Abstract
Heat shock protein 90 (HSP90) is a highly conserved molecular chaperone that plays a key role in protein synthesis, folding, denaturation prevention, and signal transduction. We cloned the complete complementary DNA (cDNA) sequence of the Laternula elliptica HSP90. The full-length cDNA was 2,823 bp in size and contained an open reading frame of 2,190 bp that was translated into 729 amino acids with a calculated molecular weight of 83.4 kDa. The deduced amino acid sequence of HSP90 showed the highest homology to Haliotis tuberculata HSP90 (83%). Reverse-transcriptase polymerase chain reaction analysis revealed the presence of HSP90 transcripts in all of the tissues examined. We also studied the transcriptional expression pattern of HSP90 exposed to thermal stress with real-time polymerase chain reaction. The relative expression level of HSP90 messenger RNA was upregulated and peaked at 12 h in the digestive gland and at 24 h in the gills, then dropped progressively.
Keywords: Antarctic, Heat shock protein 90, Laternula, Thermal stress
Introduction
Heat shock proteins (HSPs) are ubiquitous, highly conserved proteins found in all eukaryotes and prokaryotes. They play an important role in protecting organisms from damage following sublethal noxious stimuli, including oxygen radicals, toxicants, and inflammatory stress (Lindquist 1986; Lindquist and Craig 1988). HSPs can be divided into five families based on their molecular weight: HSP100, HSP90, HSP70, HSP60, and the small HSP20 (Nover and Scharf 1997). The mechanism of HSP70 molecular chaperones has been well characterized (Gething and Sambrook 1992; Johnson and Craig 1997; Saibil 2008), while the HSP90 family is the least understood in cellular function.
HSP90s are highly conserved molecular chaperones that account for 1% to 2% of all cytosolic proteins in most cells under non-stress conditions (Parsell and Lindquist 1993). They are ATP-dependent molecular chaperones, absolutely essential for cell viability under all growth conditions (Borkovich et al. 1989), especially for the refolding of denatured proteins; moreover, they prevent protein denaturation and assist in protein transport across organellar membranes (Schatz and Dobberstein 1996; Young et al. 2001). They also play key roles in the maturation of signal transduction proteins, such as hormone receptors, protein kinases, and nitric oxide synthase, by forming specific complexes (Csermely et al. 1998; Garcia-Cardena et al. 1998; Imai and Yahara 2000; Richter and Buchner 2001). Although more than 100 genes belonging to the HSP90 family exist in various organisms (Chen et al. 2006), little is known about HSP90 genes in mollusks (Tomanek and Somero 1999; Ochoa et al. 2002; Farcy et al. 2007; Gao et al. 2007). To the best of our knowledge, full-length complementary DNA (cDNA) sequences of HSP90s have been cloned from only five mollusk species, Haliotis tuberculata, H. asinina, Chlamys farreri, Crassostrea gigas, and Argopecten irradians (Farcy et al. 2007; Gao et al. 2007, 2008; Gunter and Degnan 2007; Choi et al. 2008).
The bivalve Laternula elliptica, which is endemic to the Antarctic, is one of the most abundant macro-benthic species in the Antarctic coastal region and has evolved under a cold and thermally stable environment for many millions of years (Ahn et al. 1996). In this paper, we report the molecular cloning of a full-length cDNA encoding HSP90 from L. elliptica and compare the expression patterns in transcriptional induction of HSP90 by semiquantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR).
