Abstract
Experimental evidence suggesting that heat shock protein 70 (Hsp70) gene or associated genes are responsible for the pathophysiology of hypertension is accumulating. In this study, we focused on five polymorphisms in three genes (HSPA1A, HSPA1B, and HSPA1L) of Hsp70 family to explore the genetic contribution, alone and in combination, of these polymorphisms to essential hypertension risk in a Uygur population. Genotyping was performed using PCR-RFLP and direct sequencing techniques. Data were analyzed using haplotype and multifactor dimensionality reduction (MDR) methods. Genotype distributions of all the polymorphisms satisfied the Hardy–Weinberg proportions in cases and controls. Statistical significance was only observed in the genotype (P = 0.0028) and (P = 0.0146) allele distributions of −110A/C polymorphism, with the −110C allele conferring a 1.45- and 2.83-fold of relative risk, assuming the additive and recessive models, respectively, and in 1267A/G genotype distribution (P = 0.0106) with the 1267G allele conferring a 44% reduced risk. The interaction information analysis indicated that polymorphisms −110A/C and 1267A/G had a strong synergistic effect, while polymorphisms 2074G/C and 2437T/C had a moderate synergistic effect. Haplotype analyses further strengthened the interaction information. Using the haplotype H1 as a reference, haplotype H4 had a 40% reduced risk, while haplotypes H5 and H8 had a significantly 5.00- and 3.75-fold increased risk for essential hypertension, respectively. Taken together, our results supported strong genetic interaction of the studied polymorphisms with the risk of having essential hypertension in Uygur ethnicity. Functional studies are warranted to confirm or refute these findings. This is the first study to evaluate the genetic interaction information of the Hsp70 in Uygur ethnicity, which represents one of the major nationalities in China with high homogeneity and unique lifestyles. Moreover, we employed the haplotype and MDR methods to explore the potential interaction of Hsp70 genetic polymorphisms in the pathogenesis of essential hypertension in Uygur.
Keywords: Heat shock protein 70, Uygur, Haplotype, Interaction, Single-locus, MDR
Introduction
Recently, the prevalence of hypertension has been rapidly escalating in China, and hypertension is recognized as a leading cause of morbidity and mortality among Chinese adults aged 40 years or older (He et al. 2005). Given that hypertension is an important risk factor for diseases of the circulatory system and appends a major burden on health and healthcare costs, an understanding of its potential pathogenetic mechanisms is of scientific interest. Essential hypertension, accounting for 90% of hypertensive population, is regarded as the end phenotype of a complex interaction between an individual's inherited background and various environmental factors. Recently, Cowley (2006) has written an excellent review on the genetic underpinnings of essential hypertension. Causal mutations in various biochemical mediators such as renin–angiotensin–aldosterone system that is involved in vascular remodeling and electrolyte balance have been identified and characterized. However, mutations in these mediators do not explain the occurrence of this disorder completely. Thus, it reinforces the need to investigate other logical genetic components using a candidate gene approach.
Heat shock protein 70 (Hsp70) family is a main component of heat shock proteins (HSPs) whose expression is triggered when organisms are exposed to stress or injury, including infections, mechanical stress, oxidants, and cytokine stimulation. In response of these responses, the arterial wall cells produce high levels of HSPs to protect themselves against these unfavorable conditions (Benjamin and McMillan 1998). As a dominant chaperone, Hsp70 plays an important role in the assembly and transport of newly synthesized proteins within cells, as well as in the removal of denatured proteins. Evidence is accumulating suggesting the involvement of Hsp70 in the pathogenesis of many clinical endpoints, including, for example, atherosclerosis (Xu 2002), Parkinson's disease (Wu et al. 2004), high altitude illness (Zhou et al. 2005), aging (Singh et al. 2006), ischemic stroke (Liu et al. 2007), and so on. Moreover, a series of biological or clinical results support the hypothesis that induction of Hsp70 expression in the arterial wall occurs as a physiological response to acute hypertension, i.e., hemodynamic stress or biomechanical stress (Xu et al. 1996; House et al. 2001; Chang et al. 2001). These lines of evidence may lend support for the potential involvement of Hsp70 in essential hypertension.
In this study, we focused on five common polymorphisms in three intronless genes of Hsp70 family, viz., HSPA1A (190G/C and −110A/C), HSPA1B (2074G/C and 1267A/G), and HSPA1L (2437T/C), which span in tandem on chromosome 6p21.3 within major histocompatibility complex class III region and explored the genetic contribution of these polymorphisms, alone and in combination, to essential hypertension in a Uygur population, which is characterized by the habits of drinking strong wine with big bowls and eating large cubes of meat.
