Abstract
MAPKAPK-2 (MK2) is a protein kinase activated downstream of p38-MAPK which phosphorylates the small heat shock proteins HSP27 and αB crystallin and modulates p38-MAPK cellular distribution. p38-MAPK activation is thought to contribute to myocardial ischemic injury; therefore, we investigated MK2 effects on ischemic injury and p38 cellular localization using MK2-deficient mice (KO). Immunoblotting of extracts from Langendorff-perfused hearts subjected to aerobic perfusion or global ischemia or reperfusion showed that the total and phosphorylated p38 levels were significantly lower in MK2−/− compared to MK2+/+ hearts at baseline, but the ratio of phosphorylated/total p38 was similar. These results were confirmed by cellular fractionation and immunoblotting for both cytosolic and nuclear compartments. Furthermore, HSP27 and αB crsytallin phosphorylation were reduced to baseline in MK2−/− hearts. On semiquantitative immunofluorescence laser confocal microscopy of hearts during aerobic perfusion, the mean total p38 fluorescence was significantly higher in the nuclear compared to extranuclear (cytoplasmic, sarcomeric, and sarcolemmal compartments) in MK2+/+ hearts. However, although the increase in phosphorylated p38 fluorescence intensity in all compartments following ischemia in MK2+/+ hearts was lost in MK2−/− hearts, it was basally elevated in nuclei of MK2−/− hearts and was similar to that seen during ischemia in MK2+/+ hearts. Despite these differences, similar infarct volumes were recorded in wild-type MK2+/+ and MK2−/− hearts, which were decreased by the p38 inhibitor SB203580 (1 μM) in both genotypes. In conclusion, p38 MAPK-induced myocardial ischemic injury is not modulated by MK2. However, the absence of MK2 perturbs the cellular distribution of p38. The preserved nuclear distribution of active p38 MAPK in MK2−/− hearts and the conserved response to SB203580 suggests that activation of p38 MAPK may contribute to injury independently of MK2.
Keywords: Ischemia, MAPKAPK-2, p38 MAPK, HSP27, αB-crystallin
Introduction
The p38 mitogen-activated protein kinase (p38 MAPK) is a member of the mitogen- and stress-activated protein kinases family (MAPKs/SAPKs) and is activated by a number of mitogenic and stress stimuli, including ischemia–reperfusion (Bogoyevitch et al. 1996). p38 MAPK plays an important role in many processes including inflammation, wound healing, cell growth, differentiation, and apoptosis. In the heart, p38 MAPK has been implicated in the regulation of cardiac gene expression, myocyte hypertrophy, heart failure, energy metabolism, contractility, and apoptosis (see (Bassi et al. 2008; Clark et al. 2007) for recent reviews). Activation of p38 MAPK has also been linked to both myocardial injury, i.e., contributing to infarct size following prolonged ischemia/reperfusion or cardioprotection, i.e., being involved in the protection afforded by the phenomenon of ischemic preconditioning, whereby a brief ischemic episode leads to an adaptive tolerance to a longer, injurious ischemic insult (Bassi et al. 2008). This apparent contradiction has led to much controversy in the field. However, it is generally agreed that the intraischemic activation of p38(α)MAPK contributes to myocardial injury, since infarct size following ischemia/reperfusion can be reduced by the pyridinyl imidazole compound SB203580 which is a potent and selective inhibitor of p38 MAPK. However, the underlying intracellular mechanism(s) leading to p38 MAPK activation during ischemia and its role in injury remain to be well clarified (Bassi et al. 2008; Clark et al. 2007; Saurin et al. 2000; Mackay and Mochly-Rosen 1999; Ma et al. 1999).
The cellular distribution of p38 MAPK in the heart under control conditions is still unclear. In intact heart preparations, total p38 MAPK was detected in the cytosolic, nuclear, and mitochondrial compartments (Maulik et al. 1996; Ballard-Croft et al. 2005). However, reports on possible cellular translocation of activated p38 MAPK upon ischemic insult are limited and controversial. For example, Maulik et al (1996) reported p38 translocation to the nucleus and myofibrillar striations. On the other hand, Fryer et al. (2001) showed no change. Total p38 MAPK cellular distribution and expression levels are thought to be modulated by its own downstream highly expressed substrate MAPK-activated protein kinase-2 (MAPKAPK2 or MK2; Zu et al. 1997). Activated p38 MAPK phosphorylates nuclear MK2 and forms a complex whereby an MK2 nuclear export signal is unmasked, resulting in its rapid export from the nucleus (Maulik et al. 1996; Engel et al. 1995; Ben Levy et al. 1998). In the cytoplasm, MK2 phosphorylates the small heat shock proteins (HSP) 25/27 (Stokoe et al. 1992; Freshney et al. 1994; Rouse et al. 1994) and αB-crystallin (Hoover et al. 2000; Ito et al. 2001; Kato et al. 1998). The phosphorylation of HSP25/27 induces its dissociation from large aggregates into dimers and monomers (Kato et al. 1994). The phosphorylation-dependent dissociation of HSP27 in human glioma cells appears to be mediated by two parallel cascades (Kato et al. 2001), a PKC-dependent pathway and a p38 MAPK/MK2-dependent pathway. Ischemia appears to induce p38 MAPK activation which is sensitive to SB203580 (Tanno et al. 2003). Since during ischemia, HSP25/27 phosphorylation is also SB203580-sensitive, HSP25/27 phosphorylation may be mediated through p38 MAPK/ MAPKAPK-2, rather than PKC.
