Abstract
The hand is the site of a great variety of benign lesions and, rarely, of malignant lesions. Acrometastasis can be the first manifestation of occult malignancy. Frequently, these lesions present in a similar way to benign conditions leading to erroneous diagnosis and inappropriate treatment. When located in the finger, the most frequent cause is lung cancer, while in the toes it is due to genito-urinary tumours. Awareness of the possibility of metastatic disease during orthopaedic assessment is essential to decrease patient morbidity. A case that was referred to our institution with a single metastasis in a digit from occult gastric adenocarcinoma is used to illustrate the way these lesions are managed. The diagnostic difficulties are summarised and an overview of literature was performed to determine management pathways to aid others in the treatment of these case
Keywords: Acrometastasis, Gastric carcinoma, Hand metastasis
Metastatic lesions to the hand are rare and account for 0.1% of all metastasis.1 The most common primary site is the lung, with breast and genito-urinary tract following next.1–3 In some cases, acrometastases are the first indication of malignant disease, usually presenting late. The distal phalanx, especially the thumb, is the most common site for metastasis in the hand, and the carpus is the rarest. In the foot, metastases are more common in tarsal bones, calcaneum and metatarsals.2,4
Here is a summary of the literature and the recommendations to find the primary tumour and achieve local control. A case treated at our institution has been used to illustrate acrometastasis presenting with occult gastric carcinoma.
Clinical presentation
Acrometastases usually occur in patients with wide-spread dissemination of metastases but, occasionally, it can be the first presenting sign of an occult carcinoma. Failure to recognise these lesions can lead to delay in diagnosis and inappropriate local control and treatment of the primary.
Acrometastases are commonly noticed as a palpable mass or an enlarging digit. The presenting sign is often pain usually deep, intermittent, unaffected by activity and unrelieved by ordinary analgesics. The lesion itself is usually not tender but, as it progresses, it resembles an inflammatory process with swelling and erythema. Alternatively, the patient may present with mechanical dysfunction in the hand causing impairment of activities of daily living.
Acrometastases occur twice as commonly in the dominant hand. They are more frequent in the hand rather than on the feet.3 It has been suggested that increased blood flow and trauma, or both, can be a predisposing factor for metastasis on the dominant side.3,5 Osseous metastases to the hand are most commonly caused by bronchogenic carcinoma, whereas foot metastases are more often seen with tumours originating in the gastrointestinal or genito-urinary tract.3,6
Laboratory findings in metastatic carcinoma are usually unhelpful, although hypercalcaemia is frequently seen with multiple lesions.
X-ray appearance usually shows a destructive permeative lesion. Thyroid and renal carcinomas frequently produce lytic lesions, and prostate carcinoma is classically associated with blastic secondaries. Mixed lytic and blastic deposits are most often seen with malignancies of the lung and breast.
Staging
This part of the management is essential, since the extent of spread of the primary tumour plays an important part in the prognosis of the patient. It should be remembered that 50% of patients presenting with an acrometastasis have pulmonary involvement at presentation.3
In patients with an unknown primary tumour who are suspected of having metastatic disease, a bone scan is generally used to assess the presence or absence of osseous abnormalities and multiplicity of lesions.
False-negative examinations can still occur in very aggressive bone destruction or in debilitated patients who are unable to mount a bony response to the presence of the tumour. The hand is also a site of unremembered past trauma, and false-positive results that are radiologically negative and clinically asymptomatic are common.7
Positron emission tomography (PET) is now used in the staging of malignancies. FDG-PET has been shown to improve specificity compared to bone scintigraphy in patients with breast cancer.8
Computed tomography (CT) scans of the hand have a limited value since they lack resolution in this limited space. Magnetic resonance imaging is useful in evaluating marrow disease and extra-osseous extension of the tumour.
Tissue diagnosis should be obtained by fine needle aspiration cytology (FNA).9 FNA has many advantages. It is easy to perform and has a negligible risk of tumour spread. It is highly sensitive and specific allowing adequate pre-operative planning. Incisional biopsy should be rarely performed and a core biopsy should be attempted beforehand.
Treatment
The treatment should be focused on relief of symptoms and restoration of functional capacity with a short rehabilitation time and allowing early treatment of the primary tumour.
Acrometastases have been treated with various modalities including systemic chemotherapy, curettage, radiotherapy and amputation.
In the hand, the resection is limited by inadequate volume of normal tissue around the tumour mass. Most acrometastases occur in the distal phalanx which is part of the larger digital compartment. The flexor tendon and its sheath is part of the compartment as far proximally as the midpalmar space.7 The extensor tendon goes only as far as the metacarpophalangeal joint. Metastases become quickly extracompartmental and, in the majority of cases, a minimum radical disarticulation of the ray is required to achieve a good resection margin.
