Abstract
HspBP1 is a co-chaperone that binds to and regulates the chaperone Hsp70 (Hsp70 is used to refer to HSPA1A and HSPA1B). Hsp70 is known to be elevated in breast tumor tissue, therefore the purpose of these studies was to quantify the expression of HspBP1 in primary breast tumors and in serum of these patients with a follow-up analysis after 6 to 7 years. Levels of HspBP1, Hsp70, and anti-HspBP1 antibodies in sera of breast cancer patients and healthy individuals were measured by enzyme-linked immunosorbent assay. Expression of HspBP1 was quantified from biopsies of tumor and normal breast tissue by Western blot analysis. The data obtained were analyzed for association with tumor aggressiveness markers and with patient outcome. The levels of HspBP1 and Hsp70 were significantly higher in sera of patients compared to sera of healthy individuals. HspBP1 antibodies did not differ significantly between groups. HspBP1 levels were significantly higher in tumor (14.46 ng/μg protein, n = 51) compared to normal adjacent tissue (3.17 ng/μg protein, n = 41, p < 0.001). Expression of HspBP1 was significantly lower in patients with lymph node metastasis and positive for estrogen receptors. HspBP1 levels were also significantly lower in patients with a higher incidence of metastasis and death following a 6 to 7-year follow-up. The HspBP1/Hsp70 molar ratio was not associated with the prognostic markers analyzed. Our results indicate that low HspBP1 expression could be a candidate tumor aggressiveness marker.
Keywords: Breast cancer, Co-chaperone, HspBP1, Hsp70, Prognostic markers
Introduction
Co-chaperones are proteins that bind and regulate the activity of chaperone proteins. The Hsp70 co-chaperone HspBP1 was first isolated from a human heart cDNA library using the yeast two-hybrid system with the ATPase domain of Hsp70 as bait (Raynes and Guerriero 1998). HspBP1 is classified as a nucleotide exchange factor and has been shown to either inhibit or stimulate Hsp70 ATPAse depending on assay conditions (Raynes and Guerriero 1998; Shomura et al. 2005). HspBP1 binding to Hsp70 results in a change in the conformation of the Hsp70 ATPase domain, and this is followed by inhibition of Hsp70-associated protein folding (McLellan et al. 2003). HspBP1 homologues can be found in other eukaryotic organisms; for example, Fes1 is the yeast cytoplasmic homologue of HspBP1 which can promote nucleotide dissociation from Ssa1p, the yeast homologue of Hsp70 (Kabani et al. 2002). Initial results have demonstrated that HspBP1 does not exhibit strict tissue specific expression; HspBP1 mRNA is expressed in all tissues examined with the highest levels in heart, brain, skeletal muscle, and pancreas (Raynes and Guerriero 1998).
Elevated levels of Hsp70 have been reported in a number of tumors including breast (Torronteguy et al. 2006), lung (Volm et al. 1995), cervical (Ralhan and Kaur 1995), prostate, and renal (Jaattela 1999; Jolly and Morimoto 2000). Hsp70 appears to play dual and opposite roles in cancer, promoting survival of tumor cells while contributing to tumor immunity. It was previously shown that HspBP1 levels were elevated in two mouse tumor models and that the molar ratio of HspBP1 to Hsp70 was within a small range in the normal and tumor tissues examined (Raynes et al. 2003). This ratio was considerably below the HspBP1 to Hsp70 ratio of 4.0 estimated to be needed for 50% inhibition of Hsp70-mediated refolding of a partially denatured protein (Raynes et al. 2003). However, it is possible that localized concentrations of HspBP1 in the cell could result in regional Hsp70 inhibition. Although both Hsp70 and HspBP1 levels increase and maintain the same molar ratio in normal tissues and tumor lines, these two proteins are not expressed in a coordinate manner (Gottwald et al. 2006).
Proteins identified in tissue and circulating blood proteins have the potential of profiling various disease states and therefore have tremendous diagnostic and treatment value. A major focus in cancer research is to identify proteomic markers for risk assessment and early detection in individuals. Nothing is known about the expression of HspBP1 in human primary tumors. In this study, the expression of HspBP1 and Hsp70 in breast cancer patient samples, both in tumor and normal adjacent tissue, was compared. In addition, the concentration of these two proteins, as well as anti-HspBP1 antibody, in the serum of patients and normal individuals was measured. The data were analyzed to determine if the expression of HspBP1, or the HspBP1/Hsp70 molar ratio, associates with aggressiveness tumor markers, as well as patient outcome. These results verified that HspBP1 is increased in human tumor tissue and found that low levels are related to a poor patient outcome.
