Abstract
The chaperone protein heat shock protein (HSP) 70 has been shown to protect against obesity-associated insulin resistance. Induction of HSPs is thus considered an exciting therapeutic strategy for diabetes (DM). The aims of this study were to (1) determine HSP levels in plasma, hepatic, and pancreatic tissues of type 2 DM primates and (2) assess the relationship between chaperone proteins of the HSP family and cellular protection. We collected plasma from 24 type 2 DM and 25 normoglycemic control (CTL) cynomolgus macaques. A subset of DM monkeys had liver and pancreas samples available which were compared to a second group of CTL monkeys. We found that DM monkeys had 32% lower HSP70 in circulation which remained significant even after adjustment for the greater age and bodyweight of these monkeys (p < 0.001). The liver demonstrated a similar reductions in both HSP70 and 90 that was related to 50% lower levels of the transcription factor, heat shock factor 1 (HSF1; p = 0.03). Pancreatic tissue had the opposite expression pattern with significantly higher HSF1 (p = 0.004) and accordingly higher HSP70 and 90. Pancreas from DM monkeys had less nitrosative oxidation (p = 0.03) which was unaccounted for by superoxide dismutases and was negatively associated with HSP levels (r = −0.57, p = 0.009). HSF1/HSP deficiency exists in DM liver which may contribute to hepatic insulin resistance and this deficiency was reflected in lower circulating concentrations. Pancreas maintains HSP levels despite hyperglycemia, likely in an attempt to protect vulnerable beta cells from exocrine pancreatic damage and from stress associated with insulin hypersecretion.
Keywords: Chaperones, Heat shock proteins, Type 2 diabetes, Monkey, Pancreas, Liver
Introduction
Heat shock proteins (HSPs) are the largest family of transcriptionally regulated chaperone proteins that respond to cellular stress in order to aid in repair of protein damage and survival of normal cellular functions (Hooper 2007). Heat shock factor 1 (HSF1) mediates the bulk of transcriptional activation by binding to a heat shock element found in the promoter region of the HSPs, with HSP70 being most abundant (Park and Liu 2001). Chaperone proteins have been shown to protect against obesity-associated insulin resistance (IR) by limiting cellular stress in obese rodent models (Ozcan et al. 2006; Chung et al. 2008). Induction of HSF1/HSPs is thus considered an exciting therapeutic strategy for diabetes (DM) and metabolic syndrome, as increasing the stress response will reduce the low-grade inflammation that is associated with excess bodyweight and IR (McCarty 2006).
Chaperone proteins decrease with both aging and IR (Gutsmann-Conrad et al. 1999; Jin et al. 2004) and this reduction has been implicated in age-related disease processes as cells without adequate protection accumulate oxidation products (Linton et al. 2001; Verbeke et al. 2001; Horowitz and Robinson 2007). Patients with IR and their first degree relatives have significantly decreased muscle levels of HSP70 (Kurucz et al. 2002; Bruce et al. 2003; Chung et al. 2008) and are also prone to the accumulation of oxidation products (Rea et al. 2001). The protective attributes of HSPs are further demonstrated by increased survival in people and DM rats that have not shown decreases in HSP70 as they aged or became hyperglycemic (Swiecki et al. 2003; Jin et al. 2004; Marini et al. 2004).
Induction of HSPs not only protects de novo proteins being translated through the endoplasmic reticulum (ER) or proteins currently oxidized but is also thought to up-regulate intracellular antioxidant mechanisms, such as glutathione peroxidase and superoxide dismutase (SOD), and down regulate pro-oxidant enzymes such as inducible nitric oxide synthase and NADPH oxidase (Feinstein et al. 1996; Burkart et al. 2000; Okada et al. 2004). Inadequate tissue HSPs may induce a vicious cycle where more cellular protection is required in hyperglycemic, pro-oxidant conditions and insufficient chaperone proteins are available for oxidized proteins and protection of newly translated proteins through the ER. Collectively, preclinical and in vitro studies suggest that (a) restoration of HSP levels in insulin sensitive tissues can reverse IR and associated inflammatory, pro-oxidant IR state and (b) HSP reductions may mediate beta cell loss during transition from IR to DM.
Reduced HSP70 in the skeletal muscle of IR and DM people (Kurucz et al. 2002; Bruce et al. 2003; Chung et al. 2008) affects the tissue’s ability to dispose of the bulk of circulating glucose (DeFronzo 2004). The liver produces the majority of glucose measured in the circulation under fasted conditions and is controlled by insulin released from the pancreatic beta cells. However, the chaperone protein profiles of these tissues in DM human or non-human primates is not currently known and rodent studies have reported conflicting results (Atalay et al. 2004; Najemnikova et al. 2007). The primary aim of this study was to determine HSP levels in plasma, hepatic and pancreatic tissue samples taken from type 2 DM non-human primates because these organs have central roles in the development of obesity-associated IR and type 2 DM. Further, a secondary aim was to assess the relationship between chaperone proteins of the HSP family and pancreatic cellular protection, which is known to be dependent on the maintenance of normal HSPs levels.