The phosphorylation of HSP25/27 is causally related to the regulation of actin microfilament dynamics following oxidative stress and the alteration of the redox state of actin, as well as some actin-regulatory proteins. Prolonged ischemia and oxidant stress disturb the structure and spatial organization of actin, the major constituent of the cell cytoskeleton, resulting in actin filament fragmentation and plasma membrane blebbing. Overexpression of HSP25/27 has been shown to confer protection against oxidative stress or ischemia in rat cardiomyocytes (Huot et al. 1996; Martin et al. 1997), but protection is independent of its phosphorylation state (Martin et al. 1997, 1999). Furthermore, transgenic expression of both wild-type (WT) and non-phosphorylatable mutants of HSP27 in mice have been shown to be cardioprotective against ischemia/reperfusion injury, suggesting that phosphorylation is not necessary for the protective effect of HSP27 (Hollander et al. 2004). Activation of HSP25/27 prevents oxidative stress-induced fragmentation of actin, increasing the tolerance of the cytoskeleton to stress (Guay et al. 1997). Ischemia also induces the translocation of αB-crystallin from the Triton-soluble to the Triton-insoluble compartment (Eaton et al. 2000) consisting of myofilaments and the cytoskeleton. It has been proposed that αB-crystallin and HSP25/27 mediate cardioprotection by binding to proteins of the cytoskeleton and the myofilaments to attenuate ischemic injury (Eaton et al. 2000). Overexpression of αB-crystallin has been shown to attenuate ischemic injury in cultured cardiac myocytes (Bluhm et al. 1998) and in transgenic mice (Ray et al. 2001), and the cytoprotection appears dependent on the phosphorylation of the Ser-59 residue, which in turn is dependent on MAPKAPK-2 (Kato et al. 1998) and p38 MAPK (Morrison et al. 2003).
Since phosphorylation may or may not be necessary for the cardioprotection afforded by HSP25/27 and αB-crystallin, we hypothesized that MAPKAPK-2 may play a critical role in determining the outcome of ischemic injury, either through phosphorylation of its downstream targets and/or by modulating the cellular distribution of activated p38 MAPK. Although there is indirect evidence to suggest a role for MK2 in limiting ischemic injury, the lack of a specific MK2 inhibitor has been a major obstacle to fully elucidating the role of this kinase in the signaling cascade. We, therefore, utilized a mouse line with targeted disruption of the MK2 allele, postulating that MK2 deficient mice would demonstrate increased sensitivity to ischemia.
Materials and methods
Generation of MAPKAPK-2 deficient mice
Mice homozygous for the targeted disruption of both mitogen-activated protein kinase-activated protein kinase 2 (MK2) alleles were obtained from an original source (Kotlyarov et al. 1999). Genotyping was performed on extracted genomic DNA (Qiagen, Germany) using specific primers straddling the gene and the inserted Neo cassette described previously (Kotlyarov et al. 2002).
Perfusion of isolated murine hearts
Experiments were performed in accordance with the Home Office “Guidance on the Operation of Animals (Scientific Procedures) Act 1986” published by HMSO (London). Male mice homozygous for the disrupted MK2 allele (MK2−/−) were compared with sex and weight-matched wild-type (MK2+/+) littermates, using the protocols in Fig. 1. Mice were anesthetized and their hearts rapidly excised and perfused as described previously (Tanno et al. 2003; Saurin et al. 2002; Gorog et al. 2003). Retrograde perfusion with modified Krebs–Henseleit (K–H) buffer at 85 mm Hg for a stabilization period of 40 min was followed by 30 min global ischemia (zero flow) and 2 h reperfusion. To assess the contribution of p38 MAPK to the MK2-mediated signaling cascade, one cohort was perfused with K–H buffer containing 1 μmol/L SB203580 in 0.01% DMSO for 10 min prior to ischemia, while the control group received K–H buffer containing 0.01% DMSO vehicle. At the end of the protocol, hearts were stained with 1% triphenyl tetrazolium chloride (Sigma, UK) as previously described (Tanno et al. 2003; Gorog et al. 2003) and stored at −70°C until analysis.
Fig. 1.
Langendorff heart perfusion experimental protocols in wild-type (WT) and knockout (KO) mice to investigate sensitivity to ischemia–reperfusion with and without SB203580
Infarct size
Thawed hearts were immersed in 2.5% glutaraldehyde for 1 min, set in 5% agarose, sectioned with a Vibratome 1000 plus (Products International Inc, USA) and fixed in 10% formaldehyde overnight at room temperature, followed by transfer into phosphate buffered saline for 24 h at 4°C. The slices were photographed with a digital camera, the infarct area delineated with planimetry using image analysis software (NIH Image v1.61) and surface area transformed to volume by multiplication with tissue depth. The infarct size was expressed as the percentage of area at risk, defined as the sum of total ventricular area minus cavities.
Western blot analysis
To investigate the role of MAPKAPK-2 in signal transduction during ischemia, hearts perfused in the Langendorff mode were harvested after stabilization at 0, 10, and 20 min ischemia, freeze clamped in liquid nitrogen, and stored at −70°C until analysis. Western blot analyses were performed as previously described (Saurin et al. 2002) using the following primary antibodies: mouse monoclonal to dual phospho-p38 MAPK (Thr 180/Tyr 182; Sigma Biosciences, UK); rabbit polyclonal to total p38 MAPK, goat polyclonal to total HSP27 (both Santa Cruz Biotechnology, CA, USA), mouse monoclonal to phosphor-Ser82 of HSP25/27 (Martin et al. 2001; which also recognizes mouse HSP25 phospho-Ser86), rabbit polyclonal to phosphoserine 59 of αB-crystallin (Inaguma et al. 2001), rabbit polyclonal to histone H1 (Santa Cruz), and rabbit polyclonal to GAPDH (Santa Cruz). These were detected with the appropriate peroxidase-conjugated antispecies secondary antibody (all DAKO, Denmark). Densitometric analyses were performed to quantify the degree of phosphorylation using NIH image analysis software. Myocardial content of p38 MAPK varied by MK2 genotype, necessitating normalization of phospho-protein densities by their corresponding total protein density.