Methods and materials
Patients
Samples of tumor and normal tissue were collected from 51 patients with primary breast cancer in 2001 and 2002. All patients were submitted to complete excision of tumor or total mastectomy. Exclusion criteria were previous radio or chemotherapy, familial cancer, hormonal therapy, and use of antidepressants or corticosteroids. Serum samples of 27 of these patients were available, and control serum samples were collected from 16 female healthy individuals. Samples were stored at −20°C. All individuals enrolled in the study signed an informed consent form, and the study protocol was previously approved by the Ethics Committee of the Sao Lucas Hospital.
ELISAS
HspBP1 enzyme-linked immunosorbent assays (ELISAs) were performed with slight modifications from what was previously described (Papp et al. 2005; Raynes et al. 2006). Briefly, for anti-HspBP1 analysis, individual wells of a 96-well microplates (Nalge Nunc, Rochester, NY, USA) were coated overnight at 4°C with 100 μL of 1 μg/mL HspBP1 (amino acids 84–359). Plates were blocked in 100 μL of blocking buffer (1% gelatin, 2% BSA, 0.01%Tween 20) for 1 h at room temperature with shaking. Serum samples were added at a 1:5 dilution in duplicates and incubated overnight at 4°C. Secondary antibody (HRP-conjugated goat anti-human IgG; Organon Teknika, West Chester, PA, USA) was used at a 1:500 dilution and incubated for 1 h at room temperature with shaking.
To measure HspBP1, plates were coated overnight at 4°C with 100 μL of 1 μg/mL sheep anti-HspBP1 antibody (Novus Biologicals, Littleton, CO, USA) in PBS. Plates were blocked with 100 μL of blocking buffer for 1 h at room temperature, and serum samples were diluted 1:5, followed by serial dilution in blocking buffer. The standard curve contained HspBP1 (amino acids 84–359) serially diluted from 2.5 to 0.15 ng in blocking solution. Aliquots, 100 μL, of the dilutions were added per well in duplicates, and the plate was incubated for 2 h at room temperature with shaking. Rabbit anti-HspBP1 (100 μL per well at 0.1 μg/mL; Delta Biolabs, Campbell, CA, USA) was added in blocking solution and incubated at room temperature for 1 h with shaking. Goat anti-rabbit IgG HRP conjugated (Zymed, San Francisco, CA, USA), 100 μL per well of 1:10.000 dilution was added, and the plate was incubated at room temperature for 1 h with shaking.
To measure Hsp70 in serum, plates were coated with anti-Hsp70 mouse (SPA-810, Assay Designs, Ann Arbor, MI, USA) at 2 μg/ml. Plates were blocked with 5% nonfat milk in PBS + 0.05% Tween for 2 h at room temperature. Sera were diluted at 1:5, and the standard curve was started at 1,000 ng/ml. Rabbit anti-Hsp70 rabbit (SPA-812, Assay Designs) was used at 1:2,000 dilution followed by anti-rabbit IgG (Zymed) at 1:5,000 dilution.
Plates were then rinsed six times and developed with 100 μL ready-to-use TMB (BioRad, Hercules, CA, USA) per well with incubation for 10–15 min at room temperature. To stop the reaction, 100 μL of 1 N HCl was added per well. Absorbance was read at 450 nm using an Anthos Zenyth 340r microplate reader. Standard curves were plotted using the GraphPad Prism software, and unknowns were determined from the standard curve. The molar ratio HspBP1/Hsp70 was calculated in the sera of breast cancer patients and healthy individuals based on a molecular weight of 70 kD for Hsp70 and 40 kD for HspBP1.
Western blots
Fresh tissue samples from the invasive margins of the tumors and adjacent normal breast tissue, as determined macroscopically, were retrieved from each patient and placed in Roswell Park Memorial Institute media for immediate processing. Total protein was extracted as previously described, and the measurement of Hsp70 in normal and tumor tissue was done in 2003 and 2004 (Torronteguy et al. 2006). The protein concentrations of the samples were estimated by Bradford assay (Dye Reagent Concentrate BIO-RAD, cat. no. 500-0006) and adjusted for loading on gels. Hsp70 blots from some patients were repeated to confirm sample integrity and when this was not observed, samples were excluded. HspBP1 expression in tumor and normal tissue was determined by Western blots. A total of 3 μg of tissue lysate of each sample were analyzed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, purified HspBP1 (full length) was included for a standard curve using twofold serial dilutions beginning at 0.5 μg. The membrane was blocked with 5% nonfat dry milk in PBS for 30 min at room temperature and incubated with sheep anti-HspBP1 at a concentration of 1 μg/mL for 1 h. The blot was incubated with the secondary antibody (1:1,000 HRP-Rabbit anti-sheep IgG, Zymed, San Francisco) overnight at 4°C. Actin expression was used as a control for basal constitutive protein synthesis, using an anti-human actin antibody (A4700, Sigma, St. Louis, MO, USA). Samples with nondetectable actin were discarded. For detection, blots were incubated with the ECL system (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) for 5 min and exposed to X-ray film (Kodak, Rochester, NY, USA) at different times. The concentration of HspBP1 in tumor and normal tissue bands was determined according to the area of these bands in relation to a curve of areas plotted by the Imagemaster software from Pharmacia. Data was expressed as concentration of HspBP1 per microgram of total protein. Quantitations were repeated a minimum of three times. The molar ratio HspBP1/Hsp70 was calculated in tumor and normal tissues of breast cancer patients based on a molecular weight of 70 kD for Hsp70 and 40 kD for HspBP1.