Material and methods
All experimental procedures involving animals during this study were carried out in accordance with The Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985) and in compliance with state and federal laws, standards of the US Department of Health and Human Services, and the instructions laid out by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences.
Twenty four cynomolgus macaques (Macaca fascicularis) housed at the Wake Forest University Primate Center were diagnosed with diabetes (DM) by repeated documentation of fasting hyperglycemia as defined by the American Diabetes Association (2007). Monkeys were maintained on twice daily subcutaneous insulin administration (Novolin 70/30, Novo Nordisk, Princeton, NJ, USA) to achieve a post-prandial glucose level approximating 8 mmol/l. Insulin doses were adjusted based on post-prandial glucose concentrations checked twice weekly by glucometer readings from a tail stick (Precision QID, Abbott Laboratories, Alameda, CA, USA). All monkeys had been kept stable on insulin for at least 6 months prior to collection of samples analyzed in this study. A second group of healthy control cynomolgus macaques (n = 25, CTL) were identified. All monkeys were maintained on a chow diet fed ad libitum (Purina Mills International, Brentwood, MO, USA). Age and bodyweight (BW) of the study animals are reported in Table 1.
Table 1.
Characteristics (mean ± SEM) of type 2 diabetic and control monkeys
| CTL | DM | p-value | ||
|---|---|---|---|---|
| Age | years | 15.93 ± 3.31 | 19.16 ± 4.70 | 0.02 |
| Bodyweight | kg | 4.87 ± 0.15 | 8.15 ± 0.65 | <0.001 |
| Glucose | mmol/l | 3.27 ± 0.09 | 14.43 ± 1.22 | <0.001 |
| Fructosamine | μmol/l | 185.51 ± 8.59 | 434.14 ± 33.43 | <0.001 |
| Insulin | pmol/l | 97.23 ± 9.72 | 632 ± 132.00 | <0.001 |
| C-peptide | nmol/l | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.10 |
| Pro-insulin | pmol/l | 43.05 ± 4.92 | 60.47 ± 11.81 | 0.38 |
| HOMA Index | 1.64 ± 0.15 | 51.00 ± 12.40 | <0.001 | |
| Free fatty acids | mEq/l | 0.75 ± 0.04 | 1.50 ± 0.14 | <0.001 |
Monkeys were sedated with ketamine hydrochloride (15 mg/kg, intramuscularly) after an overnight (18 h) fast and blood samples were collected between 8:30 and 9:30 am. Diabetic monkeys received only regular insulin 17 h prior to sampling to ensure, based on pharmacokinetic estimates, that they had no exogenous insulin circulating at the time of sampling (Plum et al. 2000). Blood samples were processed and plasma stored at −80°C for later analysis. Circulating endpoints measured from collected plasma included fasting blood glucose concentrations (FBG) as determined by colorimetric assay (Roche, Basel, Switzerland), with a CV% (inter- and intra-assay) of <5%. Insulin and pro-insulin concentrations were determined by ELISA (Mercodia, Uppsala, Sweden), with a CV% (inter- and intra-assay) of <10%. Fructosamine concentrations were used as an indicator of average glycemic control (Roche) and C-peptide (C-pep) of endogenously produced insulin was used to additionally confirm pancreatic insufficiency (Mercodia). The adipocytokine adiponectin (ADPN) was quantitated by ELISA supplied by Linco Research (St. Charles, MO, USA) with expected CV <10%. Homeostasis model assessment (HOMA) was calculated from the product of glucose (mmol/l) and insulin (IU/l)/22.5, and used as an indicator of insulin resistance (Bonora et al. 2000). To characterize the inflammatory and oxidant condition of these monkeys, circulating interleukin-6 (IL6; R&D Systems, Minneapolis, MN, USA), C-reactive protein (CRP, ALPCO Diagnostics, Salem, NH, USA), HSP70 (StressGen, Victoria, BC, Canada), and oxidized ApoB (ox-LDL, Mercodia) were measured by ELISA.
