain and the C-terminal substrate-binding domain of the protein are required to inhibit apoptosis induced by heat (Mosser et al. 2000). However, other similar studies have reported that Hsp72 chaperone activity is not required and that the C-terminal substrate-binding domain is sufficient to protect cells from stress. For example, (Li et al. (1995) observed that the C-terminal fragment of Hsp72 was capable of protecting Rat-1 cells from heat-induced cell death, even though it failed to enhance the repair of transcription and translation. This study neatly illustrates that the inhibition of cell death does not always require the chaperone repair function of Hsp72. While Li et al. measured cell death rather than apoptosis, other studies have reported that the substrate-binding domain is sufficient to inhibit apoptosis (Sun et al. 2006; Volloch et al. 1999). In the absence of any confirmed anti-apoptotic Hsp72-binding targets, the mechanism of how Hsp72 inhibits apoptosis may provide vital clues to how this protein protects cells from stress.
To clarify whether or not Hsp72 chaperone activity is required for the inhibition of stress-induced apoptosis, we measured both stress-induced apoptosis and chaperone activity in L929 cells constitutively expressing different Hsp72 constructs. These cell lines expressed either wild-type Hsp72 or mutants lacking ATPase or substrate-binding activity. To test the requirement for the ATPase domain and/or ATPase activity for the inhibition of apoptosis, we employed both the 381-640 deletion mutant, lacking the ATPase domain (Freeman et al. 1995), and the K71E point mutant that retains the ATPase domain but lacks ATPase activity (Rajapandi et al. 1998). We also expressed the 1-611 C-terminal deletion mutant that retains a portion of the substrate-binding domain but lacks the ability to bind protein substrates (Freeman et al. 1995).
Our results confirmed that the Hsp72 substrate-binding domain is sufficient to inhibit heat-induced apoptosis in L929.3 cells as described previously in Rat-1 fibroblasts (Volloch et al. 1999). Both the 381-640 Δ-ATPase mutant and the K71E point mutant protected cells in an equivalent fashion to the full length protein, while cells expressing the 1-611 fragment, lacking an active substrate-binding domain, apoptosed at an equivalent rate to base vector control cells. We also confirmed that the Hsp72 substrate-binding domain was capable of conferring a long-term survival advantage to heated cells and not just delay the onset of cell death. The 381-640 Δ-ATPase proved as efficient as wild-type Hsp72 in blocking extrinsic apoptotic signaling through TNFα. The K71E point mutant was also effective, but marginally less so, possibly due to the lower levels of the K71E protein present. In comparison, the 1-611 deletion mutant again failed to provide any protection compared to the base vector control cells. The inhibition of apoptosis is clearly linked to the presence of a functional substrate-binding domain as the 381-640 and K71E Hsp72 mutants are both capable of binding protein substrates, while the 1-611 Hsp72 fragment, that fails to inhibit apoptosis, lacks this capacity (Freeman et al. 1995; Rajapandi et al. 1998).
Thermally labile luciferase was used to determine the ability of wild-type and mutant Hsp72 to protect and/or repair proteins damaged by thermal denaturation in the same L929.3 cells used to assay apoptosis. Only the wild-type Hsp72 was capable of promoting a significant increase in the repair of thermally denatured luciferase, compared to the base vector control cells. All three of the mutant Hsp72 proteins did appear to provide some improvement in the repair of denatured luciferase, but this was statistically insignificant when compared to the control cells. This small increase in the rate of luciferase refolding is likely to be non-specific in nature as the three proteins contain no functional domains in common (Minton et al. 1982). Unlike previous studies (Freeman et al. 1995; Frydman et al. 1994; Herbst et al. 1997; Schumacher et al. 1994), Hsp72 expressed in L929.3 cells was not capable of protecting luciferase from thermal denaturation. This failure appeared to be specific to the L929.3 cells as Hsp72 did offer a degree of thermal protection to luciferase when expressed in 4T1.2 cells. Thermotolerant L929 cells that express endogenous Hsp72, showed significant protection against luciferase denaturation. The increased protection of luciferase activity in thermotolerant cells compared to cells expressing transgenic Hsp72 has already been noted (Nollen et al. 1999) and is probably related to the presence of additional chaperone and co-chaperone proteins in thermotolerant cells (Craig 1985).