Lesions may sometimes be so large at presentation that they are unresectable without disfiguring amputation and, in some cases, an unacceptable degree of functional disability (Table 1). In these circumstances, radiotherapy, curettage or limited marginal excision with adjuvant radiotherapy are all options to be considered.10,11 In the series reported by Healey et al.,3 one of five patients treated with curettage developed recurrent local symptoms and needed a second surgical procedure, while one in four avoided amputation.
Table 1.
Aims of treatment
| Widespread | Solitary | |
|---|---|---|
| High grade | ||
| Aim | Short-term symptom control | Possible long-term symptom control and possible cure |
| Surgical strategy | Conservative measures | Wide local excision to gain control |
| Low grade | ||
| Aim | Long-term symptom control | Long-term control with minimum morbidity |
| Surgical strategy | Intralesional/marginal resection | Precise, but marginal resection |
After ray resection, cosmesis is usually pleasing. Functionally, depending on the digit, the patients do not loose small objects from the hand. However, grip strength is usually weaker.12,13
Metastatic lesions to the hand are rare and account for 0.1% of all metastasis.1 The most common primary sites are found in the lung, with breast and kidney following next in frequency.1,3 The distal phalanx, specially the thumb, is the most common site for metastasis in the hand, and the carpus is the rarest. Foot metastases most frequently involve the tarsal bones and metastases to the phalanges are rare.2,4
The importance of acrometastases is that they can be a manifestation of occult silent primary tumour. These usually present as acute inflammatory lesions10 and are frequently misdiagnosed. They can mimic other pathology such as cysts, gout, ganglia, osteomyelitis, tuberculous dactylitis, pyogenic granuloma and primary skin tumours.1,5,14,15 The surgeon must always be suspicious of metastatic disease when presented with an enlarging mass or lytic lesion in hand or feet.
In previous studies, there was no statistical significance in the survival of the patients according to the different sites of the acrometastasis or the histological diagnosis. The age and number of acrometastases also were not significantly correlated with the duration of survival.3
An exception are patients with renal cell carcinoma. Patients with solitary, metachronous renal bone metastases are appropriate candidates for aggressive surgical resection to prolong survival.16
With recent advances in the management of cancer and prolonged survival, the clinical course of patients with metastatic disease is more likely to be prolonged and accompanied by morbidity. The complexity of the clinical problem requires adequate staging and a multidisciplinary approach for adequate management of the primary tumour and to achieve local control of the metastases.
Of particular importance in the hand, the patient and the surgeon must set their priority between local control versus preservation of function. In tumours with poor response to radiotherapy and chemotherapy, a more aggressive surgical strategy should be adopted.
Case report
We describe a case of an 82-year-old, right-handed man who presented to casualty with a 3-week history of a swollen, erythematous, tender, enlarging mass over the distal phalanx of his right ring finger (Fig. 1). There was no history of trauma. The patient was being treated with antibiotics by his general practitioner who suspected an underlying infective cause for the lesion. Casualty referred the case as an osteomyelitic ring finger.
Figure 1.
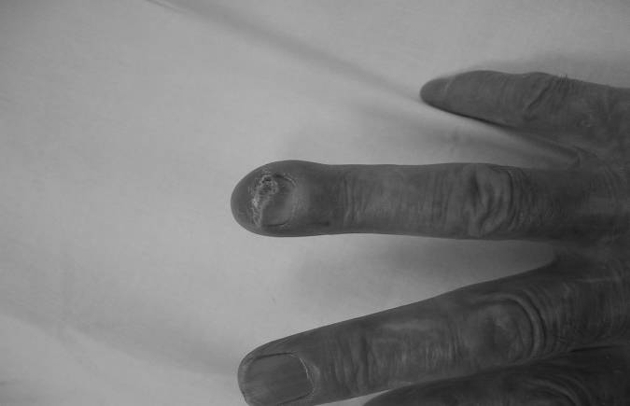
Clinical presentation.
Further questioning revealed no additional features. There were no systemic manifestations such as weight loss, rigors or altered bowel habit.
The primary investigations undertaken were blood tests. His full blood count, renal and liver profile were within normal range. Alkaline phosphatase (146 U/l) was slightly raised and he had an erythrocyte sedimentation rate of 2 mm/h.
