Abstract
Oxidative stress can be a significant cause of cell death and apoptosis. We performed studies in HepG2 cells to explore whether prior exposure to oxidative stress (“oxidative preconditioning”) and geldanamycin (GA) treatment can protect the cell from damage caused by subsequent oxidative insults. The cells were treated with 10 nM GA for 24 h before oxidative stress. Oxidative preconditioning was achieved by 2 h exposures to H2O2 (50 μM) separated by a 10-h recovery period in normal culture medium. Oxidative stress was induced by exposure to 500 μM H2O2 for 24 h. The effects of GA and oxidative preconditioning were investigated on the formation of Hsp90, vimentin, insoluble vimentin aggregates, and cleavage of vimentin in a cell culture model of oxidative stress. GA treatment leads to enhanced expression of Hsp90 and vimentin and to inhibition of vimentin protein aggregation. Similar results were obtained by oxidative preconditioning. It is confirmed that low concentrations of GA protected HepG2 cells from subsequent oxidative stress by increasing the levels of Hsp90 and by alleviating the extent of cell apoptosis induced by oxidative stress, which is similar to oxidative preconditioning. However, in contrast to preconditioning, GA treatment obviously changed binding activity of Hsp90 to vimentin cleavages. All the above indicated that low concentrations of GA treatment triggered cell protection from oxidative stress. Both the level of Hsp90 and its ability to bind with vimentin were changed by low concentrations of GA and might contribute to oxidative stress protection.
Keywords: Hsp90, Geldanamycin, Oxidative stress, Preconditioning, Vimentin
Introduction
Oxidative stress constitutes a major threat to organisms living in an aerobic environment, and in humans, it is believed to play a causative role in many disease states, as well as in the aging process. Exposure of cells to various stresses (e.g., oxidative stresses, hypoxia, exposure to amino acid analogs, and heavy metal derivatives) induces cell death. An initial, non-lethal stress provides a temporary protection against subsequent lethal stress, known as preconditioning. Oxidative preconditioning can protect against apoptosis after oxidative stress (Hong et al. 2001). However, the molecular mechanisms of cell preconditioning protection that lead to stress tolerance are incompletely understood.
Cells induce an overexpression of a family of heat shock proteins (Hsps) that protect cells from stress-induced death. Heat shock responses are remarkably well conserved throughout evolution. Heat shock responses include a transient blockage of protein synthesis, overexpression of Hsps, and induction of thermotolerance. Hsp90 is the most abundant soluble cytosolic protein (∼1% of total protein, 10–150 μM) even before heat shock (Lai et al. 1984; Nollen and Morimoto 2002), and it is poorly induced. Hsp90 is a dimer and binds to several cellular proteins including steroid receptors and protein kinases (Jakob and Buchner 1994; Kimmins and MacRae 2000; Soti et al. 1998). Hsp90 promotes folding of proteins (without adenosine triphosphate (ATP); Shaknovich et al. 1992; Wiech et al. 1992) and prevents protein unfolding and aggregation (Miyata and Yahara 1992; Jakob et al. 1995a, b) by binding early unfolding intermediates (Jakob et al. 1995a) and preventing their aggregation. Hsp90 also plays a very important role in quality control: it is required for degradation of certain misfolded substrates (McClellan et al. 2005).
Hsp90 interacts with and regulates the conformation and the activity of a large variety of cell signaling molecules, transcription factors, and the cytoskeleton. Intermediate filaments (IFs) are key components of the cytoskeleton and help cells tolerate different forms of stresses from mechanical stress to exposure to heat, viruses, toxins, apoptosis inducing ligands, and other extrinsic cellular stresses (Pekny and Lane 2007; Diana et al. 2005; Coulombe and Wong 2004). IF mutations cause or predispose to more than 30 human diseases, including skin diseases, muscular dystrophies, premature aging, amyotrophic lateral sclerosis, and end-stage liver disease (Omary et al. 2004). IF inclusions are characteristically seen in association with several liver, neuronal, or muscle disorders (Kurt et al. 2004; Goldfarb et al. 2004; Cairns et al. 2004). Among the large protein family of IFs, vimentin is one of the most familiar members, as it is the major IF protein in mesenchymal cells. Vimentin filaments could work as a binding platform for signaling determinants, acting in the same regulatory pathway, and hence modifying the transduction of a signal. It shows dynamically altered expression patterns during different developmental stages and high sequence homology throughout all vertebrates, suggesting that the protein is physiologically important (Diana et al. 2005). Under stress condition, there have been several reports describing vimentin cleavage by caspases during apoptosis (Belichenko et al. 2001; Byun et al. 2001; Jiro et al. 2003; Muller et al. 2001; Prasad et al. 1998). Furthermore, vimentin has been shown to associate physically with Hsp90, and Hsp90 may play a pivotal role in maintaining vimentin intermediate filament structure and in the formation of the cellular network due to protection of soluble vimentin subunits (Zhang et al. 2006).