Materials and methods
Animal collection and heat exposure
Specimens of L. elliptica (shell length, ca. 80 mm) were hand-collected by SCUBA divers from depths of 20–30 m in Marian Cove, near King Sejong Station on the northern Antarctic Peninsula (62°13′ S, 58°47′ W) in January 2006. The temperature treatment experiment was performed as described previously (Park et al. 2007). Briefly, the samples were divided into two groups and acclimated under conditions equivalent to those of the field temperature (ca. 1.0°C) with a constant air supply. After acclimation for 2 days, one group was transferred to water at 10 ± 1°C to induce acute heat stress, and the other group was kept at the control temperature (1.0 ± 1°C) with well-aerated natural seawater and without feeding.
cDNA library construction and nucleotide sequencing analysis
Total RNA was isolated using the TRIzol procedure (Invitrogen, Frederick, MD, USA) and then precipitated in ethanol. The messenger RNA (mRNA) was isolated from total RNA using Oligotex mRNA spin columns (Qiagen, Valencia, CA, USA). The cDNA library was constructed using the ZAP-cDNA synthesis kit and ZAP-cDNA Gigapack III Gold Cloning Kit (Stratagene, La Jolla, CA, USA) following the manufacturer’s instructions. In total, 2,592 randomly collected clones were sequenced using an ABI Prism 3730 automated sequencer (Applied Biosystems, Foster City, CA, USA). The partial HSP90 cDNA sequences were obtained from this expressed sequence tag (EST) library (Park et al. 2008).
Cloning the full-length HSP90 cDNA
Sequences obtained from the EST library were used to design specific oligonucleotides to perform 3′- and 5′-RACE for HSP90 (5′-CCT CGA GCT CAA CCC CGA CCA-3′ and 5′-TCT CCT CTA GGT ACT CGG CCT GAT-3′, respectively). The RACE reactions were performed according to the instructions provided with the Capfishing full-length cDNA kit (Seegene, Seoul, South Korea). Full-length first-strand cDNAs were synthesized using an oligo (dT)-annealing control primer (ACP) under the following amplification conditions: 0.2 mM of each dNTP, 10 mM Tris–HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.2 μM of gene-specific primers, 0.2 μM of 5′/3′-RACE primers, and 1 U Taq DNA polymerase. The program for PCR amplification was as follows: 1 cycle at 94°C for 5 min, 50°C for 1 min, and 72°C for 2 min; 35 cycles at 94°C for 30 s, 65°C for 30 s, 72°C for 3 min, and a final extension at 72°C for 10 min. The resulting products were separated on a 1.5% agarose gel and subcloned into pCR2.1-TOPO (Invitrogen).
Phylogenetic analysis
Phylogenetic trees were constructed using the amino acid sequences from various organisms, including vertebrates and invertebrates. The amino acid sequences were aligned using Clustal W software (Thompson et al. 1997). Molecular phylogenetic trees were constructed using the neighbor-joining method from the phylogenetic component of the MEGA4 software (Center for Evolutionary Functional Genomics, Tempe, AZ, USA). Support for major nodes was evaluated by bootstrapping; 1,000 bootstrap replicates of the whole data set were generated.
Tissue expression analysis
The expression of HSP90 mRNA in various tissues was examined by RT-PCR. Samples (2 μg) of total RNA from the gonads, digestive gland, mantle, gill, and intestine were reverse-transcribed with an oligo (dT) primer. The amplification of HSP90 mRNAs, and of β-actin mRNA (GenBank accession number EF198331), which was used as an internal PCR control, was performed using two pairs of specific primers: HSP90F (5′-AGA GAA CCG CAC AAT GAC CA-3′) and HSP90R (5′-TTG TCG GCA ACA AGG TAA GC-3′); and actF (5′-GGT CGT ACC ACA GGT ATT GT-3′) and actR (5′-CAT CAG GTA GTC GGT CAA AT-3′). The PCR products were visualized on a UV transilluminator after electrophoresis on a 1.5% agarose gel that contained ethidium bromide (0.5 μg/μl).
