Abstract
Phenotypic modulation of vascular smooth muscle cells (VSMCs) plays a critical role in the pathogenesis of a variety of proliferative vascular diseases. Recently, we have found that microRNA-145 (miR-145) is the most abundant miRNA in normal vascular walls and in fresh isolated VSMCs; however, the role of miR-145 in VSMC phenotypic modulation and vascular diseases is currently unknown. Here we find that miR-145 is selectively expressed in VSMCs of the vascular wall and its expression is significantly downregulated in the vascular walls with neointimal lesion formation and in cultured dedifferentiated VSMCs. More importantly, both in cultured rat VSMCs in vitro and in balloon-injured rat carotid arteries in vivo, we demonstrate that the noncoding RNA miR-145 is a novel phenotypic marker and a novel phenotypic modulator of VSMCs. VSMC differentiation marker genes such as SM α-actin, calponin, and SM-MHC are upregulated by pre-miR-145 or adenovirus expressing miR-145 (Ad-miR-145), but are downregulated by miR-145 inhibitor, 2'OMe-miR-145. We have further identified that miR-145-mediated phenotypic modulation of VSMCs is through its target gene KLF5 and its downstream signaling molecule, myocardin. Finally, restoration of miR-145 in balloon-injured arteries via Ad-miR-145 inhibits neointimal growth. We conclude that miR-145 is a novel VSMC phenotypic marker and modulator that is able of controlling vascular neointimal lesion formation. These novel findings may have extensive implications for the diagnosis and therapy of a variety of proliferative vascular diseases.
Keywords: MicroRNAs, vascular smooth muscle cells, phenotype, vascular disease
Vascular smooth muscle cells (VSMCs) are not terminally differentiated and possess the ability to modulate their phenotype in response to changing local environmental cues (1). It is well established that the transition of VSMCs from a differentiated phenotype to a dedifferentiated state plays a critical role in the pathogenesis of a variety of proliferative cardiovascular diseases such as atherosclerosis, hypertension, restenosis after angioplasty or bypass, diabetic vascular complications, and transplantation arteriopathy (1, 2). The phenotypic modulation in VSMCs is accompanied by accelerated migration, proliferation, and production of extracellular matrix components. Eventually, these cellular events result in the formation of vascular neointima, which is the common pathological lesion in these proliferative vascular diseases. It has been demonstrated that the differentiation state of VSMCs is affected by numerous environmental cues, including growth factors, cell-cell contacts, extracellular matrix components, and neuronal input. However, the final common pathway of most of these environmental influences is the change in expression of VSMC phenotypic genes. Therefore, completely understanding the regulatory mechanisms underlying VSMC phenotypic gene expression is currently the most important issue in the research area of phenotypic modulation.
Recently, the most important breakthrough regarding gene expression regulation is the discovery of microRNAs (miRNAs) (3). miRNAs comprise a novel class of endogenous, small, non-coding RNAs that negatively regulate gene expression via degradation or translational inhibition of their target mRNAs. More importantly, one miRNA is able to regulate the expression of multiple genes because it can bind to its mRNA targets as either an imperfect or perfect complement. Thus, one miRNA is functionally important as a transcription factor (4). As a group, miRNAs may directly regulate at least 30% of the genes in a cell (5).
In our recent study, we have found that miR-145 is the most abundant miRNA in vascular walls (6). However, the functions of miR-145 in VSMCs and vascular disease are completely unknown. The objective of the current study is to determine whether miR-145 can be used as a novel phenotypic marker for VSMCs and whether this abundant miRNA has any potential regulatory effects on the VSMC phenotype and vascular neointimal lesions using both cultured VSMCs in vitro and balloon-injured rat carotid arteries in vivo.
Materials and Methods
An expanded Materials and Methods section is provided in the online data supplement at http://circres.ahajournals.org.
Cell culture
VSMCs and Endothelial cells (ECs) were obtained from the rat aortas as described (6).
Oligo transfection, knockdown or overexpression of miR-145 and KLF5, in cultured VSMCs
Oligo transfection was performed according to an established protocol (6–8). miR-145 was knocked down via miR-145 inhibitor, 2'OMe-miR-145, and was overexpressed via pre-miR-145. KLF5 knockdown was performed using its siRNA (si-KLF5, 100 nM). For KLF5 upregulation, adenovirus expressing KLF5 (Ad-KLF5) was applied (30 MOI). Vehicle, oligo controls (Ambion, Inc.), siRNA control (si-control, Invitrogen), and adenovirus control (Ad-GFP) were applied.
RNA analysis by quantitative real-time PCR (qRT-PCR)
qRT-PCR for miR-145, SM-MHC, calponin, SM α-actin, myocardin, and KLF5 was performed with a Roche Lightcycler 480 Detection System as described (8).The sequences of the primers used are shown in online table I.
Northern blot analysis of miRNA
Northern blot analysis of miR-145 and pre-miR-145 was performed as described (6, 7).
Western blot analysis
Standard western blot analysis was conducted using SM-MHC, calponin, SM α-actin and KLF5 antibodies
Construction of the adenovirus
The adenovirus expressing miR-145 (Ad-miR-145), Ad-KLF5, and Ad-GFP were generated as described (8).
Luciferase assay
A construct in which a fragment of the 3’-UTR of KLF5 mRNA containing the putative miR-145 binding sequence and a firefly luciferase reporter construct and transfected into HEK 293 cells with either vehicle (Vehicle control), an empty plasmid (pDNR-CMV) (0.2 µg/ml), a plasmid expressing miR-145 (pmiR-145) (0.2 µg/ml), or a control plasmid expressing an unrelated miRNA, miR-31 (pmiR-31) (0.2 µg/ml). Relative luciferase expression was measured on a scintillation counter by using a dual luciferase reporter system (8).
Immunocytochemistry, immunofluorescence and in situ hybridization
In situ hybridization of miR-145 was performed in 10-µm vessel sections as described. (8). VSMCs were stained by immunofluorescence of SM α-actin in these sections. In addition, SM α-actin in cultured VSMCs was also determined by immunocytochemistry.
Rat carotid artery balloon injury model and adenovirus mediated gene transfer into the injured vascular wall
Carotid artery balloon injury and adenovirus mediated gene transfer were performed in male Sprague-Dawley rats as described (8).
Morphometric analysis for neointimal lesion formation
Morphometric analysis via computerized image analysis system was performed in sections stained with Masson's trichrome staining as described (6, 8).