Geldanamycin (GA) is a naturally occurring anti-tumor drug that has been shown to be active in tumor cell lines as well as in mouse models (Supko et al. 1995). Biochemical and structural studies have demonstrated that GA binds specifically to the heat shock protein Hsp90, thereby inhibiting its chaperone function (Whitesell et al. 1994; Prodromou et al. 1997; Stebbins et al. 1997). Inhibition of Hsp90 function by GA causes degradation of the substrate proteins (Smith et al. 1995; Schneider et al. 1996). Geldanamycin also activates a heat shock response in mammalian cells by binding to Hsp90 (Sittler et al. 2001). However, whether GA treatment might act as preconditioning protection, which leads to stress tolerance, has not been reported.
The present study was therefore designed to determine whether GA treatment can simulate oxidative preconditioning and protect against cell injury in a human HepG2 cell model and, if so, to instigate whether Hsp90 and vimentin are involved in the cytoprotective pathways.
Materials and methods
Cell cultures
Human hepatoma carcinoma cells (HepG2) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU of penicillin, and 100 U of streptomycin at 37°C in a humidified chamber supplemented with 5% CO2. Cells were passaged regularly and subcultured to ∼90% confluence before experimental procedures.
Transfection of siRNA and plasmid
pSilencer 2.0-U6 siRNA Expression Vectors (Ambion Inc. USA; Brummelkamp et al. 2002), which provide high levels of constitutive expression across a variety of cell types, were used to generate small interference RNA (siRNA) sequences targeting Hsp90. The 21-nt siRNA sequences targeting Hsp90 corresponded to coding regions 5′-AAUCCGGUAUGAAAGCUUGAC-3′, (Elbashir et al. 2001) as instructed by the manufacturer. All constructs were verified by sequencing.
The HepG2 cell line was stably transfected either with a vector-induced small interference RNA of human Hsp90α or with the corresponding control vector (neo). DNA (8 μg) was electroporated (975 μF, 0.21 kV, 20 ms) into 5 × 106 cells by a Gene Pulser II Electroporation (Bio-Rad) and cells were selected in G418 (0.5 mg/ml, Calbiochem, San Diego, CA, USA) containing medium. The levels of Hsp90 were analyzed by immunofluorescence and Western blotting. Individual subclones stably expressing low levels of human Hsp90 (HSP90 siRNA) were isolated and cultured, as described above.
Antibodies
The following antibodies were used for western blot (WB), coimmunoprecipitation (co-IP), and/or immunofluorescence analysis (IF): rabbit monoclonal Ab anti-HSP90 (SPA-840, StressGen, goat polyclonal antibody anti-Hsp90 (sc-1055, Santa Cruz, CA, USA), mouse monoclonal Ab anti-vimentin (MS-129-PO, NeoMarkers), HRP-bovine–anti-rabbit–IgG (sc-2371, Santa Cruz), HRP-bovine–anti-mouse–IgG (sc-2370, Santa Cruz), rhodamine–bovine–anti-goat–IgG (sc-2349, Santa Cruz), and FITC–bovine-anti-mouse–IgG (sc-2366, Santa Cruz).
GA treatment
GA was purchased from Sigma-Aldrich Company (G 3381) in the form of a lyophilized powder. It was stored in dark, airtight containers at 4°C and reconstituted in DMSO (10 nM) immediately before use. Cells were grown with 10 nM geldanamycin for 24 h before oxidative treatment.
Oxidative preconditioning and oxidative stress treatments
Preconditioning (P) was achieved by 2 h exposures to H2O2 (50 μM) separated by a 10-h recovery period in normal culture medium. Oxidative stress (S) was induced by exposure to 500 μM H2O2 for 24 h. Measurements were obtained in cells at time points immediately after the final preconditioning or after 24 h exposure to H2O2.
Cell viability and apoptosis assay
The cell viability was measured using the 3[4,5-dimethylthiazole-2-yl]2,5-diphenyltetrazolium bromide assay. Cells (1 × 105 cells) were plated and counted at the indicated time using a hemocytometer. For the MTT assay, 3,000 cells/200 μl were seeded in 96-well plates, cultured for 48 h, and then incubated with MTT (50 μg/200 μl) for 4 h at 37°C. After adding 100 μl of 0.04 N HCl in isopropanol and mixing thoroughly to dissolve the dark blue crystal, the MTT reduction was measured with a microplate reader (Bio-Rad; wavelength of 570 nm). The data were presented as percent post-treatment recovery (percent live cells) where the absorbance from the control, non-treated cells was defined as 100% live cells.