Methods
Study population
A hospital-based cross-sectional study design was carried out during 2006–2007 from two outpatient clinics of the First Affiliated Hospital of Xinjiang Medical University and the Affiliated Tumor Hospital of Xinjiang Medical University in Urumqi city, Xinjiang, China. The Ethics Committee of Xinjiang Medical University reviewed and approved this study protocol. All studied subjects are of Uygur ethnic minority, which has its own spoken and written languages and belongs to the Turki Austronesian of Altai Phylum. A total of 415 subjects were enrolled in this study and provided written informed consent. Thereof, 211 (52.1%) were found to have essential hypertension and satisfied the following criteria: (1) with no consanguinity at study entry, (2) onset of hypertension aged 35–60 years, (3) three consecutive averaged readings of blood pressure (BP) of at least 140 mm Hg systolic or 90 mm Hg diastolic, (4) without pharmacological treatment for hypertension, and (5) free of secondary causes of hypertension through extensive clinical examination. The remaining 204 (47.9%) normotensive subjects were selected as healthy controls according to matched age, gender, and region, with systolic/diastolic BP less than 135/85 mm Hg and without a family history of cardiovascular disease.
Demographic measurement
BP was measured at the right arm with a conventional mercury sphygmomanometer on three occasions of at least 5-min intervals by certified examiners according to a standard protocol recommended by the American Heart Association (Perloff et al. 1993). Body weight and height were recorded without shoes, and body mass index (BMI) was calculated from the weight and height measures (weight/height2). Moreover, the doctors administered a questionnaire to collect information on smoking habits and alcohol intake. The status of cigarette smoking and alcohol drinking was defined at the time of the survey. Two categories were constructed for smoking and drinking: nonsmoker or current smoker and nondrinker or current drinker, respectively.
Clinical assessment
Fasting venous blood samples (5 mL) were obtained from all subjects and heparinized with the serum simultaneously isolated and frozen for biochemical assay. Genomic DNA was extracted from peripheral leukocytes using proteinase K/phenol/chloroform purification, followed by ethanol precipitation, and stored in 10 mM Tris–HCl, 1 mM Na2–EDTA, pH 8.0. Plasma triglyceride, total cholesterol (TC), and high-density lipoprotein cholesterol (HDLC) concentrations were determined enzymatically using available kits and auto analyzer in Xinjiang Medical University.
Genotyping
Genotyping of the five studied polymorphisms was assessed by using PCR-RFLP technique. With respect to the HSPA1L 2437T/C polymorphism, the sequences of the forward and reverse primers were “GTC CCT GGG GCT GGA GAC GG” and “GTG ATG ATA GGG TTA CAC ATC TGC T”, respectively. PCR amplification was carried out in a PTC-200 MJ Research Peltier Thermal Cycler (Bio-Rad Lab., MA, USA) for 35 cycles with denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 45 s. All amplifications were performed with 50–100 ng of DNA in a total volume of 25 μL containing 10 pmol of each primer, 200 μmol/L dNTPs, 2.0 mmol/L MgCl2, 2.5 mmol/L KCl, and 0.5 units Taq DNA polymerase. The restriction enzyme NcoI (Promega, Beijing, China) recognized the amplified fragment (627 bp) and digested it into 354 and 273 bp when 2437T was present. After restriction enzyme treatment, the reaction mixture was separated on 2% agarose gels. The primers and amplification conditions for the other polymorphisms were according to a report of Wu et al. (2004). The accuracy of our screening method was confirmed by direct sequencing of amplified DNA from randomly selected samples (10%), with no difference observed in results between the two methods (data not shown).
Statistical analysis
Simple statistical analysis was done using SAS version 9.1.3 (Institute Inc., Cary, NC, USA). Means of continuous variables were compared by unpaired t test. The χ2 test was used to assess the goodness-of-fit between the observed allele frequencies and the expected counterparts by Hardy–Weinberg equilibrium and to evaluate the differences in genotype and allele distributions between cases and controls. Each genotype was assessed by logistic regression analysis assuming additive (major homozygotes vs. heterozygotes vs. minor homozygotes), dominant (major homozygotes vs. heterozygotes plus minor homozygotes), and recessive (major homozygotes plus heterozygotes vs. minor homozygotes) models of inheritance respectively with and without adjusting for other conventional risk factors. P < 0.05 was considered statistically significant. The linkage disequilibrium degree and blocks were identified by the Haploview software 3.2 available at http://www.broad.mit.edu/mpg/haploview (Barrett et al. 2005). The linkage disequilibrium coefficients were shown as D' on the basis of four gamete color scheme.