A subset of the DM monkeys (n = 11) had liver and pancreas tissue samples available at necropsy. Liver and pancreas tissue was available for comparison from necropsies of other normoglycemic control monkeys (n = 11). Tissues were collected freshly, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. Liver and pancreatic tissue was homogenized in protein extraction and lysis buffer (GBiosciences, St. Louis, MO, USA) supplemented with EDTA, DTT, and protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) with a PT 2100 Polytron electric blender (Kinematica, Littau-Lucerne, CH). The homogenate was centrifuged for 30 min at 14,000 ×g and the supernatant retained for protein analysis (BCA, Pierce Biotechnology, Rockford, IL, USA) and electrophoresis on a polyacrylamide gel (Invitrogen, Carlsbad, CA, USA). A heat shocked HeLa cell lysate (StressGen) was included as a positive control. Proteins were then transferred to a nitrocellulose membrane (Whatman, Sanford, ME, USA) and blocked in 5% dry milk overnight. The membranes were probed with antibodies to HSF1, HSP70, and HSP90 (StressGen). Additionally, pancreatic tissue was probed for the major isoforms of superoxide dismutase (CuZnSOD and MnSOD, StressGen). Beta-actin was probed for as a loading control to which all quantifications were normalized to (Calbiochem, Gibbstown, NJ, USA). Tissue protein s-nitrosylation was estimated by the biotin switch method according to the manufacturer’s directions (Cayman Chemicals, Ann Arbor, MI, USA). S-nitrosylation is mediated by higher oxidative states of nitric oxide and indirectly reflects the oxidant state within the tissues. A positive control sample was prepared by incubating an aliquot of tissue lysate pooled from all study samples with sodium nitroprusside (5 µM) overnight at 4°C. Biotinylation of s-nitrosylated protein bands in the positive control and sample tissue homogenates was detected by development with an avidin conjugate and detection by chemiluminescence (ECLplus, GE Healthcare, Giles, UK). Membranes were scanned with a STORM 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA, USA) and analyzed using ImageQuant 5.2 software (Molecular Dynamics). A composite nitrosylation score was calculated as the sum of band densities at 28, 70, and 90 kDa consistent with the pattern of nitrosylation seen in the positive control.
The data from tissue endpoints were compared using statistical software (Statistica, Statsoft, Tulsa, OK, USA) by two-sample t test and for the circulating parameters by ANCOVA using age and BW as covariates (HSP70, CRP, ADPN, leptin, ox-LDL, IL-6). Associations were made with Pearson’s correlation and predictions with multiple linear regression with backward stepwise removal of parameters. Significance was set with α ≤ 0.05.
Results
Characteristics of DM and CTL monkeys are shown in Table 1. Consistent with the presentation of type 2 DM in human patients, monkeys were significantly older (p < 0.001) and heavier (p < 0.001) than the normoglycemic control group. Elevated fructosamine concentrations confirmed average hyperglycemia (p < 0.001) and hyperinsulinemia with elevated HOMA index values confirmed the presence of insulin resistance (p < 0.001). Pro-insulin and C-peptide levels in the circulation were not different (p = 0.38 and 0.10, respectively) although both trended as expected, with pro-insulin being non-significantly higher and C-peptide tending to be lower in DM. Free fatty acid levels were elevated (p < 0.001) and highly associated with the degree of fasting hyperglycemia (r = 0.82, p < 0.001).
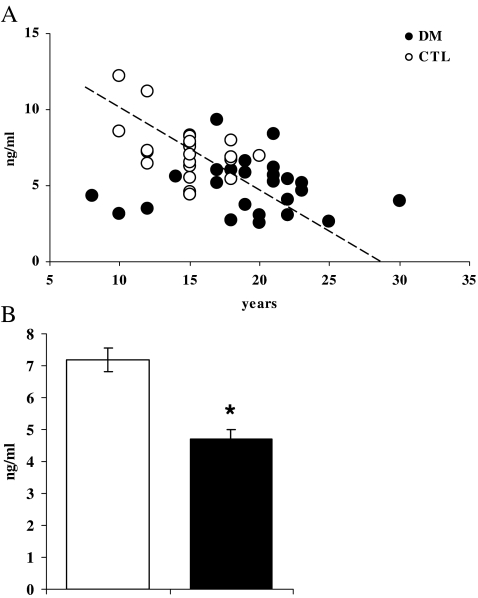
As has been described in people, levels of circulating HSP70 decreased with age in both CTL and DM monkeys (overall correlation r = −0.40, p = 0.004, Fig. 1a). Similarly, HSP70 concentrations were significantly and negatively associated with glucose, insulin concentrations (r = −0.39 and −0.40, respectively; p < 0.01 for both) and HOMA index and fructosamine (r = −0.34, p = 0.01 and r = −0.37, p = 0.009, respectively). HSP70 concentrations measured in the circulation were significantly reduced by 32% (p < 0.001) in DM monkeys (Fig. 1b). As ageing per se is associated with increased oxidation and insulin resistance, age and BW were used as covariates in analysis. After adjustment for the increased age and BW in the DM monkeys, the reduction in HSP70 levels remained significant (p = 0.01) whereas these factors accounted for higher CRP and lower ADPN concentrations (Table 2). Ox-LDL and IL-6 were not different between groups.