Results
miR-145 is the most abundant miRNA in normal rat carotid arteries that is selectively expressed in VSMCs
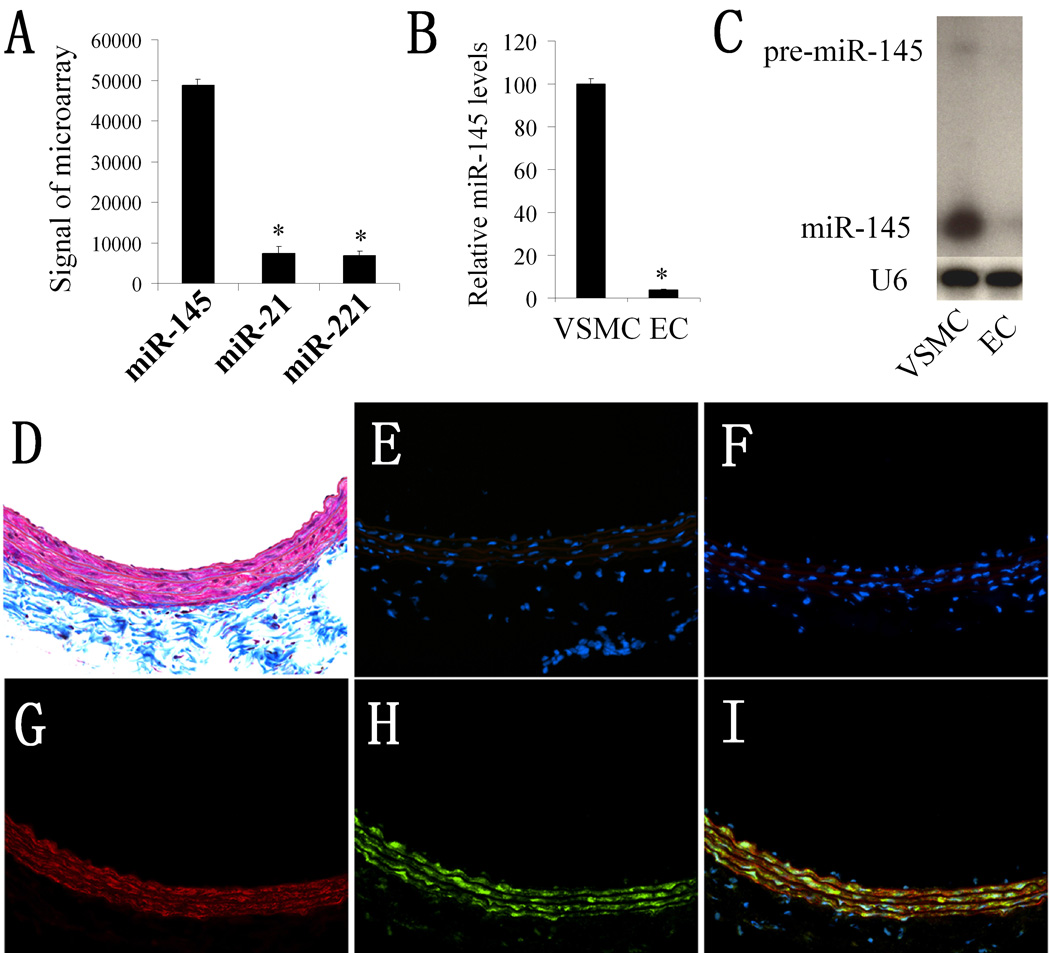
Our microarray analysis revealed that miR-145 is the most abundant miRNA in normal rat carotid arteries (6). Recent studies demonstrated that miR-21 and miR-221 are also two abundant miRNAs in vascular walls (6, 8–10). We thus compared their expression with that of miR-145. As shown in Figure 1A, microarray analysis demonstrated that the expression of miR-145 was much higher than that of miR-21 and miR-221.
Figure 1. miR-145 is selectively expressed in VSMCs of the vascular wall.
(A). The expression levels of miR-145, miR-21, and miR-221 in normal rat carotid arteries. Note: n=6; *P<0.05 compared with miR-145. (B). The relative expression of miR-145 in rat VSMCs and ECs determined by qRT-PCR. Note: n=6; *P<0.05 compared with that in VSMCs. (C). The expression of miR-145 and pre-miR-145 in rat VSMCs and ECs determined by northern blot analysis. (D). Masson's trichrome staining of rat carotid artery. (E). Negative control of In situ hybridization (no miRNA probe). (F). Scrambled probe control. (G). Immunofluorescence with the smooth muscle marker SM α-actin (red color). (H). In situ hybridization of miR-145 (green color). (I). Merged images of (G) and (H). Note: blue color is the cell nuclear staining by DAPI.
VSMCs and ECs are two of the major cell types in normal vascular walls. To determine the distribution of miR-145 expression in the vascular wall, we isolated VSMCs and ECs from rat arteries and measured miR-145 levels in these cells. As shown in Figure 1B and 1C, both qRT-PCR and northern blot analysis demonstrated that miR-145 was highly expressed in VSMCs; it was, however, almost undetectable in ECs. In addition, our unpublished microarray data revealed that miR-145 is also the most abundant miRNA in fresh isolated VSMCs and its expression was much higher than that of miR-21 and miR-221 (online Table II).
To further confirm the cellular distribution of miR-145 in the vascular wall, we performed in situ hybridization on normal rat carotid artery. Vessel structure was demonstrated via Masson's trichrome staining in frozen sections as shown in Figure 1D. In situ hybridization of miR-145 (green color) showed that it was expressed primarily in the vessel media where VSMCs were localized (Figure 1H). In contrast, no fluorescence signal was detected in the vasculature intima where endothelial cells (ECs) were localized (Figure 1H and 1I). To confirm that miR-145 was localized in VSMCs, we performed co-immunofluorescence with the smooth muscle marker SM α-actin (red color). As expected, SM α-actin was observed in VSMCs that were located in the media (Figure 1G). Interestingly, miR-145 was clearly co-localized with VSMCs (Figure 1I, merged color with red and green). Again, no expression of miR-145 was demonstrated in intimal cells (endothelial cells) (Figure 1I). Also, there was no miR-145 signal in 2 control sections: negative control (Figure 1E, no miRNA probe), and scrambled probe control (Figure 1F).