For detection of apoptotic cells, apoptotic nuclear morphology was observed by staining with Hoechst 33342. Cells were seeded onto sterile glass coverslips placed in 24-well plates at 5 × 104 cells per well and managed as described above. After treatment, the medium was removed and the cells were washed with phosphate-buffered saline (PBS) three times and stained with Hoechst 33342 solution (final concentration 0.002% (Sigma) in HBSS for 30 min). Following two washes with PBS, the coverslips were mounted onto slides using anti-fade mounting medium (Beyotime). Morphologic changes in the apoptotic nuclei were observed under a fluorescence microscope (Olympus IX50).
Preparation of protein extracts
Cell lysis and preparation of the soluble and insoluble protein fractions were performed as described by Sittler et al. (1998). For preparation of whole cell extracts, cell lysis was performed on ice for 30 min in buffer containing protease inhibitors, and nucleic acids were digested with 125 U/ml Benzonase (Merck). Protein concentration was determined by the Bio-Rad assay.
Immunoprecipitations
Cell monolayers were washed twice with PBS and lysed with lysis buffer A [50 mM Na2HPO4, 1 mM sodium pyrophosphate, 20 mM NaF, 2 mM EDTA, 2 mM EGTA, 1% Triton-X-100, 1 mM DTT, 20 μM benzamindine,40 μM leupeptin, 300 μM PMSF] (Gao and Newton 2002) at 4°C for 30 min. Cells were scraped into 1.5-ml microcentrifuge tubes and incubated at 4°C with rotation for 30 min, and then centrifuged at 15,000×g at 4°C for 30 min. The protein concentration of the supernatants was determined using the Bio-Rad protein assay kit. Immunoprecipitations were performed using 1 mg of protein from total cell lysates as starting material. Reaction volumes were equalized using buffer A. For Hsp90 precipitation, 4 mg of anti-Hsp90 goat polyclonal antibody (sc-1055, Santa Cruz Biotechnology) was added to each sample. Immunoprecipitation of samples with preimmune sera was used as a control. After 1–4 h of rotating incubation at 4°C, 80 ml of Protein G-Agarose (sc-2002, Santa Cruz) was added. Tubes were incubated overnight at 4°C with rotation. At the end of the incubation, samples were centrifuged at 1,500×g at 4°C for 2 min, and the supernatant was discarded. Beads were washed three times with buffer A and three times with buffer B (buffer A + 300 mM NaCl). After the final wash, 30 ml of Laemmli buffer was added, and the samples were boiled for 5 min. After a 3-min spin at 1,000×g, supernatants were loaded onto a 12% SDS-PAGE gel.
Polyacrylamide gel electrophoresis and Western blot analysis
For Western blot analysis of total protein, lysis buffer [10 mM Tris, pH 8.0, 150 mM NaCl, 0.1% SDS, 1 mM EDTA, 1% NP-40, and complete protease inhibitor cocktail (Roche, Mannheim, Germany)] was used. The protein concentration was quantified using a protein assay kit (Bio-Rad) according to the manufacturer’s instructions. Equal amounts of protein extracts (20 μg) were fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) using 12% polyacrylamide gel. After electrophoresis, the proteins were transferred onto PVDF (Bio-Rad) and blocked with 2% bovine serum albumin (BSA) in Tris-buffered saline Tween-20 (TBST; 20 mM Tris/HCl, pH 7.5, 137 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated with primary antibody HSP90 (SPA-840, StressGen rabbit monoclonal Ab, 1:1,000, diluted in TBST with 1% BSA), or with primary antibody vimentin (MS-129-PO, NeoMarkers, rabbit monoclonal Ab, 1:1,000). After washing with TBST, the membranes were incubated with the appropriate secondary antibody (1:10,000) for 1 h at room temperature and visualized using an ECL kit (Amersham) according to the manufacturer’s instructions. Filters were washed three times in wash solution and the protein bands were detected using enhanced chemiluminescence (ECL, Amersham or Supersignal, Pierce) in conjunction with Fuji RX film. Blots were scanned by UMAX Magic Scan 4.3 program, and the intensity of protein bands was quantified by Molecular Analyst software.
