Abstract
Forkhead box protein A1 (Foxa1) is an evolutionarily conserved winged helix transcription factor that was traditionally considered to be involved in embryonic development and cell differentiation. However, little is known about the role of Foxa1 in oxidative-stress-induced apoptosis. In this study, hydrogen peroxide (H2O2)-induced apoptosis, upregulation of Foxa1, and the role of Foxa1 in the regulation of bcl2 gene expression were studied in A549 type II pneumocytes. H2O2 upregulated Foxa1 mRNA and protein in a time- and dose-dependent manner. Overexpression of Foxa1 promoted apoptosis, whereas Foxa1 deficiency, induced by antisense oligonucleotides, decreased A549 cell apoptosis induced by H2O2, as shown by flow cytometry. Moreover, Foxa1 overexpression decreased the expression of bcl2, while Foxa1 depletion increased the expression of bcl2. Electrophoretic mobility shift assay and chromatin immunoprecipitation revealed that Foxa1 bound to bcl2 promoter, and H2O2 promoted its DNA binding activity. Luciferase reporter showed that Foxa1 also decreased the transcription activity of bcl2 promoter under normal conditions and oxidative stress. These results indicate that Foxa1 plays a pro-apoptotic role by inhibiting the expression of anti-apoptotic gene bcl2.
Keywords: Apoptosis, bcl2; Forkhead box A1, Gene expression, Oxidative stress
Introduction
Forkhead box A [Foxa; previously termed hepatocyte nuclear factor (HNF)-3] transcription factors comprise a subfamily of forkhead transcription factors that share >90% homology in the winged helix DNA binding domain. Foxa proteins are expressed primarily in endodermally derived tissues, in which they influence embryonic patterning, and cell differentiation and function (Hromas and Costa 1995). The Foxa family includes Foxa1, Foxa2, and Foxa3 (previously termed HNF3α, HNF3β, and HNF3γ, respectively). Recently, it has been reported that the Foxa family plays an important role in regulation of apoptotic cell death in various diseases. For example, upregulation of Foxa2 through C/EBPα (CCAAT/enhancer binding protein alpha) promotes apoptosis of the H538 lung carcinoma cell line (Halmos et al. 2004). Foxa2 has also been shown to promote prostatic cell apoptosis (Jones et al. 2006). Foxa3 is involved in 2-acetylaminofluorene-induced bile duct cell apoptosis (Bisgaard et al. 1996). Wolf et al. (2007) have reported that Foxa1 regulates expression of p27Kip1, and forced expression of Foxa1 inhibits clonal growth of breast cancer cell lines, which suggest that Foxa1 plays a role in growth inhibition. Recently, it has been reported that Foxa1 and Foxa2 attenuate NKX2.1 [otherwise known as thyroid transcription factor (TTF)-1]-dependent transcription of surfactant protein A (SP-A), an inhibitor of pulmonary II type epithelial cell apoptosis in vivo (Vazquez de Lara et al. 2000; White et al. 2001; Minoo et al. 2007). In addition, our previous studies have demonstrated type II pneumocytes apoptosis in vivo in an oleic-acid-induced acute lung injury model, and Foxa1 expression was upegulated in lung tissue. However, the potential role of Foxa1 in apoptosis and regulation of apoptosis-related gene expression has not been completely elucidated.
Apoptotic processes are regulated by several proteins including bcl2, which is a critical intracellular checkpoint of apoptosis within a distal common cell death pathway. Overexpression of bcl2 promotes cells survival in vitro and in vivo (Garcia et al. 1992; Allsopp et al. 1993; Pinon et al. 1997).
By using Matinspector Professional program at www.genomatix.de and the Transcription Element Search System (TESS) at www.cbil.upenn.edu, we found that several apoptosis-related genes, including bcl2, bax, contained putative Foxa1-binding sites in their promoters. However, the direct effect of Foxa1 on the expression of these apoptosis-related genes remains unknown.
In this study, hydrogen peroxide (H2O2)-induced apoptosis and expression of Foxa1 were investigated in A549 type II pneumocytes, and the role of Foxa1 in the regulation of bcl2 gene expression was further investigated.
Materials and methods
Cell culture and challenge
Human A549 type II pneumocytes were maintained in DMEM nutrient mixture (Gibco), which contained 10% fetal bovine serum and 1% penicillin–streptomycin, at 37°C and 5% CO2. The cells were challenged with H2O2 (Xilong Chemical Factory, China). Cells were harvested at indicated time points after treatment.
RNA extraction, reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted by TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed by the reverse transcription kit (Fermentas) and PCR was performed using iCycler Apparatus (Biometra). For PCR amplification, the following primers were used: GAPDH (21 cycles), 5′-AAG CCC ATC ACC ATC TTC CA-3′ (forward) and 5′-CCT GCT TCA CCA CCT TCT TG-3′ (reverse); Foxa1(28 cycles), 5′-GTG GGT CCA GGA TGT TAG GA-3′ (forward) and 5′-CCG CAG TCA TGC TGT TCA T-3′ (reverse); bcl2 (28 cycles), 5′-CGA CGA CTT CTC CCG CCG CTA CCG C-3′ (forward) and 5′-AGA TCA TCT CTG CCT GAG TAT GCT T-3′ (reverse).