EH/EH+ program (Zapata et al. 2001) was employed to estimate and calculate the haplotype frequencies between hyper- and normotensive subjects by χ2 test from a series of 2 × 2 contingency tables by combining other haplotypes. The selection criteria of haplotypes follow the haplotype frequencies greater than 1% in either hyper- or normotensive group. The rigorous Bonferroni correction was applied in multiple testing. In addition, to adjust for multiple-testing association, we employed the false discovery rate (FDR) method with an FDR q value threshold of 0.20 as suggested by Smith et al. (2007).
Interaction analysis was implemented in the open-source MDR software package (v.1.0.0) available from http://www.epistasis.org. Using MDR constructive induction function, we constructed all possible combinations of one to five polymorphisms. Then, we used a Bayes classifier in the context of tenfold cross-validation to estimate the testing accuracy of each best model. A single best model was selected that maximized the testing accuracy. This is the model that is likely to generalize to independent datasets. We also report the cross-validation consistency that measures the number of times of ten divisions of the data that the best model was found. Statistical significance was evaluated using a 1,000-fold permutation test to compare observed testing accuracies with those expected under the null hypothesis of no association. Permutation testing corrects for multiple testing by repeating the entire analysis on 1,000 datasets that are consistent with the null hypothesis. Models were considered significant at the 0.05 level. Finally, we collapsed the genetic data into two categories, high and low risk in each cell. Interaction dendrogram was used to visualize the nature of the dependencies.
Results
The baseline characteristics of the study population are shown in Table 1. No differences were found in age and gender distributions (P > 0.05). The levels of BMI, SBP, DBP, smoking and drinking, as well as the concentrations of plasma triglyceride and TC, were significantly higher in hyper- than in normotensives, while the plasma HDLC level was significantly lower (P = 0.0028).
Table 1.
The baseline characteristics of the study population
| Characteristics | HTs (n = 211) | NTs (n = 204) | Pa |
|---|---|---|---|
| Age (years) | 48.62 ± 10.33 | 48.49 ± 9.97 | 0.5149 |
| Gender (M, %) | 50.71 | 50.49 | 0.9641b |
| BMI (kg/m2) | 25.94 ± 3.07 | 23.26 ± 2.99 | <0.0001 |
| SBP (mmHg) | 166.61 ± 15.57 | 119.52 ± 10.11 | <0.0001 |
| DBP (mmHg) | 102.95 ± 9.64 | 75.24 ± 8.60 | <0.0001 |
| Smoking (%) | 55.45 | 43.63 | 0.0160b |
| Drinking (%) | 58.77 | 45.59 | 0.0072b |
| Triglyceride (mg/dL) | 160.22 ± 67.93 | 119.26 ± 62.98 | <0.0001 |
| TC (mg/dL) | 201.59 ± 48.03 | 180.04 ± 37.96 | <0.0001 |
| HDLC (mg/dL) | 49.61 ± 10.75 | 54.47 ± 11.60 | 0.0028 |
Data are expressed as mean ± SD or percentage
M males, HTs hypertensives, NTs normotensives, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, HDLC high-density lipoprotein cholesterol
aP was calculated using unpaired t test
bP was calculated using χ2 test
The genotype frequencies for all the studied polymorphisms respected the Hardy–Weinberg equilibrium in both hyper- and normotensive groups (P > 0.05). As shown in Table 2, statistical significant difference was only observed in the genotype (P = 0.0028) and (P = 0.0146) allele distributions of −110A/C polymorphism and in 1267A/G genotype distribution (P = 0.0106). In addition, the similar genotype/allele distributions were noted by gender stratification (data not shown). The relative risk associated with the −110C allele was 1.45 (95%CI, 1.09 to 1.94; P = 0.0124) under an additive model and 2.83 (95%CI, 1.56 to 5.37; P = 0.0009) under a recessive model. With respect to the 1267A/G polymorphism, the 1267G allele was associated with a 44% reduced risk (OR = 0.56; 95%CI, 0.37 to 0.84; P = 0.0058) of essential hypertension assuming a dominant model. After adjusting for age, gender, BMI, smoking and drinking status, triglyceride, TC, and HDLC, no obvious differences were noted for the relative risk of having essential hypertension.
Table 2.