miR-145 is a novel phenotypic marker for VSMCs in vitro in cultured cells
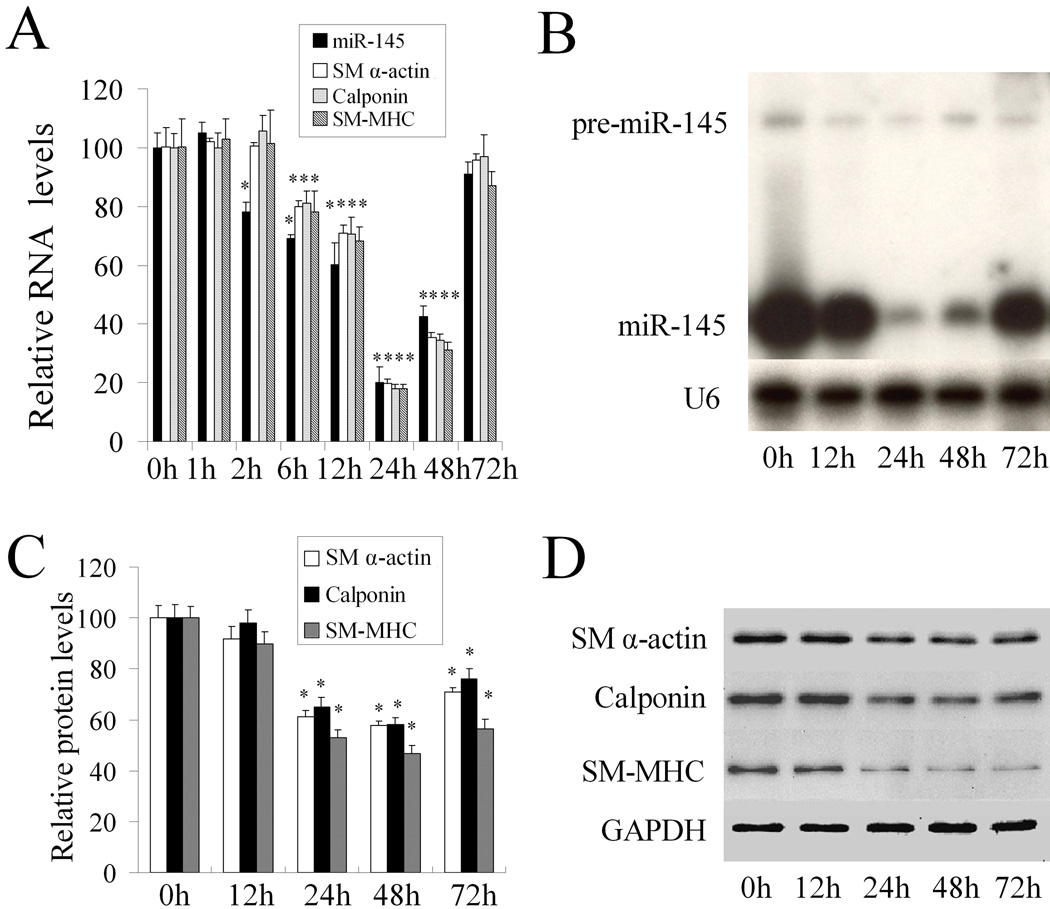
To explore the relationship between miR-145 and the VSMC phenotype, we applied a well established VSMC model for phenotypic modulation in which VSMC dedifferentiation was induced by platelet-derived growth factor (PDGF) (11). As shown in Figure 2A, PDGF-BB (20 ng/ml) caused a time-dependent suppression of the mRNA levels of VSMC differentiation marker genes such as SM α-actin, calponin, and SM-MHC. Interestingly, the similar expression pattern was found in miR-145 levels in VSMCs after PDGF stimulation. PDGF-BB (20 ng/ml) caused a rapid decrease in miR-145 expression as demonstrated by qRT-PCR (Figure 2A) and northern blot (Figure 2B). In contrast to the high expression of mature miR-145, pre-miR-145 expression was very low in VSMCs both at basal and PDGF-stimulated conditions (Figure 2B). As miRNA controls, we also determined the expression levels of miR-221 and miR-221 after PDGF-stimulation. Unlike miR-145, the expression of miR-221 and miR-222 was increased by PDGF (8). The protein levels of VSMC differentiation marker genes after PDGF-stimulation were displayed in Figure 2C and 2D. These results demonstrated that miR-145 was a novel phenotypic marker for VSMCs.
Figure 2. miR-145 is a phenotypic marker for VSMCs in vitro in cultured cells.
(A). PDGF-BB (20 ng/ml) caused a time-dependent suppression of miR-145 and VSMC differentiation marker genes such as SM α-actin, calponin, and SM-MHC as determined by qRT-PCR. (B). miR-145 and pre-miR-145 in PDGF-BB (20 ng/ml)-treated VSMCs as determined by northern blot analysis. (C). Quantitative analysis of VSMC differentiation marker genes by western blot. (D). Representative western blots in VSMCs treated with PDGF. Note: n=6; *P<0.05 compared with 0 h group.
miR-145 is also a phenotypic marker for VSMCs in the vascular wall in vivo
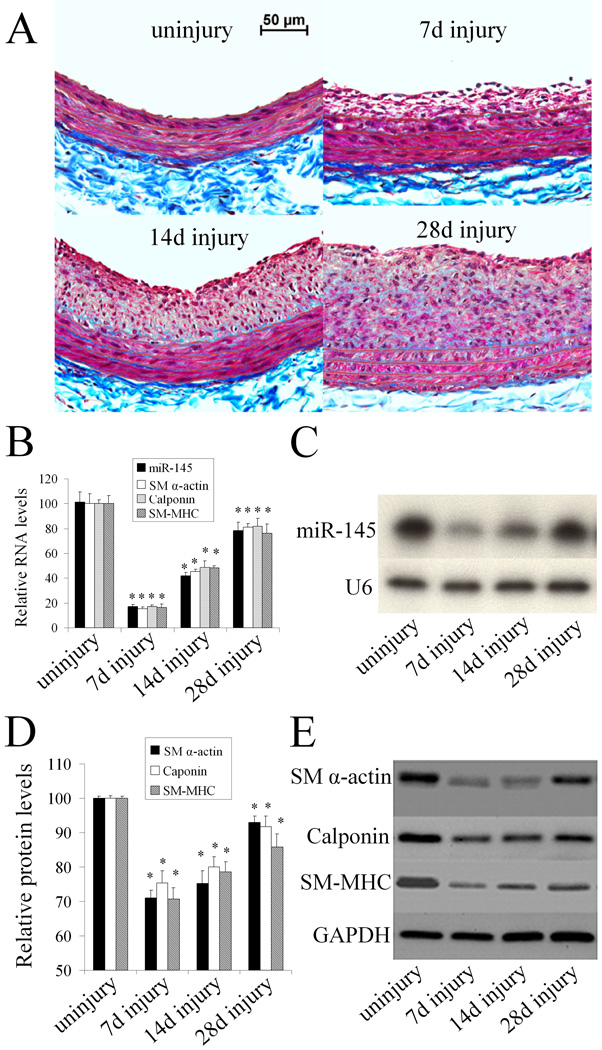
To investigate whether miR-145 is a phenotypic marker for VSMCs in the vascular wall in vivo, we applied a well-established balloon-injury model of the rat carotid artery, as described (6). In this experiment, we isolated the rat right carotid arteries at 7, 14, and 28 days after angioplasty. Uninjured arteries were used as controls. As shown in Figure 3A, balloon-injury resulted in time-dependent neointimal lesion formation in rat carotid arteries. Accordingly, the VSMC differentiation marker genes such as SM α-actin, calponin, and SM-MHC were downregulated both at the mRNA (Figure. 3B) and protein levels (Figure 3D and 3E); however, there was a partial recovery at 28 days after injury. The results suggest that VSMCs in the vascular wall quickly changed from a differentiated phenotype to a dedifferentiated state after angioplasty. qRT-PCR (Figure 3B) and northern blot analysis (Figure 3C) showed that miR-145 expression was significantly downregulated, with a similar time course to the VSMC differentiation marker genes. These results suggest that miR-145 is a phenotypic marker for VSMCs in the vascular wall in vivo.
Figure 3. miR-145 is a phenotypic marker for VSMCs in the vascular wall in vivo.
