Abstract
Oxidative stress is one of the main causes of myocardial injury, which is associated with cardiomyocyte death. Mitochondria play a key role in triggering the necrosis and apoptosis pathway of cardiomyocytes under oxidative stress. Although prohibitin (PHB) has been acknowledged as a mitochondrial chaperone, its functions in cardiomyocytes are poorly characterized. The present research was designed to investigate the cardioprotective role of PHB in mitochondria. Oxidative stress can increase the PHB content in mitochondria in a time-dependent manner. Overexpression of PHB in cultured cardiomyocytes by transfection of recombinant adenovirus vector containing PHB sense cDNA resulted in an increase of PHB in mitochondria. Compared with the non-transfection cardiomyocytes, PHB overexpression could protect the mitochondria from oxidative stress-induced injury. The mitochondria-mediated apoptosis pathway was consistently suppressed in PHB-overexpressed cardiomyocytes after hydrogen peroxide (H2O2) treatment, including a reduced change in mitochondrial membrane permeability transition and an inhibited release of cytochrome c from mitochondria to cytoplasma. As a result, the oxidative stress-induced cardiomyocyte apoptosis was suppressed. These data indicated that PHB protected the cardiomyocytes from oxidative stress-induced damage, and that increasing PHB content in mitochondria constituted a new therapeutic target for myocardium injury.
Keywords: Apoptosis, Cardiomyocyte, Mitochondria, Oxidative stress, Prohibitin
Introduction
Prohibitin is a multifunctional, highly conserved protein involved in mitochondrial structure, function, and inheritance (Ikonen et al. 1995; Berger and Yaffe 1998; Thompson et al. 1999, 2003; Nijtmans et al. 2000, 2002; Gregory-Bass et al. 2008; Kasashima et al. 2008; Merkwirth et al. 2008; Schleicher et al. 2008), cell cycle regulation (Nuell et al. 1991; Wang et al. 1999a, b), transmembrane signal transduction (Terashima et al. 1994; Montano et al. 1999; Rajalingam and Rudel 2005; He et al. 2008), and control of life span (Coates et al. 1997). To date, the best described function of mitochondrial PHB is as a chaperone protein for the stabilization of newly synthesized subunits of mitochondrial respiratory enzymes (Nijtmans et al. 2002). PHB is also essential for normal mitochondrial development, and its deficiency in Caenorhabditis elegans is associated with inhibition of mitochondrial biogenesis and senescence (Artal-Sanz et al. 2003). Recently, Vessal and colleagues have found that PHB existing in extracellular fluid of cultured adipocytes can be translocated to mitochondria and modulates mitochondrial fuel metabolism by inhibition of pyruvate carboxylase (Vessal et al. 2006). Previous studies in our laboratory (Liu et al. 2004) using proteomics technology have found PHB expression in mitochondria increased dramatically when cardiac structures and functions were injured by chronic restraint stress. However, the function of PHB in cardiomyocytes is still far from clear.
Oxidative stress is considered a causative agent in inducing cardiomyocyte death. In an attempt to understand the role of PHB, we investigated the effect of PHB on cardiac and mitochondrial functions after oxidative stress. Here we report that mitochondrial content of PHB in cardiomyocytes increases gradually when the duration of H2O2-treated time is prolonged. Furthermore, overexpression of PHB can relieve H2O2-induced cardiomyocyte injury by elevating ATP synthesis, stabilizing mitochondrial membrane potential (MMP) and delaying the onset of apoptosis through inhibiting the release of cytochrome c from mitochondria.
Materials and methods
Preparation and culture of cardiomyocytes
Methods for primary culture of neonatal rat cardiac myocytes have been described previously (Simpson and Savion 1982). Briefly, enriched cultures of myocyte cells were obtained from 3-day-old neonatal rats by stepwise trypsin dissociation and plated at a density of 4 × 106 cells/60 mm dish or 3 × 105 cells/well on four-well glass dishes (Nunc, Denmark) in modified Eagle’s medium (MEM) (GIBCO, USA) supplemented with 20% fetal bovine serum (TBD, China), 0.01 mmol/l Brdu, 100 U/ml penicillin, and 100 μg/ml streptomycin. After 5 days, the cultures contained 90% cardiac myocytes contracting at >140 beats/min. The cells were seeded in the absence or presence of 200 μM H2O2 and incubated at 37°C in a water-saturated atmosphere containing 5% CO2 in air. Cells were harvested at various time points after seeding.