The comparison of the genotype and allele distributions between hypertensive patients and normotensive controls as well as the three genetic models of inheritance
| Variation | Genotype/allele | HTs (n = 211) | NTs (n = 204) | χ2 | Pa | OR; 95%CI; Pb | OR; 95%CI; Pc |
|---|---|---|---|---|---|---|---|
| 2074G/C | GG | 78 | 71 | 0.96; 0.73–1.26; 0.7439 | 0.95; 0.71–1.25; 0.7418 | ||
| GC | 98 | 99 | 0.23 | 0.8912 | 0.91; 0.61–1.36; 0.6463 | 0.91; 0.60–1.36; 0.6462 | |
| CC | 35 | 34 | 0.99; 0.59–1.67; 0.9828 | 1.00; 0.62–1.69; 0.9925 | |||
| G/C | 0.60/0.40 | 0.59/0.41 | 0.11 | 0.7421 | |||
| 1267A/G | AA | 84 | 55 | 0.78; 0.58–1.03; 0.0772 | 0.76; 0.55–1.00; 0.0665 | ||
| AG | 91 | 116 | 9.09 | 0.0106 | 0.56; 0.37–0.84; 0.0058 | 0.55; 0.36–0.85; 0.0053 | |
| GG | 36 | 33 | 1.07; 0.64–1.79; 0.8088 | 1.04; 0.69–1.73; 0.8164 | |||
| A/G | 0.61/0.39 | 0.55/0.45 | 3.06 | 0.0804 | |||
| 190G/C | GG | 113 | 116 | 1.16; 0.87–1.56; 0.3204 | 1.16; 0.86–1.55; 0.3200 | ||
| GC | 76 | 73 | 1.31 | 0.5204 | 1.14; 0.78–1.69; 0.4984 | 1.15; 0.80–1.65; 0.4839 | |
| CC | 22 | 15 | 1.47; 0.74–2.97; 0.2742 | 1.49; 0.78–2.90; 0.2496 | |||
| G/C | 0.72/0.28 | 0.75/0.25 | 1.08 | 0.2998 | |||
| −110A/C | AA | 75 | 83 | 1.45; 1.09–1.94; 0.0124 | 1.47; 1.12–1.99; 0.0100 | ||
| AC | 95 | 105 | 11.76 | 0.0028 | 1.24; 0.84–1.85; 0.2815 | 1.25; 0.84–1.86; 0.2743 | |
| CC | 41 | 16 | 2.83; 1.56–5.37; 0.0009 | 2.84; 1.55–5.62; 0.0008 | |||
| A/C | 0.58/0.42 | 0.66/0.34 | 5.97 | 0.0146 | |||
| 2437T/C | TT | 130 | 135 | 1.17; 0.83–1.66; 0.3695 | 1.20; 0.85–1.73; 0.2975 | ||
| TC | 73 | 62 | 0.94 | 0.6252 | 1.22; 0.82–1.82; 0.3334 | 1.23; 0.85–1.86; 0.3328 | |
| CC | 8 | 7 | 1.11; 0.39–3.22; 0.8445 | 1.13; 0.43–3.19; 0.8256 | |||
| T/C | 0.79/0.21 | 0.81/0.19 | 0.79 | 0.3742 |
Genotype data are expressed as number and allele data as percentage. OR, 95%CI, and P were calculated using logistic regression analysis with the top for additive model, the middle for dominant model, and the bottom for recessive model of inheritance with (b) and without (c) adjusting for age, gender, BMI, smoking and drinking status, triglyceride, TC, and HDLC
HTs hypertensives, NTs normotensives, OR odds ratio, 95%CI 95% confidence interval
aP was calculated using χ2 test of 3 × 2 contingency table for the genotype distribution and 2 × 2 contingency table for allele distribution
The degrees of linkage disequilibrium between 2074G/C and 2437T/C (D' = 0.88 vs. 0.69) polymorphisms, as well as between 1267A/G and −110A/C (D' = 0.88 vs. 0.71), were stronger in hypertensive group than in normotensive group (data not shown). The same trend was also found between 190G/C and −110A/C polymorphisms (D' = 0.63 in hypertensives vs. 0.23 in normotensives).
Table 3 summarizes the results of the exhaustive MDR analysis that evaluated all possible combinations of the studied polymorphisms. The best model of each order was shown along with its testing accuracy, cross-validation consistency, and significance level as determined by permutation test. The overall best model included −110A/C and 1267A/G polymorphisms. This model had a maximal testing accuracy of 0.846 and a maximal cross-validation consistency of 10 out of 10. This model was significant at the 0.001 level, which indicates that a model this good or better was observed only one time out of 1,000 permutations and was thus unlikely under the null hypothesis of no association.
Table 3.