Western blot analysis
Proteins in the whole cell lysate were resolved on 12% SDS-PAGE and then transferred onto PVDF membranes (Schleicher & Schuell). The membranes were blocked overnight in phosphate-buffered saline containing 10% non-fat dry milk and 0.5% Tween-20, and incubated with primary antibodies for 2 h. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. The immunoreactive bands were visualized using DAB (Boster Biological Technology). Anti-GAPDH was used to normalize for equal amounts of proteins and calculate the relative induction ratio. The following antibodies were used: rabbit anti-Foxa1 polyclonal antibody (abcam); rabbit anti-bcl2 polyclonal antibody (Santa Cruz); mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Sigma); HRP-conjugated anti-mouse and anti-rabbit IgG (Boster Biological Technology).
Foxa1 expression plasmid construction
Oligonucleotide primers were designed to amplify the coding sequence of human Foxa1 cDNA, yielding a 1.4-kb product. The oligonucleotide primers are as follows: FoxA1, 5′-CCG GAA TTC AGG GTG GCT CCA GGA TGT TAG-3′ (forward) and 5′-CCC AAG CTT GAA GTG TTT AGG ACG GGT ATG-3′ (reverse). The PCR product was electrophoresed onto 0.9% agarose and the 1.4-kb fragment was purified with the purification system (Qiagen). The fragment was then inserted into the pcDNA3.1 vector (Stratagene) and sequenced commercially (Invitrogen).
Lipofectamine-mediated gene transient transfection
Transient transfection of A549 cells was performed according the manufacturer’s instructions (lipofectamine 2000™, Invitrogen). Briefly, about 5 × 105 cells per bottle containing 5 ml of appropriate complete growth medium were seeded and incubated at 37°C with 5% CO2 until the cells were 70% to 80% confluent (24 h). After the cells were rinsed with serum-free and antibiotics-free medium, the cells were transfected separately with 10 μg pcDNA3.1-Foxa1 per 20 μl lipofectamine (experimental group) or 10 μg pcDNA3.1 per 20 μl lipofectamine (vector control), followed by incubation at 37°C in a CO2 incubator for 6 h. The medium was then changed to regular medium with 10% fetal bovine serum. Cell samples were harvested for corresponding experiments.
Loss-of-function assay with morpholino oligonucleotides
A Foxa1 morpholino antisense oligonucleotide was designed to target the initiation site for FoxA1 translation (Foxa1-AS, tcttcacagttcctaacat) and was synthesized commercially (Invitrogen). Morpholinos were transfected into A549 cells with lipofectamine according to the manufacturer’s instructions (lipofectamine 2000™, Invitrogen) 24 h after plating. The specificity of the antisense oligo was validated by employing a control oligo (Foxa1-Ctrl, gcggagccaggtctagctt) and a group treated only with lipofectamine (Lipo). Cell samples were harvested for corresponding experiments.
Analysis of apoptosis by flow cytometry
The apoptosis rate was measured using flow cytometry. Briefly, A549 cells were washed with PBS (pH 7.4), fixed in cold 70% (v/v) ethanol, and incubated at −20°C for at least 2 h. The fixed cells were harvested by centrifugation at 250 ×g for 5 min. Cell pellets were resuspended in 1 ml of PBS at room temperature for 10 min, centrifuged and resuspended in 500 μl of PBS containing 0.2 g/l RNase A, and incubated at 37°C for 30 min. After incubation, the cells were stained with 20 g/l propidium iodide at 4°C for 30 min. Cellular fluorescence was measured using a FACSCalibur flowcytometer (BD BioSciences, San Jose, CA, USA). The relative DNA content indicated the cell cycle distribution of a population of cells. Apoptotic cells resulted in the appearance of a subdiploid peak in the cell cycle profile. The percentage of apoptotic cells was determined using BD CellQuest software (BD BioSciences).
Nuclear extract preparation and electrophoretic mobility shift assay (EMSA)
A549 cells were cultured under serum-free conditions for 24 h prior to preparation of the nuclear extracts. Cells were incubated with 0.5 mM H2O2 for 30 min. After treatment, cells were harvested and washed twice with cold PBS. Briefly, the cell pellet was resuspended in 400 μl cold buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF). The cells were allowed to swell on ice for 15 min, then 25 μl of a 10% solution of Nonidet P-40 (NP-40) was added, and the tube was vortexed vigorously for 10 s. The homogenate was centrifuged at 10,000 ×g for 30 s, and the nuclear pellet was resuspended in 50 μl ice-cold buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF). After vigorous rocking at 4°C for 15 min on a shaking platform, the nuclear extract was centrifuged at 10,000 ×g for 5 min at 4°C in a microcentrifuge, and the supernatant was frozen in aliquots at −80°C. The protein content of the different fractions was determined by the Bradford method.
EMSA were performed using nuclear extracts from A549 type II pneumocytes, according to the instructions of the Chemiluminescent Nucleic Acid Detection Module (Pierce). Supershift antibody to Foxa1 was incubated with nuclear extracts for 30 min at 4°C prior to adding the biotin-labeled oligonucleotide. DNA probes were also generated according to the Foxa1 sites at positions −835 to −829 bp, −538 to −532 bp, and −280 to −272 bp of the human bcl2 promoter, as double-stranded, biotin-labeled oligonucleotides that corresponded to the wild-type (5′-CGCGCGTGTGTGCGCGCG-3′, 5′-TATGCATTTGTTTTGGTT-3′ and 5′-TTATTAGTTTGTTTTTTCTT-3′, respectively) and mutant sequences for the positions −538 to −532 bp and −280 to −272 bp (5′-AATTCAATAGTAGG-3′ and 5′-TTATTAGTTACAAATTTCTT-3′, respectively).