Fig. 1.
a HSP70 concentrations decreased significantly with increasing age (r = −0.4, p = 0.004). b Mean (±SEM) circulating HSP70 concentrations are significantly (p < 0.001) reduced in type 2 diabetic monkeys (■) as compared to normoglycemic control monkeys (□)
Table 2.
Mean (±SEM) concentrations of circulating parameters indicating oxidation and inflammation in type 2 diabetic and control monkeys
| CTL | DM | p-value | p-value adjusted for age and BW | ||
|---|---|---|---|---|---|
| HSP70 | ng/ml | 7.17 ± 0.37 | 4.88 ± 0.35 | <0.001 | 0.01 |
| C-reactive protein | ng/ml | 301.43 ± 32.01 | 402.13 ± 33.04 | 0.03 | NS |
| Adiponectin | pg/ml | 24.80 ± 3.77 | 11.59 ± 3.53 | 0.001 | NS |
| Oxidized low density lipoprotein | U/l | 49.90 ± 2.55 | 49.10 ± 3.42 | NS | NS |
| Interleukin-6 | pg/ml | 4.42 ± 0.79 | 2.22 ± 0.24 | NS | NS |
Predictive regression modeling was employed to further elucidate the relationship of HSP70 with other metabolic parameters. Backward stepwise removal of variables (12 variables included age, BW, insulin, pro-insulin, FBG, c-pep, fructosamine, ox-LDL, IL-6, CRP, ADPN, and FFA) resulted in a model that was significant overall (p < 0.0001) and accounted for 39% of the variance in circulating HSP70 concentrations (Table 3). The parameters that remained significantly predictive in the model included age, ox-LDL, and FBG. Age and glucose contributed the most, uniquely accounting for 14.8% and 11.5% of the variance in HSP70, respectively. Further, HSP70 levels were not correlated with markers of inflammation (CRP, IL6, ADPN, ox-LDL) but were correlated with diabetes indices (FGB, INS, HOMA, fructosamine), suggesting that their decrease in levels is related to hyperglycemia rather than the chronic inflammation associated with obesity and diabetes.
Table 3.
Predictive regression modeling for circulating HSP70 concentrations
| β coefficient | p-value | Variance in HSP70 concentration accounted for by model parameter | ||
|---|---|---|---|---|
| Age | Years | −0.43 | 0.001 | 14.8% |
| Ox-LDL | U/l | −0.32 | 0.011 | 5.2% |
| FBG | mmol/l | −0.27 | 0.037 | 11.5% |
Overall, a significant model included variables of age, ox-LDL concentrations, and fasting blood glucose (FBG; model excluded the parameters bodyweight, fasting insulin, pro-insulin, c-peptide, fructosamine, interleukin-6, c-reactive protein, adiponectin, and free fatty acid concentrations)
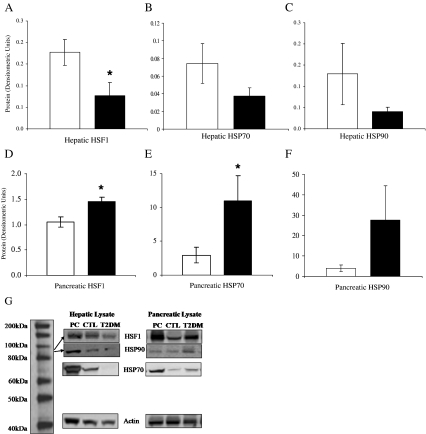
Consistent with the reduction in HSP70 measured in the circulation, liver from diabetic monkeys had 50% lower HSF1 (the main transcription factor for HSP70) than CTL liver (p = 0.03; Fig. 2a). The reduction in HSF1 transcriptional activation was reflected in DM liver HSP70 being approximately half that of CTL (p = 0.17; Fig. 2b) and HSP90 in DM liver being 69% lower than control (p = 0.07; Fig. 2c). Accordingly, correlation between HSF1 and HSP70 or 90 was high (r = 0.70 and 0.81, respectively; p < 0.01 for both). In contrast, pancreatic tissue from DM monkeys had elevated HSF1 (p = 0.004; Fig. 2d) and consequentially more than 3-fold elevation in HSP70 (p = 0.05; Fig. 2e) and 7-fold elevation in HSP90 (p = 0.09; Fig. 2f) levels despite being exposed to the same level of hyperglycemia as the liver.
Fig. 2.