Tissue fractionation
Myocardial tissue samples were fractionated according to Mizukami et al. (1997). Samples were homogenized in two volumes of STE buffer (0.32 M sucrose, 10 mM tris HCl, pH 7.4, 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM 2-mercaptoethanol, 20 μM leupeptin, 0.15 mM pepstatin A, 0.2 mM PMSF, 50 mM NaF, 1 mM Na orthovanadate, 0.4 nM microcystin) using a mechanical homogenizer (Ystral, Germany). Homogenates were centrifuged at 5,000×g for 10 min at 4°C. The supernatant was removed and centrifuged at 100,000×g for 60 min at 4°C to yield a cytosolic fraction (100,000×g supernatant) and a membrane fraction (100,000×g pellet). The 5,000×g pellet was washed twice and resuspended in 1% Triton X-100, 150 mM NaCl, 10 mM Tris.HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.2 mM Na orthovanadate, 20 mM leupeptin A, 0.2 mM PMSF, 50 mM NaF, 0.4 nM microcystin, and centrifuged at 15,000×g for 30 min at 4°C to yield a nuclear fraction (15,000×g supernatant). Representative samples of each fraction were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted as described above.
Isolation of mouse cardiac myocytes
Hearts were excised and perfused for 5 min with Tyrode solution (NaCl 130 mM, KCl 5.4 mM, MgCl2 1.4 mM, NaH2PO4 0.4 mM, HEPES 4.2 mM, Glucose 10 mM, Taurine 20 mM, Creatine 10 mM, pH 7.3 at 37°C, and bubbled with 100% O2) containing 750 mM Ca2+ in Langendorff mode. The hearts were then perfused with Tyrode solution containing 100 mM EGTA (Sigma) for 3 min and then with Tyrode solution containing 100 mM of Ca2+ and 1 mg/ml collagenase (275 U/mg, Worthington) for 8 min. The ventricles were cut into small pieces in the collagenase solution and bubbled in a water bath at 37°C for 5 min until fully digested. The content was then filtered using a nylon mesh (and placed into a water bath at 37°C for 8 min). Supernatant was removed and the pellets were immediately resuspended and incubated for further 10 min with Tyrode solution containing 0.5 mM Ca2+ and 1% bovine serum albumin (BSA; Sigma). Finally the cells were resuspended with 10 ml of Tyrode solution containing 1 mM Ca2+.
Immunofluorescence and laser confocal microscopy
After control perfusion or ischemia, at the appropriate times, WT and KO hearts were embedded in OCT compound, rapidly frozen in ice-cooled isopentane in liquid N2 and then stored at –80°C. Twelve-micrometer thick longitudinal ventricular cryostat sections were fixed with ice-cold 4% formaldehyde in physiological-buffered saline (PBS; 15 min at room temperature), permeabilized with 0.2% Triton-X-100 in PBS (20 min), and then incubated for 2 h with blocking buffer (containing in PBS; 2% bovine serum albumin, 0.2% TritonX-100, 5% goat serum). Sections were first incubated at 4°C overnight with primary antibody, polyclonal rabbit anti-alpha p38 MAPK, or phospho-p38 MAPK diluted 1:100 or 1:30, respectively. Secondary antibody (goat anti-rabbit IgG Alexa 488 FITC, 1:200; molecular Probes) was applied for 1 h at room temperature. Nuclei were identified using the fluorescent nucleic acid binding dye TO-PRO-3 (Molecular Probes; 1:500) added with the secondary. Sections were then mounted with Vectashield fluorescence mounting medium (Vectashield, Vector Laboratories, Inc), viewed and analyzed at ×40 magnification using a Zeiss© LSM 510 laser-scanning confocal microscope. Images of heart sections were taken at a focal plane from the middle of the cell using a z-stack of ten images with 1-μm spacing. Total p38 or P-p38 mean nuclear fluorescence intensity was calculated as mean fluorescence intensity of green within the nucleus stained with TO-PRO-3. For each experiment, average intensities of 20 nuclei per section of a total of three sections per experiment were calculated. Cytoplasmic colocalization was assessed by averaging the fluorescence intensity of 20 areas of interest in every section. In each experiment, three sections were imaged and analyzed.
Freshly isolated adult mouse cardiac myocytes were cultured on laminin-coated individual dishes for 1 h in culture media. The media was removed and cells were washed with phosphate buffered saline (PBS) and fixed by 4% paraformaldehyde (Analar BDH) in PBS for 15 min. This was followed by three 5-min washes with PBS. The cells were permeabilized with 0.2% Triton X-100 in PBS followed by a 5-min wash with PBS and blocked with 100 ml/dish of 5% Normal Goat Serum. Rabbit polyclonal anti-total (pan) p38 (Cell Signaling) was diluted 1:50 plus primary mouse anti-α actinin (diluted 1:500) in buffer containing Trizma 20 mM, NaCl 155 mM, EGTA 2 mM, MgCl2 2 mM, pH 7.5, and 1% BSA. The dishes were placed into a humid chamber and incubated overnight at 4°C. The following day, the cells were washed in PBS as before and incubated over night at 4°C with secondary antibodies; anti-mouse Cy3a diluted 1:500 (ML Jackson Stratech Scientific), anti-rabbit Cy2a diluted 1:100 (ML Jackson Stratech Scientific), and DAPI diluted 1:100 (Sigma). The cells were then washed as before and mounted with a droplet of Mounting Medium (0.03 M Tris, pH 9.5; 70% glycerol; 5% (w/v) n-propylgallate) and covered with cover slips (VWR 30 mm diameter, Science Warehouse). The edge of each dish was removed with a hot wire and coverslips sealed with nail varnish. Each dish was then glued onto a microscope slide and kept at 4°C until analysis.
Statistical analysis
Morphological characteristics and infarct sizes were compared using one-way analysis of variance (ANOVA), followed where appropriate by the Newman–Keuls post hoc comparison, and hemodynamic parameters compared using two-way repeat measures ANOVA with a multivariate linear model to correct for the differing group sizes. The relationship between infarct volume and total heart volume was interrogated with simple linear regression and the regression lines compared by analysis of covariance (ANCOVA) using the SigmaStat statistical package with an Excel plug-in (Ferris State University, USA). Results are shown as mean ± SEM, except where otherwise indicated and p < 0.05 was taken as significant.