Chromatin immunoprecipitation (ChIP)
ChIP analysis was performed using the EZ ChIP Kit (Upstate, Charlottesville, VA, USA) according to the manufacturer’s protocol. To cross-link proteins to DNA, formaldehyde (final concentration 1%) was added to the culture medium and incubated for 10 min at room temperature. Then, a final concentration of 0.125 M glycine was added to stop fixation, and cells were scraped and collected by centrifugation at 700 ×g for 5 min at 4°C. Cell pellets were treated with SDS lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris, pH 8.1) that contained protease inhibitors. Aliquots of cell lysates were sonicated to shear DNA into 0.2–1.0-kb fragments, and cellular debris was removed by centrifugation at 14,000 ×g for 15 min at 4°C. The resultant chromatin-containing solutions were aliquoted (100 μl) and stored at −80°C until use. Chromatin aliquots were pre-cleared with 60 μl of 50% protein G agarose suspension. Samples were then incubated with anti-Foxa1 antibody (Abcam) or normal rabbit IgG (Santa Cruz Biotechnology) (as a control) overnight at 4°C with rotation. Immune complexes were mixed with 60 μl of 50% protein G agarose suspension, followed by incubation for 1 h at 4°C with rotation. Beads were collected by brief centrifugation and the immunocomplexes were eluted by freshly prepared elution buffer (100 mM NaHCO3, 1% SDS). Chromatin was then de-cross-linked for 5 h at 65°C. After treatment with proteinase K, DNA was purified with a Qiaquick PCR Purification Kit (Qiagen) and finally eluted in 50 μl of TE.
An aliquot (2 μl) of each sample was subjected to PCR analysis using HotStar Taq DNA polymerase (Qiagen) (32 cycles). Two pairs of primers to amplify the proximal region of the bcl2 promoter that contained the Foxa1 binding site were: primers that contained the −538 to −532 Foxa1 binding site, 5′-ACA TTT CTG TGA AGC AGA AGT C-3′ and 5′-CTG GAA ATT AAA TTT ACT CGA A-3′, and primers that contained the −280 to −272 Foxa1 binding site, 5′-TAA ATT TAA TTT CCA GGC AGC T-3′ and 5′-TTC TTG GAC GAG GGG GGA GAC T-3′.
Construction of pGL-3 bcl2 promoter–reporter gene and luciferase reporter gene assay
The human bcl2 promoter region (−1000 to +10, −532 to +10, and −272 to +10) was amplified by PCR of human genomic DNA. PCR products were digested with HindIII and KpnI and cloned into pGL3-Basic, and authenticity was verified by sequencing (data not shown). The luciferase reporter gene assay was performed according to the instruction of the Dual Luciferase Reporter System (Promega). Exponentially growing A549 cells were seeded in 24-well culture dishes. Transfection was performed as described above. All transfection was performed in triplicate from at least three independent experiments. Each transfection experiment contained 500 ng pGL3-bcl2 promoter reporter construct, with 500 ng pcDNA3.1-Foxa1 vector and 20 ng pRL-null vector (Promega) as an internal transfection control.
Statistical analysis
All data were expressed as the mean ± SEM. Continuous parametric data were subjected to analysis of variance followed by the Student–Newman–Keuls post hoc test for determining differences between groups. A χ2 test was used to determine statistical differences in the rate of hypoploid cells. P < 0.05 was considered statistically significant.
Results
H2O2 induces expression of Foxa1 in A549 type II pneumocytes
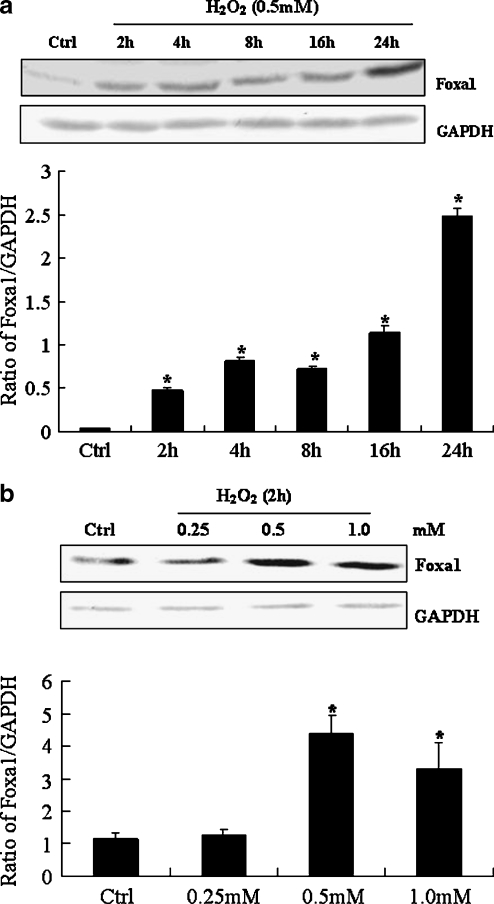
As shown in Fig. 1a, H2O2 treatment of A549 type II pneumocytes led to a sustainable increase in the protein levels of Foxa1 from 2 to 24 h. Fig. 1b shows a dose–response study in A549 type II pneumocytes treated with H2O2 for 2 h. As shown, there was a dose-dependent increase in Foxa1 protein levels.
Fig. 1.