Mean (±SEM) HSF1 protein levels (a) were significantly decreased in the liver samples of diabetic monkeys (■) as compared to normoglycemic control monkeys (□). The main product of HSF1 transcriptional activation is HSP70 production. Consistent with the significant reduction in HSF1, we saw approximately 50% the level of HSP70 protein (p = 0.17) in the diabetic monkeys as compared to normoglycemic control monkeys (b, mean ± SEM). Liver HSP90 (c, mean ± SEM) also results from HSF1-mediated transcription and was similarly found to be less than a third of the levels of control monkeys (p = 0.07). d, e, and f show the mean (±SEM) levels of HSF1, HSP70, and HSP90, respectively, from total pancreas lysate of type 2 diabetic (■) and control (□) monkeys. In contrast to the expression pattern of these proteins in the liver, pancreas showed significantly elevated HSF1 (p < 0.01) and HSP70 (p = 0.05). HSP90 concordantly demonstrated a trend towards elevation (p = 0.09). g includes representative samples from Western blots of positive control (PC), liver and pancreas lysate of control (CTL), and diabetic (T2DM) monkeys
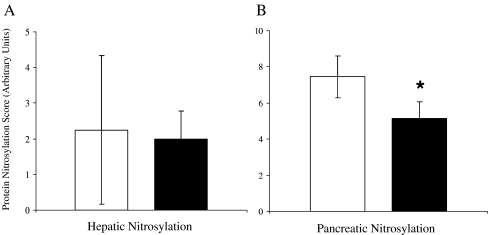
To assess whether this tissue-specific difference in chaperone protein response led to differences in cellular protection, protein nitrosylation of DM and CTL liver and pancreas was quantitated. Liver nitrosylation was variable and not different between DM and CTL (p = 0.33, Fig. 3a) whereas pancreatic nitrosylation was significantly lower in DM (p = 0.03; Fig. 3b). It appears that the chaperone protein response is related to protection as pancreatic nitrosylation was negatively correlated with HSF1 levels (r = −0.57, p = 0.009). No relationship was detected between liver HSF1 and nitrosylation. To further account for the reduction in pancreatic protein nitrosylation, levels of MnSOD and CuZnSOD were quantitated as they account for much of cellular antioxidant defense and were not found to be different between groups (Table 4).
Fig. 3.
Mean (±SEM) nitrosylation of tissue proteins in the liver (a) and pancreas (b) from control (□) and from type 2 diabetic (■) monkeys. Nitrosylation indicates reaction of free thiol groups of proteins with oxidized derivatives of nitric oxide (nitrates and nitrites), and was significantly (p = 0.03) reduced in pancreas from diabetic monkeys compared to that from control animals
Table 4.
Quantification of superoxide dismutase (mean ± SEM, normalized for beta actin [SOD]) protein isoforms in pancreas
| CTL | DM | p-value | |
|---|---|---|---|
| CuZnSOD | 13.3 ± 0.43 | 14.5 ± 0.85 | 0.20 |
| MnSOD | 448,210 ± 29,335 | 441,600 ± 53,543 | 0.91 |
Pancreatic levels of SOD isoforms were not different between control and type 2 diabetic monkeys. Measurements are in arbitrary densitometric units, corrected for actin
Discussion
In the current study, we show for the first time that reduced HSP70 is detectable in the circulation of type 2 DM primates. Regression modeling suggests a role for hyperglycemia in this lowering, rather than inflammation of which measured biomarkers were not associated with circulating HSP70. Lower HSF1 levels in the liver lead to similar magnitude of reductions in hepatic HSPs, HSP70 and 90, as that seen in plasma. However, the pancreas from DM monkeys had up-regulated HSF1 and HSPs that were associated with lower tissue oxidation. Our finding of significant and DM-specific reductions in circulating HSP70 in non-human primates corroborates with findings of decreased tissue HSP70 in diabetic people (Kurucz et al. 2002; Bruce et al. 2003; Chung et al. 2008). A significant and strong negative association between higher glucose concentrations and tissue gene expression of HSP70 in DM patients has been demonstrated (Patti et al. 2003) with the magnitude of reduction (42%) being similar to the 32% reduction in plasma HSP70 in DM non-human primates described herein. The role of glucose in mediating reductions of these chaperones is supported by a study by Febbraio et al. (2004) which showed that ingestion of glucose blunted exercise-related increase in HSP70 concentrations in the circulation of healthy people. This reduction was calculated to be resultant from decreased release of HSP70 from the hepatosplanchnic circulation rather than from peripheral muscle tissue, which is in line with data from these hyperglycemic primates.
We confirm in our non-human primate model that reduced hepatic HSP70 is associated with hyperglycemia and, further, is likely the result of decreased HSF1 levels, although there are many steps in HSF1 activation that may play a role. HSF1 is cleaved by the same proteases as those that process the SREBP-1 cholesterol-sensing transcription factor, trimerizes, and becomes phosphorylated by JNK-1 under conditions of stress prior to translocation to the nucleus (Park and Liu 2001; Hooper 2007). Typically, both JNK1 activity and cholesterol are elevated in obesity and diabetes which suggests that processes associated with HSF1 activation would also be increased. Our limited inflammatory panel included CRP which was higher in DM and suggests increased JNK-1 would be present in DM tissues; however, our data suggest that HSF1 levels are more critical than subsequent activation steps in ensuring adequate chaperone levels. The mechanism behind reductions in HSF1 levels of liver is not known; however, the unfolded protein response that is characteristic of ER stress results in reduced protein translation, which may in turn decrease HSF1 (Scheuner et al. 2005).