Results
Effect of MAPKAPK-2 (MK2) on sensitivity to ischemia-reperfusion
Morphologic and hemodynamic characteristics are shown in Table 1. There were no statistically significant differences in these characteristics between MK2−/− and MK2+/+ hearts or between hearts treated with SB203580 or vehicle. However, there was a trend toward reduced coronary flow in the presence of SB203580 at 60 and 120 min in WT but not KO hearts and reduced LV developed pressure in KO hearts in the presence or absence of SB203580. Despite these nonsignificant trends, treatment with SB203580 reduced the sensitivity to ischemia similarly in MK2−/− and MK2+/+ hearts (Fig. 2). Sensitivity to ischemia was directly related to heart volume, and similar in MK2−/− and MK2+/+ hearts (results not shown).
Table 1.
Morphological and hemodynamic characteristics of wild type (WT) and knockout (KO) hearts subjected to ischemia-reperfusion following pretreatment with SB203580 or vehicle
| SB203580 | WT | WT | KO | KO | ANOVA |
|---|---|---|---|---|---|
| – | + | – | + | p | |
| Body weight (g) | 29 ± 3 | 29 ± 2 | 30 ± 1 | 30 ± 3 | 0.9 |
| Wet heart weight (mg) | 139 ± 40 | 128 ± 15 | 134 ± 11 | 138 ± 15 | 0.8 |
| Heart volume (mm3) | 77 ± 15 | 91 ± 14 | 98 ± 14 | 98 ± 14 | 0.1 |
| Baseline coronary flow (ml/min) | 3 ± 0.6 | 3.2 ± 0.7 | 3.6 ± 0.6 | 3.2 ± 0.9 | 0.5 |
| Baseline LV developed pressure (mm Hg) | 57 ± 4 | 62 ± 8 | 64 ± 10 | 57 ± 6 | 0.3 |
| Coronary flow at 60 min reperfusion (ml/min) | 2.8 ± 1.2 | 1.6 ± 0.8 | 2.1 ± 0.6 | 2.1 ± 0.3 | 0.1 |
| LV developed pressure at 60 min reperfusion (mm Hg) | 12 ± 8 | 11 ± 5 | 7 ± 2 | 7 ± 2 | 0.3 |
| Coronary flow at 120 min reperfusion (ml/min) | 2.5 ± 1.0 | 1.7 ± 0.7 | 2.1 ± 0.7 | 2.3 ± 0.5 | 0.2 |
| LV developed pressure at 120 min reperfusion (mm Hg) | 12 ± 7 | 12 ± 6 | 9 ± 6 | 7 ± 3 | 0.4 |
Values are mean ± SD; n = 6 per group
Fig. 2.
Infarct size expressed as a percentage of area at risk in wild-type (WT) and knockout (KO) mice subjected to 30 min global ischemia and 2 h reperfusion with (SB) and without (vehicle, DMSO) pretreatment with SB203580. Error bars represent mean ± SD
Signal transduction during ischemia
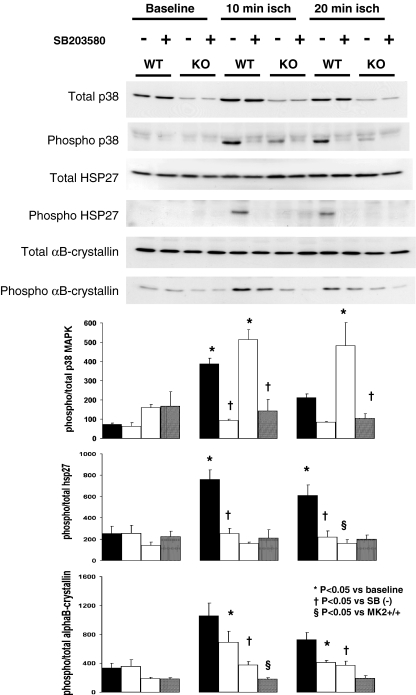
Total p38 MAPK was markedly reduced in MK2−/− compared to MK2+/+ hearts as demonstrated by western blotting (Fig. 3). Total HSP25/27 and αB-crystallin contents were similar in MK2−/− and MK2+/+ hearts. Ischemia induced p38 MAPK phosphorylation in both MK2+/+ and MK2−/− hearts. In wild-type mice, activation of p38 MAPK was reflected by the downstream phosphorylation of HSP25 (Ser86) and αB-crystallin (Ser59). Activation of p38 MAPK in MK2−/− mice was not associated with downstream phosphorylation of HSP25, nor significant phosphorylation of αB-crystallin compared to wild-types. SB203580 pretreatment inhibited p38 MAPK and HSP25 phosphorylation and partially inhibited downstream αB-crystallin phosphorylation in both MK2+/+ and MK2−/− hearts.
Fig. 3.
Upper panel: Western blots of total and phosphorylated p38 MAPK, HSP25, and αB-crystallin in vehicle (−) and SB203580 (+) treated-hearts of wild-type (WT) and knockout (KO) mice subjected to 10 or 20 min ischemia. Lower panel: Quantitative data (arbitrary units), directly corresponding to genotype and protocol of western blots above, showing densitometrically derived ratios of phosphorylated vs. total p38 MAPK, HSP25/27, and αB-crystallin (mean ± SEM, n = 3/group). Groups compared with one-way ANOVA with Newman–Keuls post hoc analysis
Since total p38 MAPK is reduced in MK2−/− compared to MK2+/+ hearts, we interrogated activation of p38 MAPK by calculating the ratio of phosphorylated/total p38 MAPK by densitometry. Although the absolute level of p38 MAPK phosphorylation observed in MK2−/− compared to MK2+/+ hearts during ischemia was lower, the relative increase in phosphorylated p38 MAPK was similar in MK2+/+ and MK2−/− hearts at 10 min ischemia (315 ± 22 vs. 352 ± 37, p = 0.4, expressed here as the ratio of total, arbitrary densitometric units as a difference from baseline) and at 20 min ischemia (139 ± 27 vs. 323 ± 113, p = 0.2). Furthermore, SB203580 reduced the ratio of phosphorylated/total p38 MAPK by a similar extent in both MK2+/+ and MK2−/− hearts at 10 min ischemia (a reduction of 294 ± 23 vs. 371 ± 66, p = 0.3) and at 20 min ischemia (a reduction of 128 ± 16 vs. 378 ± 114, p = 0.1).