Expression of Foxa1 in H2O2-stimulated type II pneumocytes. a type II pneumocytes were stimulated with 0.5 mM H2O2 for various times. Foxa1 protein level was determined by Western blotting. b Foxa1 protein in H2O2-stimulated type II pneumocytes at indicated doses for 2 h was determined by Western blotting. The relative values of all results were determined and expressed as means ± SEM of three duplicate experiments. *Statistically significant difference from the control group, P < 0.05
Foxa1 influences H2O2-mediated apoptosis of A549 type II pneumocytes
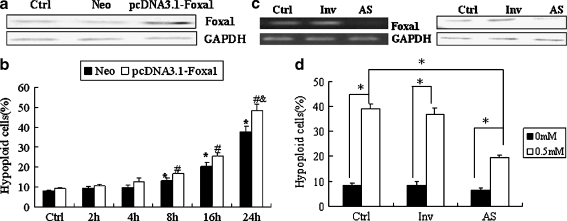
In order to determine the role of Foxa1 in H2O2-mediated apoptosis of type II pneumocytes, A549 cells were transfected with pcDNA3.1-Foxa1. Successful overexpression of Foxa1 was assessed by RT-PCR and Western blotting (Fig. 2a). The efficiency of transfection was 50–80% detected by flow cytometry. Following overexpression of Foxa1, H2O2-mediated apoptosis was significantly increased as compared to control groups at 24 h (Fig. 2b). The level of apoptosis was assessed following inhibition of endogenous Foxa1 expression in response to H2O2 treatment. As shown in Fig. 2c and d, antisense-mediated inhibition of basal Foxa1 expression decreased the rate of H2O2-mediated apoptosis in A549 cells at 24 h.
Fig. 2.
Effect of Foxa1 overexpression or deficiency on A549 cell apoptosis induced by H2O2. a A549 cells were transfected with pcDNA3.1-Foxa1 and the expression levels of Foxa1 were assessed by Western blotting. Ctrl A549 cells treated with lipofectamine only, Neo vector control group, pcDNA3.1-Foxa1 Foxa1 overexpression group. b Effect of Foxa1 overexpression on H2O2-induced apoptosis of A549 cells. Ctrl A549 cells not treated with H2O2. Results were expressed as mean ± SEM of three duplicate experiments. Cells were treated with H2O2 (0.5 mM) for indicated times. *Statistically significant difference between the Neo group and controls, P < 0.05; #statistically significant difference between the pcDNA3.1-Foxa1 group and the controls, P < 0.05; &statistically significant difference from the Neo group at 24 h, P < 0.05. c A549 cells were transiently transfected with Foxa1 morpholino antisense oligonucleotides. Expression of Foxa1 was assessed by RT-PCR and Western blotting in order to identify basal Foxa1 inhibition. d Effect of Foxa1 deficiency on apoptosis of A549 cells induced by H2O2 (0.5 mM for 24 h). Ctrl A549 cells treated with lipofectamine only, Inv A549 cells transiently transfected with random Foxa1 oligonucleotides, AS A549 cells transiently transfected with Foxa1 morpholino antisense oligonucleotides. *P < 0.05
Foxa1 decreases upregulation of bcl2 induced by H2O2 in A549 type II pneumocytes
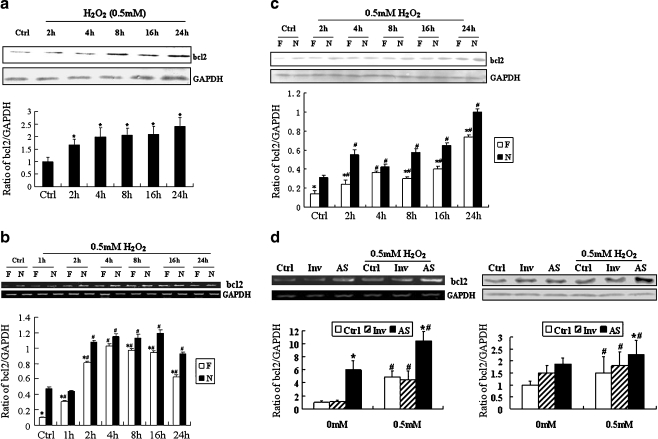
As shown in Fig. 3a, bcl2 was also increased from 2 to 24 h after H2O2 stimulation. The expression change of Foxa1 and bcl2 might suggest a potential relationship between these two genes. Therefore, we hypothesized that this transcription factor may influence the expression of bcl2, an important anti-apoptosis gene. We overexpressed Foxa1 in A549 type II pneumocytes using a pcDNA3.1-Foxa1 construct (Fig. 2a). As demonstrated in Fig. 3b, the levels of bcl2 mRNA were increased significantly in A549 cells in response to H2O2. However, the level of bcl2 mRNA was significantly lower in the Foxa1 overexpression group than in the vector control group. We further investigated the effect of Foxa1 overexpression on the bcl2 protein levels by Western blotting. Figure 3c shows that the basal level of bcl2 protein after Foxa1 overexpression was decreased. H2O2 stimulation significantly increased the expression of bcl2 protein, which was inhibited by Foxa1 overexpression.
Fig. 3.