Histology
Tumor samples were fixed in formaldehyde 10%, embedded in paraffin, sectioned (4 μm), and applied to histology slides. Antigen recovery was performed in Tris–ethylenediaminetetraacetic acid buffer pH 9.0 by microwave for 25 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide and 0.1% sodium azide. Additional blocking was performed with 5% nonfat dry milk. Incubation with primary antibody 1:200 (sheep anti-HspBP1 or normal sheep serum) was performed overnight at room temperature, followed by incubation with anti-sheep IgG-HRP (Zymed). Slides were developed with DAB and hydrogen peroxide. The slides were analyzed in a BX50 Olympus Microscope, and the pictures were acquired with a DXC 107A/P CCD Iris Sony camera using the Image pro-plus 4.5.1 Software (Media Cybernetics).
Statistical analysis
Statistical analysis was performed using the SPSS software version 10 (SPSS, Chicago, IL, USA) and GraphPad prism 4 (San Diego, CA, USA). The data were tested for normality of distribution using Shapiro–Wilk’s W test and Kolmogorov–Smirnov. None of the data were found to be normally distributed. The Mann–Whitney U (between two groups) and Krustal–Wallis (three or more groups) tests were used to assess differences between patients and healthy individuals as well as normal tissue and tumor breast tissue. Also, these tests were used to assess the relationship between the data with the prognostic markers and patient’s outcome. All statistical tests were two sided, and p < 0.05 was considered to be statistically significant. Correlations between the data were evaluated using Spearman rank correlation coefficients. Kaplan–Meier survival curves were calculated from the data using the percent survival of the patients with a long-rank test.
Results
Patient and tumor characteristics
The mean age of patients was 58.39 ± 17.01 years old, and 49.3% had a menopause status. The mean age for healthy individuals was 36 ± 11 years. The mean of tumor size was 2.63 ±1.5 cm. Tumor characteristics, such as histological type of the tumor, tumor size, lymph node invasion by tumor, grade, staging (TNM classification), and lymphocyte infiltrate and estrogen and progesterone receptors status are summarized in Table 1.
Table 1.
Tumor characteristics
| Size (mean ± SD) | 2.63 ± 1.5 cm |
| Auxiliary lymph node status | |
| Node negative | 48.9% |
| 1–3 | 31.3% |
| 4–10 | 11.1% |
| >10 | 8.9% |
| Staging | |
| I | 29.5% |
| IIA | 26.2% |
| IIB | 19.7% |
| IIIA | 4.9% |
| IIIB | 8.2% |
| IIIC | 8.2% |
| IV | 3.3% |
| Tumor histological type | |
| Invasive ductal | 70.3% |
| Invasive lobular | 18.8% |
| Invasive tubule—lobular | 6.3% |
| Intraductal | 3.1% |
| Mucinous | 1.6% |
| Tumor differentiation | |
| Grade I | 10.4% |
| Grade II | 64.6% |
| Grade III | 25.0% |
| Lymphocyte infiltrate | |
| Absent | 23.8% |
| Present | 76.2% |
| Estrogen receptor | |
| Negative | 13.3% |
| Positive | 86.7% |
| Progesterone receptor | |
| Negative | 43.3% |
| Positive | 56.7% |
HspBP1 and Hsp70 are elevated in sera of patients compared to controls
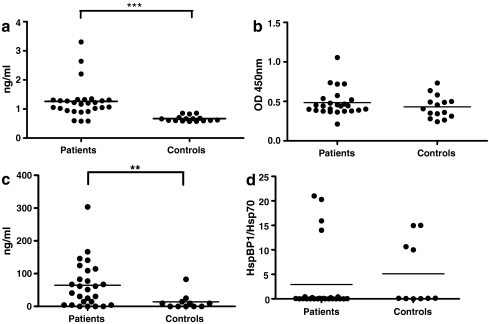
The levels of serum HspBP1 and anti-HspBP1 antibody in patients and normal individuals were measured. HspBP1 was significantly higher in sera of patients compared with sera of healthy individuals with means of 1.25 and 0.66 ng/ml, respectively (Fig. 1A). The levels of anti-HspBP1 antibodies did not differ significantly between groups (Fig. 1b). Hsp70 levels were also measured to test if the serum HspBP1/Hsp70 molar ratio differed between patients and controls. In general, the mean levels of serum Hsp70 (63.87 ng/ml) were much higher than the mean levels of serum HspBP1 (1.25 ng/ml) and the mean Hsp70 levels (63.87 ng/ml) were significantly higher in sera of patients compared to healthy individuals (13.69 ng/ml; Fig. 1C). However, the serum HspBP1/Hsp70 molar ratio was not different between patients with breast cancer tumors and healthy individuals (Fig. 1D).