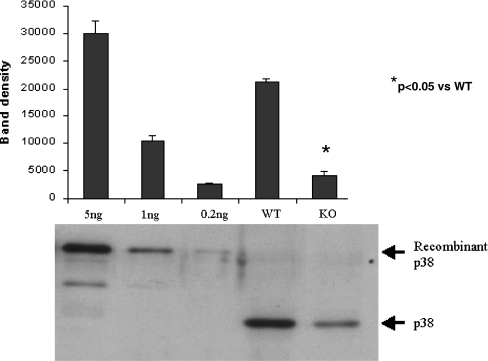
Quantification of the effect of MK2 genotype on myocardial p38 MAPK content
Since total p38 MAPK is markedly reduced in MK2−/− compared to MK2+/+ hearts, total p38 MAPK was quantitated by immunoblotting baseline samples of ventricular tissue (MK2−/−, n = 6 and MK2+/+, n = 6) against known quantities of GST-tagged (Sigma, UK) recombinant p38 MAPK as shown in Fig. 4. A fivefold reduction in total p38 MAPK level was detected in MK2 deficient mice (total p38 MAPK, 4.5 ± 0.1 vs. 0.9 ± 0.1 ng/μg of soluble myocardial protein, p < 0.05; n = 6/group).
Fig. 4.
Densitometric analysis of westerns blots of total p38 MAPK expression in hearts from wild-type (WT) and knockout (KO) mice, immunoblotted against known quantities of GST-tagged recombinant p38 MAPK (n = 6/recombinant protein or heart sample). Error bars represent SEM. A representative western blot is shown in the lower panel
Analysis of cellular distribution of total and phospho-p38 MAPK by cellular fractionation
We also examined the changes in phosphorylation status and distribution of p38 using a cell fractionation approach. Homogenates of WT or KO hearts following aerobic perfusion (control) or global ischemia were fractionated into cytosolic and nuclear fractions by differential centrifugation. As shown in Fig. 5, the results were similar to the immunoblotting data. Total p38 levels were decreased in the cytosolic and nuclear fractions of KO hearts compared to WT. Ischemia induced an increase in cytosolic phospho-p38 in both WT and KO hearts to a similar level. The overall level of p38 phosphorylation in nuclear fractions was much lower than for cytosolic p38. The fidelity of the cytosolic fraction and the nuclear component of the particulate fraction was determined by immunoblotting theses samples against a cytosolic marker (GAPDH) and a nuclear marker (histone H1). Because the total p38 antibody recognizes predominantly p38alpha (results not shown), this suggests that p38alpha may constitute the major species in the nuclear/myofibrillar/cytoskeletal fractions following ischemia.
Fig. 5.
Myocardial tissue samples from wild-type (WT) and knockout (KO) hearts under control perfusion (C) and following 20 mins global ischemia (I) were homogenized and fractionated into cytosolic and nuclear fractions by differential centrifugation. Samples were then separated by SDS-PAGE and western blotted. The fidelity of the cytosolic and nuclear fractions was determined by probing blots for GAPDH (cytosolic) and histone H1 (nuclear). The relative distribution of total p38-MAPK and phospho-p38-MAPK was determined using antibodies specific for these forms
Analysis of cellular distribution of total and phospho-p38 MAPK by immunofluorescence
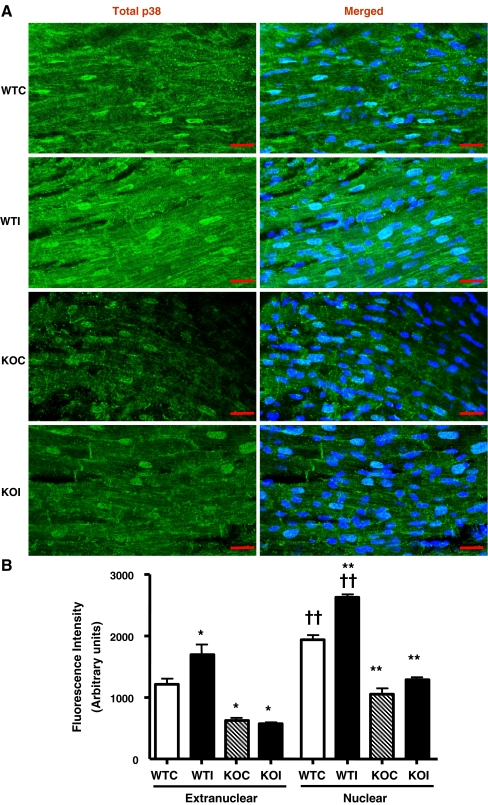
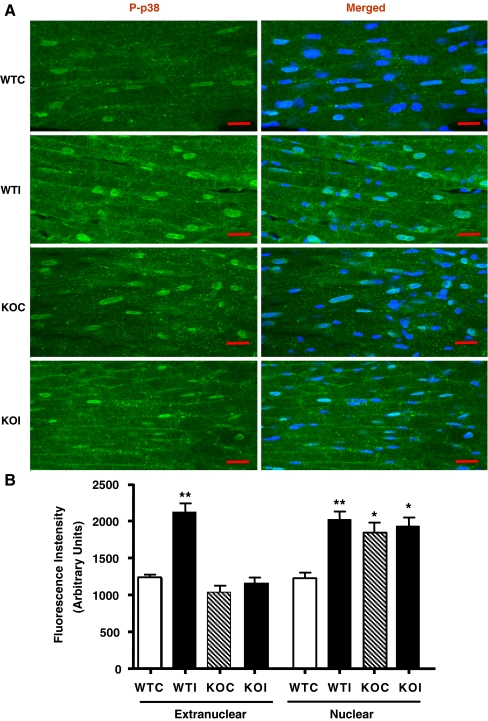
Since the ratio of phosphorylated/total p38 was similar in both MK2+/+ and MK2−/− following ischemia (WTI and KOI, respectively), we sought to further assess cellular distribution of total and phosphorylated p38 MAPK in MK2+/+ and MK2−/− hearts after control perfusion (WTC and KOC, respectively) and 20 min of global ischemia (WTI and KOI, respectively) by immunofluorescence. Total p38 was detected and quantified in both extranuclear and nuclear compartments in all heart sections. As shown in Fig. 6, the nuclear fluorescence intensity of total p38 was significantly higher than the extranuclear fluorescence in all hearts. Moreover, the total p38 levels were significantly reduced in KO hearts compared to the wild-type hearts which is in agreement with our western blot data as well as previous reports. Interestingly, the mean measured fluorescence intensity of extranuclear total p38 was increased in WTI compared to WTC (p ≤ 0.05). Also, the nuclear total p38 fluorescence intensity was significantly higher in WTI compared to WTC (p ≤ 0.001). This increase in fluorescence intensity was associated with a marked translocation of p38-MAPK to the z-lines of the sarcomere, the intercalated discs, and the sarcolemmal membrane and was due to increased localization on specific structures rather than an increase in p38-MAPK levels, per se, in that in control hearts, the p38 fluorescence is more difuse. In contrast, no increase in the overall fluorescence intensity was observed in KOI compared to KOC, although translocation to the z-lines and intercalated discs was still observed. As shown in Fig. 7, phospho-p38 (representative of the active p38 fraction) increased in the nuclear and extranuclear compartments upon ischemia (WTI vs. WTC: p ≤ 0.001 and p ≤ 0.001, respectively). However, this was associated with increased fluorescence intensity of phospho-p38 in the nucleus and sarcolemma but not the z-lines or intercalated discs. In contrast, there was no overall increase in cytosolic or nuclear phospho-p38 in the knockout hearts (KOI vs. KOC), although sarcolemmal phospho-p38 increased upon ischemia and nuclear phospho-p38 was significantly higher at baseline (KOC vs. WTC).
