Abstract
Neurophysiological recordings of brain activity during behavior in awake animals have traditionally been performed in primates because of their evolutionary close relationship to humans and comparable behavioral skills. However, with properly designed behavioral tasks, many fundamental questions about how the brain controls behavior can also be addressed in small rodents. Today, the rapid progress in mouse neurogenetics, including the development of mouse models of human brain disorders, provides unique and unparalleled opportunities for the investigation of normal and pathological brain function. The development of experimental procedures for the recording of neuronal activity in awake and behaving mice is an important and necessary step towards neurophysiological investigation of normal and pathological mouse brain function. Here we describe a method for stereotaxic recordings of neuronal activity from head-restrained mice during fluid licking. Fluid licking is a natural and spontaneous behavior in rodents, which mice readily perform under head-restrained conditions. Using a head-restrained preparation allows recordings of well-isolated single units at multiple sites during repeated experimental sessions. Thus, a large number of neurons can be tested for their relationship with behavior and detailed spatial maps of behavior related neuronal activity can be generated as exemplified here with recordings from lick-related Purkinje cells in the cerebellum.
Keywords: Awake mouse, Neurophysiology, Behavior, Fluid licking, Purkinje cell, Brain map, Brain disorder
1. Introduction
A central goal of brain research is to understand how the brain controls behavior and how the brain’s ability to generate normal behavior is affected by brain disorders. Studies of behavior related neuronal activity in awake and behaving animals yield the most promising method of accurately assessing the complex interactions of multiple cell types and networks during the generation of behavior. Although traditionally a domain of primate research, many fundamental questions about normal and pathological brain function can be successfully studied in small rodents. In fact, the development of genetic mouse models of human brain disorders now offers unparalleled opportunities to study the neuronal and behavioral defects associated with brain disorders. While the use of anesthesia alters the brain’s mode of operation, sometimes producing results irrelevant for our understanding of the function of the conscious brain (Schonewille et al., 2006; Bengtsson and Jorntell, 2007), recordings in awake, behaving mice avoid this issue.
Recordings of neuronal activity in awake mice have been successfully performed with permanently implanted electrodes to study hippocampal long term potentiation (LTP) based on local field potentials (LFPs) and multi-unit signals (Errington et al., 1997; Davis et al., 1997; Jones et al., 2001; Koranda et al., 2008). Advantages of this established technique are that it allows users to make long term observations of neuronal activity at one location and animals can behave more naturally as they move around freely. Limitations include that implanted electrode recordings are not suitable for mapping studies and that stable single unit recordings are more reliably and easily obtained in a head-restrained paradigm because of reduced tissue movements and because micromanipulators can be used for micrometer precise electrode placement. Furthermore, not all brain areas are as amenable to recordings with implanted electrodes as the neocortex and particularly the hippocampus. We attempted to use implanted electrodes in the cerebellum and failed to get stable single unit recordings. Anatomical examination of the brains revealed large lesions around the implanted electrodes, which may have resulted from movement of the cerebellar tissue relative to the stationary electrodes (unpublished observations). Using the head-restrained preparation described here overcomes these problems and additionally allows the use of optical imaging methods and intracellular recording techniques in the awake mouse. Optical and physiological recordings from head-restrained mice have been performed by a small number of labs (Cheron et al., 2004; Goossens et al., 2004; Schonewille et al., 2006; Ferezou et al., 2007) and so far no comprehensive description of the experimental procedures involved is available in the literature. The experimental technique described here was initially developed to perform cerebellar recordings in awake behaving rats (Heck et al., 2002, 2007) and has been adapted for the performance of stereotaxic recordings from behaving mice during head fixation. The behavioral spectrum of head-restrained mice is limited, but the animals will perform spontaneous orofacial behaviors such as whisker movements (Ferezou et al., 2007) and rhythmic fluid licking (Hayar et al., 2006). These behaviors also yield a large number of repetitions, which is an important prerequisite for electrophysiological investigations of the underlying neuronal processes. Here, we investigated the neuronal representation of rhythmic fluid licking behavior in the simple and complex spike activity of Purkinje cells in the mouse cerebellar cortex. Fluid licking has been studied in a variety of contexts, including studies of taste preference (Lewis et al., 2005), chronic or acute drug treatment (Hsiao and Spencer, 1983; Genn et al., 2003), and to phenotype mouse models of autism spectrum disorders such as Angelman syndrome (Heck et al., 2008).