Fig. 1.
Levels of serum HspBP1, Hsp70, anti-HspBP1 antibody, and serum HspBP1/Hsp70 ratios in breast cancer patients and controls. Serum samples of breast cancer patients (n = 27) and healthy individuals (n = 16) was used to quantify a Serum HspBP1 (Mann–Whitney, p < 0.001); b Anti-HspBP1; c Hsp70 (Mann–Whitney, p < 0.001); d Molar ratio of HspBP1/Hsp70. The ratio was calculated based on a molecular weight of 70 kD for Hsp70 and 40 kD for HspBP1
HspBP1 expression by tumor and normal tissues
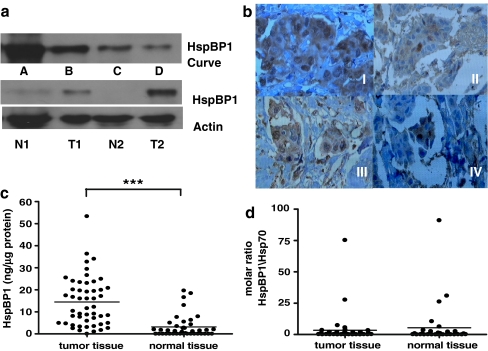
It has previously been determined that Hsp70 is elevated in tumor tissue of breast cancer patients (Torronteguy et al. 2006). HspBP1 and Hsp70 are both elevated in two mouse tumor cell lines (Raynes et al. 2003); however, to date, nothing is known on the expression of HspBP1 in human primary tumors. HspBP1 expression was quantified in tumor and normal tissue by western blot analysis in breast cancer patient samples. Figure 2A shows a typical result for HspBP1 expression in normal and tumor tissue of patients. A summary of the data are presented in Fig. 2C, showing that tumor tissue presented a significantly higher expression of HspBP1 (mean value of 14.46 ng/μg protein) than normal adjacent tissue (mean value of 3.17 ng/μg protein; p < 0.001). However, the mean molar ratio (HspBP1/Hsp70) did not differ between tumor and normal tissue (Fig. 2D).
Fig. 2.
HspBP1 levels in normal and tumor tissue. Tissue samples were analyzed by Western blot for HspBP1 expression and quantified using a standard curve of purified HspBP1. a Standard curve for HspBP1. Lanes A–D are serial dilutions of HspBP1 starting at 0.5 μg. T tumor tissue; N, normal tissue, probed with anti-HspBP1 or anti-actin. Numbers next to these letters denote the patient. b Tissue samples of breast cancer were analyzed by immunohistochemistry for HspBP1. I Tumor tissue with high HspBP1; II Tumor tissue with low HspBP1; III Tumor tissue with high molar ratio; IV Tumor with low molar ratio HspBP1/Hsp70. Magnification 400×. c Levels of HspBP1 in normal (n = 41) and tumor tissue (n = 51) of breast cancer patients quantified by Western blot analysis. d Data were analyzed using a t test (p < 0.001) and the distribution is normal. The molar ratio of HspBP1/Hsp70 was calculated as described in Fig. 1
Histology
HspBP1 levels are elevated in tumor tissue; therefore, it is possible that the distribution pattern of HspBP1 differs in tumors samples from patients with high versus low HspBP1 levels. Tumor sections from these two groups of patients were sectioned and analyzed for HspBP1. Also, tumors from patients with the two highest and two lowest HspBP1/Hsp70 molar ratios were examined for HspBP1 distribution. The results, shown in Fig. 2B, confirm what was reported by Western blot—expression of HspBP1 is higher in tumor cells and low or absent in surrounding normal tissue (see Fig. 2b I and II). Tumors with high and low expression of HspBP1 (determined by Western blot) do not show differences in the distribution pattern of the protein that is localized in both the nucleus and the cytoplasm. This is similar to what has been described for Hsp70 in these patients. The same was observed for patients that presented a high (Fig. 2D III) versus a low (IV) HspBP1/Hsp70 molar ratio.
Associations between serum and tissue levels
The data obtained for HspBP1 and Hsp70 in the sera of patients and controls was analyzed for correlations with the data obtained in tissue samples. A Spearman correlation test was performed and the results are shown in Table 2. Serum levels of HspBP1, Hsp70 or anti-HspBP1 antibody did not significantly correlate with tissue expression of HspBP1 or Hsp70. Interestingly, we found that HspBP1 in normal tissue correlated negatively with Hsp70 in tumor tissue (p < 0.05). As expected, molar ratios correlated negatively with HspBP1 expression, and positively with Hsp70 expression, but only in the compartment (tissue or serum) analyzed.