Fig. 6.
A Laser confocal Immunofluorescence images from 12 μm longitudinal cryostat wild-type and knockout left ventricular heart sections in control (WTC, KOC) and after 20 min ischemia (WTI and KOI) double-labeled with anti-Total-p38 (green; FITC) and nuclear stain TO-PRO 3 (blue; Cy5; merged image). B The mean fluorescence intensity of extranuclear and nuclear Total-p38, measured from the corresponding cryostat heart sections. Error bars represent MEAN ± SEM; n = 3 (*p ≤ 0.05 vs. corresponding WT and †p ≤ 0.05 vs. corresponding extranuclear compartment groups compared with one-way ANOVA with Newman–Keuls post hoc analysis)
Fig. 7.
A Laser confocal Immunofluorescence images from 12-μm cryostat heart sections, showing the cellular distribution of Phospho-p38 (green; FITC) and costained with nuclear stain TO-PRO 3 (blue; Cy5; merged image) of hearts perfused with control KH solution for 30 min (WTC, KOC) or subjected to 20 min global ischemia (WTI, KOI; 20 μm). B The mean extranuclear and nuclear fluorescence intensity of Phospho-p38 measured from the corresponding cryostat heart sections (error bars represent SEM; n = 3; *p ≤ 0.05 vs. WTC; Groups compared with one-way ANOVA with Newman–Keuls post hoc analysis)
Although the cell fractionation and immunofluorescence data are broadly similar, there is a slight discrepancy in that the immunofluorescence data suggest that in the KO hearts, the p38 associated with the cytoskeleton is not activated by ischemia. Furthermore, the nuclear phospho-p38 levels were similar to WT controls despite there being less total p38, whereas the fractionation data shown in Fig. 5 suggest that phospho-p38 was not elevated at baseline in the nuclei of KO hearts, in contrast to the immunofluorescence data, although did appear to increase further following ischemia. However, the immunofluorescence data also suggest that the majority of phosphorylated p38 is nuclear.
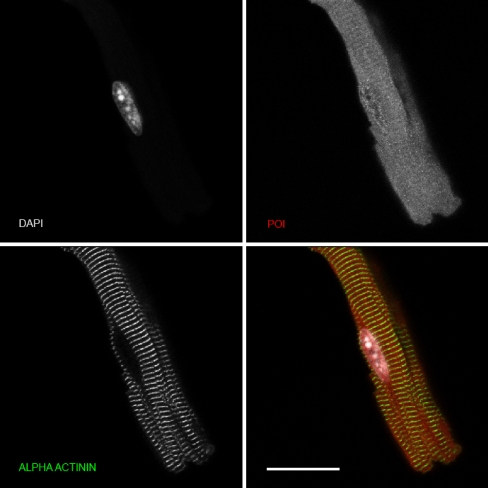
To confirm the accuracy of the p38 immunostaining in heart sections, we investigated the subcellular localization of total p38 by confocal immunofluorescence analysis in isolated mouse myocytes. Figure 8 shows the distribution of total p38 (predominantly representing p38α) in freshly isolated control myocytes. The total p38 distribution was difuse and present in the cytosol and nucleus but was not found in the sarcomeres (as determined by costaining for α-actinin), sarcolemma, or intercalated discs. This distribution agrees with that shown in Fig. 6 for total p38 in WT control hearts.
Fig. 8.
Confocal immunofluorescence analysis of the subcellular distribution of total p38 (corresponding to p38α) in isolated adult mouse cardiac myocytes. Freshly isolated mouse ventricular cardiomyocytes were fixed and subjected to immunofluorescence using total p38 antibody (labeled as POI: protein of interest). Myocytes were costained with an anti α-actinin antibody to visualise the sarcomeres (z-lines) and with DAPI to visualise the nuclei. Scale bar represents 10 μM
Discussion
In this study, we report that the absence of MK2 abolishes HSP25 phosphorylation during ischemia, but this does not influence infarction volume. The absence of MK2 with the presence of SB203580 abolishes αB-crystallin phosphorylation, but this diminishes rather than increases infarct size. We also observed that phosphorylated p38 is redistributed in MK2−/−, and its nuclear intensity was higher than in MK2+/+. Furthermore, the increase in sarcomeric and intercalated disc localization seen in wild-type hearts following ischemia is consistent with translocation in response to ischemia and was lost in MK2−/− hearts. Taken together, these observations suggest that neither αB-crystallin nor HSP25 phosphorylation are important for protection against ischemic injury.