(A). Representative Masson's trichrome staining in uninjured and injured rat carotid arteries at 7, 14, and 28 days after angioplasty. (B). The expression of miR-145, VSMC differentiation marker genes, S M α-actin, calponin, and SM-MHC in uninjured and injured rat carotid arteries determined by qRT-PCR. (C). miR-145 expression in the vascular walls determined by northern blot analysis. (D). Quantitative analysis of VSMC differentiation marker genes by western blot. (E). Representative western blots of VSMC differentiation marker genes. Note: n=6; *P<0.05 compared with uninjured control group.
miR-145 is a phenotypic modulator for VSMCs in vitro in cultured cells
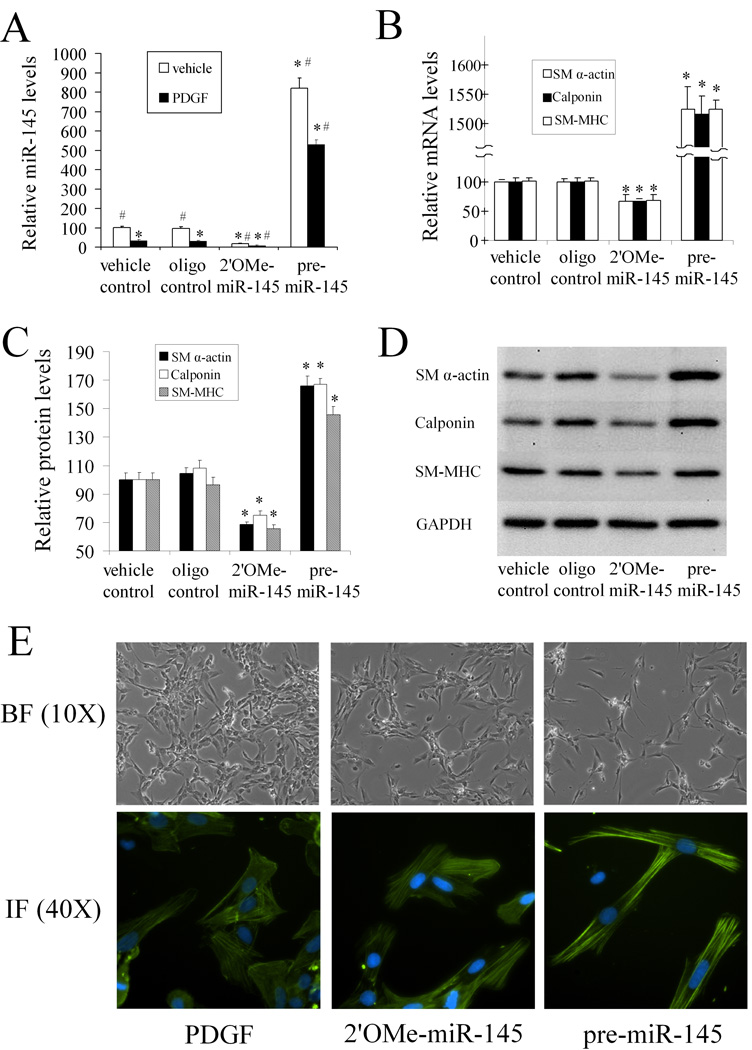
To determine whether miR-145 affects VSMC phenotype, we addressed the following two questions in cultured VSMCs. First, in a PDGF-induced phenotypic modulation model, we tested whether or not miR-145 has an effect on the VSMC phenotype. If the first experiment was positive, then the next question was whether or not modulating the miR-145 level itself is sufficient to elicit phenotypic changes in quiescent, non-stimulated VSMCs.
To modulate miR-145 in cultured VSMCs, we applied both gain-of-function and loss-of-function approaches. For the miR-145 knockdown, the miR-145 inhibitor (2'OMe-miR-145) was added to the culture media at final oligonucleotide concentrations of 1, 3, 10, 30, and 100 nmol/L. 2'OMe-miR-145 was the miR-145 antisense oligonucleotide which was modified at each nucleotide by an O-methyl moiety at the 2'-ribose position. As shown in online Figure IA, 2'OMe-miR-145 decreased miR-145 expression in a dose-dependent manner. For miR-145 upregulation, pre-miR-145 was added to the culture media at final oligonucleotide concentrations of 1, 3, 10, 30, and 100 nmol/L. As shown in online Figure IB, pre-miR-145 increased miR-145 expression in a dose-dependent manner. It should be noted that 30–100 nM of pre-miR-145 is able to increase miR-145 expression to the level that is similar to fresh isolated, differentiated VSMCs. In addition, the effects of both 2'OMe-miR-145 and pre-miR-145 on miR-145 expression were miR-145 specific, as no effects were found on other miRNAs such as miR-125b and miR-352 (online Figure II).
As shown in Figure 4A, 2'OMe-miR-145 (100 nmol/L) decreased miR-145 expression in VSMCs with or without PDGF-stimulation. In contrast, miR-145 was increased by pre-miR-145 (100 nM) under these experimental conditions. Consistent with the expression changes in miR-145, 2'OMe-miR-145 strengthened the PDGF-mediated effects on VSMC dedifferentiation. However, pre-miR-145 inhibited the PDGF-mediated effects on VSMC dedifferentiation as shown by the expression changes of VSMC differentiation marker genes detected at the mRNA (Figure 4B) and protein levels (Figure 4C and 4D). These results indicate that miR-145 has a regulatory effect on the VSMC phenotype.
Figure 4. miR-145 modulates VSMC phenotype in vitro in cultured cells.
The VSMCs were pre-treated with vehicle, control oligo, 2'OMe-miR-145, or pre-miR-145 for 4h followed by PDGF or vehicle for 24 h. (A). Modulation of miR-145 expression by 2'OMe-miR-145 (100 nM) and pre-miR-145 (100 nM) in VSMCs with or without PDGF (20 ng/ml). n=6; *P<0.05 compared with oligo control group treated with vehicle. # p<0.05 compared with oligo control group treated with PDGF. (B). 2'OMe-miR-145 strengthened, whereas pre-miR-145 inhibited PDGF-mediated effects on VSMC maker genes as determined by qRT-PCR. n=6; *P<0.05 compared with oligo control. (C). 2'OMe-miR-145 strengthened, whereas pre-miR-145 inhibited PDGF-mediated effects on VSMC maker genes as determined by western blot. n=6; *P<0.05 compared with oligo control. (D). Representative western blots of VSMC differentiation marker genes. (E). Up panel: Representative morphological changes of primary cultured VSMCs treated with PDGF (20 ng/ml), 2'OMe-miR-145 (100 nM), or pre-miR-145 (100 nM) for 48 hours. Bottom panel: Representative immunofluorescence images of the VSMCs via anti-SM α-actin antibody (green color). Note: blue color is the cell nuclear staining by DAPI.
To test whether modulating the miR-145 itself is sufficient to elicit phenotypic changes, we determined the effect of altering miR-145 on the VSMC phenotype in quiescent, non-stimulated VSMCs. As shown in online Figure III, 2'OMe-miR-145 (100 nM) suppressed the expression of VSMC differentiation marker genes. In contrast, these marker genes were enhanced by pre-miR-145 (100 nM). These results demonstrate that modulating of miR-145 alone is sufficient to elicit phenotypic changes. Thus, the miR-145-mediated effect on the VSMC phenotype is not limited to PDGF-induced phenotypic modulation and miR-145 might be a causative regulator of the VSMC phenotype.