Table 2.
Correlations between the data (Spearman correlation)
| Serum HspBP1 | Serum Hsp70 | Anti-HspBP1 | Serum HspBP1/Hsp70 | HspBP1 in normal tissue | HspBP1 in tumor tissue | HspBP1/Hsp70 molar ratio tumor tissue | HspBP1/Hsp70 molar ratio normal tissue | |
|---|---|---|---|---|---|---|---|---|
| Serum HspBP1 | 1 | 0.353 | 0.053 | −0.1 | −0.285 | −0.184 | 0.173 | −0.277 |
| Serum Hsp70 | 0.353 | 1 | 0.36 | −0.941** | −0.349 | 0.268 | 0.169 | 0.105 |
| Anti-HspBP1 | 0.053 | 0.36 | 1 | −0.302 | −0.503 | 0.088 | 0.02 | −0.378 |
| Serum HspBP1/Hsp70 | −0.1 | −0.941** | −0.302 | 1 | 0.16 | −0.332 | −0.091 | −0.209 |
| HspBP1 in normal tissue | −0.285 | −0.349 | −0.503 | 0.16 | 1 | 0.255 | 0.478** | 0.666** |
| HspBP1 in tumor tissue | −0.184 | 0.268 | 0.088 | −0.332 | 0.255 | 1 | 0.634** | 0.212 |
| Hsp70 in tumor tissue | −0.214 | −0.058 | 0.26 | −0.025 | −0.400* | −0.005 | −0.719** | −0.530** |
| Hsp70 in normal tissue | −0.075 | 0.058 | 0.224 | −0.077 | −0.137 | −0.042 | −0.342* | −0.796** |
*p < 0.05
**p < 0.01
Association with tumor aggressiveness markers
The serum levels of these proteins, as well as the HspBP1/Hsp70 molar ratios, were analyzed for association with tumor aggressiveness markers (progesterone and estrogen receptors, auxiliary lymph node status, tumor size, stage, histology grade, and lymphocyte infiltrate). Mann–Whitney and Kruskall–Wallis analysis of the means of tumors with different characteristics were performed. None of the tested parameters in the sera were significantly associated with tumor aggressiveness markers (data not shown).
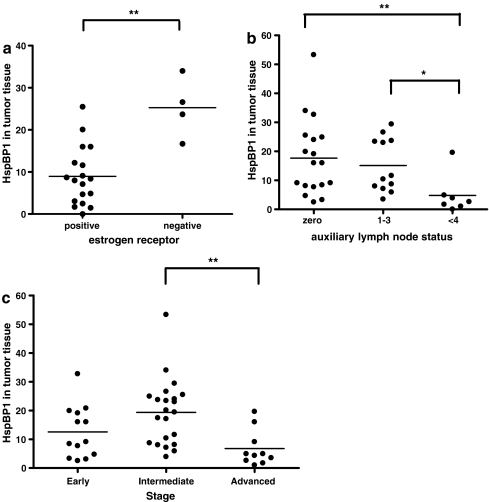
Associations between the protein levels and tumor characteristics (progesterone and estrogen receptors, auxiliary lymph node status, tumor size, stage, histology grade, and lymphocyte infiltrate) were analyzed. Mean levels of HspBP1 in tumor samples were significantly lower in patients with tumors that were positive for estrogen receptor, at an advanced stage, or with metastatic auxiliary lymph nodes. The graphs in Fig. 3 detail the significant differences for each of these parameters. HspBP1 levels were significantly lower in tumors of patients with an increased number of compromised lymph nodes (Fig. 3b). Also, HspBP1 levels were significantly lowest in patients with tumors in an advanced stage with statistical significance when compared to intermediate stage tumors (Fig. 3C). This trend was present in early stage tumors but it was not statistically significant. The other tumor characteristics were not significantly associates with HspBP1 levels in tumor tissue (data not shown)
Fig. 3.