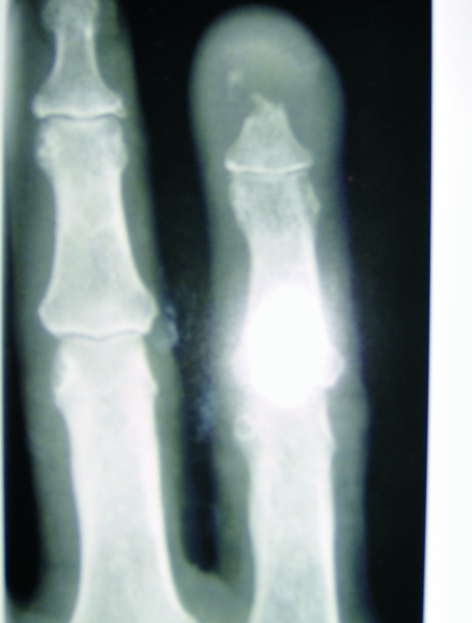
Radiographs, which showed a destructive lesion at the tip of the distal phalanx, were shown to one of the senior authors in passing in casualty (Fig. 2). He examined the patient finding a mobile mass. A metastasis or primary tumour was suspected and a tru-cut biopsy and staging investigation arranged including CT of chest, abdomen and pelvis. Histology showed a mixed adeno/squamous carcinoma picture. The CT of the abdomen showed a 4.2 × 2.6 × 2.1 cm mass in the posterior wall of the stomach (Fig. 3). The features were suggestive of a gastric carcinoma with evidence of transmural invasion, regional lymphadenopathy, and possible lung metastasis. A bone scan was also performed looking for metastatic lesions; this was negative.
Figure 2.

Clinical presentation.
Figure 3.
X-ray of the lesion.
While undergoing staging, the lesion was clinically increasing in size to involve the finger more proximally to the proximal PIP joint crease. A ray excision of the ring finger was performed. Histology showed a moderately differentiated metastatic adenocarcinoma involving the dermis, consistent with a gastric primary. This was confirmed on gastric biopsy (mucinous adenocarcinoma moderate-to-poorly differentiated)
One year later, the patient, having refused chemotherapy, is completely asymptomatic with a good function in his dominant right hand, and no restriction to his activity.
References
- 1.Kerin R. Metastatic tumours of the hand – a review of the literature. J Bone Joint Surg Am. 1983;65:1331–5. [PubMed] [Google Scholar]
- 2.Gall RJ, Sim FH, Pritchard DJ. Metastatic tumours to the bones of the foot. Cancer. 1976;37:1492–5. doi: 10.1002/1097-0142(197603)37:3<1492::aid-cncr2820370335>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases – a study of twenty-nine patients osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68:743–6. [PubMed] [Google Scholar]
- 4.Zindrick MR, Young MP, Daley RJ, Light TR. Metastatic tumours of the foot: case report and literature review. Clin Orthop. 1982;170:219–25. [PubMed] [Google Scholar]
- 5.Mulvey RB. Peripheral bone metastases. AJR Am J Roentgenol. 1964;91:155–60. [PubMed] [Google Scholar]
- 6.Baran R, Tosti A. Metastatic carcinoma to the terminal phalanx of the big toe: report of two cases and review of the literature. J Am Acad Dermatol. 1994;31:259–63. doi: 10.1016/s0190-9622(94)70159-8. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF. Musculoskeletal Tumour Surgery. vol 1. Edinburgh: Churchill Livingstone; 1983. [Google Scholar]
- 8.Ohta M, Tokuda Y, Suzuki Y, Kubota M, Makuuchi H, Tajima T, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun. 2001;22:875–9. doi: 10.1097/00006231-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Knapp D, Abdul-Karim FW. Fine needle aspiration cytology of acrometastasis. A report of two cases. Acta Cytol. 1994;38:589–91. [PubMed] [Google Scholar]
- 10.Carvalho Hde A, Tsai PW, Takagaki TY. Thumb metastasis from small cell lung cancer treated with radiation. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:283–6. doi: 10.1590/s0041-87812002000600007. [DOI] [PubMed] [Google Scholar]
- 11.Yaparpalvi R, Mahadevia PS, Gorla GR, Beitler JJ. Radiation therapy for the salvage of unresectable subungual squamous cell carcinoma. Dermatol Surg. 2003;29:294–6. doi: 10.1046/j.1524-4725.2003.29065.x. [DOI] [PubMed] [Google Scholar]
- 12.Nuzumlali E, Orhun E, Otzturk K, Cepel S, Polatkan S. Results of ray resection and amputation for ring avulsion injuries at the proximal interphalangeal joint. J Hand Surg Br. 2003;28:578–81. doi: 10.1016/s0266-7681(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 13.Peimer C, Wheeler D, Barrett A, Goldschmidt PG. Hand function following a single ray amputation. J Hand Surg Am. 1999;24:1245–8. doi: 10.1053/jhsu.1999.1245. [DOI] [PubMed] [Google Scholar]
- 14.Bevan DA, Ehrlich GE, Gupta VP. Metastatic carcinoma simulating gout. JAMA. 1977;237:2746–7. [PubMed] [Google Scholar]
- 15.Okada H, Qing J, Ohnishi T, Watanabe S. Metastasis of gastric carcinoma to a finger. Br J Dermatol. 1999;140:749–82. [PubMed] [Google Scholar]
- 16.Jung ST, Ghert MA, Harrelson JM, Scully SP. Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop. 2003;409:223–31. doi: 10.1097/01.blo.0000059580.08469.3e. [DOI] [PubMed] [Google Scholar]