The causative role of miR-145 in VSMC modulation made us test one of the most important issues in vascular biology: whether the miR-145 is able to keep differentiated morphometry in primary cultured cells. In this experiment, primary cultured cells were treated with PDGF (20 ng/ml), 2'OMe-miR-145 (100 nM), or pre-miR-145 (100 nM) for two days. As shown in Figure 4E (up panel), PDGF elicited a quickly flattened morphology that reflected a dedifferentiated state. 2'OMe-miR-145 led to a similar morphological change although it was less pronounced. In contrast, pre-miR-145 kept a spindle-like shape that reflected a differentiated state. The morphological changes were further confirmed by immunofluorescence using anti-SM α-actin antibody (Figure 4E, bottom panel).
miR-145 is a phenotypic modulator of VSMCs in the vascular wall in vivo
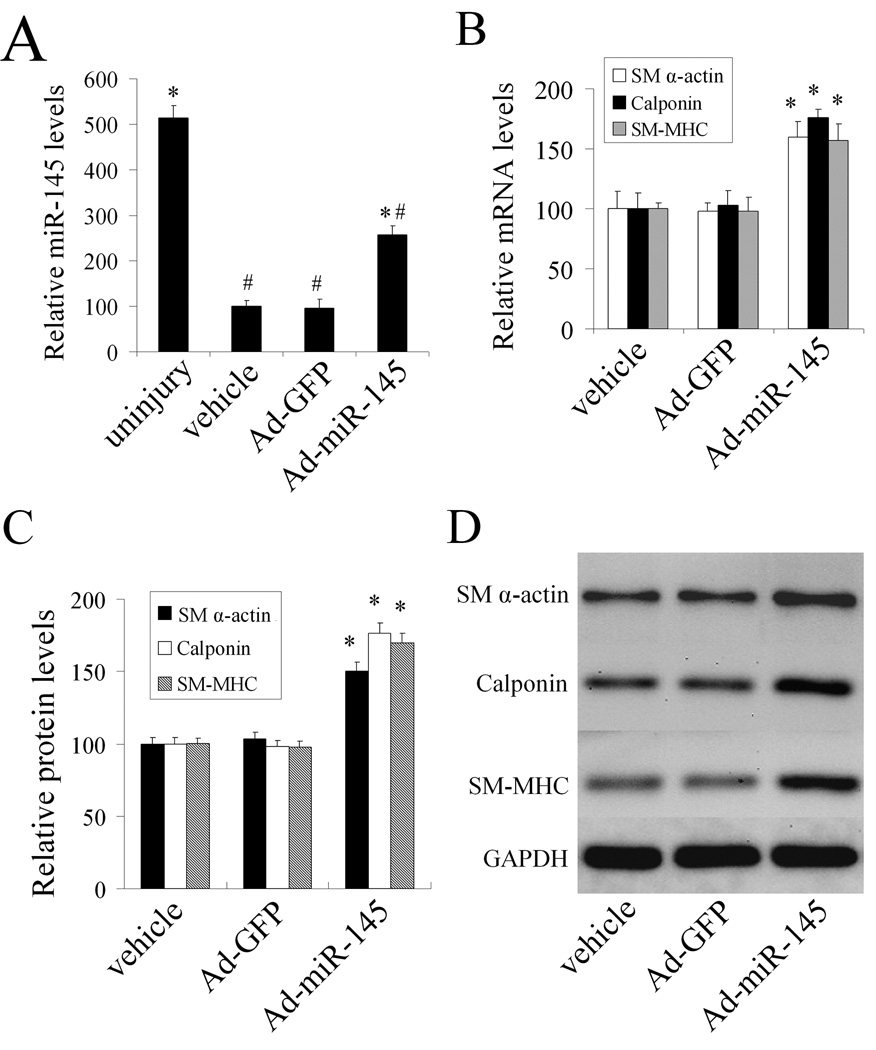
To reverse the decreased miR-145 expression in the injured vascular wall, Ad-miR-145 was used. As shown in Figure 5A, at 7 days after balloon injury, miR-145 expression was significantly increased in balloon-injured rat carotid arteries treated with Ad-miR- 145 compared with those in balloon-injured arteries treated with either vehicle or Ad-GFP as determined by qRT-PCR. However, the miR-145 could only be partially restored due to the limited transfection of the adenovirus (Figure 5A). Interestingly, adenovirus-mediated miR-145 expression significantly increased the VSMC differentiation marker genes compared with those from vehicle or control adenovirus-treated vessels as determined by qRT-PCR and western blot analysis (Figure 5B–5D).
Figure 5. miR-145 modulates the VSMC phenotype in vivo in balloon injured rat carotid arteries.
(A). miR-145 expression in uninjured arteries, and in balloon-injured arteries at 7 days after angioplasty, which were treated with vehicle, Ad-GFP, or Ad-miR-145. (B). Ad-miR-145 increased the VSMC differentiation marker genes in injured arteries as determined by qRT-PCR. (C). Ad-miR-145 increased the VSMC differentiation marker genes in injured arteries as determined by western blot. (D). Representative western blots of VSMC differentiation marker genes. Note: n=6; *P<0.05 compared with Ad-GFP control. #p<0.05 compared with uninjured control group.
KLF5 is the critical target gene of miR-145 that is responsible for miR-145-mediated effects on VSMC phenotypic modulation
Based on the cellular effect of miR-145, our bioinformatic analysis suggested that KLF5 could be a potential gene target for miR-145. Computational analysis suggested that KLF5 had a miR-145 binding site in its 3'-untranslated region (3'-UTR) that was conserved, as shown in online Figure IV.