In organs with high secretory capacity such as the liver and the pancreas, the HSP family is vital to effecting normal physiology (Hooper 2007). In diabetes, cellular exposure to high levels of glucose and free fatty acids signals the need for increased metabolism (DeFronzo 2004). Inadequate cellular machinery to manage the increased flux of nutrients signals the translation of more proteins through the ER to perform these functions. An overload of proteins passing through the ER overwhelms HSPs’ chaperoning capacity, already stressed by the glucose-mediated oxidation of cytosolic and membrane proteins (Hotamisligil 2005). A healthy cell will respond by increasing the translation of chaperone proteins; however, in the diabetic state, we see inadequate, if not reduced, levels, as shown by the hepatic profile from these monkeys. Diabetes has been referred to as accelerated aging, as toxic glucose metabolites and glycoxidation products accumulate in tissues (Aronson 2003). Our data support this concept, since the young diabetic monkeys in our study had HSP70 levels comparable with those measured from control monkeys of advanced age. The cellular mechanism behind HSP70 reductions with advancing age, seen both in vitro and in vivo, are unknown (Gutsmann-Conrad et al. 1999; Rea et al. 2001; Verbeke et al. 2001; Jin et al. 2004). While others have shown that age may play a role in the ability of HSF1 to effect transcription via heat shock element activation (Gutsmann-Conrad et al. 1999), HSF1 levels in hepatocytes taken from our DM primates were significantly reduced independent of age, suggesting a specific effect of diabetes on this transcription factor. This reduction is likely to contribute largely to the reduced HSP levels as their associations were strong and reduced HSF1 has been seen to result in lower HSP transcription in genetically modified animals, associated with accelerated aging and shortened lifespan (Hsu et al. 2003).
Our findings in non-human primates differ from some reports of diabetic rodent models in which hepatic HSP70 has been reported to be undetectable, unchanged, or even increased (Yamagishi et al. 2001; Swiecki et al. 2003; Atalay et al. 2004; Najemnikova et al. 2007). Rodent models of diabetes examined have been predominantly hyperglycemic secondary to insulinopenia induced by streptozotocin injection, which is toxic to pancreatic beta cells via oxidative damage (Ohkuwa et al. 1995; Lubec et al. 1998). The addition of this toxin may explain differences in stress protein responses reported in rodents as compared to the primates evaluated. Variable diabetes duration prior to sampling may additionally account for the differing responses reported (Wachlin et al. 2002), as illustrated by levels of HSP70 in rat inflammatory cells and exudate being decreased after 1 week of diabetes duration, while circulating levels of HSP70 do not significantly decline until after 4 weeks (Bitar et al. 1999; McMurtry et al. 1999). The time course for reductions in rodent tissue HSP levels is unknown. Potentially, reductions in HSPs secondary to DM are sub-acute to chronic changes in this animal model or reductions are masked by the effect of streptozotocin, whereas data from people and non-human primates seem to suggest a close temporal relationship between circulating glucose and HSP levels (Patti et al. 2003; Febbraio et al. 2004) (Kavanagh et al. 2008, unpublished observations).
Tissue-specific expression patterns of HSPs have been reported in rodent studies, with the weight of evidence suggesting lower levels of HSPs in liver, skeletal, and cardiac muscle result from hyperglycemia (Yamagishi et al. 2001; Atalay et al. 2004; Ooie et al. 2005). Lower muscle HSP70 is consistently reported in hyperglycemic people (Kurucz et al. 2002; Bruce et al. 2003; Chung et al. 2008), while HSP90 is unchanged in skeletal muscle of IR people (Chung et al. 2008) and DM rodents (Yamagishi et al. 2001). HSF1-mediated up-regulation of HSP70 but not HSP90 has been seen in skeletal muscle cell culture, supporting the observations made from human patients and suggests yet another tissue-specific effect (Chung et al. 2008).
Pancreatic HSF1 and HSP levels are yet to be reported in primate tissues; however, islets isolated from human pancreata have significantly higher HSP70 than rodents (Eizirik et al. 1994; Welsh et al. 1995). Human type 2 diabetic islets have greater immunostaining for HSP70 than do islets from non-diabetic humans, which is consistent with our data (Laybutt et al. 2007). The preservation of HSP responses in the diabetic pancreas despite the glucose-related reductions in other insulin sensitive tissues may be attributed to the need for protection from the exocrine pancreatic enzyme, trypsin. HSP70 prevents the activation of trypsinogen, and thus cellular autodigestion by trypsin (Bhagat et al. 2000).