Quantification of HSP90 mRNA expression by real-time PCR
The levels of HSP90 mRNA were measured by real-time quantitative RT-PCR. Amplifications were performed in 25-μl reactions containing cDNA generated from 2 μg of the original RNA template, 0.2 μM each of the gene-specific forward and reverse primers, HSP90F and HSP90R, and 12.5 μl of Brilliant II SYBR Green QPCR mix (Stratagene). The amplified signals were monitored continuously with the Mx3000P QPCR System (Stratagene), and the amplification protocol was as follows: initial denaturation and enzyme activation at 95°C for 15 s, followed by 45 cycles at 95°C for 5 s, 52°C for 15 s, and 72°C for 15 s. The β-actin gene of L. elliptica was used as a reference to normalize the expression levels between samples. All data are expressed relative to β-actin to compensate for any difference in reverse transcriptase efficiency. All experiments were performed in triplicate. The relative fold change in gene expression was determined by the 2-ddCt method (Livak and Schmittgen 2001). All data are expressed as the mean ± SD and were analyzed by an unpaired Student’s t test after normalization. Differences were considered statistically significant at P < 0.05.
Results
Identification and characterization of HSP90
Based on the EST sequences, two specific primers, 5′HSP90 and 3′HSP90, were designed to clone the full-length cDNA sequence of the HSP90 gene. Using a RACE PCR approach, two fragments corresponding to the 5′- and 3′-ends of the HSP90 cDNA were amplified. The full-length HSP90 cDNA of L. elliptica (designated as leHSP90) was 2,823 bp in size and contained an open reading frame (ORF) of 2,190 bp, which translated into 729 amino acids (Fig. 1). The theoretical molecular weight of leHSP90 based on the deduced amino acid sequence was calculated to be 83.435 kDa, with an isoelectric point (pI) of 4.86. The leHSP90 cDNA included a 5′-untranslated region (UTR) located 90 bp upstream of the putative start codon (ATG) and a 3′-UTR of 563 nucleotides that ended in a poly(A) tail. The 3′-UTR region contained a consensus signal sequence for polyadenylation (AATAAA) and five ATTTA consensus sequence elements. The cDNA sequence of the leHSP90 gene was deposited in GenBank (accession number EU831278).
Fig. 1.
cDNA and deduced amino acid sequences of Laternula elliptica heat shock protein (HSP) 90. An asterisk marks the termination codon. The 3′- and 5′-untranslated regions are in lowercase. Five ATTTA sequence motifs in the 3′-untranslated region are underlined and the polyadenylation sequence (AATAAA) is double-underlined
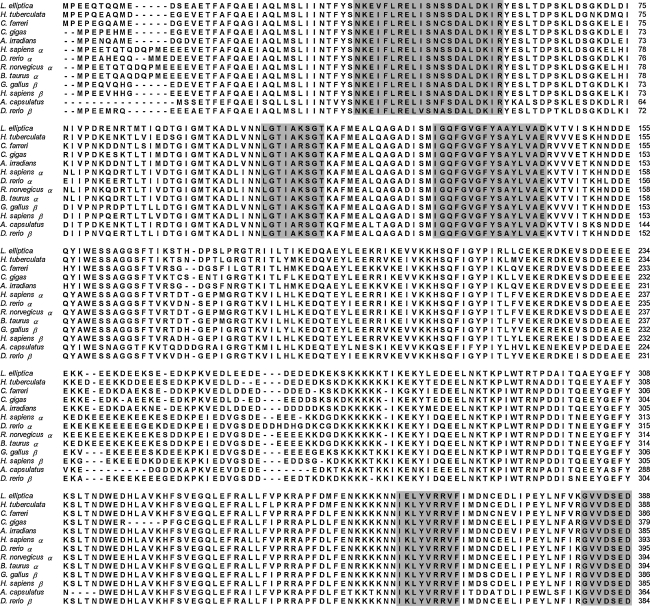
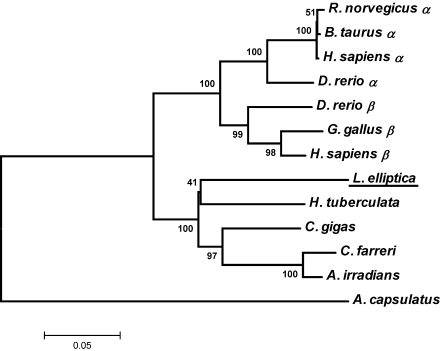