Immunofluorescence studies of colocalization of Hsp90 and vimentin
HepG2 cell were fixed and permeabilized with methanol for 5 min at −20°C (Dou et al. 2001). After fixation, cells were blocked with 10% BSA for 1 h at 37°C. Samples were incubated with goat anti-Hsp90 polyclonal Ab (sc-1055, Santa Cruz, 1:1,000), mouse anti-vimentin monoclonal Ab (MS-129-PO, NeoMarkers, 1:1,000), at 4°C overnight. The secondary Abs, rhodamine-conjugated (red) anti-goat Ab, and FITC-conjugated (green) anti-rabbit Ab, were used at a 1:100 dilution and incubated at room temperature for 1 h. Cells were observed on an Olympus IX50 microscope. On average, 15 to 20 fields (five to 20 cells each) were evaluated on each coverslip and three to four fields were photographed with a digital camera at the same exposure time to generate the raw data. Experiments were repeated at least twice.
Statistical analysis
The data are presented as the mean ± SD, with n representing the number of independent samples. The mean values among groups were compared by one-way ANOVA and χ2 test. All statistical tests were operated with the program SPSS and p < 0.05 was considered statistically significant.
Results
Low concentrations of GA activates a protective response in HepG2 cells during oxidative stress similar to oxidative preconditioning
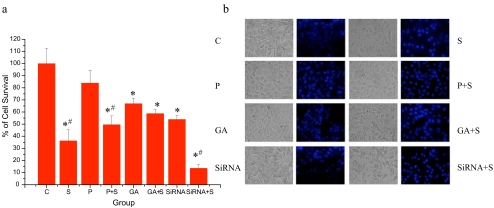
Oxidative stress can cause significant cell death and apoptosis. We performed studies in HepG2 cells to explore whether prior exposure to oxidative stress (“oxidative preconditioning”) and GA treatment can protect against the damage caused by subsequent oxidative insults (Fig. 1). The cells were treated with 10 nM GA for 24 h. The concentration of 10 nM was chosen for these experiments because it caused a significant reduction of cell viability. Changes of cell viability indicated that GA also triggered a preconditioning response, similar to oxidative preconditioning (Fig. 1a).
Fig. 1.
Oxidative preconditioning and GA treatment caused changes of cell viability and apoptosis in HepG2 cells during oxidative stress. a Effect of oxidative preconditioning and GA treatment on cell viability. Cell survival of HepG2 cells untreated (C) or treated with oxidative preconditioning (P: preconditioned with 2 h exposure to 50 μM H2O2, followed by 10 h recovery), geldanamycin (GA, 10 nM, 24 h), or small interference RNA of Hsp90 (siRNA) after oxidative stress (S: exposure to 500 μM H2O2 for 24 h), presented by MTT assay as percent of cell survival, where 100% survival is that of HepG2 cells that did not undergo oxidative stress (control). Results are from eight independent experiments (mean ± SE; *, #p < 0.05; asterisk each group vs control; number sign with oxidative stress vs without oxidative stress). b Effects of oxidative preconditioning and GA treatment on apoptotic morphology. Phase-contrast images (left and fluorescent images (right) were taken. The nuclear morphological changes were observed by Hoechst 33342 staining. In the control group, the nuclei of most HepG2 cells (>90%) were round and homogeneously stained. Cells treated with GA (10 nM) and oxidative preconditioning showed slightly increased cell numbers of typical morphologic features of apoptosis, such as chromatin condensation and fragmented fluorescent nuclei, which did not increased significantly compared to the directly oxidative stressed cells
Using phase-contrast microscopy, oxidative stressed cells exhibited apparent swelling (Fig. 1b). As to the Hoechst 33342 staining, the nuclei of control cells without H2O2 for 24 h had a homogeneous pattern of staining. After 24 h of oxidative stress, most of the cells were observed to have brightly stained condensed nuclei. Treatment with oxidative stress or GA reduced these changes in apoptotic morphology. In contrast, an Hsp90 siRNA cell line which expressed low levels of Hsp90 presented an increasing number of apoptotic cells (Fig. 1b).
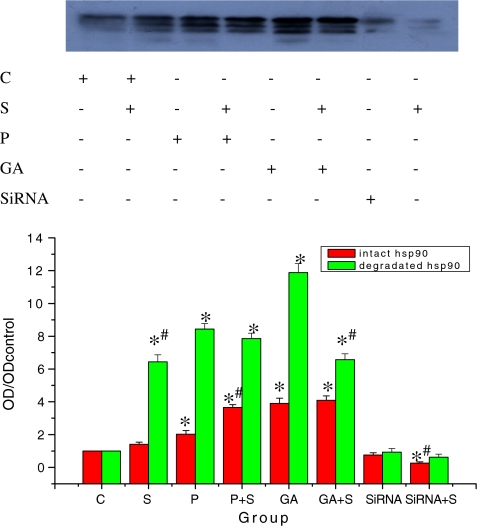
GA caused significant induction and degradation of Hsp90
We observed significant induction of Hsp90 in GA-treated cells similar to oxidative preconditioning (Fig. 2). GA (without H2O2 treatment) induced increasing levels of intact Hsp90 accompanied by increasing degradation of Hsp90 (Fig. 2). However, the degradation of Hsp90 was obvious immediately after 24 h of oxidative stress (Fig. 2). Oxidative preconditioning and GA treatment reduced the degradation of Hsp90 induced by stress. The degradation of Hsp90 might relate to Hsp90 level as the Hsp90 siRNA cell line showed to decrease the portion of degraded Hsp90.