Associations between HspBP1 levels in breast tumors and prognostic markers. a Estrogen receptor (negative and positive; Mann–Whitney, p < 0.05). b Auxiliary lymph node status based on pathological classification of TNM (zero, 1–3, more than 4; Krustal–Wallis with Dunn’s pos hoc test, p < 0.05) and c Stage (Early = Stage I; Intermediate = Stage IIa and IIb; Advanced = Stage IIIa, IIIb, IIIc, and IV; Krustal–Wallis with Dunn’s pos hoc test, p < 0.05)
Follow-up
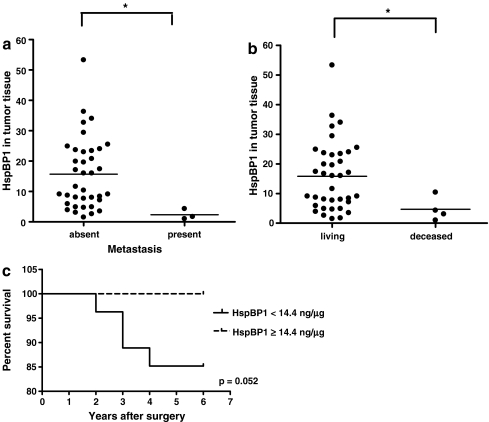
For all patients enrolled in this study 80% of patients have been under clinical follow-up for 6 to 7 years, and the other 20% do not return to clinical following in the São Lucas Hospital. All patients in the clinical follow-up received surgical treatment and tamoxifen for 5 years after the surgery. Some patients received chemotherapy and radiotherapy based on stage and treatment response. Their clinical data was obtained at the time of this analysis to determine if the levels of HspBP1 as well as the other measurements performed in this study were associated with the patients’ clinical outcome. Associations were analyzed (levels of HspBP1 and Hsp70 in serum, levels of anti-HspBP1, levels of HspBP1 and HspBP1/Hsp70 in tumor and normal tissue) with the presence of distance metastasis, tumor relapse, and patient death. It was found that tumor mean HspBP1 levels were significantly lower in patients that had metastasis and died. Figure 4A and B shows graphs detailing the significant differences for each parameter. This result suggested that a low HspBP1 expression in primary tumors can be an indicator of a poor prognosis. None of the other parameters were significantly associated with the patients’ clinical outcome (data not shown). Survival curves for the patients based on their tissue and serum expression of HspBP1, as well as their HspBP1/Hsp70 molar ratios were made. To categorize high and low expression of HspBP1, patients were divided into two groups: below, equal, or above the mean levels. For HspBP1/Hsp70 molar ratio, patients were categorized in groups with molar ratios that were equal or above 4 (value known to result in inhibition of 50% of Hsp70 ATPase activity), versus patients with molar ratios below 4. None of the differences were statistically significant (data not shown), however, all patients with high levels of HspBP1 in tumor tissue survived, and the difference was borderline significant (p < 0.052; Fig. 4C).
Fig. 4.
Associations between HspBP1 levels in breast tumors and patient outcomes. a Presence or absence of metastasis (Mann–Whitney, p < 0.05). b Deceased or alive (Mann–Whitney, p < 0.05). c Kaplan–Meier survival curve of HspBP1 in tumor tissue (the mean was 14.4 ng/ml and groups were either below, or equal and above mean levels)
Discussion
To our knowledge, this is the first report of HspBP1 levels in human primary tumors. The results showed that HspBP1 expression was increased both in tumor tissue and sera of breast cancer patients compared to normal adjacent tissue and healthy normal individuals. These data agree with previous work on two mouse tumors models that demonstrated high expression of HspBP1 in the examined tumors (Raynes et al. 2003). Interestingly, Hsp70 is also highly expressed both in murine (Raynes et al. 2003) as well as human tumors (Kaur and Ralhan 1995; Ralhan and Kaur 1995; Torronteguy et al. 2006). This is the first study to demonstrate that serum Hsp70 levels are also increased in breast cancer patients compared to healthy individuals. A previous other study reported serum Hsp70 was elevated in lung cancer patients (Suzuki et al. 2006).
Nevertheless, no significant differences were observed when comparing the soluble HspBP1/Hsp70 molar ratio in patients and controls. Also, no differences were observed when we compared the HspBP1/Hsp70 molar ratio in primary neoplasic breast tissue and adjacent normal breast tissue. The fact that both Hsp70 and HspBP1 levels were elevated in tumor samples could at least in part explain why we did not observe any significant differences when comparing the levels of HspBP1/Hsp70 molar ratios in the groups analyzed. Overall, the HspBP1/Hps70 molar ratio in serum as well as in breast tissue was distributed within a small range; just a few samples had molar ratio greater than four (the amount previously determined to inhibit 50% of Hsp70 activity). This finding agrees with what was previously demonstrated by Raynes et al. (2003), who found that molar ratios in murine tumor lines also varied within a small range.
The serum levels of HspBP1, Hsp70, or anti-HspBP1 antibody did not significantly correlate with tumor or healthy breast tissue expression of HspBP1 or Hsp70. We hypothesized that the proteins found in sera could be released by other, nonanalyzed tissue, since it has previously been shown that HspBP1 mRNA is expressed in different tissues (Raynes and Guerriero 1998). Serum HspBP1 and anti-HspBP1 antibody were previously demonstrated to be present in healthy individuals (Raynes et al. 2006), HspBP1 is present in human serum at concentrations ranging between 0.74 to 3.98 ng/mL. Also, it was demonstrated that there is no significant correlation between HspBP1 and anti-HspBP1 (Raynes et al. 2006), thus agreeing with our present study.