The alignments of the deduced amino acid sequence of leHSP90 with HSP90s of other species are shown in Fig. 2. The deduced amino acid sequence of leHSP90 was highly similar to HSP90s of invertebrates and vertebrates (more than 76% similarity in all cases), in particular H. tuberculata (83%), C. farreri (82%), and C. gigas (81%). The multiple sequence alignment of leHSP90 with other known HSP90 amino acid sequences revealed that they were highly conserved, especially in the regions of HSP90 family signatures. All five conserved amino acid blocks defining the HSP90 protein family signature (NKE[V/I]FLRELISN[S/A]SDALDKIR, LGTIAKSGT, IGQFGVGFY[A/S]AYLVAD, IKLYVRRVF, and GVVDSEDLPLNISRE) and the consensus sequence MEEVD at the C-terminus were highly conserved in the leHSP90 sequence (shadow in Fig. 2). An unrooted phylogenetic tree was created based on the amino acid distances of the aligned sequences using the neighbor-joining method with 1,000 bootstrap replications. The phylogenetic tree exhibited clustering of all vertebrates into two branches (HSP90α and HSP90β isoforms) and a cluster containing all mollusks (Fig. 3). The relationships displayed in the phylogenetic tree were in general agreement with traditional taxonomy.
Fig. 2.
Alignment of the leHSP90 amino acid sequence of known HSP90s. The five HSP90 family signature sequences and the consensus sequence MEEVD at the C-terminus are in shadow. The amino acids are numbered along the right margin. The common names, species names, and GenBank accession numbers are as follows: oyster, Crassostrea gigas, ABS18268; Zhikong scallop, Chlamys farreri, AAR11781; bay scallop, Argopecten irradians, ABS50431; abalone, Haliotis tuberculata, CAK95235; zebrafish, Danio rerio, NP_571403 (HSP90α), O57521 (HSP90β); chicken, Gallus gallus, CAA49704 (HSP90β); human, Homo sapiens, NP_005339 (HSP90α), NP_031381 (HSP90β); rat, Rattus norvegicus, NP_786937 (HSP90α), P34058 (HSP90β); bovine, Bos taurus, NP_001012688 (HSP90α)
Fig. 3.
Unrooted phylogeny showing the relationships between the leHSP90 and HSP90s of other organisms. Branch lengths are proportional to estimates of evolutionary change. The number associated with each internal branch is the local bootstrap probability, which is an indicator of confidence
Tissue distribution of leHSP90 mRNA
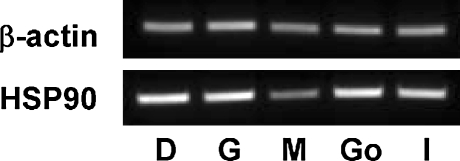
To examine the tissue distribution profile of the leHSP90 gene, 2 μg of total RNA from the gonads, digestive gland, mantle, gill, and intestine was reverse-transcribed with an oligo (dT) primer. The leHSP90 mRNAs were then amplified sequentially using RT-PCR analysis of different tissues with primers HSP90F and HSP90R. As an internal PCR control, the primers actF and actR were used to amplify a fragment of the housekeeping β-actin gene. A 189-bp fragment of the HSP90 gene was amplified in all tissues examined (Fig. 4).
Fig. 4.