Effect of MAPKAPK-2 deficiency and SB203580 on downstream signaling during ischemia
Since the downstream phosphorylation targets of MK2 are involved in stabilization of the cytoskeleton and cardioprotection, we expected the hearts of mice deficient in MK2 to exhibit enhanced sensitivity to ischemia. Instead, despite absence of ischemia-induced HSP25 phosphorylation in MK2−/− hearts, infarct size was similar to that in MK2+/+ hearts, implying that HSP25 phosphorylation is not critical to outcome in ischemia. Earlier studies had indicated that activation of p38 MAPK and MK2 are necessary for the phosphorylation of αB-crystallin on Ser-59 (Kato et al. 1998; Ito et al. 1997). In the absence of MK2, phosphorylation of αB-crystallin on Ser-59 was reduced in response to ischemia but was not completely abolished, implying that other kinases may act in concert with MK2 to achieve maximal αB-crystallin phosphorylation. Furthermore, the similar infarct burden, despite the reduced αB-crystallin phosphorylation in MK2−/− hearts, indicates that either phosphorylation of αB-crystallin does not contribute significantly to cardioprotection or that even this limited phosphorylation is sufficient to maintain cell viability. However, this latter interpretation is unlikely since further abolition of the residual phosphorylation in MK2−/− hearts with SB203580 reduces, rather than increases, infarct volumes.
Effect of MAPKAPK-2 deficiency on p38 MAPK expression
The markedly reduced level of total p38 MAPK in MK2−/− compared with MK2+/+ hearts has been described previously (Kotlyarov et al. 2002). Thus, the effects of MK2 deficiency may be underestimated due to the confounding effects on p38 MAPK content. Since ischemic activation of p38 MAPK is thought to contribute to myocardial injury (Saurin et al. 2000; Mackay and Mochly-Rosen 1999; Ma et al. 1999), the reduced p38 MAPK expression would be expected to attenuate ischemic damage. Although total p38 MAPK was reduced, activation was readily detectable, and the relative amount of p38 MAPK phosphorylation in response to ischemia (as percent of baseline) was similar in MK2−/− and MK2+/+ hearts. This implies that it may be the proportion of p38 MAPK that becomes phosphorylated rather than the absolute level of phosphorylated p38 MAPK, which determines the extent of infarction. This view is supported by the observation that despite the reduced absolute amount of phosphorylated p38 MAPK, SB203580 reduced infarction similarly in MK2+/+ and MK2−/− hearts and also that despite decreased levels of total p38 MAPK in MK2−/− hearts, infarct sizes were similar in both genotypes in the absence of SB203580. The only other interpretation of this observation is that the infarct reduction by SB203580 is due to an off-target pharmacological effect.
Role of MAPKAPK-2 in determining infarct size
The phosphorylation of HSP25/27 has been shown to confer protection against ischemia and enhance cytoskeletal tolerance to stress (Huot et al. 1996; Guay et al. 1997; Martin et al. 1997). However, there are contradictory data apart from our own. For example, the protective activity of HSP25/27 can be associated with macroaggregates and, hence, phosphorylation is not required for protection against TNFα-mediated injury (Mehlen et al. 1997; Preville et al. 1998). Similarly, the absence of MAPKAPK-2 decreased, rather than increased, injury in a mouse model of transient or sustained myocardial (Shiroto et al. 2005) and cerebral ischemia (Wang et al. 2002). In addition, Martin et al. and others have shown that although the overexpression of wild-type HSP27 protected cardiac myocytes from simulated ischemia, this protection did not depend on serine phosphorylation (Martin et al. 1997; Armstrong et al. 1999).
Effect of MAPKAPK-2 deficiency on p38 MAPK cellular distribution
The pattern of basal total p38 MAPK distribution in control MK2+/+ mouse ventricular sections was similar to previous report in rat heart (Maulik et al. 1996). In addition to the depression of total p38 in MK2−/−, p38 distribution was altered compared to MK2+/+. The increase in association of p38 with specific compartments such as the z-lines of the sarcomeres, the intercalated discs, and the sarcolemmal membrane following ischemia in wild-type hearts was abolished in MK2−/− hearts. On the other hand, the basal nuclear phospho-p38 MAPK fluorescence intensity in MK2−/− hearts was higher than in MK2+/+ and similar to the ischemic MK2+/+. This supports the conclusion that MK2 is the main protein which mediates p38 nuclear export. It is not known whether this redistribution of p38 MAPK is affected by SB203580, but SB203580 binds equally well to active and inactive p38 MAPK (Clark et al. 2007).
However, since this redistribution is lost in MK2−/− hearts, this does not explain the protective effect of SB203580. However, the majority of p38-MAPK associated with the cytoskeletal components appears to be inactive, since the level of total p38-MAPK in this fraction as determined by cell fractionation was disproportionate to the level of phosphorylation associated with the nucleus. So far, the mechanisms by which MK2 and p38 interact and affect p38 expression is poorly understood; however, it is possible that nuclear p38 may stimulate a specific transcription factor which is associated with p38 expression or that p38 which is not chaperoned by MAPKAPK2 is targeted for degradation. In conclusion, MAPKAPK-2 is an unlikely downstream mediator for intraischemic p38 MAPK-induced myocardial injury. Instead, the residual effects of SB203580 on the MK2−/− background suggest an alternative p38 substrate or SB203580 target yet to be identified.
Acknowledgements
DA Gorog and AMN Kabir were supported by fellowships (Fellowships FS/2001 016 and FS/2001 043, respectively) and the project by grant 02/105/14432 all from the British Heart Foundation. RIJ was supported by Wellcome Trust project grant 065021/Z/01/Z.