Fig. 2.
Effect of Hsp90 on cell cycle distribution of HepG2 cells. Adherent growing HepG2 cells were kept either untreated or were irradiated (500 μM H2O2, 24 h). Immediately after oxidative stress, cells were lysed and intact and degraded Hsp90 was assayed by Western blotting using anti-Hsp90 (Stressgen, SPA-840). Equal amounts (20 μg) of protein were loaded. The signal intensity obtained from HepG2 cells without added GA was arbitrarily set to 1.0 (control). Quantitative data represent averages of three experiments (mean ± SE; *, #p < 0.05; asterisk each group vs control; number sign: with oxidative stress vs without oxidative stress)
Change of Hsp90 induced by GA related to cell viability and apoptosis
GA-treated cells similar to oxidative preconditioning expressed high levels of Hsp90 and showed higher cell viability after 24 h H2O2 treatment (Fig. 1a). In contrast, the Hsp90 siRNA cell line with low Hsp90 levels presented low cell viability and apparent apoptotic morphology (Fig. 1). All the above showed that the level of Hsp90 paralleled cell viability in HepG2 cells after oxidative stress.
GA treatment induced an increased level of vimentin and reduced vimentin cleavage in oxidative stress
Like preconditioning, GA treatment increased the level of both soluble and insoluble vimentin. However, the change caused by GA is more apparent than H2O2 preconditioning. Insoluble vimentin increased dramatically in all groups after oxidative stress, whereas it was slightly increased in GA and H2O2 treatment without the following stress (Fig. 3b).
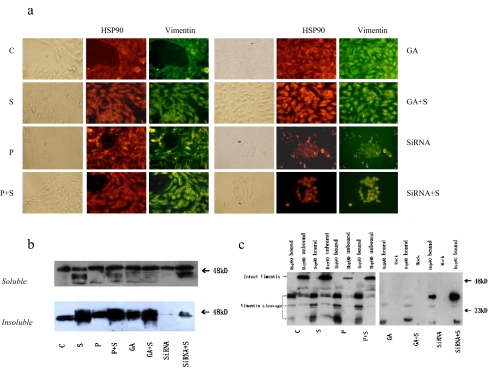
Fig. 3.
Identification of vimentin cleavage fragments as HSP90 binding protein. a Subcellular colocalization of vimentin and Hsp90. Cells were doubly stained for vimentin and Hsp90. b Adherent growing HepG2 cells were kept either untreated or were irradiated (500 μM H2O2, 24 h). Immediately after oxidative stress, cells were lysed and soluble and insoluble vimentin were assayed by Western blotting using anti-vimentin. Equal amounts (20 μg) of protein were loaded. c HepG2 cells were immunoprecipitated with anti-Hsp90 antibodies. The resulting pellets were subjected to SDS-PAGE and analyzed by immunoblotting with anti-vimentin antibodies. The signal intensity obtained from HepG2 cells without added GA was arbitrarily set to 1.0 (control)
Oxidative stress induced the apparent cleavage of vimentin, which is indicated by low molecular bands stained by Ab anti-vimentin (MS-129-PO, NeoMarkers, immunologically recognized both intact and degraded vimentin). Although GA and H2O2 treatment without oxidative stress caused a slightly cleavage of vimentin, these treatments obviously reduced vimentin cleavage after oxidative stress (Fig. 3b).
GA treatment reduced the combination of Hsp90 and cleavaged vimentin
To investigate the possibility of a relationship between HSP90 and vimentin cleavage, we applied immunofluorescence and coimmunoprecipitation to confirm the combination of Hsp90 and vimentin. Immunofluorescence results showed a similar distribution of Hsp90 and vimentin in HepG2 cells (Fig. 3a).