Expression of leHSP90 mRNA in various tissues of Laternula elliptica, as determined by RT-PCR. The β-actin RNA was used as an internal control. Go gonad, D digestive gland, M mantle, G gill, I intestine
Quantification of leHSP90 mRNA expression after temperature treatment
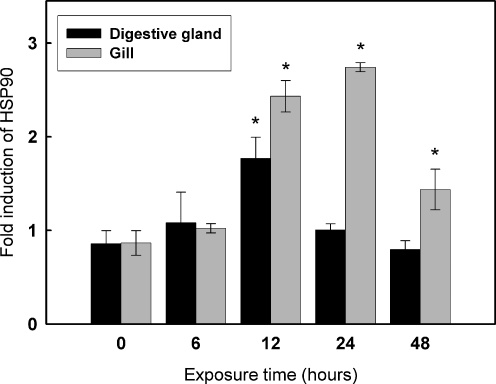
Real-time RT-PCR was employed to measure the temporal expression of leHSP90 mRNA in response to external temperature stimulus. As shown in Fig. 5, the leHSP90 transcript was significantly upregulated (1.7-fold) in the digestive gland at only 12 h of thermal exposure (P < 0.05). With prolonged exposure time, the expression level of leHSP90 mRNA dropped back down to the control level. However, significant differences in the expression levels of leHSP90 in the gills were observed at 12, 24, and 48 h compared to the control. The expression of leHSP90 transcripts was gradually upregulated and reached the highest level (2.7-fold) at 24 h in the gills, and then dropped to 1.5-fold at 48 h of thermal exposure.
Fig. 5.
Relative leHSP90 mRNA expression levels at different time points after heat treatment. Transcript levels for all samples were assessed by semiquantitative RT-PCR with SYBR Green, and the relative expression levels of leHSP90 were obtained relative to β-actin expression. Values are expressed as means ± SD of the relative variations (fold induction) between each treatment (10°C) and the control sample (1°C); asterisks above the bars indicate statistically significant differences (*P < 0.05)
Discussion
The heat shock proteins are a group of proteins that respond to sudden increases in temperature or exposure to a variety of other stresses. The HSP90 family is ubiquitous and its sequences are highly conserved among all species. Hence, the HSP90 family provides a useful model system for evolutionary studies (Gupta 1995). In addition, the response of these proteins to environmental stresses, such as changes in temperature (Palmisano et al. 2000; Hermesz et al. 2001; Landais et al. 2001), hyperosmotic shock (Pan et al. 2000), hypoxia (Li et al. 2008), metal ions (Ali et al. 1996), polychlorinated biphenyls (Wiens et al. 2000), and also diseases (Ramaglia et al. 2004) has been examined. Despite of these various studies, only five complete cDNA sequences have been reported from mollusks (Farcy et al. 2007; Gunter and Degnan 2007), including three HSP90 cDNA sequences in bivalves (Gao et al. 2007, 2008; Choi et al. 2008).
In this study, the complete cDNA sequence of an Antarctic bivalve HSP90 is reported. The full-length cDNA was 2,823 bp in size, including an ORF of 2,190 bp that encodes 729 amino acids. The leHSP90 sequence displayed five ATTTA motifs in the 3′-UTR. The ATTTA consensus sequence elements are reported to mediate RNA instability (Sachs 1993), and these multiple ATTTA consensus sequences suggest functional properties in maintaining higher levels of HSP90 transcripts. The deduced amino acid sequence from the leHSP90 cDNA showed high homology with HSP90 sequences from other organisms, including invertebrates and higher vertebrates. In bivalve species, only three HSP90 sequences are known, which belong to the Zhikong scallop (C. farreri), bay scallop (A. irradians), and Pacific oyster (C. gigas). The HSP90 of the Antarctic bivalve L. elliptica displayed high homology with those of abalone and the Zhikong scallop at 83% and 82%, respectively. The leHSP90 of the Antarctic bivalve contained five well-conserved signature peptide sequences, and the consensus sequence MEEVD at the C-terminus shared by all cytosolic proteins was identified.
In vertebrates, two isoforms of HSP90, HSP90α and HSP90β, were found, characterized by the structure of the glutamine-rich sequence (QTQDQ) at the N-terminus (Gao et al. 2007, 2008). To date, only one HSP90 isoform has been identified in invertebrates and found to be close to the vertebrate β-isoform. The leHSP90 is also more closely related to the vertebrate β-isoforms due to its lack of glutamine-rich sequences. Expression of leHSP90 mRNA was observed in all five of the organs examined. HSP90 is abundant at 1–2% of the cellular proteins within tissues under normal, unstressed conditions (Parsell and Lindquist 1993), and HSP90 is deduced to have basic physiological roles in various organs.