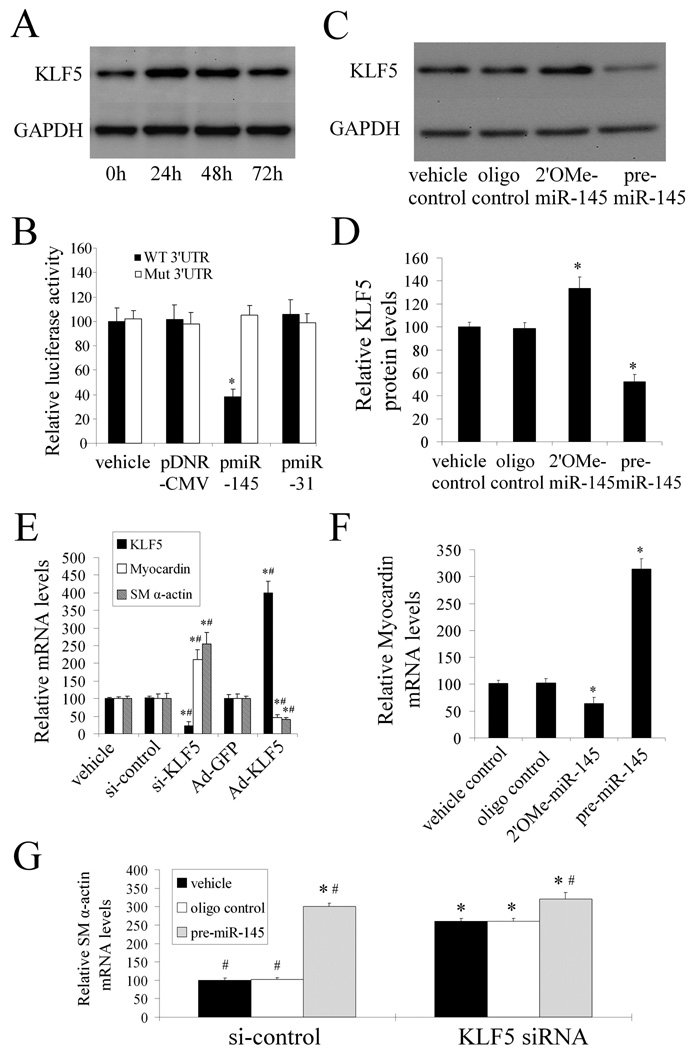
If KLF5 is a target gene for miR-145, its expression should be upregulated in cultured dedifferentiated VSMCs stimulated with PDGF, because miR-145 expression is downregulated by PDGF. As shown in Figure 6A, KLF5 expression was indeed increased in PDGF-stimulated VSMCs.
Figure 6. KLF5 is the critical target gene of miR-145 that is responsible for miR-145-mediated effect on VSMC phenotypic modulation.
(A). KLF5 expression was increased in VSMCs after PDGF (20 ng/ml)-stimulation. (B). pmiR-145, but not pDNR-CMV, or unrelated pmiR-31 inhibited luciferase activity. In the mutated control group, the inhibitory effect of pmiR-145 on luciferase activity was abrogated. n=5; *P<0.05 compared with vehicle control. (C). Representative western blots of KLF5 in miR-145 modulated VSMCs. (D). 2'OMe-miR-145 increased, whereas pre-miR-145 decreased KLF5 expression in cultured VSMCs. n=6; *P<0.05 compared with oligo control. (E).The effects of KLF5 on the expression of myocardin and SM α–actin. n=5; * P<0.05 compared with siRNA control (si-control) and, # P<0.05 compared with Ad-GFP. (F). The effect of miR-145 on the expression of myocardin. n=6; *P<0.05 compared with oligo control. (G). The effects of pre-miR-145 on the expression of SM α–actin in VSMCs with or without KLF5 depletion. Note: n=6; *P<0.05 compared with oligo control in siRNA control (si-control)-treated groups. #P<0.05 compared with oligo control in KLF5 siRNA-treated groups.
To determine whether miR-145 is able to bind to and inhibit KLF5 expression directly, we performed the luciferase assay, in which a construct in which a fragment of the 3’- UTR of KLF5 mRNA containing the putative miR-145 binding sequence was used. As we expected, pmiR-145, but not pmiR-31 and pDNR-CMV, increased miR-145 expression in HEK 293 cells (online Figure V). Moreover, pmiR-145, but not pDNR-CMV or pmiR-31 inhibited luciferase activity (Figure 6B). In the mutated control groups, the inhibitory effect of pmiR-145 on luciferase activity disappeared (Figure 6B). The results suggest that miR-145 can bind to KLF5 directly and inhibit its expression.
The regulatory effect of miR-145 on KLF5 in VSMCs was determined using both loss-of-function and gain-of-function approaches. As shown in Figure 6C and 6D, KLF5 expression was upregulated by 2'OMe-miR-145 and was downregulated by pre-miR-145 at the protein level, but not at the mRNA level (online Figure VI). The results suggest that KLF5 is a target gene of miR-145 in VSMCs.
We then determined the effect of KLF5 on VSMC phenotype. As shown in Figure 6E, KLF5 was knocked-down by its siRNA (si-KLF5) and was upregulated by Ad-KLF5. Interestingly, downregulation of KLF5 increased, whereas upregulation of KLF5 decreased VSMC differentiation marker gene, SM α-actin.
One recent report indicated that myocardin, a key modulator for differentiation marker genes (12), might be a downstream molecule of KLF5 (14). As shown in Figure 6E, we found that downregulation of KLF5 increased, whereas upregulation of KLF5 decreased the expression of myocardin in VSMCs. In addition, as shown in Figure 6F, 2'OMe-miR-145 (100 nM) decreased, whereas pre-miR-145 (100 nM) increased the expression of myocardin.
Finally, to further verify that KLF5 was a functional target gene related to miR-145-induced effects on the VSMC phenotype, we depleted KLF5 expression in cultured VSMCs via its siRNA. As shown in Figure 6G, knocking-down of KLF5 and upregulation of miR-145 induced a similar effect on VSMC differentiation marker gene, SM α-actin. In the target gene, KLF5 depleted VSMCs, pre-miR-145-mediated additional effect on SM α-actin were significantly attenuated (Figure 6G).
miR-145 modulates KLF5 expression and vascular neointimal lesion formation in vivo in balloon-injured rat carotid arteries
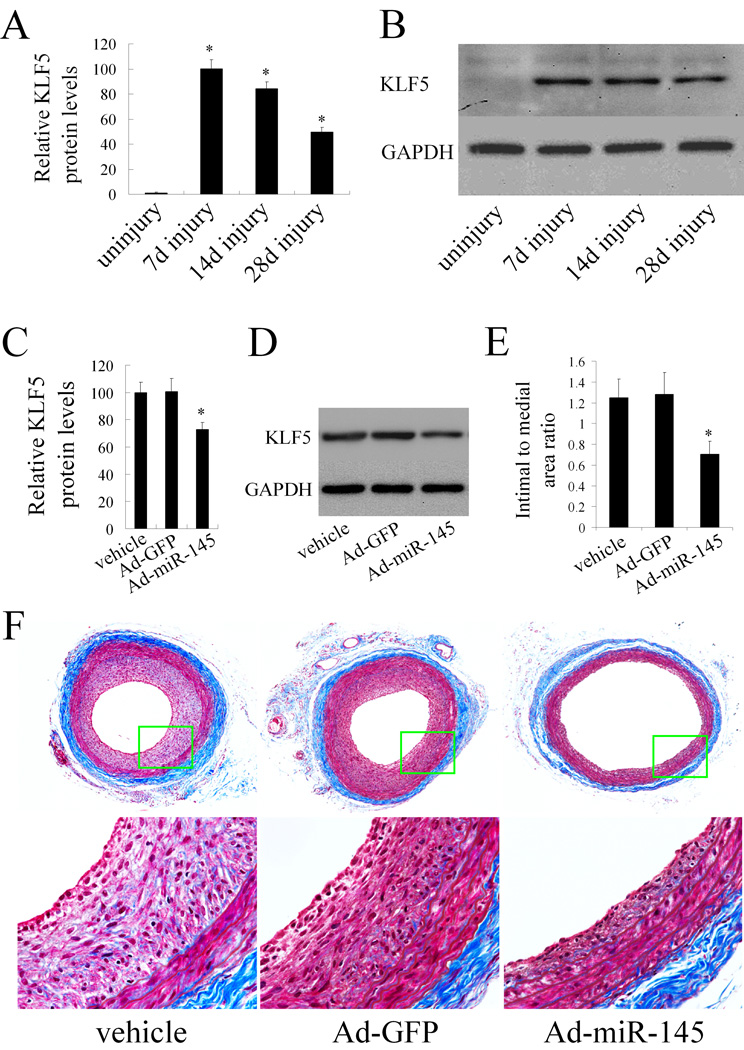
In this experiment, we first determined the expression levels of KLF5 in rat carotid arteries with or without injury. As shown in Figure 7A and 7B, in uninjured vessels, the expression of KLF5 was very low. However, after angioplasty, the expression of KLF5 was significantly upregulated. We then modulated miR-145 level via Ad-miR-145 in balloon-injured vessels. As shown in Figure 7C and 7D, KLF5 was downregulated by Ad-miR-145 in vivo. The results suggest that KLF5 is indeed a target gene of miR-145 in vivo.