Western blot
Cultured cardiomyocytes were washed twice with PBS and harvested with 1 ml ice-cold RIPA lysis buffer and incubated for 1 h at 4°C. The insoluble material was removed by centrifugation at 10,000 ×g for 10 min at 4°C. Protein content was determined by Folin-phenol method with BSA as a standard substance. The proteins were then placed in diluted SDS sample buffer and denatured for 5 min at 99°C before being subjected to 12.5% SDS-PAGE. Forty micrograms of total protein was loaded in each lane, subjected to electrophoresis, and subsequently transferred to PVDF membranes (Millipore Corp., Bedford, MA, USA) by electroblotting. The membranes were blocked in TBS buffer (25 mM Tris–HCl, 150 mM NaCl, pH 7.5) containing 0.05% Tween-20 and 5% milk and incubated with a mouse monoclonal antibody against rat PHB (NeoMarkers, CA, USA), α-tubulin (Boster, China), CoxII (Invitrogen, USA), and cytochrome c (Santa Cruz, CA, USA) in blocking solution. Membranes were washed in TBS buffer containing 0.05% Tween-20. Immunoreactive proteins were visualized by enhanced chemiluminescence (ECL) reagents (Santa Cruz) using a horseradish peroxidase-conjugated secondary antibody (1:5,000) (Santa Cruz). Results of representative chemiluminescence were scanned and densitometrically analyzed using ImageMaster VDS system (Amersham, UK) with the help of the Imagequant TL site program.
Generation of recombinant adenoviral vectors and transfection of cardiomyocytes
Procedures for construction of recombinant adenoviral vectors were as described previously (Chowdhury et al. 2006). Briefly, prohibitin gene PHB cDNA was amplified by PCR using total RNA from rat heart tissue. The specific primers used were: (5′-GTCGACCATGGCTGCCAAAGT-3′, 5′-AAGCTTGGGGTGGGAGCAGAAGGAA-3′) containing SalI and HindIII sites (underlined), respectively. The PCR product was subcloned into the multiple cloning site of the shuttle plasmid pAdTrack-CMV (with GFP gene). The purified recombinant plasmids were linearized and co-electroporated with pAdEasy-1 adenoviral backbone vector into Escherichia coli BJ5183. The complete adenovectors were linearized and used for transfection of 293 cells, where viral particles were further amplified, purified, and titered according to GFP-positive units.
Cardiomyocytes were cultured on a six-well culture dish in MEM medium and infected with adenoviruses (multiplicity of infection of 25–50 plaque-forming units/cell) for 2 h. Subsequently, MEM medium with 10% fetal bovine serum was added to each dish, and 24 h after transfection, the media was removed and replaced with fresh media for 12 h. The cells were then incubated in the absence or presence of 200 μM H2O2 for different time periods.
Functional assay of cardiomyocytes
LDH activity assay
Cells were seeded at a density of 5 × 104 cells/ml in 200 μl medium per well in 96-well plates (TPP, Switzerland). Protein samples were diluted with 0.9% NaCl to a concentration of 45 μM and added to the cells to yield a final concentration of 15 μM. Cells were grown in the presence of 200 μM H2O2 for 3, 6, 12, and 24 h, and the medium was removed and collected for detection of LDH activity in the supernatants according to the supplier’s protocol. Briefly, 200 μl supernatant and 1,000 μl LDH reagent were added to a new 1-cm colorimetric cylinder following 30 min of incubation at room temperature in the dark. After incubation, the absorbance was read at a wavelength of 340 nm in a U-2100 spectrophotometer (Hitachi, Japan).
Creatine kinase MB (CK-MB) viability assay
Cells were seeded and grown under identical conditions as described above for the LDH. The CK-MB viability assay was performed according to the supplier’s protocol. Briefly, 200 μl supernatant and 1,000 μl CK-MB reagent were added to a new 1-cm colorimetric cylinder following 30 min of incubation at room temperature in the dark. After incubation, the absorbance was read at a wavelength of 340 nm in a U-2100 spectrophotometer (Hitachi, Japan).
MTT cell viability assay
Cells were treated as described above for the LDH and CK-MB assay. After 24 h, the medium was removed and 200 μl MTT medium (0.5 mg/ml MTT reagent in fresh medium) was added to each well. After incubation for 4 h, the MTT reagent was removed and 150 μl dimethyl sulfoxide was added to each well following 10 min of gentle shaking. The absorbance was read at a wavelength of 490 nm in a MULTISKAN MK3 (Thermo Electron Corporation, The Netherlands).
Analysis of cardiomyocyte apoptosis with flow cytometry
The detection of cardiomyocytes apoptosis was based on DNA content evaluation with the use of propidium iodide and flow cytometry (Nicoletti et al. 1991). Cardiomyocytes were washed twice with PBS, fixed with 70% ethanol for 30 min at 4°C, and then, after washing with PBS, were stained with propidium iodide (Sigma, 50 μg/ml) overnight at 4°C. The cardiomyocyte suspensions were analyzed with a FACSCalibur flow cytometer (Becton Dickinson, USA). The percentage of cells containing subdiploid DNA content, a characteristic of apoptosis, was quantified.