Extracellular Hsp70 released from tumor cells undergoing necrosis, as well as circulating in the bloodstream was associated with enhancement of tumor growth (Asea et al. 2000; Calderwood et al. 2005). Also, Hsp70 expression in tumor tissue was associated with a poor prognosis in breast cancer patients (Ciocca et al. 1993; Elledge et al. 1994; Thanner et al. 2003; Torronteguy et al. 2006). However, we were not able to see any significant association between serum Hsp70 levels and prognostic markers. Tissue HspBP1 levels was associated with prognostic markers, HspBP1 levels were significantly lower in patients who had advanced stage and metastasis in auxiliary lymph nodes. Involvement of auxiliary lymph nodes is the most reliable and reproducible prognostic indicators for primary breast cancer; in general, 50% to 70% of patients with positive lymph nodes have a relapse (Fisher et al. 1983). This indicates that lower expression of HspBP1 in primary breast tissue could be related with poor patient’s outcome. Mean levels of HspBP1 were also significantly lower in patients who had positive estrogen receptors (ER). The relationship with prognosis of ER expression in breast cancer is still controversial. Although ER positive tumors are more often well differentiated, associated with favorable prognostic characteristics and are predictors of a favorable response to endocrine therapy (Osborne 1998), some studies have suggested that very high levels of receptor may be associated with poor prognosis (Fisher et al. 1988; Thorpe et al. 1993; Hilsenbeck et al. 1998). Receptor data may be of greater value when combined with other prognostic factors. For this reason, it is still too early to draw conclusions from the association of HspBP1 with estrogen receptors; however, we believe that in this study, the significantly lower mean of HspBP1 in patients with positive estrogen receptor may be related to tumor aggressiveness, since two other important tumor aggressiveness markers were correlated with HspBP1, namely, staging and lymph node status.
After a 6- to 7-year follow-up, we found that patients that had metastasis and died presented significantly lower mean levels of HspBP1 in their primary tumors. This result reinforced the hypothesis that the lower expression of HspBP1 in primary breast tissue could be related to poor patient outcome. Although we were not able to see a statistically significant difference between the groups with low or high HspBP1 using the Kaplan–Meier survival curve, a trend could be observed since the results are on the verge of significance (p < 0.051). Studies with a higher patient number will enable us to test if HspBP1 levels in primary tumors are associated with survival. The molecular mechanisms responsible for overexpression of HspBP1 in tumor cells are unknown and may be tumor specific.
The observations that primary tumor cells have elevated levels of HspBP1 and that the levels of HspBP1 are associated with poor prognostic markers therefore raise intriguing questions regarding whether this protein confers a selective prosurvival advantage to such cells, contributing to the process of tumorigenesis. There are a number of reports indicating that Hsp70 enhances tumorigenic potential, and several candidate mechanisms have been suggested based on the molecular evidence. We demonstrated that HspBP1 was localized in cytoplasm and nucleus of breast tumor tissue cells, the same localization already described for Hsp70 expression in tumors (Vargas-Roig et al. 1998; Torronteguy et al. 2006; Ramp et al. 2007). It remains to be tested if HspBP1 can be involved in tumor survival in a manner associated with Hsp70 and if there are any differences between the activities of this protein found in the nucleus or cytoplasm.
Breast cancer is the most common malignancy for women throughout the industrialized world. In general, most individuals with cancer do not die from the tumor in the primary site, but rather from local invasion and/or distant metastasis (Woodhouse et al. 1997). Breast cancer is a heterogeneous disease, and there is a continual drive to identify markers that will aid in predicting prognosis and response to therapy. To date, relatively few markers have established prognostic power (Payne et al. 2008). This present study has shown that HspBP1 is elevated in breast cancer tumors, and lower levels are associated with poor prognosis. This inverse relationship could be a candidate for a new cancer marker.
Acknowledgements
We wish to thank the patients that consented to participate in this study; Tiago Giuliane Lopes in the Pathology Department for the expert technical support with the histology studies; Dr. Moises Evandro Bauer for statistical support. Also, we wish to thank the financial support from FAPERGS, CNPq and the National Institute of General Medical Sciences (GM072628–02 (V.G.)).
Footnotes
This work was supported by FAPERGS, CNPq, and the National Institute of General Medical Sciences grant number GM072628-02 (V.G.)
The expression of HspBP1 (an Hsp70 co-chaperone) was analyzed in tumor samples and sera from breast cancer patients. HspBP1 is over expressed in these tumors and a seven year follow-up analysis found an association with a poor prognosis. Chaperones have been shown to play important roles in tumor biology and immunology; therefore, we believe the data in this study will serve as a basis for the formulation of a new hypothesis on chaperone-co-chaperone interactions and their role in tumor growth.