The summary of MDR analysis
| No. of loci in model | Variants included in the best combination in each model | Cross-validation consistency | Testing accuracy | P value |
|---|---|---|---|---|
| 1 | −110A/C | 7 | 0.717 | 0.0230 |
| 2 | −110A/C, 1267A/G | 10 | 0.846 | 0.0010a |
| 3 | −110A/C, 1267A/G, 2074G/C | 10 | 0.818 | 0.0010 |
| 4 | −110A/C, 1267A/G, 2074G/C, 2437T/C | 10 | 0.825 | 0.0010 |
| 5 | −110A/C, 190G/C, 1267A/G, 2074G/C, 2437T/C | 9 | 0.846 | 0.0107 |
aThe overall best MDR model
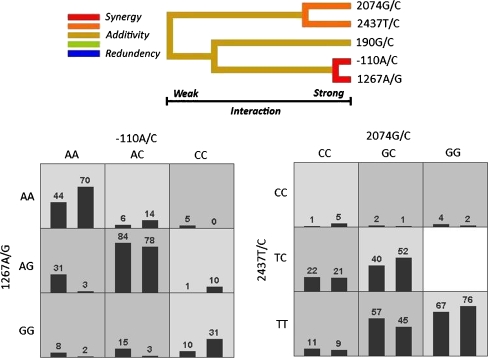
Shown was an interaction dendrogram highlighting the amount of information gained about case–control status by putting two polymorphisms together using the MDR function (Fig. 1). The interaction information analysis indicated that polymorphisms −110A/C and 1267A/G, connected with a red line, had a strong synergistic effect, while polymorphisms 2074G/C and 2437T/C with an orange line had a moderate synergistic effect. Each set of these two interacting polymorphisms was connected with the other by a yellow line, suggesting that the effects of each pair are independent of the other pair. The pattern of high- (dark gray) and low-risk (light gray) genotype combinations was nonlinear across the nine two-locus genotype cells, which represents interaction as suggested by the interaction analysis.
Fig. 1.
Interaction dendrogram for all studied polymorphisms (the upper panel) and the distribution of cases (left bars) and controls (right bars) for the two sets of polymorphisms with strong and moderate interaction. A red or orange line connecting two polymorphisms suggests a positive information gain, which can be interpreted as a synergistic or nonadditive relationship, while a blue or green line suggests a loss of information, which can be interpreted as redundancy or correlation (e.g., linkage disequilibrium). A yellow line indicates independence or additivity. Boxes were labeled as high-risk if the ratio of the number of cases (n = 211) to controls (n = 204) met or exceeded the threshold of 211/204≈1.03, and boxes were labeled as low risk if the threshold was not exceeded
The omnibus haplotype test showed significant association with essential hypertension (P < 0.0001; Table 4). Of the ten different haplotypes with frequency greater than 1% in either group for the five polymorphisms tested, the frequency of haplotype H4 was significantly lower (7.8% vs. 13.9%) in hypertensive group than in normotensive group, while that of haplotypes H5 (5.9% vs. 1.4%) and H8 (4.5% vs. 1.4%) were significantly higher even after the Bonferroni correction and FDR method. Using the most common haplotype H1 as a reference, haplotype H4 had a 40% reduced risk, while haplotypes H5 and H8 had a significantly 5.00- and 3.75-fold increased risk for essential hypertension, respectively.
Table 4.
The haplotype distribution of the five studied polymorphisms
| Haplotype | Allele combination | HTs (%) | NTs (%) | χ2 | Pa | FDR q | OR; 95%CI; Pb |
|---|---|---|---|---|---|---|---|
| H1 | G–A–G–A–T | 0.292 | 0.314 | 0.48 | 0.4903 | 0.6129 | Ref. |
| H2 | C–G–C–C–T | 0.146 | 0.155 | 0.13 | 0.7168 | 0.7964 | 1.01; 0.66–1.55; 0.9724 |
| H3 | G–G–G–C–T | 0.106 | 0.112 | 0.08 | 0.7810 | 0.7810 | 1.02; 0.63–1.65; 0.9439 |
| H4 | C–G–G–A–C | 0.078 | 0.139 | 8.01 | 0.0047 | 0.0235 | 0.60; 0.39–0.98; 0.0416 |
| H5 | C–A–G–C–T | 0.059 | 0.014 | 11.83 | 0.0006 | 0.0060 | 5.00; 2.00–15.20; 0.0015 |
| H6 | G–G–C–C–T | 0.051 | 0.074 | 1.88 | 0.1705 | 0.3410 | 0.73; 0.39–1.33; 0.3088 |
| H7 | G–A–G–C–T | 0.047 | 0.030 | 1.61 | 0.2042 | 0.3403 | 1.65; 0.77–3.63; 0.2001 |
| H8 | G–A–G–C–C | 0.045 | 0.014 | 6.91 | 0.0086 | 0.0287 | 3.75; 1.44–11.62; 0.0113 |
| H9 | G–A–C–A–T | 0.040 | 0.031 | 0.49 | 0.4843 | 0.6919 | 1.39; 0.63–3.11; 0.4155 |
| H10 | C–G–G–C–T | 0.040 | 0.022 | 2.22 | 0.1359 | 0.3398 | 2.08; 0.88–5.30; 0.1042 |
The alleles in haplotype are in the order of 2074G/C, 1267A/G, 190G/C, −110A/C, and 2437T/C polymorphisms
HTs hypertensives, NTs normotensives
aP was calculated using χ2 test from a series of 2 × 2 contingency tables by combining the other haplotypes listed
bOR, 95%CI, and P were calculated by logistic regression analysis using the most common haplotype H1 as a reference.