Effect of Foxa1 on expression of bcl2 during oxidative stress in type II pneumocytes. a H2O2 (0.5 mM)-induced bcl2 level was determined by Western blotting. *Statistically significant difference from the control group, P < 0.05. b Effect of Foxa1 overexpression on level of bcl2 in type II pneumocytes was determined by RT-PCR and Western blotting (c). N vector control group, F Foxa1 overexpression group. d Effect of Foxa1 inhibition on the level of bcl2 measured by RT-PCR and Western blotting. Ctrl type II pneumocytes treated only with lipofectamine, Inv type II pneumocytes transiently transfected with random oligonucleotide of Foxa1, AS type II pneumocytes transiently transfected with morpholino antisense oligonucleotide of Foxa1. The relative values of all results were determined and expressed as means ± SEM of three duplicate experiments. *Statistically significant difference from the vector control group (N) or lipofectamine control group, P < 0.05; #statistically significant difference form the group without H2O2 stimulation, P < 0.05
In the above experiments, we observed that the expression of both Foxa1 and bcl2 increased during oxidative stress and the overexpression of Foxa1 could reduce bcl2 induction. The possible reason is that though Foxa1 could reduce bcl2 induction, there were still many other causes which might promote bcl2 expression in oxidative stress. The expression level of bcl2 would be higher without the presence/induction of endogenous Foxa1. So, we concluded that the elevated basal levels of Foxa1 reduced bcl2 induction by oxidative stress.
In order to observe the effect of Foxa1 depletion on the expression of bcl2, morpholino antisense oligonucleotide of Foxa1 was transfected into A549 type II pneumocytes. Expression of Foxa1 was detected by RT-PCR and Western blotting for identification of basal FoxA1 inhibition (Fig. 2d). After basal expression of FoxA1 was inhibited, expression of bcl2 was determined by RT-PCR and Western blotting. As shown in Fig. 3d, after Foxa1 depletion, the basal and H2O2-induced (0.5 mM, 24 h) expression of bcl2 was increased as compared to that in the control oligonucleotide group.
We also investigated the effect of Foxa1 overexpression on the bax expression. The results shown that Foxa1 overexpression did not change the expression of bax both in basic and H2O2-stimulated conditions. Moreover, in H2O2-stimulated condition, the bax: bcl2 ration was increased (data not shown), which indicated that the changes in bcl2 resulted in apoptosis in our model.
Foxa1 regulates bcl2 promoter in A549 type II pneumocytes
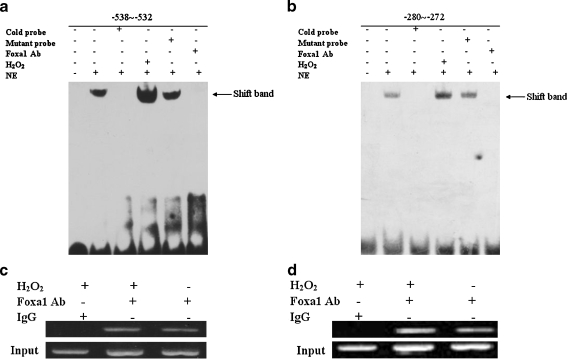
Foxa1 is a transcription factor that can bind DNA and thereby regulates the expression of various target genes. The promoter sequence of key genes involved in apoptosis (bcl2, bax, etc.) was analyzed, using Matinspector Professional program at www.genomatix.de and TESS at www.cbil.upenn.edu, to predict the Foxa1-binding elements in the promoter. Three Foxa1-binding elements were found at −835 to −829, −538 to −532, and −280 to −272 bp in the promoter of bcl2. To determine whether Foxa1 was capable of binding to the potential Foxa1 binding sites on the bcl2 promoter, EMSA was performed. Figure 4 shows that the biotin-labeled probe designed according to the bcl2 promoter (site at −538 to −532 and −280 to −272 bp) could bind to the Foxa1 protein in the nuclear extract of A549 type II pneumocytes. Specificity of binding was verified by using mutant cold oligonucleotides, which failed to compete for binding with Foxa1, and by supershift studies with Foxa1 antibody. The site at −835 to −829 bp showed no binding activity with Foxa1 protein (data not shown). To determine the binding activity of Foxa1 to the promoter of bcl2 in response to H2O2, the nuclear proteins were extracted from A549 type II pneumocytes after 30-min stimulation with H2O2. It was shown that the binding activity of Foxa1 to the promoter of bcl2 was significantly increased after H2O2 stimulation in A549 type II pneumocytes.
Fig. 4.
Association of Foxa1 with bcl2 promoter in type II pneumocytes shown by EMSA and ChIP. Foxa1 bound to the Foxa1 binding element in the region −538 to −532 bp (a) and −280 to −272 bp (b) in bcl2 promoter, as shown by EMSA. H2O2 promoted the binding of Foxa1 to the corresponding probes that contained the Foxa1 binding element. Cold probe: competition with cold probe (200-fold excess concentration); mutant probe: competition with mutant cold probe (200-fold excess concentration); Foxa1 Ab: supershift group by Foxa1 antibody; H2O2: cells stimulated by H2O2 (0.5 mM) for 30 min; NE: nuclear extract. c, d Association of Foxa1 with bcl2 promoter shown by ChIP. Chromatin was extracted and binding of Foxa1 to the bcl2 promoter was analyzed by ChIP using a pair of primers that contained the Foxa1 binding site at −538 to −532 bp (c) or the Foxa1 binding site at −280 to −272 bp (d), which specifically targeted the human bcl2 proximal promoter region. A549 cells treated with H2O2 (0.5 mM) for 24 h or normal control cells were harvested and used for ChIP. IgG: immunoprecipitated chromatin with an irrelevant rabbit IgG as negative control; Foxa1 Ab: immunoprecipitated chromatin with anti-Foxa1 antibody; Input: input lanes verified by equal amounts of DNA used for the initial immunoprecipitation. The image is representative of three independent experiments
To investigate the endogenous relevance of Foxa1 with bcl2 promoter, we performed ChIP assays on A549 cells that were treated with or without H2O2. The DNA isolated through IgG ChIP was used as a negative control. Input DNA, obtained from chromatin that was cross-link reversed similarly to the sample, served as a positive control for PCR effectiveness. Compared to control IgG, Foxa1 bound to the bcl2 promoter and H2O2 treatment increased Foxa1 binding to bcl2 promoter (Fig. 4c, d).