Insulin resistance characterizes type 2 DM, which typically presents initially in people as hyperinsulinemia (DeFronzo 2004). Expression of HSPs increase with insulin secretion, as insulin levels are controlled at the translational level. The greater demand for insulin is coupled with greater demand for chaperones at the ER to prevent stress and initiation of apoptosis resultant from misfolded newly translated proteins (Scheuner et al. 2005). Pancreatic HSP70 is induced alongside other indicators of ER stress by thiazolidinedione drugs, which suppresses inflammatory signaling that occurs with hyperglycemia (Weber et al. 2004). This protective effect may explain the beta cell preservation and diabetes prevention that is seen following treatment of IR people with this class of drugs (Decker et al. 2008). Our data in hyperinsulinemic, insulin-requiring DM monkeys might suggest that the up-regulated HSF1/HSP response is inadequate protection for beta cells. Beta cells comprise only about 3% of pancreatic mass, thus this tissue-specific preservation of the stress response in pancreas should not be assumed to represent only islet biology despite consistent reports of high beta cell HSPs in DM people and rodents (Laybutt et al. 2002, 2007).
Nitric oxide in the oxidative hyperglycemic milieu of DM forms reactive peroxynitrite and peroxynitrate which are able to nitrosylate proteins, potentially modifying their functions (Turko and Murad 2002). Pancreatic beta cell survival depends on normal production of HSP70 to prevent initiation of apoptosis (Burkart et al. 2000; Laybutt et al. 2002). Beta cells eventually apoptose as a response to chronic or severe hyperglycemia and apoptosis rate is thought to determine progression from IR to type 2 DM (Ritzel et al. 2006). The up-regulation of the heat shock response in the pancreas appears to confer protection from damage, as assessed by protein oxidative nitrosylation and may be part of the proliferative response of beta cells prior to apoptosis and diabetes (Laybutt et al. 2003). This cellular protection was not resultant of increased SOD as has been seen in rodent pancreas exposed to chronic hyperglycemia (Laybutt et al. 2002). Nitrosative damage is of particular relevance to beta cells where nitric oxide released from cytokine stimulated inducible nitric oxide synthase is detrimental to beta cell function and induces apoptosis (Eizirik et al. 1994; Burkart et al. 2000). Conversely, in our study, the DM liver did not exhibit elevated protein oxidation indicative of adequate antioxidant capacity despite lower HSP levels. Oxidative damage is not correlated with metabolic derangement as evidenced by restoration of HSP levels in DM rodents, which leads to improved glucose metabolism, hepatic insulin sensitivity, and reversal of hepatic steatosis (Ozcan et al. 2006).
In summary, we show that HSP proteins are reduced in the liver and circulation of spontaneously type 2 DM primates but the pancreatic response is different. The mechanisms behind the tissue-specific regulation of non-human primate heat shock proteins are not clear but this study provides further evidence that up-regulation of HSPs through HSF1 may be an effective strategy to minimize tissue damage resultant of hyperglycemia and restore insulin sensitivity in tissues such as the liver, which are central to the pathogenesis of IR.
Acknowledgements
Funding for this study was provided by NIH Cardiovascular Pathology Training Grant 5T32HL07115 (KK), Wake Forest University School of Medicine, and Pfizer. The authors gratefully recognize Mr. Mickey Flynn for his editorial assistance.
Abbreviations
- ADPN
adiponectin
- BW
bodyweight
- c-pep
C-peptide
- CRP
C-reactive protein
- CTL
control
- DM
diabetes
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic reticulum
- FBG
fasting blood glucose
- IR
insulin resistance
- HSF1
heat shock factor 1
- HSP
heat shock protein
- IL-6
interleukin-6
- NADPH
nicotinamide adenine dinucleotide phosphate
- ox-LDL
oxidized ApoB lipoprotein
- SOD
superoxide dismutase
- SEM
standard error of the mean
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(1):S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala NK, Laaksonen DE, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97:605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest. 2000;106:81–89. doi: 10.1172/JCI8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar MS, Farook T, John B, Francis IM. Heat-shock protein 72/73 and impaired wound healing in diabetic and hypercortisolemic states. Surgery. 1999;125:594–601. [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Burkart V, Liu H, Bellmann K, et al. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;275:19521–19528. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M, Hofflich H, Elias AN. Thiazolidinediones and the preservation of beta-cell function, cellular proliferation and apoptosis. Diabetes Obes Metab. 2008;10(8):617–625. doi: 10.1111/j.1463-1326.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A. 1994;91:9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Mesa JL, Chung J, et al. Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones. 2004;9:390–396. doi: 10.1379/CSC-24R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.46.29489. [DOI] [PubMed] [Google Scholar]