Discussion
Numerous epidemiological studies have suggested that several genetic variants increase the risk for essential hypertension, but the genes underlying the genetic susceptibility to this condition remain to be identified definitively (Izawa et al. 2003). Although the candidate gene approach may not replace the genome-wide scan strategy in detecting the genetics of complex traits, it is an important alternative strategy, especially when the population studied is relatively homogeneous in nature. The present study population, albeit small in sample size, represents such one with high homogeneity for the religious and cultural influences, which can avoid confounding factors due to population stratification. As such, the genotype frequencies of the five selected polymorphisms followed Hardy–Weinberg equilibrium in each group, which can lend support for the proper selection of the study population, although it is based on a hospital-based study design. Moreover, the study population, the Uygur subjects, shared a similar dietetic habit, which is characterized by drinking strong wine and consuming more animal fat and salt and less unsaturated fatty acid and fresh vegetables. Nevertheless, the prevalence of hypertension in the Uygur population (10.3%) was much lower than that in the Kazak (17.4%) and Mongolian (20.2%) populations in Xinjiang according to the reports of the Chinese national survey on hypertension in 1991 (The Chinese Task Force of National Survey of Hypertension 1995). Thus, it has sparked off a special interest to elucidate the underlying mechanisms of essential hypertension in Uygur ethnicity.
In current study, we used candidate gene approach to explore the genetic contribution of five polymorphisms in three genes of Hsp70 family to essential hypertension risk in a Uygur population. The key finding of our study was the significant individual and interactive influences of these polymorphisms on essential hypertension. Usually, the combined effects of the two or more genes/loci on disease status cannot be accurately described as the sum of their separate effects, but only within a framework that accommodates epistasis, a phenomenon whereby the effects of a given gene/locus on a biological trait are masked or potentiated by one or more other genes/loci (Moore 2005). In this study, except the strong interaction between polymorphisms −110A/C and 1267A/G whose association with essential hypertension was significant in single-locus analysis, we also extended the interaction information to another two polymorphisms 2074G/C and 2437T/C, which had negative results in single association analysis, but had moderate synergism and were independent of the two aforementioned polymorphisms in MDR function. Moreover, the linkage patterns within each set of these two interacting polymorphisms differed remarkably between cases and controls, which add another indication for the genetic implication of these polymorphisms in essential hypertension.
The small sample size in this study should be recognized as a major limitation, while development of MDR method can statistically overcome this since its nonparametric and genetic model-free nature in design. In addition, MDR method has been successfully applied to detect and characterize high-order gene–gene and gene–environment interactions in case–control and discordant-sib-pair studies with relatively small samples (Ritchie et al. 2001). Furthermore, empirical studies with both simulated and real data indicate that MDR has good power for identifying high-order gene–gene interactions (Ritchie et al. 2001; Moore and Williams 2002). To a certain extent, application of MDR can strengthen the validity of our results from the statistical point of view. However, MDR program itself has some underlying limitations such as computational intensiveness, indistinct interpretation, lack of sensitivity, and heterogeneity-free assumption as summarized by Manuguerra et al. (2007). Thus, MDR analysis may represent the first step in providing clues to direct further research.
Promoter −110A/C polymorphism in HSPA1A ranks high among the genetic variation most frequently studied in relation to a wide range of clinical endpoints such as Parkinson's disease (Wu et al. 2004). In our study, the frequency of −110C allele in control group was 33.6%, which was almost equal to that identified in other areas in Taiwanese at 33.2% (Wu et al. 2004). In contrast, the identification in France by Bolla et al. (1998) yielded an exceedingly high frequency of 61.6%. However, a literature search did not find any information on the genetic association of Hsp70 family with essential hypertension. Experimental evidence is accumulating, suggesting that Hsp70 gene or associated genes are responsible for the pathophysiology of hypertension (Hamet et al. 1990; Lodwick et al. 1993; Xu et al. 1996; Chan et al. 2003; Lakshmikuttyamma et al. 2006; Armutcu et al. 2008). Thus, the selection of Hsp70 gene as candidate in this study is founded on strong biological hints, which are crucial to the reliability of candidate gene approach. Importantly, our results indicated that −110C allele was associated with a 2.83-fold increased risk of essential hypertension under the recessive model, and meanwhile, 1267G allele was associated with a 44% reduced risk under the dominant model. On the contrary, the study by Giacconi et al. (2005) examining the association of 1267A/G polymorphism with carotid plaque rupture and cerebral ischemia in old type 2 diabetes–atherosclerotic patients found that 1267G allele conferred a 1.86-fold increased relative risk. The inconsistency may have been explained by the different populations selected and/or the lack of statistical power due to the small sample size. Although the sample size in this study is not large enough (n = 415), we have approximately 70% power to detect 1267A/G allelic difference between cases and controls. Moreover, lack of consideration of this polymorphism with others might affect the final conclusions reached. One doable way is to perform haplotype analyses, which were shown here to enhance the visibility of the association of 1267A/G polymorphism with essential hypertension when accounting for others in other genes of Hsp70 family.