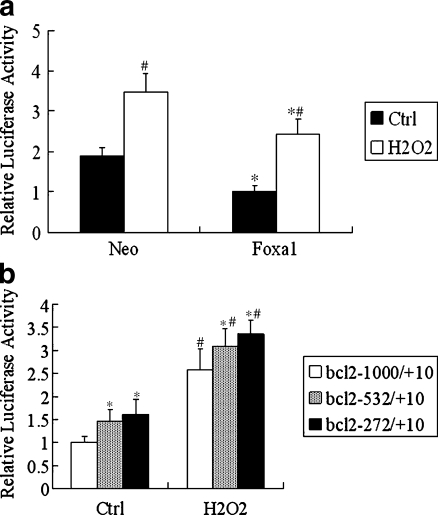
In order to understand how Foxa1 repressed bcl2, we assessed its effect on bcl2 promoter activity. The bcl2–1000 promoter–luciferase construct was transfected into A549 cells in the presence of a pcDNA3.1 empty vector or a pcDNA3.1-Foxa1 expression plasmid. As shown in Fig. 5a, there was a strong transcription inhibition effect of Foxa1 on the bcl2–1000 promoter, both under basic and H2O2-stimulated conditions. The results suggest that Foxa1 could decrease bcl2 protein levels in A549 cells, which is most likely mediated through downregulation of bcl2 gene transcription. The 1,000-bp bcl2 promoter region contains two putative Foxa1 binding sites at positions −538 to −532 and −280 to −272. To determine which Foxa1 site is responsible for Foxa1-induced repression of bcl2 promoter, truncation mutants of bcl2 promoter–luciferase constructs were transfected into A549 cells with pcDNA3.1-Foxa1 plasmid. The repression efficiencies of Foxa1 toward the truncated and full-length promoters were examined and compared. As shown in Fig. 5b, cotransfection of Foxa1 expression vector with a bcl2-532/+10 reporter that lacks the −538 to −532 Foxa1 site resulted in an increase in promoter activity in both basic and H2O2-stimulated conditions, indicating the existence of Foxa1-responsive elements between bases −1000 and −532 that might contribute to Foxa1-induced bcl2 promoter repression. Further deletion of the bcl2 promoter from −532 to −272 led to a further increase in promoter activity, suggesting the existence of Foxa1-responsive elements between bases −532 and −272 that might contribute to the repression of the promoter. The combination of the results from EMSA, ChIP, and reporter gene assays indicated the role of Foxa1-binding site in the −538 to −532 and −280 to −272 bp regions in the regulation of bcl2 expression.
Fig. 5.
Role of Foxa1 in the regulation of transcription activity of bcl2 promoter in type II pneumocytes. a Type II pneumocytes were transiently co-transfected with an expression plasmid of full-length Foxa1 (500 ng) and a reporter driven by bcl2 promoter (500 ng). The luciferase activity was detected using the Dual Luciferase Reporter System. All transfections were performed at least three times in triplicate. Cells were treated with H2O2 (0.5 mM) for 24 h. *Statistically significant difference versus the vector control group (Neo), P < 0.05. #Statistically significant difference from the relevant group without H2O2 stimulation, P < 0.05. b Transient co-transfection studies were performed in type II pneumocytes using full-length Foxa1 and a reporter driven by each of the truncate bcl2 promoter (500 ng). *Statistically significant difference from the bcl2 −1000/+10 group, P < 0.05. #Statistically significant difference from the relevant group without H2O2 stimulation, P < 0.05
Discussion
The Fox class of transcription factors, now numbering more than 100 members, is characterized by an evolutionarily conserved 110-amino-acid DNA-binding domain known as the forkhead domain (Kaestner 2000; Carlsson and Mahlapuu 2002). Three Foxa proteins, Foxa1, Foxa2, and Foxa3, are currently known and each shares a conserved structure, which consists of a DNA-binding domain at the C terminus of the protein and transactivating regions at the N terminus (Pani et al. 1992; Qian and Costa 1995; Cirillo et al. 2002). Foxa1 is expressed in the liver, pancreas, bladder, prostate, colon, lung, and mammary gland. Our previous study has indicated that Foxa1 is upregulated in rat lung tissues and pulmonary epithelial cells in acute lung injury induced by oleic acid. Since oxidative stress occurs after acute lung injury in an experimental setting and in humans (Tampo et al. 1999; Zhang et al. 2000; Yang et al. 2003), we presume that Foxa1 may play a role in oxidative stress. In the present study, we demonstrated that Foxa1 could be induced by H2O2 in A549 type II pneumocytes in a dose- and time-dependent manner.