Mitochondrial function
Synthesis activity of mitochondrial H+-ATPase
Synthesis activity of mitochondrial H+-ATPase was measured by fluorescence bioluminescence (Qian et al. 2004). Mitochondria were isolated from cardiomyocyte as described above and adjusted to a protein concentration of 1 mg/ml. Ten-microliter samples of mitochondria were mixed with 100 ml luciferin–luciferase solution (containing 200 mg luciferin–luciferase). Adding ADP triggered the ATP synthesis reaction of the mitochondria, and the intensity of emitted light was detected with a luminosity spectrophotometer (FT632, Beijing Medicine Instrument Factory, Beijing, China). Synthesis activity of mitochondrial H+-ATPase was calculated according to the intensity of emitted light of determined content of ATP in the parallel standard test and described as micromoles per minute milligram protein.
Mitochondrial transmembrane potential
The cardiomyocyte MMP in vitro was measured by flow cytometric analysis performed on a FACSCalibur fitted with an excitation/emission setting of 488 nm/525 nm. The Rh123 was used to detect changes in the MMP. Cardiomyocytes were incubated with Rh123 (0.1 nM) in a 37°C water bath for 5 min in PBS. Moreover, 10,000 cells were analyzed by flow cytometry (Ward et al. 2000). Cellular mean fluorescence intensity was analyzed using Cell Quest software programs (Phoenix Flow Systems, CA, USA).
Release of cytochrome c from mitochondria
The analysis was performed mainly according to Kluck et al. (1997). Cardiomyocytes were harvested by centrifugation at 1,000 ×g for 10 min at 4°C. The cell pellets were washed with ice-cold phosphate-buffered saline (PBS) and resuspended with 5 vol of lysis buffer (0.25 mol/l sucrose, 20 mmol/l Hepes–KOH (pH 7.5), 10 mmol/l KCl, 1.5 mmol/l MgCl2, 1 mmol/EDTA, 1 mmol/l EGTA, 1 mmol/l DTT, 0.1 mmol/l PMSF). The cells were homogenized and then centrifuged at 750 ×g for 10 min at 4°C. The supernatants were centrifuged at 10,000 ×g for 15 min at 4°C and the resulting mitochondria pellets were resuspended in 1× SDS. The supernatants of the 1,000 ×g spin were further centrifuged at 100,000 ×g for 1 h at 4°C and the resulting supernatants were the S-100 fraction. Proteins in the supernatant (15 μl) of mitochondria preparation and from the S-100 fraction were subjected to SDS-PAGE (10–15%). Immunoblots for the determination of cytochrome c release were performed using a specific cytochrome c monoclonal antibody.
Statistical analyses
All experimental data were replicated a minimum of three times and expressed as mean ± SEM. Statistical analysis was performed by one way ANOVA using SPSS version 11.0 software, and multiple comparisons were done by Student t test. Differences were considered significant at p ≤ 0.05.
Results
Prohibitin expression changes in cardiomyocytes and mitochondria during oxidative stress
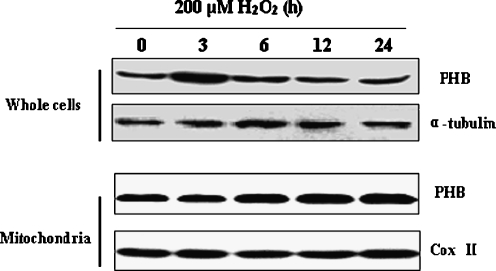
To investigate the function of PHB in cardiomyocyte, 200 μM H2O2 was used to cause cardiomyocytes to mimic the cellular effects of oxidative stress. The expression changes of PHB were examined at different treatment time periods and it was found that PHB protein expression reached maximal level at 3 h of treatment and then declined in cardiomyocytes (Fig. 1, upper bands). In contrast to this result, PHB levels in mitochondria began to increase after 6 h of treatment (Fig. 1, lower bands). These results confirmed that oxidative stress could increase the PHB content in mitochondria in a time-dependent manner. We also found that the mitochondrial level of PHB remained elevated when the whole cell content of PHB declined. These results suggested that there might be an increased translocation of PHB from cytoplasma to mitochondria upon oxidative stress.
Fig. 1.