In agreement with studies by Li et al. and Volloch et al., our results clearly show that Hsp72 protects cells from heat-induced apoptosis and heat-induced protein damage by distinctly different mechanisms (Li et al. 1995; Volloch et al. 1999). While the repair of heat-denatured luciferase required the chaperone activity of wild-type Hsp72, the substrate-binding domain of Hsp72 was sufficient to inhibit heat-induced apoptosis. Paradoxically, the survival of heated cells does not appear to be dependent on protein repair, despite heat causing extensive protein denaturation that might be expected to have a negative impact on cell survival (Lepock et al. 1993). The distinction between protein repair and the regulation of apoptosis is further highlighted by the ability of both the substrate-binding domain and wild-type Hsp72 to inhibit TNFα-induced apoptosis. TNFα induces apoptosis by activating the extrinsic apoptotic signaling pathway and does not cause any protein damage that might require repair by protein chaperones (Steel et al. 2004).
While we and others have observed that the Hsp72 substrate-binding domain inhibits apoptosis, others have shown that chaperone activity is required to regulate apoptotic signaling (Gotoh et al. 2004; Mosser et al. 2000; Ruchalski et al. 2006). In each of these examples, Hsp72 was expressed under control of the inducible tetracycline promoter, while those studies that reported protection independent of chaperone activity used constitutive Hsp72 expression systems (Li et al. 1992; Sun et al. 2006; Volloch et al. 1999). Differences in behavior between constitutively and inducibly expressed Hsp72 have been noted previously but never adequately explained. In the only comparative study of inducible and constitutive Hsp72 expression, Mosser and co-workers reported that inducible Hsp72 inhibited apoptosis by suppressing JNK signaling while constitutively expressed Hsp72-inhibited apoptosis by a JNK-independent mechanism (Mosser et al. 1997). It appears that the requirement of Hsp72 chaperone activity for the inhibition of stress-induced apoptosis also varies depending on the mode of Hsp72 expression. The existence of chaperone-dependent and -independent regulation of stress-induced apoptosis suggests that Hsp72 may be functioning by at least two independent mechanisms, depending on the cellular context. These multiple mechanisms may in part explain the conflicting results reported by different laboratories studying Hsp72 function.
The two different modes of Hsp72 activity in the inhibition of stress-induced apoptosis provide important clues into how Hsp72 may be interacting with the apoptotic signaling pathways. In vitro studies have shown that, in the absence of a functional ATPase domain, the substrate-binding domain maintains a high affinity for peptide and protein substrates and cannot adopt the low affinity conformation normally induced by the binding of ATP (Freeman et al. 1995; Rajapandi et al. 1998). Thus, it seems likely that the inhibition of apoptosis by the substrate-binding domain is due to the formation of a stable complex between it and unknown component(s) of the apoptosis signaling pathway, rather than chaperone activity being required to remodel the structure of the signaling molecule(s), as is the case with the glucocorticoid receptor (Dittmar and Pratt 1997). Expression of the 381-640 and K71E mutants in L929 cells also resulted in a slower rate of cell growth, suggesting that the unregulated binding properties of these proteins may affect other cellular functions in addition to apoptosis. Similarly, the Hsp72 substrate-binding domain is still capable of inhibiting JNK activation when expressed transiently, even though it fails to protect cells from heat-induced apoptosis under these conditions (Mosser et al. 2000). While the equal levels of protection afforded by wild-type Hsp72 and the Hsp72 substrate-binding domain in L929.3 cells suggests that they are acting by a common mechanism, the Δ-ATPase mutants and wild-type Hsp72 possess very different substrate-binding properties (Freeman et al. 1995; Rajapandi et al. 1998). Unfortunately, there is very little information on the binding kinetics of Hsp72 to protein substrates in vivo, so it is unclear if wild-type Hsp72 is capable of forming stable protein complexes inside cells or whether the cycle of Hsp72 binding and release associated with chaperone activity is capable of producing similar outcomes as more stable binding interactions. The lack of stable complex formation between Hsp72 and apoptotic signaling molecules may explain the failure to identify Hsp72-binding target(s) responsible for regulating apoptosis.