Oxidative stress has been shown to be an important inducer of apoptosis (Li et al. 1997; Janssen et al. 1998; Wang et al. 2003), and experimental depletion of antioxidants leads to increased cell death (Brown et al. 1997). In the present study, we showed that Foxa1 was upregulated in response to H2O2, and the apoptosis rate of A549 cells was increased following Foxa1 overexpression. In contrast, inhibition of Foxa1 resulted in decreased apoptosis 24 h after H2O2 treatment. These results suggest that Foxa1 promotes apoptosis of A549 type II pneumocytes in oxidative-stress-induced apoptosis. Using bioinformatics analysis, we further found that some apoptosis-related genes including bcl2 contained putative Foxa1 binding elements in their promoters. Therefore, we hypothesized that Foxa1 might regulate the expression of bcl2 gene directly. Foxa1 overexpression or deletion by transfection with Foxa1 antisense oligonucleotides in A549 type II pneumocytes showed that the basal expression of bcl2 was decreased after Foxa1 overexpression and increased after Foxa1 depletion. These results indicate that Foxa1 can inhibit bcl2 gene expression under normal conditions. In addition, the upregulation of bcl2 induced by H2O2 was suppressed after Foxa1 overexpression, but further upregulated after Foxa1 depletion, which further demonstrates the role of Foxa1 in bcl2 repression during oxidative stress. However, the mechanism by which Foxa1 regulates bcl2 remains unclear.
It has been reported that Foxa1 can bind to the promoters of more than 100 genes associated with metabolic processes, regulation of signaling, and the cell cycle (Carlsson and Mahlapuu 2002; Hsieh et al. 2003; Tomaru et al. 2003). Using bioinformatics analysis, we found that there were three potential Foxa1 binding elements in the promoter of bcl2, i.e., −835 to −829, −538 to −532, and −280 to −272 bp. We also showed by EMSA and supershift assay that Foxa1 bound to the elements at −538 to −532 and −280 to −272 bp, and we further confirmed by ChIP that Foxa1 could bind to the bcl2 promoter. Luciferase reporter assay showed that Foxa1 inhibited the transcription of bcl2 reporter gene by binding to promoter regions of −538 to −532 and −280 to −272 bp. This showed that Foxa1 inhibited the expression of bcl2 gene, which might be one of the reasons for apoptosis of type II pneumocytes during oxidative stress/damage. Our results provided novel information about the function of Foxa1 in the regulation of bcl2 gene expression and its potential pro-apoptotic role during oxidative stress.
Inhibition of bcl2 by Foxa1 has important implication for disease. High expression of Foxa1 has been reported in various tumors, including lung, esophageal, breast cancer, and prostate cancer (Lin et al. 2002; Gao et al. 2003). Lower bcl2 expression has been correlated with poorer clinical outcome in patients with metastatic breast carcinoma (Joensuu et al. 1994; Hellemans et al. 1995; Charpin et al. 1998; Le et al. 1999). In breast cancer, current data suggest a pro-apoptotic role for Foxa1(Wolf et al. 2007). In addition, it has been reported that alterations in bcl2 expression are important in determining the susceptibility of type II pneumocytes and interstitial cells to apoptosis (Guinee et al. 1997). Our previous study has also demonstrated that Foxa1 expression is increased in lung tissues in rats with acute lung injury. In the present study, we showed increased expression of both Foxa1 and bcl2 in type II pneumocytes after exposure to H2O2 and, for the first time, the role of Foxa1 in the regulation of bcl2 gene expression, and therefore, a potential pro-apoptotic role of Foxa1 during oxidative stress. In order to understand the exact functions of Foxa1 during apoptosis, further investigations are needed.
Acknowledgments
This work was supported by funding from the National Nature Science Foundation of China (30330280), the Nature Science Foundation of Hunan Province, China (08JJ3030), and the Science Foundation of Health Department of Hunan, China (2007B090).
References
- Allsopp TE, Wyatt S, Paterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993;73(2):295–307. doi: 10.1016/0092-8674(93)90230-N. [DOI] [PubMed] [Google Scholar]
- Bisgaard HC, Nagy P, Santoni-Rugiu E, Thorgeirsson SS. Proliferation, apoptosis, and induction of hepatic transcription factors are characteristics of the early response of biliary epithelial (oval) cells to chemical carcinogens. Hepatology. 1996;23(1):62–70. doi: 10.1002/hep.510230110. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Jones DP. Ascorbate deficiency and oxidative stress in the alveolar type II cell. Am J Physiol. 1997;273(4 Pt 1):L782–788. doi: 10.1152/ajplung.1997.273.4.L782. [DOI] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250(1):1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Charpin C, Garcia S, Bonnier P, et al. bcl-2 automated and quantitative immunocytochemical assays in breast carcinomas: correlation with 10-year follow-up. J Clin Oncol. 1998;16(6):2025–2031. doi: 10.1200/JCO.1998.16.6.2025. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9(2):279–289. doi: 10.1016/S1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Gao N, Zhang J, Rao MA, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17(8):1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- Garcia I, Martinou I, Tsujimoto Y, Martinou JC. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992;258(5080):302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Guinee D, Brambilla E, Jr, Fleming M, et al. The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am J Pathol. 1997;151(4):999–1007. [PMC free article] [PubMed] [Google Scholar]