Expression changes of prohibitin in cardiomyocyte and mitochondria during oxidative stress. PHB expression was detected by Western blot method. Cultured neonatal cardiomyocytes were treated with 200 μM H2O2 for different times. Protein samples were then extracted from cardiomyocytes (whole cells) and mitochondria. Alpha (α)-tubulin levels and CoxII were measured as an internal control for whole cells and mitochondria, respectively
Overexpression of prohibitin protects cardiomyocytes from oxidative stress-induced damage
To investigate the role of PHB in cardiomyocytes during oxidative stress, a recombinant adenovirus vector containing PHB sense cDNA and eGFP (Ad-PHB) was constructed. Primary cardiomyocytes were infected with adenovirus at a MOI of 35 pfu/cell for 24 h. Immunofluorescence microscopy was used to observe PHB-eGFP expression in infected cells. At a MOI of 35 pfu/cell, PHB-eGFP expression was detected in greater than 95% of cultured cells. Next, the expression levels of exogenous PHB in the infected cardiomyocytes were examined by using Western-blotting techniques. As expected, PHB levels increased dramatically in cells infected with adenovirus sense PHB cDNA, while cells infected with vector without PHB only showed a thin band (Fig. 2a).
Fig. 2.
Effects of recombinant adenovirus directed overexpression of prohibitin in cardiomyocytes on cell injury induced by oxidative stress. a Overexpression of PHB in cardiomyocytes by transient infection with recombinant adenovirus constructs containing PHB coding cDNA (MOI = 35 pfu/cell). Cardiomyocytes were infected with the adenoviral PHB constructs for 2 h and cultured for 24 h. Then, PHB content in cardiomyocytes and mitochondria was detected by Western blot method. Alpha (α)-tubulin levels and CoxII were measured as an internal control. b–d Cardiomyocytes were infected with adenovirus with PHB-coding (Ad-PHB) or non-coding (Ad) constructs at a MOI of 35 pfu/cell for 2 h and maintained in culture media for 24 h. Then, cardiomyocytes were cultured in fetal bovine serum-free media for 12 h and treated with 200 μM H2O2 for indicated time periods (0, 3, 6, 12, and 24 h). The survival rate of cardiomyocytes (b) and the enzyme activity of LDH (c) and CK-MB (d) in culture media were assayed subsequently. The bar graphs represented the mean ± SEM of results from six replicate experiments (b, c, and d). *p < 0.05 as compared with control. #p < 0.05 as compared with Ad + H2O2
Further experiments were performed in which 200 μM H2O2 was exposed to non-transfection and transfection cardiomyocytes to examine the association of PHB with oxidative stress-induced cardiomyocyte injury. Thus data from four groups could be obtained: without H2O2 intervention and adenovirus transfection group (control); H2O2 intervention group (H2O2); adenovirus with non-coding tranfection plus H2O2 intervention group (Ad + H2O2); and adenovirus with PHB-coding tranfection plus H2O2 intervention group (Ad-PHB + H2O2). Cardiomyocyte injuries were evaluated by an assay of cell viability, LDH activity, and CK-MB activity in culture media. According to Fig. 2b–d, when cultured cardiomyocytes were treated with 200 μM H2O2 for 12 h, cell viability assayed by MTT method apparently declined. Meanwhile, the LDH activity and CK-MB activity in culture media increased significantly. These data showed that cardiac injury was induced by H2O2. However, when cardiomyocytes were infected by Ad-PHB to elevate PHB content, the cellular injury caused by H2O2 treatment was reduced resulting in increased cell viability and reduced release of LDH and CK-MB to culture media. We also found there were no significant differences in LDH activity and CK-MB activity in culture media between the control group and the adenoviral empty vector control group. Therefore, it was confirmed that PHB played a protective role for cardiomyocytes under oxidative stress.
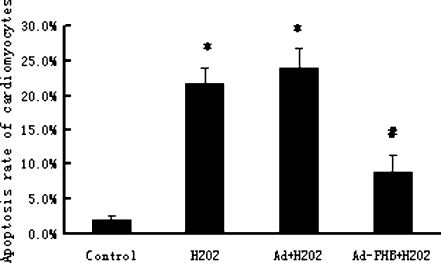
Overexpression of prohibitin inhibits oxidative stress-induced apoptosis in cardiomyocytes
In our previous study, we found that the PHB content in mitochondria increased in apoptotic cardiomyocytes induced by restraint stress (Liu et al. 2004). To determine whether PHB played a role in regulating the cardiomyocyte apoptosis induced by H2O2 intervention, the apoptotic rate of cardiomyocytes treated with 200 μM H2O2 for 12 h was examined by flow cytometry. As shown in Fig. 3, 200 μM H2O2 induced obvious apoptosis in normal cultured cardiomyocytes. But in PHB-overexpressed cardiomyocytes, the apoptotic rate induced by H2O2 decreased. Because no significant difference was found between the control group and the adenoviral empty vector control group, we concluded that overexpression of PHB could protect cardiomyocytes from H2O2 induced apoptosis.