In cells expressing inducible Hsp72, Hsp72 chaperone activity is required for the inhibition of apoptosis and therefore it may be assumed that rather than simply binding a component of the apoptotic signaling pathway, Hsp72 is actively remodeling an apoptotic signaling molecule. While there is some information available on the role that Hsp72 plays in chaperoning proteins through intracellular membranes (De Los Rios et al. 2006) and remodeling complex protein structures such as clathrin-coated pits (Prasad et al. 1994), the effect of Hsp72 on intracellular signaling remains largely undefined. Hsp72 has been shown to bind to a variety of different kinases and signaling molecules, and so may have an impact on a number of different aspects of cell regulation including the cell cycle (Diehl et al. 2003) and p53 signaling (Fourie et al. 1997).
This study has highlighted the two different modes of Hsp72 activity present in cells. In L929.3 cells that constitutively express Hsp72, chaperone activity was required for the repair of heat-denatured proteins but was not required for the inhibition of stress-induced apoptosis. In these cells, Hsp72 substrate binding was sufficient to inhibit apoptotic signaling in response to both heat and TNFα as well as producing slower rates of cell replication. Thus, it seems that the formation of stable complexes between Hsp72 and other cellular proteins is capable of influencing various aspects of cell behavior. However, other studies have shown that in cells expressing Hsp72 in a transient fashion, chaperone activity is required for the inhibition of apoptosis, presumably through the remodeling of apoptotic signaling molecules. Thus, the expression of Hsp72 under transient or chronic conditions leads to different mechanisms of Hsp72 inhibiting apoptotic signaling. As inducible expression of Hsp72 is associated with the heat shock response (Craig 1985) and constitutive expression of Hsp72 is commonly observed in cancer (Ciocca and Calderwood 2005), both models of Hsp72 activity deserve further examination.
Acknowledgments
This study was supported by a grant from the NIH/NCI CA81421. The authors wish to acknowledge the kind gifts of reagents from Dr. R. Morimoto, Dr. Lois Greene, Dr. C. Hunt, Dr. S. Jane, Dr. W. Welch, Dr. P. Darcy, and Dr. N. Hoogenraad and technical assistance from Ms. C. Restall.
References
- Agashe VR, Hartl FU. Roles of molecular chaperones in cytoplasmic protein folding. Semin Cell Dev Biol. 2000;11:15–25. doi: 10.1006/scdb.1999.0347. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by HSP70. Mol Cells. 1999;9:200–206. [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice JA, Hightower LE. A mutational study of the peptide-binding domain of Hsc70 guided by secondary structure prediction. J Biol Chem. 1997;272:24825–24831. doi: 10.1074/jbc.272.40.24825. [DOI] [PubMed] [Google Scholar]
- Brinker A, Scheufler C, Der Mulbe F, Fleckenstein B, Herrmann C, et al. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 x Hop x Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200. [DOI] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- Chappell TG, Welch WJ, Schlossman DM, Palter KB, Schlesinger MJ, Rothman JE. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986;45:3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA. The heat shock response. CRC Crit Rev Biochem. 1985;18:239–80. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Los Rios P, Ben-Zvi A, Slutsky O, Azem A, Goloubinoff P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc Natl Acad Sci U S A. 2006;103:6166–71. doi: 10.1073/pnas.0510496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Vivo C, Halicka HD, Li CJ, Bhalla K, et al. Pharmacological induction of Hsp70 protects apoptosis-prone cells from doxorubicin: comparison with caspase-inhibitor- and cycle-arrest-mediated cytoprotection. Cell Death Differ. 2006;13:1434–1441. doi: 10.1038/sj.cdd.4401812. [DOI] [PubMed] [Google Scholar]
- Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Yang W, Rimerman RA, Xiao H, Emili A. Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol Cell Biol. 2003;23:1764–1774. doi: 10.1128/MCB.23.5.1764-1774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- Dressel R, Grzeszik C, Kreiss M, Lindemann D, Herrmann T, et al. Differential effect of acute and permanent heat shock protein 70 overexpression in tumor cells on lysability by cytotoxic T lymphocytes. Cancer Res. 2003;63:8212–8220. [PubMed] [Google Scholar]