In control cells, Hsp90 exhibited normal arrangement with vimentin which was seen to traverse intricately throughout the cell. In contrast, oxidative stress caused a relocation of Hsp90 and vimentin with only a slightly increased stain throughout the cytoplasm and around the periphery of the nucleus. On the other hand, GA treatment, as in oxidative preconditioning, significantly increased the intensity of Hsp90 and vimentin in the cytoplasm and lessened the loss of Hsp90 and vimentin after oxidative stress (Fig. 3a).
Coimmunoprecipitation bands indicated that Hsp90 only bind to cleavage fragments of vimentin, not the intact vimentin (Fig. 3c). HepG2 cells without GA or siRNA of Hsp90 interference treatment showed similar Hsp90 binding bands of vimentin fragments, after slight stress (preconditioning 50 μM H2O2) or serious stress (500 μM H2O2). Interestingly, although GA-treated cells showed increasing levels of Hsp90, the vimentin fragments that bound to Hsp90 decreased dramatically, which indicated that the binding function of Hsp90 in the GA-treated cells might be inhibited. On the other hand, Hsp90 siRNA cell lines which were stably transfected with Hsp90 interference plasmid even showed an increased binding of high molecular fragments of vimentin after stress, which might be related to the increased levels of degraded vimentin of Hsp90 cells.
Discussion
Geldanamycin, a benzoquinone ansamycin antibiotic and natural product of Streptomyces geldanus, possesses anti-tumor activity and specifically binds with high affinity to ATP-binding sites of Hsp90 (Lewis et al. 2000; Katschinski et al. 2002; Nomura et al. 2004). Previous studies have established that Hsp90 inhibitors are particularly toxic to tumor cells because of a presumed increased binding affinity for these drugs and an enhanced Hsp90 reliance of genetically unstable tumor cells. But Hsp90 inhibitors such as geldanamycin and the ansamycin derivative 17-AAG have been reported to show other effects than tumor inhibition (Schumacher et al. 2007). Several data demonstrated that GA pretreatment could induce cell protection (Price et al. 2005; Gotz et al. 2006).
These results raise concerns about treating certain kinds of cancers with Hsp90 inhibitors and suggest that it will be important to understand the tissue-specific effects of Hsp90 inhibition. New Hsp90 drugs that are being developed as cancer therapeutic might also be useful for the treatment of other unrelated diseases (Richter et al. 2007).
Zou et al. (1998) have shown that GA also disrupts a complex consisting of Hsp90 and the heat shock transcription factor HSF1 and triggers the activation of a heat shock response in mammalian cells. Low GA treatment might therefore have a binary function, one to elevate the level of Hsp90 similar to preconditioning by the heat shock response mentioned above, and the other to block the binding of substrates with the association of Hsp90 which may result in reducing the degradation of stress-induced misfolded proteins (Salim and Eikenburg 2007; Young et al. 2004).
Our results also suggested that the level of Hsp90 is related to stress protection (Richter et al. 2007) demonstrated by treatment of oxidative preconditioning, GA and Hsp90 siRNA. Meanwhile, the level of Hsp90 is also related to Hsp90 degradation. Low concentrations of GA induced Hsp90 upregulation and maybe accompanied by partial inhibition of molecular chaperone function of Hsp90, which is different from oxidative preconditioning. The Hsp90 cell line, which expressed low levels of Hsp90, is different from oxidative preconditioned or GA-treated HepG2 cells that presented decreased portion of degraded Hsp90, which may elucidate a low level of Hsp90 companied with a decreased combining ability of Hsp90 with misfolded proteins (Young et al. 2004).
Compared to directly stressed cells, GA treatment and oxidative preconditioning reduced the obvious degradation of Hsp90 immediately after 24 h of oxidative stress, which suggested that GA treatment and oxidative preconditioning might keep the stability of Hsp90 and therefore maintain the protective function.
Vimentin filaments could work as a binding platform for signaling determinants, acting in the same regulatory pathway, and hence, modifying the transduction of a signal (Pekny and Lane 2007; Ivaska et al. 2007). In this study, low concentrations of GA treatment were similar to oxidative preconditioning, induced increasing levels of vimentin, and reduced vimentin cleavage in oxidative stress, which suggested an important role for vimentin in stress protection.
Hsp90 may play a pivotal role in maintaining vimentin intermediate filament structure and in the formation of its cellular network due to the protection of soluble vimentin subunits (Zhang et al. 2006). Vimentin, a major component of intermediate filaments, is degraded in response to apoptotic inducers (van Engeland et al. 1997; Prasad et al. 1998). The cleavage of vimentin by caspases (Byun et al. 2001) probably results in disruption of its filamentous structure, which may facilitate nuclear condensation and subsequent fragmentation (Morishima 1999). It has been demonstrated that proteolysis of vimentin promotes apoptosis by dismantling intermediate filaments and amplifying the cell death signal, via proapoptotic cleavage products. Our results demonstrated that vimentin cleavages, rather than intact vimentin, is a novel binding partner of Hsp90, and oxidative stress causing cleavage of vimentin was affected by Hsp90 level and function. Low concentrations of GA treatment might inhibit vimentin binding with Hsp90 and eventually reduce vimentin degradation by the proteolysis pathway, which in turn reduced apoptosis caused by oxidative stress (Byun et al. 2001).