Fig. 3.
Effects of recombinant adenovirus directed overexpression of prohibitin in cardiomyocyte on cellular apoptosis induced by oxidative stress. Cardiomyocytes were infected with adenovirus with PHB-coding (Ad-PHB) or non-coding constructs (Ad) at a MOI of 35 pfu/cell for 2 h and maintained in culture media for 24 h. Cardiomyocytes were then cultured in fetal bovine serum-free media for 12 h and treated with 200 μM H2O2 for 12 h. Cardiomyocyte apoptosis was analyzed with flow cytometry. The percentage of apoptotic cells was determined according to cells containing subdiploid DNA content. The bar graphs represented the mean ± SEM of results from six replicate experiments. *p < 0.05 as compared with control. #p < 0.05 as compared with Ad + H2O2
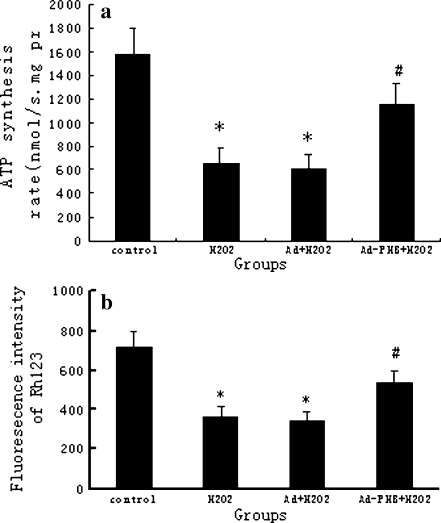
Overexpression of prohibitin increases mitochondrial H+-ATPase synthesis activity and inhibits the collapse of mitochondrial transmembrane potential in oxidative stress-injured cardiomyocytes
H+-ATPase synthesis activity is a comprehensive evaluation of the mitochondrial function. In vitro, the alteration of H+-ATPase synthesis activity in transfection and non-transfection cardiomyocytes was determined after 200 μM H2O2 treatment. Synthesis activity of mitochondrial H+-ATPase was calculated according to the intensity of emitted light of determined content of ATP in the parallel standard test. A comparative test revealed that H2O2 treatment for 12 h resulted in a significant decrease of mitochondrial H+-ATPase synthesis activity. As shown in Fig. 4a, the H+-ATPase synthesis activity in 200 μM H2O2-treated cardiomyocytes decreased to 42% of that in non-H2O2 treated cells. However, overexpression of PHB increased mitochondrial H+-ATPase synthesis activity significantly. Thus, overexpression of PHB maintained mitochondrial energy-producing function in oxidative stress-injured cardiomyocytes (no significant difference was found between the control group and the adenoviral empty vector control group).
Fig. 4.
Effect of overexpressed prohibitin on H+-ATPase synthesis activity and mitochondrial membrane potential in cardiomyocytes injured by oxidative stress. Mitochondria were isolated from four groups of cardiomyocytes including without 200 μM H2O2 intervention and adenovirus transfection group (control), 200 μM H2O2 intervention group (H22), adenovirus with non-coding tranfection plus 200 μM H2O2 intervention group (Ad + H22), and adenovirus with PHB-coding tranfection plus 200 μM H2O2 intervention group (Ad-PHB + H22). a mitochondrial H+-ATPase synthesis activity was measured by fluorescence bioluminescence and calculated according to the intensity of emitted light of determined content of ATP in the parallel standard test and described as micromole per minute milligram protein. b The cardiomyocyte mitochondrial membrane potential in vitro was measured by flow cytometry. The Rh123 was used to detect changes in the MMP and cellular mean fluorescence intensity was analyzed using Cell Quest software programs. The bar graph represents the mean ± SEM of results from six replicate experiments. *p < 0.05 as compared with control. #p < 0.05 as compared with Ad + H2O2
Mitochondrial membrane permeability transition is an important structure for maintaining mitochondrial homeostasis and membrane potential. Opening the mitochondrial membrane permeability transition pore (MPTP) can subsequently cause a series of cytological effects, including loss of mitochondrial transmembrane potentials, uncoupling of respiratory chain, leakage of mitochondrial Ca2+, excess generation of reactive oxygen species, and release of resident apoptosis-associated proteins. We examined the MMP to indicate the opening status of mitochondrial MPTP. As shown in Fig. 4b, MMP decreased almost 50% in cardiomyocytes under H2O2 intervention compared with that in normal cells. In contrast, overexpression of PHB partly inhibited the collapse of the electrochemical gradient across the mitochondrial membrane in oxidative stressed cardiomyocytes. There was no significant difference between the control group and the adenoviral empty vector control group in MMP.