- Esser C, Alberti S, Hohfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Fourie AM, Hupp TR, Lane DP, Sang BC, Barbosa MS, et al. HSP70 binding sites in the tumor suppressor protein p53. J Biol Chem. 1997;272:19471–19479. doi: 10.1074/jbc.272.31.19471. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene. 2005;24:3328–3338. doi: 10.1038/sj.onc.1208495. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Terada K, Oyadomari S, Mori M. hsp70–DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- Herbst R, Schafer U, Seckler R. Equilibrium intermediates in the reversible unfolding of firefly (Photinus pyralis) luciferase. J Biol Chem. 1997;272:7099–7105. doi: 10.1074/jbc.272.11.7099. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kume A, Li M, Doyu M, Hata M, et al. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J Biol Chem. 2000;275:8772–8778. doi: 10.1074/jbc.275.12.8772. [DOI] [PubMed] [Google Scholar]
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Dewey WC. Effect of cycloheximide or puromycin on induction of thermotolerance by heat in Chinese hamster ovary cells: dose fractionation at 45.5 degrees C1. Cancer Res. 1987;47:5960–5966. [PubMed] [Google Scholar]
- Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, Anderson RL. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–170. doi: 10.1023/A:1006689719505. [DOI] [PubMed] [Google Scholar]
- Lepock JR, Frey HE, Ritchie KP. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J Cell Biol. 1993;122:1267–1276. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Li L, Liu RY, Rehman M, Lee WM. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992;89:2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen G, Li GC. Effects of expressing human Hsp70 and its deletion derivatives on heat killing and on RNA and protein synthesis. Exp Cell Res. 1995;217:460–468. doi: 10.1006/excr.1995.1110. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton KW, Karmin P, Hahn GM, Minton AP. Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci U S A. 1982;79:7107–7111. doi: 10.1073/pnas.79.23.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshauser RC, Hu W, Wang H, Pang Y, Flynn GC, Zuiderweg ER. High-resolution solution structure of the 18 kDa substrate-binding domain of the mammalian chaperone protein Hsc70. J Mol Biol. 1999;289:1387–1403. doi: 10.1006/jmbi.1999.2776. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleros DR, Welch WJ, Fink AL. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991;88:5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Heuser J, Eisenberg E, Greene L. Complex formation between clathrin and uncoating ATPase. J Biol Chem. 1994;269:6931–6939. [PubMed] [Google Scholar]
- Rajapandi T, Wu C, Eisenberg E, Greene L. Characterization of D10S and K71E mutants of human cytosolic hsp70. Biochemistry. 1998;37:7244–7250. doi: 10.1021/bi972252r. [DOI] [PubMed] [Google Scholar]
- Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, et al. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem. 2006;281:7873–7880. doi: 10.1074/jbc.M513728200. [DOI] [PubMed] [Google Scholar]
- Schumacher RJ, Hurst R, Sullivan WP, McMahon NJ, Toft DO, Matts RL. ATP-dependent chaperoning activity of reticulocyte lysate. J Biol Chem. 1994;269:9493–9499. [PubMed] [Google Scholar]
- Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, et al. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. Br J Cancer. 1996;74:172–177. doi: 10.1038/bjc.1996.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–1557. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–51499. doi: 10.1074/jbc.M401314200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ouyang YB, Xu L, Chow AM, Anderson R, et al. The carboxyl-terminal domain of inducible Hsp70 protects from ischemic injury in vivo and in vitro. J Cereb Blood Flow Metab. 2006;26:937–950. doi: 10.1038/sj.jcbfm.9600246. [DOI] [PubMed] [Google Scholar]
- Volloch V, Gabai VL, Rits S, Sherman MY. ATPase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 1999;461:73–76. doi: 10.1016/S0014-5793(99)01428-3. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mosser DD, Bag J. Induction of HSP70 expression and recruitment of HSC70 and HSP70 in the nucleus reduce aggregation of a polyalanine expansion mutant of PABPN1 in HeLa cells. Hum Mol Genet. 2005;14:3673–3684. doi: 10.1093/hmg/ddi395. [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]