In this study, we have examined the effect of GA and oxidative preconditioning on the formation of Hsp90, vimentin, insoluble vimentin aggregates, and cleavage of vimentin in a cell culture model of oxidative stress. We found that treatment of cells with GA leads to an enhanced expression of Hsp90 and vimentin and inhibition of vimentin protein aggregation. Similar results were obtained by oxidative preconditioning. We confirmed that low concentrations of GA protected HepG2 cells from subsequent oxidative stress by increasing levels of Hsp90 and alleviating the extent of cell apoptosis, which are similar to oxidative preconditioning. However, in contrast to preconditioning, GA treatment obviously changed the binding activity of Hsp90 to vimentin cleavages. Low concentrations of GA treatment triggered cell protection from oxidative stress, which is similar to preconditioning, but not in the same way. All the above suggested that not only the level of Hsp90 but also the binding ability of Hsp90 to specific client proteins may contribute to oxidative stress protection.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30500580), the National Natural Science Foundation of Guangdong Province (5300465), and the National Basic Research Program of China, No. 2006CB504100.
Abbreviations
- 17-AAG
17-Allylamino-17-demethoxygeldanamycin
- GA
Geldanamycin
- Hsp90
Heat shock protein 90
- DMSO
Dimethyl sulfoxide
- FITC
Fluorescein isothiocyanate
- GA
Geldanamycin
- H2O2
Hydrogen peroxide
- Hsp
Heat shock protein
- MTT
3[4,5-dimethylthiazole-2-yl]2,5-diphenyltetrazolium bromide
- PBS
Phosphate-buffered saline
References
- Belichenko I, Morishima N, Separovic D. Caspase-resistant vimentin suppresses apoptosis after photodynamic treatment with a silicon phthalocyanine in Jurkat cells. Arch Biochem Biophys. 2001;390:57–63. doi: 10.1006/abbi.2001.2365. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–450. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Lee VMY, Trojanowski JQ. The cytoskeleton in neurodegenerative diseases. J Pathol. 2004;204:438–449. doi: 10.1002/path.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. doi: 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- Diana MT, Guo ZT, Aida H, Jian L. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15:608–617. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp90 to protein kinase C. J Biol Chem. 2002;277:31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Vicart P, Goebel HH, Dalakas MC. Desmin myopathy. Brain. 2004;127:723–734. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM, Schonrock N. Alzheimer's disease and frontotemporal dementia: prospects of a tailored therapy. Med J Aust. 2006;185:381–384. doi: 10.5694/j.1326-5377.2006.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Hong H, Huizhen W, Hong L, Stanley N, Zhiguo W. Oxidative preconditioning and apoptosis in L-cells—roles of protein kinase B and mitogen-activated protein kinases. J Biol Chem. 2001;276:26357–26364. doi: 10.1074/jbc.M011136200. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of hsp90 with early unfolding intermediates of citrate synthase—implications for heat shock in vivo. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- Jakob U, Meyer I, Bugl H, Andre S, Bardwell JC, Buchner J. Structural organization of prokaryotic and eukaryotic Hsp90. Influence of divalent cations on structure and function. J Biol Chem. 1995;270:14412–14419. doi: 10.1074/jbc.270.24.14412. [DOI] [PubMed] [Google Scholar]
- Jiro F, Shuji B, Yu Y, Fei W, Yuji O, Takeo Y, Toshihiko I. High molecular weight vimentin complex is formed after proteolytic digestion of vimentin by caspase-3: detection by sera of patients with interstitial pneumonia. Microbiol Immunol. 2003;47(6):447–451. doi: 10.1111/j.1348-0421.2003.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Katschinski DM, Le L, Heinrich D, Wagner KF, Hofer T, Schindler SG, Wenger RH. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J Biol Chem. 2002;277:9262–9267. doi: 10.1074/jbc.M110377200. [DOI] [PubMed] [Google Scholar]
- Kimmins S, MacRae TH. Maturation of steroid receptors: an example of functional cooperation among molecular chaperones and their associated proteins. Cell Stress Chaperones. 2000;5:76–86. doi: 10.1379/1466-1268(2000)005<0076:MOSRAE>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt Z, Conny S, Andrea F, Elke J, Helmut D. Intermediate filament protein inclusions. Methods Cell Biol. 2004;78:205–228. doi: 10.1016/S0091-679X(04)78008-5. [DOI] [PubMed] [Google Scholar]