Figure 7. miR-145 modulates KLF5 expression and vascular neointimal lesion formation in vivo in balloon-injured rat carotid arteries.
(A). The expression of KLF5 in uninjured and balloon-injured rat carotid arteries. n=6; *P<0.05 compared with uninjured control. (B). Representative western blots of KLF5 in uninjured and balloon-injured rat carotid arteries. (C).The effect of Ad-miR-145 on the expression of KLF5 in balloon-injured arteries. n=5; *P<0.05 compared with Ad-GFP. (D). Representative western blots of KLF5 in balloon-injured rat carotid arteries treated with vehicle, Ad-GFP or Ad-miR-145. (E). The effect of Ad-miR-145 on vascular neointimal lesion formation in rat carotid arteries at 14 days after angioplasty. n=9; *P<0.05 compared with Ad-GFP. (F). Representative Masson's trichrome stained photomicrographs of rat carotid arteries treated with vehicle, Ad-GFP or Ad-miR145.
Phenotypic change of VSMC plays a critical role vascular neintimal development. We thus determined the effect of miR-145 on neointimal growth. As shown in Figure 7E, restoration of the downregulated miR-145 via Ad-miR-145 in balloon-injured rat carotid arteries inhibited neointimal lesion formation significantly. Representative Masson's trichrome stained photomicrographs of rat carotid arteries from vehicle, Ad-GFP, and Ad-miR-145-treated groups were shown in Figure 7F.
Discussion
In the current study, we found that miR-145, which is the most abundant miRNA in vascular walls and in fresh isolated VSMCs, is selectively expressed in VSMCs of the vascular wall. However, its expression is almost undetectable in ECs from the normal vessels. The result is consistent with two recent reports in which no miR-145 expression is found in human ECs (13, 14). Thus, miR-145 may be used as a novel marker for VSMCs in the vascular walls.
In our recent study, we found that miR-145 expression was significantly suppressed in the vascular walls with neointimal lesion formation (6). This phenomenon was accompanied by phenotypic changes of VSMCs within the vascular lesions; thus, we tested the relationship between miR-145 expression and the VSMC phenotype. In cultured VSMCs stimulated with PDGF, we found that the expression changes of miR-145 are consistent with the expression changes of VSMC differentiation marker genes such as SM α-actin, SM-MHC, and calponin. This discovery was further confirmed in vivo using balloon injured rat carotid arteries. These results demonstrated for the first time that the non-coding RNA, miR-145, was a novel phenotypic marker for VSMCs.
We then sought to determine the potential role of miR-145 in VSMC phenotypic modulation. First, we found that overexpression of miR-145 was able to inhibit PDGF-induced VSMC dedifferentiation. In contrast, PDGF-induced VSMC dedifferentiation was strengthened by miR-145 inhibition. Our time course studies revealed that the decrease in miR-145 expression after PDGF-stimulation was earlier than that of VSMC differentiation marker genes (Figure 2A). Moreover, the process of protein expression recovery of VSMC differentiation marker genes after PDGF-stimulation was slower than that of miR-145 and mRNAs of these marker genes (Figure 2C and 2D). The result suggests that the recovery process of VSMC differentiation is from miR-145 to mRNAs of VSMC differentiation marker genes, and then to protein expression of these marker genes. Second, we investigated whether miR-145 expression plays a causative role in VSMC phenotypic modulation. We tested the effects of 2'OMe-miR-145 and pre-miR-145 on the VSMC phenotype in quiescent, non-PDGF-stimulated VSMCs. Both the gain-of-function and the loss-of-function experiments revealed that the miR-145 itself is sufficient to elicit phenotypic changes. Furthermore, the differentiated morphometry of primary cultured VSMCs was kept via overexpression of miR-145. Finally, the regulatory effect of miR-145 on VSMC phenotype was further verified in vivo in balloon-injured rat carotid arteries via Ad-miR-145. Thus, the current study revealed that miR-145 is a novel phenotypic modulator for VSMCs both in vitro and in vivo.
In the current study, computational analysis suggests that KLF5 is a miR-145 target gene. The negative co-relationship between miR-145 expression and KLF5 expression in both PDGF-stimulated VSMCs and balloon-injured arteries indicated that KLF5 could be a potential target gene of miR-145 in VSMCs. To verify this, we first confirmed that miR-145 was able to bind to KLF5 and regulate its expression directly using a construct in which a fragment of the 3’-UTR of KLF5 mRNA, containing the putative miR-145 binding sequence. We then found that miR-145 was sufficient to regulate KLF5 expression in cultured VSMCs and in balloon-injured arteries. In addition, downreguation of KLF5 and upregulation of miR-145 were able to induce a similar effect on VSMC phenotype. Furthermore, in KLF5-deleted VSMCs, pre-miR-145-mediated additional effect on VSMC phenotype was attenuated due to the lack of its target gene. For example, in siRNA control group, pre-miR-145 increased the expression of SM α- actin by about 300%. However, in KLF5-depleted cells, the expression of SM α-actin was only increased by about 30% (Figure 6). The results suggest that, although KLF5 is not a sole target gene, it is indeed a critical functional target gene that is involved in miR-145-mediated effect on the VSMC phenotype.
How does KLF5 work as a negative regulator for VSMC differentiation marker genes? Two previous reports from Dr. Owens’ group demonstrated that KLF5 is able to increase SM α-actin transcription in cultured 10T1/2 cells and NIH3T3 cells (15, 16). Obviously, the direct effect of KLF5 on SM α-actin in these non-VSMCs does not match the effect of KLF5 on SM α-actin in VSMCs as demonstrated in the current study. However, one recent study suggested that the effects of KLF5 on gene expression are cell specific (17). The direct effect of KLF5 on the transcription of SM α-actin in VSMCs should be determined in a future study. One recent study demonstrated that KLF5 has a negative effect of on myocardin (18). As myocardin is a key modulator for VSMC differentiation marker genes (12), we hypothesized that KLF5-induced downregulation of VSMC differentiation marker genes might be related to the downstream molecule, myocardin. Indeed, we found that downregulation of KLF5 increased, whereas upregulation of KLF5 decreased the expression of myocardin in VSMCs. Moreover, the expression of myocardin was also upregulated by pre-miR-145 and downregulated by miR-145 inhibitor. These results suggest that miR-145-mediated effect on VSMC phenotype is through KLF5/ myocardin pathway.