References
- Asea A, Kraeft SK, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;64:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Clark GM, Fuqua SA, Yu YY, Allred DC. p53 protein accumulation detected by five different antibodies: relationship to prognosis and heat shock protein 70 in breast cancer. Cancer Res. 1994;5414:3752–3757. [PubMed] [Google Scholar]
- Fisher B, Bauer M, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::AID-CNCR2820520902>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol. 1988;67:1076–1087. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- Gottwald E, Herschbach M, Lahni B, Miesfeld RL, Kunz S, Raynes DA, Guerriero V. Expression of the cochaperone HspBP1 is not coordinately regulated with Hsp70 expression. Cell Biol Int. 2006;30:553–558. doi: 10.1016/j.cellbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hilsenbeck SG, Ravdin PM, Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52:227–237. doi: 10.1023/A:1006133418245. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Kabani M, McLellan C, Raynes DA, Guerriero V, Brodsky JL. HspBP1, a homologue of the yeast Fes1 and Sls1 proteins, is an Hsc70 nucleotide exchange factor. FEBS Lett. 2002;531:339–342. doi: 10.1016/S0014-5793(02)03570-6. [DOI] [PubMed] [Google Scholar]
- Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer. 1995;63:774–779. doi: 10.1002/ijc.2910630604. [DOI] [PubMed] [Google Scholar]
- McLellan CA, Raynes DA, Guerriero V. HspBP1, an Hsp70 cochaperone, has two structural domains and is capable of altering the conformation of the Hsp70 ATPase domain. J Biol Chem. 2003;278:19017–19022. doi: 10.1074/jbc.M301109200. [DOI] [PubMed] [Google Scholar]
- Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998;51:227–238. doi: 10.1023/A:1006132427948. [DOI] [PubMed] [Google Scholar]
- Papp D, Prohaszka Z, Kocsis J, Fust G, Banhegyi D, Raynes DA, Guerriero V. Development of a sensitive assay for the measurement of antibodies against heat shock protein binding protein 1 (HspBP1): increased levels of anti-HspBP1 IgG are prevalent in HIV infected subjects. J Med Virol. 2005;76:464–469. doi: 10.1002/jmv.20384. [DOI] [PubMed] [Google Scholar]
- Payne SJ, Bowen RL, Jones JL, Wells CA. Predictive markers in breast cancer—the present. Histopathology. 2008;521:82–90. doi: 10.1111/j.1365-2559.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- Ralhan R, Kaur J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin Cancer Res. 1995;110:1217–1222. [PubMed] [Google Scholar]
- Ramp U, Mahotka C, Heikaus S, Shibata T, Grimm MO, Willers R, Gabbert HE. Expression of heat shock protein 70 in renal cell carcinoma and its relation to tumor progression and prognosis. Histol Histopathol. 2007;2210:1099–1107. doi: 10.14670/HH-22.1099. [DOI] [PubMed] [Google Scholar]
- Raynes DA, Guerriero V., Jr Inhibition of Hsp70 ATPase activity and protein renaturation by a novel Hsp70-binding protein. J Biol Chem. 1998;273:32883–328888. doi: 10.1074/jbc.273.49.32883. [DOI] [PubMed] [Google Scholar]
- Raynes DA, Graner MW, Bagatell R, McLellan C, Guerriero V. Increased expression of the Hsp70 cochaperone HspBP1 in tumors. Tumour Biol. 2003;24:281–285. doi: 10.1159/000076459. [DOI] [PubMed] [Google Scholar]
- Raynes DA, Thomson CA, Stroster J, Newton T, Cuneo P, Guerriero V. Human serum contains detectable levels of the Hsp70 cochaperone HspBP1 and antibodies bound to HspBP1. J Immunoassay Immunochem. 2006;27:251–264. doi: 10.1080/15321810600734935. [DOI] [PubMed] [Google Scholar]
- Shomura Y, Dragovic Z, et al. Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell. 2005;173:367–379. doi: 10.1016/j.molcel.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ito Y, et al. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1733–1737. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- Thanner F, Sutterlin MW, et al. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res. 2003;232A:1057–1062. [PubMed] [Google Scholar]
- Thorpe SM, Christensen IJ, Rasmussen BB, Rose C. Short recurrence-free survival associated with high oestrogen receptor levels in the natural history of postmenopausal, primary breast cancer. Eur J Cancer. 1993;29(A7):971–977. doi: 10.1016/S0959-8049(05)80204-7. [DOI] [PubMed] [Google Scholar]
- Torronteguy C, Frasson A, Zerwes F, Winnikov E, Silva VD, Menoret A, Bonorino C. Inducible heat shock protein 70 expression as a potential predictive marker of metastasis in breast tumors. Cell Stress Chaperones. 2006;11:34–43. doi: 10.1379/CSC-159R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–475. doi: 10.1002/(SICI)1097-0215(19981023)79:5<468::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Volm M, Mattern J, Stammler G. Up-regulation of heat shock protein 70 in adenocarcinomas of the lung in smokers. Anticancer Res. 1995;156B:2607–2609. [PubMed] [Google Scholar]
- Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(Suppl):1529–1537. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1529::AID-CNCR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]