Haplotype analyses, which study single genetic variants in their combination simultaneously, have a higher complexity level than single genetic variant analyses and have more power to explore the association between candidate genes and complex diseases (Rioux et al. 2001). As we previously indicated, haplotype analyses could provide more information about the effect of genetic interaction, especially when these alleles have a synergistic effect (Qi et al. 2008). In this study, we identified two risk-conferring haplotypes H5 (C–A–G–C–T) and H8 (G–A–G–C–C) for and one protective haplotype H4 (C–G–G–A–C) against essential hypertension in this Uygur population. On the basis of the interaction information obtained from MDR function, two possible inferences can be drawn from haplotype results. One is that the combination of 1267A/G and −110A/C polymorphisms might play a leading role since the (1267)A–(−110)C combination harbored the risk-conferring haplotypes (H5 and H8) and the alternative harbored the risk-reducing one (H4). Another inference is from the combination of 2074G/C and 2437T/C polymorphisms that haplotypes (H5 and H8) with only one mutant allele (2074C–2437T or 2074G–2437C) in both sites have an increased risk, while haplotype (H4) with two mutant alleles has a reduced risk for essential hypertension. We thus reasoned that the two co-existent mutations might offset each other in the course of the disease. This might indicate the existence of interaction. However, because statistical interaction may not automatically imply biological interaction, the jury must remain out until a larger, well-designed study confirms or refutes these findings.
Nevertheless, it is important to acknowledge several limitations of the present study. First, for the case–control nature of this study design, it inevitably suffers from the limitations of this type of study, i.e., the inability to prove the existence of a causality relationship. Second, we only genotyped five polymorphisms in three genes of Hsp70 family and did not examine other genes/variants, which might associate with hypertension, and the polymorphisms selected do not cover the genes fully and extensively. Third, other risk factors or intermediate phenotypes such as lifestyles (salt consumption and physical activity, etc.) and plasma stress-related proteins such as Hsp70 level were unavailable for analysis. Fourth, given the small number of this study population as mentioned above, our results should be considered preliminary, and confirmation in a larger study is critical.
To sum up, we demonstrated strong genetic interaction of the five studied polymorphisms in three genes of Hsp70 family with the risk of having essential hypertension in Uygur ethnicity. However, due to the complex molecular interactions, our study cannot be extrapolated directly to contribution of these results to hypertensive patients, and thus, further functional studies are warranted.
Acknowledgments
We wholeheartedly thank the expert and unfailing assistance of the faculty and doctors of the First Affiliated Hospital of Xinjiang Medical University and the Affiliated Tumor Hospital of Xinjiang Medical University in Urumqi City, Xinjiang, China. We also gratefully thank the dedicators for their outstanding commitment and cooperation.
This work was supported by the Hi-Tech Research and Development Program of China (863 Project; Grant No. 2004AA227111).