Fluctuation of the environmental temperature can lead to induction of cellular stress responses, including the HSP family in mussels (Hofmann and Somero 1995; Farcy et al. 2007; Park et al. 2007). Despite the intense interest in HSPs, few studies have addressed the HSP90 response to thermal stress by investigating the intracellular levels of HSP90 transcripts. In comparison with other heat shock proteins of this species, the expression of HSP70 at the transcriptional level was greatly upregulated by thermal stress in the Antarctic bivalve L. elliptica. Treatment of this species at 10°C resulted in a 4.6- and 3.6-fold increase in the mRNA for 12 and 24 h exposure, respectively (Park et al. 2007). In this study, the HSP90 mRNA showed an upregulation pattern similar to that of HSP70 when subjected to acute temperature elevation, but to a lesser extent (2.7-fold at 24 h in the gills and 1.7-fold in the digestive gland) compared to the expression levels of HSP70. However, Farcy et al. (2007) reported an 11-fold increase in HSP90 transcripts in the hemocytes of heat-shocked abalone H. tuberculata; in contrast, that of HSP70 increased 4-fold. The present study is the first to report the upregulation of HSP90 transcripts in response to thermal stress in molluskan tissues.
Our results demonstrated the relationship between HSP90 transcriptional change and increases in temperature in the Antarctic bivalve L. elliptica. The HSP90 transcriptional upregulation following temperature stimulus suggests that these genes are thermally inducible. Investigations are in progress to determine the role of other HSPs and novel mediated proteins in the adaptation and thermal tolerance of L. elliptica.
Acknowledgments
This study was supported by a Status and Changes of Polar Indicator Species and Coastal/Terrestrial Ecosystems grant (PE08040) funded by the Korea Polar Research Institute (KOPRI) of the Korean Ocean Research & Development Institute (KORDI).
References
- Ahn IY, Lee SH, Kim KT, Shim JH, Kim D-Y. Baseline heavy metal concentrations in the Antarctic clam, Laternula elliptica in Maxwell Bay, King George Island, Antarctica. Mar Pollut Bull. 1996;32:592–598. doi: 10.1016/0025-326X(95)00247-K. [DOI] [Google Scholar]
- Ali A, Krone PH, Pearson DS, Heikkila JJ. Evaluation of stress-inducible hsp90 gene expression as a potential molecular biomarker in Xenopus laevis. Cell Stress Chaperones. 1996;1:62–69. doi: 10.1379/1466-1268(1996)001<0062:EOSIHG>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Jo PG, Choi CY. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the Pacific oyster, Crassostrea gigas. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:286–292. doi: 10.1016/j.cbpc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Farcy E, Serpentini A, Fievet B, Lebel JM. Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: transcriptional induction in response to thermal stress in hemocyte primary culture. Comp Biochem Physiol B Biochem Mol Biol. 2007;146:540–550. doi: 10.1016/j.cbpb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gao Q, Song L, Ni D, Wu L, Zhang H, Chang Y. cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop Chlamys farreri. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:704–715. doi: 10.1016/j.cbpb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao J, Song L, Qiu L, Yu Y, Zhang H, Ni D. Molecular cloning, characterization and expression of heat shock protein 90 gene in the haemocytes of bay scallop Argopecten irradians. Fish Shellfish Immunol. 2008;24:379–385. doi: 10.1016/j.fsi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gunter HM, Degnan BM. Developmental expression of Hsp90, Hsp70 and HSF during morphogenesis in the vetigastropod Haliotis asinina. Dev Genes Evol. 2007;217:603–612. doi: 10.1007/s00427-007-0171-2. [DOI] [PubMed] [Google Scholar]
- Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol. 1995;12:1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- Hermesz E, Abraham M, Nemcsok J. Identification of two hsp90 genes in carp. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:397–407. doi: 10.1016/S1532-0456(01)00216-2. [DOI] [PubMed] [Google Scholar]
- Hofmann G, Somero G. Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J Exp Biol. 1995;198:1509–1518. doi: 10.1242/jeb.198.7.1509. [DOI] [PubMed] [Google Scholar]
- Imai J, Yahara I. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol Cell Biol. 2000;20:9262–9270. doi: 10.1128/MCB.20.24.9262-9270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Craig EA. Protein folding in vivo: unraveling complex pathways. Cell. 1997;90:201–204. doi: 10.1016/S0092-8674(00)80327-X. [DOI] [PubMed] [Google Scholar]
- Landais I, Pommet J, Mita K, Nohata J, Gimenez S, Fournier P, Devauchelle G, Duonor-Cerutti M, Ogliastro M. Characterization of the cDNA encoding the 90 kDa heat-shock protein in the Lepidoptera Bombyx mori and Spodoptera frugiperda. Gene. 2001;271:223–231. doi: 10.1016/S0378-1119(01)00523-6. [DOI] [PubMed] [Google Scholar]
- Li F, Luan W, Zhang C, Zhang J, Wang B, Xie Y, Li S, Xiang J (2008) Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia. Cell Stress Chaperones, doi:10.1007/s12192-008-0069-6 [DOI] [PMC free article] [PubMed]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD. Heat stress proteins and transcription factors. Cell Mol Life Sci. 1997;53:80–103. doi: 10.1007/PL00000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa GH, Clark YM, Matsumoto B, Torres-Ruiz JA, Robles LJ. Heat shock protein 70 and heat shock protein 90 expression in light- and dark-adapted adult octopus retinas. J Neurocytol. 2002;31:161–174. doi: 10.1023/A:1023949707669. [DOI] [PubMed] [Google Scholar]
- Palmisano AN, Winton JR, Dickhoff WW. Tissue-specific induction of Hsp90 mRNA and plasma cortisol response in Chinook salmon following heat shock, seawater challenge, and handling challenge. Mar Biotechnol (NY) 2000;2:329–338. doi: 10.1007/s101260000005. [DOI] [PubMed] [Google Scholar]
- Pan F, Zarate JM, Tremblay GC, Bradley TM. Cloning and characterization of salmon hsp90 cDNA: upregulation by thermal and hyperosmotic stress. J Exp Zool. 2000;287:199–212. doi: 10.1002/1097-010X(20000801)287:3<199::AID-JEZ2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Park H, Ahn IY, Lee HE. Expression of heat shock protein 70 in the thermally stressed Antarctic clam Laternula elliptica. Cell Stress Chaperones. 2007;12:275–282. doi: 10.1379/CSC-271.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Ahn I-Y, Kim HK, Chun JN, Kim MS (2008) Analysis of ESTs and expression of two peroxiredoxins in the thermally stressed Antarctic bivalve Laternula elliptica. Fish Shellfish Immunol, doi:10.1016/j.fsi.2008.07.017 [DOI] [PubMed]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Harapa GM, White N, Buck LT. Bacterial infection and tissue-specific Hsp72, -73 and -90 expression in western painted turtles. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:139–148. doi: 10.1016/j.cca.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Sachs AB. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-E. [DOI] [PubMed] [Google Scholar]
- Saibil HR. Chaperone machines in action. Curr Opin Struct Biol. 2008;18:35–42. doi: 10.1016/j.sbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Wiens M, Ammar MS, Nawar AH, Koziol C, Hassanein HM, Eisinger M, Muller IM, Muller WE. Induction of heat-shock (stress) protein gene expression by selected natural and anthropogenic disturbances in the octocoral Dendronephthya klunzingeri. J Exp Mar Biol Ecol. 2000;245:265–276. doi: 10.1016/S0022-0981(99)00167-7. [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]