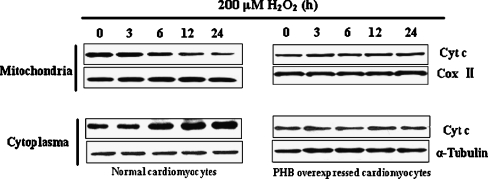
The release of cytochrome c from mitochondria to cytosol decreased in PHB-overexpressed cardiomyocyte treated with H2O2
In this study, we investigated whether overexpression of PHB inhibits cytochrome c release from the mitochondria to the cytosol in H2O2-treated cardiomyocytes. As shown in Fig. 5, the decrease of cytochrome c protein content was observed from isolated mitochondrial extract at 6 h after treatment with 200 μM H2O2 (left panel, upper bands); meanwhile, an increase in cytochrome c protein content was observed in cytosol (left panel, lower bands). This phenomenon became more apparent and continued at subsequent times, which confirmed that H2O2 induced the release of cytochrome c from the mitochondria to cytosol in cardiomyocytes. But when cardiomyocytes were transfected with adenovirus with PHB coding at a MOI of 35 pfu/cell, the tendency of cytochrome c release from mitochondria to cytosol was significantly reduced after treatment with 200 μM H2O2 (Fig. 5, right panel).
Fig. 5.
Changes of cytochrome c release from mitochondria to cytoplasma in PHB-overexpressed cardiomyocyte under H2O2 treatment. a Cultured cardiomyocytes were treated with 200 μM H2O2 for 0, 3, 6, 12, and 24 h, and cytochrome c content in mitochondrial and cytosolic extracts was assayed by Western blot. b Cultured cardiomyocytes were first infected with adenovirus with PHB-coding (Ad-PHB) at a MOI of 35 pfu/cell for 2 h and maintained in culture media for 24 h. Then, cardiomyocytes were cultured in fetal bovine serum-free media for 12 h and treated with 200 μM H2O2 for indicated time periods (0, 3, 6, 12, and 24 h). Cytochrome c content in mitochondrial and cytosolic extracts was assayed by Western blot. Alpha (α)-tubulin levels and CoxII were measured as an internal control for whole cells and mitochondria, respectively
Discussion
All living beings are continuously subjected to stress in everyday life. The cardiovascular system is the primary target organ attacked by stress. Most cardiovascular diseases such as hypertension, atherosclerosis, coronary artery disease, and myocardial infarction are closely related to the trigger of the stress response (Esch et al. 2002; McDougall et al. 2000; Irving et al. 1998; Knardahl and Hendley 1990). Our previous studies also observed that myocardial injury existed in rats exposed to restraint stress for 3 weeks. At the same time, the increased mitochondrial content of PHB in myocardium has been found by using two-dimensional electrophoresis method (Liu et al. 2004). The real roles of PHB in cardiomyocyte and mitochondria during chronic stress remain unclear, however.
PHB is a highly conserved protein in eukaryotic cells. Although it has been found in multiple cellular compartments and appears to have a diverse range of functions (Nijtmans et al. 2000; Gamble et al. 2004; Fusaro et al. 2003; Sharma and Qadri 2004), the more commonly known function of PHB is that of a chaperone for imported proteins in mitochondria. However, the functional association between PHB and cardiomyocyte injury has never been shown. Oxidative stress is considered one of the main causative agents in inducing cardiomyocyte death. In the present study, we focused on the roles of PHB in cardiomyocytes after oxidative stress. At first, we observed that PHB expression in the whole cells first increased and then declined while its mitochondrial content continued to rise after 6-h treatment when cultured cardiomyocytes were treated with 200 μM H2O2. These results confirmed that oxidative stress increased the PHB content in mitochondria in a time-dependent manner. Then another question came up. What is the function of PHB in cardiomyocytes during oxidative stress? A recombinant adenovirus vector was constituted to overexpress PHB in cardiomyocytes and mitochondria. It was found that increased content of PHB in cardiomyocytes and mitochondria relieved oxidative stress-induced cellular injury. This suggests that PHB possesses a protective function when cardiomyocytes are under oxidative stress.