Abbreviations
- HSP
Heat shock protein
- MDR
Multifactor dimensionality reduction
- BP
Blood pressure
- TC
Total cholesterol
- HDLC
High-density lipoprotein cholesterol
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- OR
Odds ratio
- 95%CI
95% confidence interval
- SD
Standard deviation
- FDR
False discovery rate
References
- Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46:785–790. doi: 10.1515/CCLM.2008.166. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Bolla MK, Miller GJ, Yellon DM, et al. Analysis of the association of a heat shock protein70-1 gene promoter polymorphism with myocardial infarction and coronary risk traits. Dis Markers. 1998;13:227–235. doi: 10.1155/1998/235151. [DOI] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Chang KF, Ou CC, Chan JY. Altered temporal profile of heat shock factor 1 phosphorylation and heat shock protein 70 expression induced by heat shock in nucleus tractus solitarii of spontaneously hypertensive rats. Circulation. 2003;107:339–345. doi: 10.1161/01.CIR.0000044942.94957.87. [DOI] [PubMed] [Google Scholar]
- Chang J, Wasser JS, Cornelussen RN, Knowlton AA. Activation of heat-shock factor by stretch-activated channels in rat hearts. Circulation. 2001;104:209–214. doi: 10.1161/hc3101.092213. [DOI] [PubMed] [Google Scholar]
- Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Caruso C, Lio D, et al. 1267 HSP70-2 polymorphism as a risk factor for carotid plaque rupture and cerebral ischaemia in old type 2 diabetes–atherosclerotic patients. Mech Ageing Dev. 2005;126:866–873. doi: 10.1016/j.mad.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Hamet P, Malo D, Hashimoto T, Tremblay J. Heat stress genes in hypertension. J Hypertens. 1990;8:S47–S52. doi: 10.1097/00004872-199003001-00010. [DOI] [PubMed] [Google Scholar]
- He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- House SD, Guidon PT, Jr, Perdrizet GA, et al. Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress & Chaperones. 2001;6:164–171. doi: 10.1379/1466-1268(2001)006<0164:EOHSSC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa H, Yamada Y, Okada T, Tanaka M, Hirayama H, Yokota M. Prediction of genetic risk for hypertension. Hypertension. 2003;41:1035–1040. doi: 10.1161/01.HYP.0000065618.56368.24. [DOI] [PubMed] [Google Scholar]
- Lakshmikuttyamma A, Selvakumar P, Sharma RK. Interaction between heat shock protein 70 kDa and calcineurin in cardiovascular systems. Int J Mol Med. 2006;17:419–423. [PubMed] [Google Scholar]
- Liu J, Cheng J, Peng J, Han S, Yu L, Nie S. Effects of polymorphisms of heat shock protein 70 gene on ischemic stroke, and interaction with smoking in China. Clin Chim Acta. 2007;384:64–68. doi: 10.1016/j.cca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lodwick D, Kaiser MA, Harris J, Privat P, Vincent M, Sassard J, Samani NJ. Failure of the heat-shock protein 70 locus to cosegregate with blood pressure in spontaneously hypertensive rat x Wistar–Kyoto rat cross. J Hypertens. 1993;11:1047–1051. doi: 10.1097/00004872-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Manuguerra M, Matullo G, Veglia F, et al. Multi-factor dimensionality reduction applied to a large prospective investigation on gene–gene and gene–environment interactions. Carcinogenesis. 2007;28:414–422. doi: 10.1093/carcin/bgl159. [DOI] [PubMed] [Google Scholar]
- Moore JH, Williams SM. New strategies for identifying gene–gene interactions in hypertension. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- Moore JH. A global view of epistasis. Nat Genet. 2005;37:13–14. doi: 10.1038/ng0105-13. [DOI] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Qi Y, Niu W, Zhou W, Hou S, Qiu C. Correlation between angiotensinogen gene polymorphisms and essential hypertension in Chinese population. J Hum Hypertens. 2008;2:147–150. doi: 10.1038/sj.jhh.1002282. [DOI] [PubMed] [Google Scholar]
- Rioux JD, Daly MJ, Silverberg MS, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet. 2001;29:223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kølvraa S, Bross P, Jensen UB, Gregersen N, Tan Q, Knudsen C, Rattan SI. Reduced heat shock response in human mononuclear cells during aging and its association with polymorphisms in HSP70 genes. Cell Stress & Chaperones. 2006;11:208–215. doi: 10.1379/CSC-184R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NL, Hindorff LA, Heckbert SR, et al. Association of genetic variations with nonfatal venous thrombosis in postmenopausal women. JAMA. 2007;297:489–498. doi: 10.1001/jama.297.5.489. [DOI] [PubMed] [Google Scholar]
- The Chinese Task Force of National Survey of Hypertension The prevalence, awareness, treatment and control of hypertension in China: the report of national sampling survey of hypertension 1991. Chin J Hypertens. 1995;3:14–18. [Google Scholar]
- Wu YR, Wang CK, Chen CM, et al. Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson's disease. Hum Genet. 2004;114:236–241. doi: 10.1007/s00439-003-1050-1. [DOI] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.ATV.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- Xu Q, Fawcett TW, Udelsman R, Holbrook NJ. Activation of heat shock transcription factor 1 in rat aorta in response to high blood pressure. Hypertension. 1996;28:53–57. doi: 10.1161/01.hyp.28.1.53. [DOI] [PubMed] [Google Scholar]
- Zapata C, Carollo C, Rodriguez S. Sampling variance and distribution of the D' measure of overall gametic disequilibrium between multiallelic loci. Ann Hum Genet. 2001;65:395–406. doi: 10.1046/j.1469-1809.2001.6540395.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Wang F, Li F, Yuan J, Zeng H, Wei Q, Tanguay RM, Wu T. Association of hsp70-2 and hsp-hom gene polymorphisms with risk of acute high-altitude illness in a Chinese population. Cell Stress Chaperones. 2005;10:349–356. doi: 10.1379/CSC-156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]