- Lai BT, Chin NW, Stanek AE, Keh W, Lanks KW. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984;4:2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu ZG. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275:10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- Morishima N. Changes in nuclear morphology during apoptosis correlate with vimentin cleavage by different caspases located either upstream or downstream of Bcl-2 action. Genes Cells. 1999;4:401–414. doi: 10.1046/j.1365-2443.1999.00270.x. [DOI] [PubMed] [Google Scholar]
- Muller K, Dulku S, Hardwick SJ, Skepper JN, Mitchinson MJ. Changes in vimentin in human macrophages during apoptosis induced by oxidized low density lipoprotein. Atherosclerosis. 2001;156:133–144. doi: 10.1016/S0021-9150(00)00641-9. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Nomura M, Nomura N, Newcomb EW, Lukyanov Y, Tamasdan C, Zagzag D. Geldanamycin induces mitotic catastrophe and subsequent apoptosis in human glioma cells. J Cell Physiol. 2004;201:374–384. doi: 10.1002/jcp.20090. [DOI] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, McLean WHI. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res. 2007;313:2244–2254. doi: 10.1016/j.yexcr.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Prasad SC, Thraves PJ, Kuettel MR, Srinivasarao GY, Dritschilo A, Soldatenkov VA. Apoptosis-associated proteolysis of vimentin in human prostate epithelial tumor cells. Biochem Biophys Res Commun. 1998;249:332–338. doi: 10.1006/bbrc.1998.9137. [DOI] [PubMed] [Google Scholar]
- Price JT, Quinn JM, Sims NA, Vieusseux J, Waldeck K, Docherty SE, Myers D, Nakamura A, Waltham MC, Gillespie MT, Thompson EW. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 2005;65:4929–4938. doi: 10.1158/0008-5472.CAN-04-4458. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Richter K, Hendershot LM, Freeman BC. The cellular world according to Hsp90. Nat Struct Mol Biol. 2007;2:90–94. doi: 10.1038/nsmb0207-90. [DOI] [PubMed] [Google Scholar]
- Salim S, Eikenburg DC. Role of 90-kDa heat shock protein (Hsp 90) and protein degradation in regulating neuronal levels of G protein-coupled receptor kinase 3. J Pharmacol Exp Ther. 2007;320:1106–1112. doi: 10.1124/jpet.106.114835. [DOI] [PubMed] [Google Scholar]
- Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:4536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JA, Crockett DK, Kojo SJ, Megan SL. Proteome-wide changes induced by the Hsp90 inhibitor, geldanamycin in anaplastic large cell lymphoma cells. Proteomics. 2007;7:2603–2616. doi: 10.1002/pmic.200700108. [DOI] [PubMed] [Google Scholar]
- Shaknovich R, Shue G, Kohtz DS. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84) Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittler A, Waelter S, Wedemeyer N, Hasenbank R, Scherzinger E, Eickhoff H, Bates GP, Lehrach H, Wanker EE. SH3GL3 associates with the Huntingtin exon 1 protein and promotes the formation of polygln-containing protein aggregates. Mol Cell. 1998;2:427–436. doi: 10.1016/S1097-2765(00)80142-2. [DOI] [PubMed] [Google Scholar]
- Sittler S, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, Hartl FU, Wanker EE. Geldanamycin activates a heat shock response and inhibits Huntingtin aggregation in a cell culture model of Huntington’s disease. Hum Mol Genet. 2001;10(12):1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C, Radics L, Yahara I, Csermely P. Interaction of vanadate oligomers and permolybdate with the 90-kDa heat-shock protein, Hsp90. Eur J Biochem. 1998;255:611–617. doi: 10.1046/j.1432-1327.1998.2550611.x. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/S0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- Engeland M, Kuijpers HJ, Ramaekers FC, Reutelingsperger CP, Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp Cell Res. 1997;235:421–430. doi: 10.1006/excr.1997.3738. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H, Buchner J, Zimmermann R, Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992;358:169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone mediated protein folding in the cytosol. Mol Cell. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- Zhang MH, Lee JS, Kim HJ, Jin D, Kim J, Lee KJ, Seo JS. HSP90 protects apoptotic cleavage of vimentin in geldanamycin-induced apoptosis. Mol Cell Biochem. 2006;281:111–121. doi: 10.1007/s11010-006-0638-x. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/S0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]