PHB is predominantly associated with mitochondria and its content in mitochondria remains higher in cardiomyocytes undergoing oxidative stress. Our previous studies also observed that the damage to mitochondrial structure and function and their secondary biological effects were important mechanisms of myocardium injury induced by stress (Liu et al. 2004). A number of stimuli induce dysfunction and structural injury in mitochondria, especially alterations in mitochondrial membrane permeability, which causes a series of cellular events and ultimately leads to apoptosis and necrosis (McDougall et al. 2000; Qian et al. 2004). We thus presume that PHB protected cardiomyocytes from oxidative stress by maintaining the integrity of mitochondrial structure and function. In this research, we found that, compared with the non-transfection cardiomyocytes, PHB overexpression protected the mitochondria from oxidative stress-induced injury and suppressed the mitochondria-mediated apoptosis pathway, including the reduced change of mitochondrial membrane permeability transition, increased energy metabolism, and inhibited release of cytochrome c from mitochondria to cytosol. As a result, the stress-induced cardiomyocyte apoptosis was suppressed. Our finding that increased PHB levels provide protection to the cell from apoptosis is in agreement with previous observations in murine interleukin-3 (IL-3)-dependent FL5.12 cell line (Vander Heiden et al. 2002), osteosarcoma cells (Fellenberg et al. 2003) and rat granulose cells. Although our results provide evidence for a functional role of PHB in maintaining mitochondrial normal function in cardiomyocytes, the molecular mechanism(s) involved are not well known. Recently, some investigators have observed that there existed a close relationship between PHB and ATP synthase (Osman et al. 2007; Ferrer et al. 2007). PHB can organize and stabilize mitochondrial cristae (Merkwirth et al. 2008) and mitochondrial nucleoids (Kasashima et al. 2008) to maintain mitochondrial function.
Together, our results demonstrate that oxidative stress can induce the increased mitochondrial levels of PHB in cardiomyocytes. PHB overexpression in cardiomyocytes and mitochondria can combat the damaging effects of oxidative stress on cardiomyocytes and suppress mitochondria-mediated apoptosis by reduced change of mitochondrial membrane permeability transition, increased energy metabolism, and inhibited release of cytochrome c from mitochondria to cytosol. These data indicated that PHB protected the cardiomyocytes from oxidative stress-induced damage, and that increasing PHB content in mitochondria may constitute a new therapeutic target for oxidative stress-induced myocardium injury.
Acknowledgments
This research was supported by the General Program of the Chinese National Natural Science Foundation (grant no. 30570753) and the Major Research Plan of the Chinese National Natural Science Foundation (grant nos. 30430590 and 30393134).
Abbreviations
- PHB
prohibitin
- MEM
modified Eagle’s medium
- Cox II
cytochrome c oxidase subunit II
- LDH
lactate dehydrogenase
- CK
creatine kinase
- MMP
mitochondrial transmembrane potential
- MPTP
membrane permeability transition pore
- H2O2
hydrogen peroxide
- PBS
phosphate-buffered saline
Footnotes
XiaoHua Liu and Zhe Ren contributed equally to this work.
● Prohibitin is an evolutionarily conserved and ubiquitously expressed protein involved in mitochondrial structure, function, and inheritance whose function in cardiomyocyte is not known. In this study, we found oxidative stress could induce increased expression in cardiomyocytes and mitochondrial translocation of PHB, and PHB can protect against oxidative stress in cultured neonatal cardiomyocyte.
References
- Artal-Sanz M, Tsang WY, Willems EM, Grivell LA, Lemire BD, vander Spek H, Nijtmans LG, Sanz MA. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem. 2003;278:32091–32099. doi: 10.1074/jbc.M304877200. [DOI] [PubMed] [Google Scholar]
- Berger KH, Yaffe MP. Prohibitin family members interact genetically with mitochondrial inheritance components in Saccharomyces cerevisiae. Mol Cell Biolm. 1998;18:4043–4052. doi: 10.1128/mcb.18.7.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Xu W, Stiles JK, Zeleznik A, Yao X, Matthews R, Thomas K, Thompson WE. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus directed overexpression of prohibitin. Endocrinology. 2006;148:206–217. doi: 10.1210/en.2006-0187. [DOI] [PubMed] [Google Scholar]
- Coates PJ, Jamieson DJ, Smart K, Prescott AR, Hall PA. The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr Biol. 1997;7:607–610. doi: 10.1016/S0960-9822(06)00261-2. [DOI] [PubMed] [Google Scholar]
- Esch T, Stefano GB, Fricchione GL, Benson H. Stress-related diseases—a potential role for nitric oxide. Med Sci Monit. 2002;8:RA103–RA118. [PubMed] [Google Scholar]