There appear to be dramatic differences in mRNA expression between PDGF-treated and untreated cells that have overexpression of miR-145 (Figure 4B and online Figure IIIA). We think this phenomenon may be induced by PDGF-mediated sensitization. However, the baseline levels of the marker mRNAs are very low in PDGF-treated cells, the absolute amounts of these marker mRNAs after overexpression of miR-145 are not hugely different between PDGF-treated and untreated cells. This may explain, at least in part, why the protein levels of these marker genes are not very different in these two groups (Figure 4C and Figure IIIB). The results also suggest that there may be unknown underlying mechanisms in normal cells that limit a gene from over responding to a miRNA.
Dedifferentiated VSMCs often have accelerated proliferation that plays a critical role in vascular neintimal development. Indeed, we also found that PDGF-induced VSMC proliferation was significantly inhibited by overexpression of miR-145 (Online Figure VII). Finally, we determined the role of miR-145 in neointimal growth via adenovirus-mediated miR-145 gene transfer. As expected, we found that miR-145 is an important negative controller for vascular neointimal lesion formation. It should be noted, Ad-miR-145 was majorly transfected into VSMCs in the balloon-injury model, because ECs had been removed during the angioplasty procedure.
Our microarray data have revealed that multiple miRNAs are aberrantly expressed in the vascular walls after balloon injury (6). Until now, we have found, miR-21, miR-221/222, and miR-145 have a modest effect on neointimal growth (6, 8). Based on our limited data, we have found that their major cellular effects are anti-apoptosis, pro-proliferation, and anti-dedifferentiation respectively. Although the interactions among the aberrantly expressed miRNAs after angioplasty have not been studied, we predict that they may have additive, synergistic, or antagonistic interactions in the process of neointimal formation. In the current study, we have prioritized the effect of miR-145, miR-21 and miR-221 on VSMC phenotype. As shown in online Figure VIII, pre-miR-145 induced the largest increase in the expression of VSMC differentiation marker gene, SM α-actin. Although pre-miR-21 also increases the expression of SM α-actin, the effect is much smaller than that induced by pre-miR-145. In contrast, pre-miR-221 decreased the expression of SM α-actin. Thus, among these 3 miRNAs, miR-145 is the most important miRNA in keeping the VSMC differentiation. In addition, we have compared the effect on VSMC differentiation induced by pre-miR-145 alone, with that induced by pre-miR-145 and pre-miR-221. We have found that pre-miR-145-induced increased in SM α-actin expression was significantly decreased via adding of pre-miR-221 (online Figure IX). Clearly, there is an antagonistic interaction between miR-miR-145 and miR-221 in modulating VSMC phenotype. Other interactions between miRNAs in regulating cellular functions and neointimal formation should be investigated in future studies.
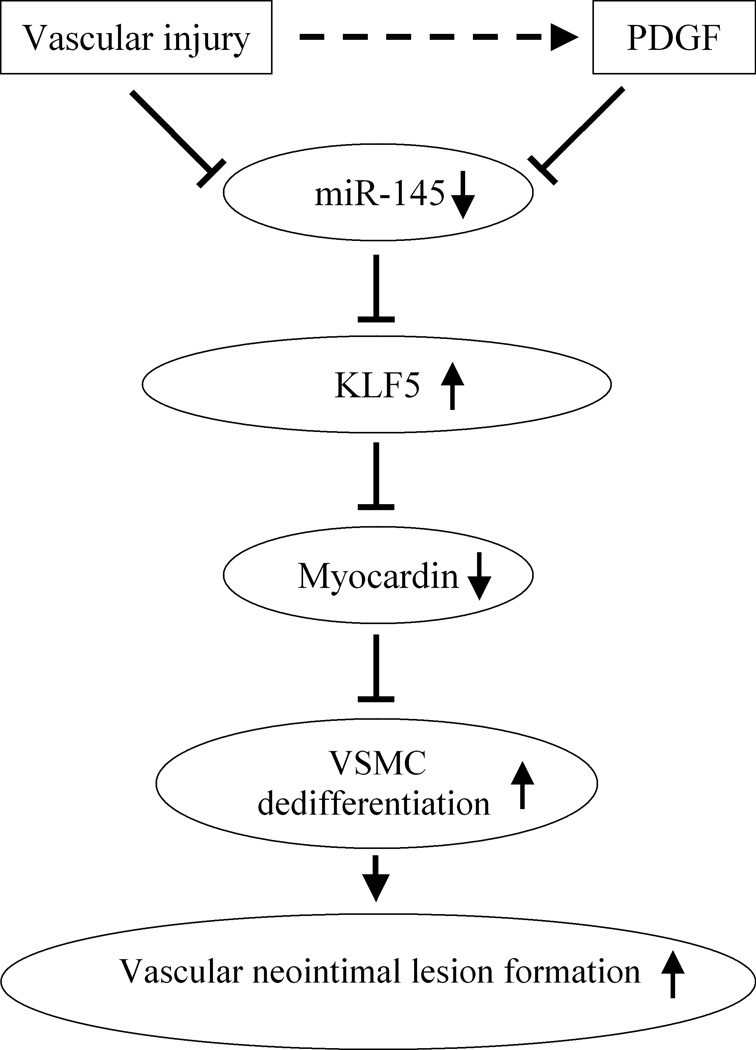
In summary, as shown in Figure 8, the current study reveals that vascular injury or PDGF is able to quickly decrease miR-145 expression in VSMCs. The decreased miR-145 will increase the expression of its target gene KLF5. The increased KLF-5 will then decrease VSMC differentiation marker genes via its downstream molecule, myocardin. The dedifferentiated VSMCs will have increased proliferation and result in the increased neointimal lesion formation. The findings that the non-coding RNA, miR-145, is a novel phenotypic marker and modulator of VSMCs that controls vascular neointimal lesion formation, may have extensive implications for the diagnosis and therapy of a variety of proliferative cardiovascular diseases.
Figure 8. Molecular mechanisms of miR-145-mediated effects on VSMC phenotypic modulation and vascular neointimal lesion formation.
Acknowledgments
Sources of Funding
This work was supported by a NIH Grant HL080133.
Footnotes
Disclosures
None
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45:A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-222 and miR-221 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-BB-mediated activation of Akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J Biol Chem. 2003;278:39830–39838. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 12.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 14.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Sinha S, Owens G. A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278:48004–48011. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- 16.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 17.Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, Maemura K, Kubota N, Kadowaki T, Nagai R. SUMOylation of Krüppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008;14:656–666. doi: 10.1038/nm1756. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth and inhibition of apoptosis. J Biol Chem. 2009;284:9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]