- Halmos B, Basseres DS, Monti S, et al. A transcriptional profiling study of CCAAT/enhancer binding protein targets identifies hepatocyte nuclear factor 3 beta as a novel tumor suppressor in lung cancer. Cancer Res. 2004;64(12):4137–4147. doi: 10.1158/0008-5472.CAN-03-4052. [DOI] [PubMed] [Google Scholar]
- Hellemans P, Dam PA, Weyler J, Oosterom AT, Buytaert P, Marck E. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995;72(2):354–360. doi: 10.1038/bjc.1995.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromas R, Costa R. The hepatocyte nuclear factor-3/forkhead transcription regulatory family in development, inflammation, and neoplasia. Crit Rev Oncol Hematol. 1995;20(1–2):129–140. doi: 10.1016/1040-8428(94)00151-I. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Chang MS, Chen JY, Yen JJ, Lu IC, Chou CM, Huang CJ. Cloning of zebrafish BAD, a BH3-only proapoptotic protein, whose overexpression leads to apoptosis in COS-1 cells and zebrafish embryos. Biochem Biophys Res Commun. 2003;304(4):667–675. doi: 10.1016/S0006-291X(03)00646-6. [DOI] [PubMed] [Google Scholar]
- Janssen YM, Soultanakis R, Steece K, Heerdt E, Singh RJ, Joseph J, Kalyanaraman B. Depletion of nitric oxide causes cell cycle alterations, apoptosis, and oxidative stress in pulmonary cells. Am J Physiol. 1998;275(6 Pt 1):L1100–1109. doi: 10.1152/ajplung.1998.275.6.L1100. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Pylkkanen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994;145(5):1191–1198. [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Li X, Gray PD, et al. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175(3):505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab. 2000;11(7):281–285. doi: 10.1016/S1043-2760(00)00271-X. [DOI] [PubMed] [Google Scholar]
- Le MG, Mathieu MC, Douc-Rasy S, Bihan ML, Adb El All H, Spielmann M, Riou G. c-myc, p53 and bcl-2, apoptosis-related genes in infiltrating breast carcinomas: evidence of a link between bcl-2 protein over-expression and a lower risk of metastasis and death in operable patients. Int J Cancer. 1999;84(6):562–567. doi: 10.1002/(SICI)1097-0215(19991222)84:6<562::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem. 1997;272(33):20646–20649. doi: 10.1074/jbc.272.33.20646. [DOI] [PubMed] [Google Scholar]
- Lin L, Miller CT, Contreras JI, et al. The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1), on chromosome band 14q13 is amplified and overexpressed in esophageal and lung adenocarcinomas. Cancer Res. 2002;62(18):5273–5279. [PubMed] [Google Scholar]
- Minoo P, Hu L, Xing Y, et al. Physical and functional interactions between homeodomain NKX2.1 and winged helix/forkhead FOXA1 in lung epithelial cells. Mol Cell Biol. 2007;27(6):2155–2165. doi: 10.1128/MCB.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L, Overdier DG, Porcella A, Qian X, Lai E, Costa RH. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Mol Cell Biol. 1992;12(9):3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon LG, Middleton G, Davies AM. Bcl-2 is required for cranial sensory neuron survival at defined stages of embryonic development. Development. 1997;124(20):4173–4178. doi: 10.1242/dev.124.20.4173. [DOI] [PubMed] [Google Scholar]
- Qian X, Costa RH. Analysis of hepatocyte nuclear factor-3 beta protein domains required for transcriptional activation and nuclear targeting. Nucleic Acids Res. 1995;23(7):1184–1191. doi: 10.1093/nar/23.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampo Y, Tsukamoto M, Yonaha M. Superoxide production from paraquat evoked by exogenous NADPH in pulmonary endothelial cells. Free Radic Biol Med. 1999;27(5–6):588–595. doi: 10.1016/S0891-5849(99)00110-0. [DOI] [PubMed] [Google Scholar]
- Tomaru Y, Kondo S, Suzuki M, Hayashizaki Y. A comprehensive search for HNF-3alpha-regulated genes in mouse hepatoma cells by 60K cDNA microarray and chromatin immunoprecipitation/PCR analysis. Biochem Biophys Res Commun. 2003;310(2):667–674. doi: 10.1016/j.bbrc.2003.08.148. [DOI] [PubMed] [Google Scholar]
- Vazquez de Lara L, Becerril C, Montano M, et al. Surfactant components modulate fibroblast apoptosis and type I collagen and collagenase-1 expression. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L950–957. doi: 10.1152/ajplung.2000.279.5.L950. [DOI] [PubMed] [Google Scholar]
- Wang L, Medan D, Mercer R, et al. Vanadium-induced apoptosis and pulmonary inflammation in mice: role of reactive oxygen species. J Cell Physiol. 2003;195(1):99–107. doi: 10.1002/jcp.10232. [DOI] [PubMed] [Google Scholar]
- White MK, Baireddy V, Strayer DS. Natural protection from apoptosis by surfactant protein A in type II pneumocytes. Exp Cell Res. 2001;263(2):183–192. doi: 10.1006/excr.2000.5120. [DOI] [PubMed] [Google Scholar]
- Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer. 2007;120(5):1013–1022. doi: 10.1002/ijc.22389. [DOI] [PubMed] [Google Scholar]
- Yang C, Moriuchi H, Takase J, Ishitsuka Y, Irikura M, Irie T. Oxidative stress in early stage of acute lung injury induced with oleic acid in guinea pigs. Biol Pharm Bull. 2003;26(4):424–428. doi: 10.1248/bpb.26.424. [DOI] [PubMed] [Google Scholar]
- Zhang H, Slutsky AS, Vincent JL. Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Med. 2000;26(4):474–476. doi: 10.1007/s001340051185. [DOI] [PubMed] [Google Scholar]