- Gutsmann-Conrad A, Pahlavani MA, Heydari AR, Richardson A. Expression of heat shock protein 70 decreases with age in hepatocytes and splenocytes from female rats. Mech Ageing Dev. 1999;107:255–270. doi: 10.1016/S0047-6374(98)00132-8. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Insulin signaling, GSK-3, heat shock proteins and the natural history of type 2 diabetes mellitus: a hypothesis. Metab Syndr Relat Disord. 2007;5:220–230. doi: 10.1089/met.2007.0005. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Robinson SD. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res. 2007;162:433–446. doi: 10.1016/S0079-6123(06)62021-9. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–S78. doi: 10.2337/diabetes.54.suppl_2.S73. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jin X, Wang R, Xiao C, et al. Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones. 2004;9:69–75. doi: 10.1379/477.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Kaneto H, Hasenkamp W, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes. 2002;51:413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278:2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- Linton S, Davies MJ, Dean RT. Protein oxidation and ageing. Exp Gerontol. 2001;36:1503–1518. doi: 10.1016/S0531-5565(01)00136-X. [DOI] [PubMed] [Google Scholar]
- Lubec B, Hermon M, Hoeger H, Lubec G. Aromatic hydroxylation in animal models of diabetes mellitus. FASEB J. 1998;12:1581–1587. doi: 10.1096/fasebj.12.14.1581. [DOI] [PubMed] [Google Scholar]
- Marini M, Lapalombella R, Canaider S, et al. Heat shock response by EBV-immortalized B-lymphocytes from centenarians and control subjects: a model to study the relevance of stress response in longevity. Exp Gerontol. 2004;39:83–90. doi: 10.1016/j.exger.2003.09.023. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66:527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]
- McMurtry AL, Cho K, Young LJ, Nelson CF, Greenhalgh DG. Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res. 1999;86:36–41. doi: 10.1006/jsre.1999.5700. [DOI] [PubMed] [Google Scholar]
- Najemnikova E, Rodgers CD, Locke M. Altered heat stress response following streptozotocin-induced diabetes. Cell Stress Chaperones. 2007;12:342–352. doi: 10.1379/CSC-292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuwa T, Sato Y, Naoi M. Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci. 1995;56:1789–1798. doi: 10.1016/0024-3205(95)00150-5. [DOI] [PubMed] [Google Scholar]
- Okada M, Hasebe N, Aizawa Y, Izawa K, Kawabe J, Kikuchi K. Thermal treatment attenuates neointimal thickening with enhanced expression of heat-shock protein 72 and suppression of oxidative stress. Circulation. 2004;109:1763–1768. doi: 10.1161/01.CIR.0000124226.88860.55. [DOI] [PubMed] [Google Scholar]
- Ooie T, Kajimoto M, Takahashi N, et al. Effects of insulin resistance on geranylgeranylacetone-induced expression of heat shock protein 72 and cardioprotection in high-fat diet rats. Life Sci. 2005;77:869–881. doi: 10.1016/j.lfs.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Liu AY. JNK phosphorylates the HSF1 transcriptional activation domain: role of JNK in the regulation of the heat shock response. J Cell Biochem. 2001;82:326–338. doi: 10.1002/jcb.1163. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum A, Agerso H, Andersen L. Pharmacokinetics of the rapid-acting insulin analog, insulin aspart, in rats, dogs, and pigs, and pharmacodynamics of insulin aspart in pigs. Drug Metab Dispos. 2000;28:155–160. [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- Swiecki C, Stojadinovic A, Anderson J, Zhao A, Dawson H, Shea-Donohue T. Effect of hyperglycemia and nitric oxide synthase inhibition on heat tolerance and induction of heat shock protein 72 kDa in vivo. Am Surg. 2003;69:587–592. [PubMed] [Google Scholar]
- Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Clark BF, Rattan SI. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Radic Biol Med. 2001;31:1593–1602. doi: 10.1016/S0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Wachlin G, Heine L, Kloting I, Dunger A, Hahn HJ, Schmidt S. Stress response of pancreatic islets from diabetes prone BB rats of different age. Autoimmunity. 2002;35:389–395. doi: 10.1080/0891693021000014989. [DOI] [PubMed] [Google Scholar]
- Weber SM, Chambers KT, Bensch KG, Scarim AL, Corbett JA. PPARgamma ligands induce ER stress in pancreatic beta-cells: ER stress activation results in attenuation of cytokine signaling. Am J Physiol Endocrinol Metab. 2004;287:E1171–E1177. doi: 10.1152/ajpendo.00331.2004. [DOI] [PubMed] [Google Scholar]
- Welsh N, Margulis B, Borg LA, et al. Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med. 1995;1:806–820. [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Nakayama K, Wakatsuki T, Hatayama T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci. 2001;69:2603–2609. doi: 10.1016/S0024-3205(01)01337-6. [DOI] [PubMed] [Google Scholar]