Footnotes
Diana A Gorog and Rita I Jabr made equal contributions to this work.
References
- Armstrong SC, Delacey M, Ganote CE. Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31(3):555–567. doi: 10.1006/jmcc.1998.0891. [DOI] [PubMed] [Google Scholar]
- Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM, Jr, Lasley RD. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am J Physiol Heart Circ Physiol. 2005;288(3):H1359–H1366. doi: 10.1152/ajpheart.01006.2004. [DOI] [PubMed] [Google Scholar]
- Bassi R, Heads R, Marber MS, Clark JE. Targeting p38-MAPK in the ischaemic heart: kill or cure. Curr Opin Pharmacol. 2008;8(2):141–146. doi: 10.1016/j.coph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Ben Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8(19):1049–1057. doi: 10.1016/S0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Martin JL, Mestril R, Dillmann WH. Specific heat shock proteins protect microtubules during simulated ischemia in cardiac myocytes. Am J Physiol. 1998;275(6 Pt 2):H2243–H2249. doi: 10.1152/ajpheart.1998.275.6.H2243. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79(2):162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- Clark JE, Sarafraz N, Marber MS. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116(2):192–206. doi: 10.1016/j.pharmthera.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Eaton P, Awad WI, Miller JI, Hearse DJ, Shattock MJ. Ischemic preconditioning: a potential role for constitutive low molecular weight stress protein translocation and phosphorylation? J Mol Cell Cardiol. 2000;32(6):961–971. doi: 10.1006/jmcc.2000.1136. [DOI] [PubMed] [Google Scholar]
- Engel K, Schultz H, Martin F, Kotlyarov A, Plath K, Hahn M, Heinemann U, Gaestel M. Constitutive activation of mitogen-activated protein kinase-activated protein kinase 2 by mutation of phosphorylation sites and an A-helix motif. J Biol Chem. 1995;270(45):27213–27221. doi: 10.1074/jbc.270.45.27213. [DOI] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78(6):1039–1049. doi: 10.1016/0092-8674(94)90278-X. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281(3):H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- Gorog DA, Tanno M, Kabir AM, Kanaganayagam GS, Bassi R, Fisher SG, Marber MS. Varying susceptibility to myocardial infarction among C57BL/6 mice of different genetic background. J Mol Cell Cardiol. 2003;35(6):705–708. doi: 10.1016/S0022-2828(03)00082-8. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110(23):3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000;275(31):23825–23833. doi: 10.1074/jbc.M003864200. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56(2):273–279. [PubMed] [Google Scholar]
- Inaguma Y, Ito H, Iwamoto I, Saga S, Kato K. AlphaB-crystallin phosphorylated at Ser-59 is localized in centrosomes and midbodies during mitosis. Eur J Cell Biol. 2001;80(12):741–748. doi: 10.1078/0171-9335-00203. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem. 1997;272(47):29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276(7):5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem. 1994;269(15):11274–11278. [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273(43):28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Iwamoto I, Lida K, Inaguma Y. Protein kinase inhibitors can suppress stress-induced dissociation of Hsp27. Cell Stress Chaperones. 2001;6(1):16–20. doi: 10.1379/1466-1268(2001)006<0016:PKICSS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1(2):94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, Lin LL, Gaestel M. Distinct cellular functions of MK2. Mol Cell Biol. 2002;22(13):4827–4835. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99(13):1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274(10):6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96(12):4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- Martin JL, Hickey E, Weber LA, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Expr. 1999;7(4–6):349–355. [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Avkiran M, Quinlan RA, Cohen P, Marber MS. Antiischemic effects of SB203580 are mediated through the inhibition of p38alpha mitogen-activated protein kinase: Evidence from ectopic expression of an inhibition-resistant kinase. Circ Res. 2001;89(9):750–752. doi: 10.1161/hh2101.099504. [DOI] [PubMed] [Google Scholar]
- Maulik N, Watanabe M, Zu YL, Huang CK, Cordis GA, Schley JA, Das DK. Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett. 1996;396(2–3):233–237. doi: 10.1016/0014-5793(96)01109-X. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo AP. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241(1):187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Yoshioka K, Morimoto S, Yoshida K. A novel mechanism of JNK1 activation. Nuclear translocation and activation of JNK1 during ischemia and reperfusion. J Biol Chem. 1997;272(26):16657–16662. doi: 10.1074/jbc.272.26.16657. [DOI] [PubMed] [Google Scholar]
- Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92(2):203–211. doi: 10.1161/01.RES.0000052989.83995.A5. [DOI] [PubMed] [Google Scholar]
- Preville X, Schultz H, Knauf U, Gaestel M, Arrigo AP. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide-induced cell death. J Cell Biochem. 1998;69(4):436–452. doi: 10.1002/(SICI)1097-4644(19980615)69:4<436::AID-JCB5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15(2):393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, Wang Y, Marber MS. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14(14):2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- Saurin A, Pennington D, Raat N, Latchman D, Owen M, Marber M. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res. 2002;55(3):672. doi: 10.1016/S0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- Shiroto K, Otani H, Yamamoto F, Huang CK, Maulik N, Das DK. MK2−/− gene knockout mouse hearts carry anti-apoptotic signal and are resistant to ischemia reperfusion injury. J Mol Cell Cardiol. 2005;38(1):93–97. doi: 10.1016/j.yjmcc.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313(3):307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA, Marber MS. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation. evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93(3):254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu L, Wang H, Young PR, Gaestel M, Feuerstein GZ. Mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 deficiency protects brain from ischemic injury in mice. J Biol Chem. 2002;277(46):43968–43972. doi: 10.1074/jbc.M206837200. [DOI] [PubMed] [Google Scholar]
- Zu YL, Ai Y, Gilchrist A, Maulik N, Watras J, Sha'afi RI, Das DK, Huang CK. High expression and activation of MAP kinase-activated protein kinase 2 in cardiac muscle cells. J Mol Cell Cardiol. 1997;29(8):2159–2168. doi: 10.1006/jmcc.1997.0449. [DOI] [PubMed] [Google Scholar]