- Fellenberg J, Dechant MJ, Ewerbeck V, Mau H. Identication of drug-regulated genes in osteosacoma cells. . Int J Cancer. 2003;105:636–643. doi: 10.1002/ijc.11135. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Perez E, Dalfó E, Barrachina M. Abnormal levels of prohibitin and ATP synthase in the substantia nigra and frontal cortex in Parkinson’s disease. Neurosci Lett. 2007;415:205–209. doi: 10.1016/j.neulet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Gamble SC, Odontiadis M, Waxman J, Westbrook JA, Dunn MJ, Wait R, Lam EW, Bevan CL. Androgens target prohibitin to regulate proliferation of prostate cancer cells. Oncogene. 2004;23:2996–3004. doi: 10.1038/sj.onc.1207444. [DOI] [PubMed] [Google Scholar]
- Gregory-Bass RC, Olatinwo M, Xu W, et al. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int J Cancer. 2008;122:1923–1930. doi: 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O’Malley BW. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol. 2008;22:344–360. doi: 10.1210/me.2007-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, Fiedler K, Parton RG, Simons K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett. 1995;358:273–277. doi: 10.1016/0014-5793(94)01444-6. [DOI] [PubMed] [Google Scholar]
- Irving RJ, White J, Chan R. The influence of restraint on blood pressure in the rat. J Pharmacol Toxicol Methods. 1998;38:157–162. doi: 10.1016/S1056-8719(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Kasashima K, Sumitani M, Satoh M, Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp Cell Res. 2008;314:988–996. doi: 10.1016/j.yexcr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bal-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Knardahl S, Hendley ED. Association between cardiovascular reactivity to stress and hypertension or behavior. Am J Physiol. 1990;259:H248–H257. doi: 10.1152/ajpheart.1990.259.1.H248. [DOI] [PubMed] [Google Scholar]
- Liu XH, Qian LJ, Gong JB, Shen J, Zhang XM, Qian XH. Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics. 2004;4:3167–3176. doi: 10.1002/pmic.200300845. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Paull JRA, Widdop RE, Lawrence AJ. Restraint stress: differential cardiovascular responses in Wistar–Kyoto and spontaneously hypertensive rats. Hypertension. 2000;35:126–129. doi: 10.1161/01.hyp.35.1.126. [DOI] [PubMed] [Google Scholar]
- Merkwirth C, Dargazanli S, Tatsuta T, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–88. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. Proc Natl Acad Sci U S A. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by a propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- Nijtmans LG, Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, vander Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans LG, Artal Sanz M, Grivell LA, Coates PJ. The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, aging and degenerative disease. Cell Mol Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuell MJ, Stewart DA, Walker L, et al. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol Cell Biol. 1991;11:1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C, Wilmes C, Tatsuta T, Langer T. Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol Biol Cell. 2007;18:627–635. doi: 10.1091/mbc.E06-09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian LJ, Song XL, Ren HR, Gong JB, Cheng SQ. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress & Chaperones. 2004;9:281–293. doi: 10.1379/CSC-20R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalingam K, Rudel T. Ras–Raf signaling needs prohibitin. Cell Cycle. 2005;4:1503–1505. doi: 10.4161/cc.4.11.2142. [DOI] [PubMed] [Google Scholar]
- Schleicher M, Shepherd BR, Suarez Y, et al. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol. 2008;180:101–112. doi: 10.1083/jcb.200706072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- Terashima M, Kim KM, Adachi T, Nielsen PJ, Reth M, Kohler G, Lamers MC. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WE, Powell JM, Whittaker JA, Sridaran R, Thomas KH. Immunolocalization and expression of prohibitin, a mitochondrial associated protein within the rat ovaries. Anat Rec. 1999;256:40–48. doi: 10.1002/(SICI)1097-0185(19990901)256:1<40::AID-AR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Ramalho-Santa J, Sutovsky P. Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod. 2003;69:254–260. doi: 10.1095/biolreprod.102.010975. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Choy JS, VanderWeele DJ, et al. Bcl-x (L) complements Saccharomyces cerevisiae genes that facilitate the switch from glycolytic to oxidative metabolism. J Biol Chem. 2002;277:44870–44876. doi: 10.1074/jbc.M204888200. [DOI] [PubMed] [Google Scholar]
- Vessal M, Mishra S, Moulik S, Murphy LJ. Prohibitin attenuates insulin-stimulated glucose and fatty acid oxidation in adipose tissue by inhibition of pyruvate carboxylase. FEBS J. 2006;273:568–576. doi: 10.1111/j.1742-4658.2005.05090.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–3510. doi: 10.1038/sj.onc.1202684. [DOI] [PubMed] [Google Scholar]
- Wang S, Nath N, Fusaro G, Chellappan S. Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol Cell Biol. 1999;19:7447–7460. doi: 10.1128/mcb.19.11.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MW, Rego AC, Frenguelli BG, Nicholls DG. Mitochondrial membrane potential and glutamate excitototoxicity in cultured cerebellar granule cells. J Neurosci. 2000;20:7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]