The rhythm or inter-lick-interval (ILI) of fluid licking in mice is strain-specific (Horowitz et al., 1977; Boughter et al., 2007). The experimental paradigm described here can therefore be useful for the investigation of strain differences in the neuronal mechanisms controlling the licking rhythm. This approach can be readily adapted for recordings from other brain areas of the mouse while monitoring either licking or other behaviors, like movements of the mystacial whiskers.
2. Materials and methods
2.1. Surgical procedures and head-fixation assembly
All experiments adhered to procedural guidelines approved by the University of Tennessee Health Science Center Animal Care and Use Committee. Principles of laboratory animal care (NIH publication No. 86–23, rev. 1996) were followed.
The following procedures were successfully performed with adult mice from four different strains (C57BL/6J, DBA/2J, Cbln1−/− and Lurcher mice). Here we present data examples obtained from C57BL/6J mice. Prior to surgery, mice were weighed and then anesthetized with isoflurane or tribromoethanol (Avertin). Tribromoethanol was used for anesthesia in Lurcher mice because this strain had a very low survival rate with isoflurane. With Tribromoethanol as anesthetic (250 mg/kg, IP), survival rates for Lurcher mice went up to around 90%. For all other strains, that tolerated it well, isoflurane was used because duration and depth of the anesthesia could be more accurately controlled with a vaporizer. Isoflurane anesthesia was induced in a chamber filled with a mixture of 3% isoflurane in oxygen (isoflurane from Baxter Pharmaceutical Products, Deerfield IL) and maintained during surgery (via nose-cone) at 1.0–2.5%. Isoflurane concentration was controlled with a vaporizer (Ohio Isoflurane vaporizer, Highland Medical Equipment, CA). Survival rates dropped markedly if the surgeries lasted significantly longer than 1 h. Thus, considerable effort was made to reduce the time in surgery through careful preparation and practice of all critical steps. Also, during the last 10–15 min of the surgery, i.e. application of the acrylic cement and injections of fluid supplement and analgesic, isoflurane concentration was always lowered to 1%. This measure reduced the time it took the mice to wake up from anesthesia.
The anesthetized mouse was mounted in a stereotaxic instrument with non-rupture ear bars (Zygoma ear cups, David Kopf Instruments, Tujunga, CA). Body temperature was monitored with an electronic rectal thermometer and maintained at 37–38.0 °C using a feedback controlled heating pad (FHC Inc., Bowdoinham, ME). The scalp hair at the surgical site was removed with a depilatory and the skin was treated with iodine solution (Xenodine, Veterinary Products Laboratory, Phoenix, AZ). A scalpel was used to make a 1–1.5 cm long midline incision in the scalp. The skin was retracted to expose the skull bone which was then cleaned of all tissue with a dental scraper and sterile cotton swaps. Three holes were drilled in the parietal and interparietal bones at the approximate positions indicated by the arrowheads in Fig. 1, using a 0.7 mm spherical stainless steel burr (SS White, Lakewood, NJ). Small machine screws (1/8′ dome head, 0.8 mm diameter, 2 mm long, part number: MX-000120-01B, Small Parts, Inc., Florida, USA) were carefully fastened about 1 mm deep into the holes. To provide access to the brain area of interest, a circular opening of 34 mm diameter was drilled into the lateral interparietal bone, exposing part of the cerebellar hemisphere. The same size drill bit was used to drill the screw holes and the skull opening. If bleeding occurred, it was immediately stopped with either an electric cauterizer (Aaron medical, St. Petersburg, FL) or Gelfoam (Pfizer Inc., NY, NY). Great care was taken to leave the dura intact. Immediately after removing the bone the dura was covered with Triple Antibiotic Ointment (Walgreens, US) to prevent drying and infections.
Fig. 1.
Line drawing of the experimental setup for extracellular recordings from the cerebellum of an awake head-restrained mouse during an experiment involving licking behavior. The recording chamber and headpost are embedded in acrylic cement and are firmly attached to the mouse’s skull (see text and Fig. 2). The top of the headpost is inserted into the headpost clamp and held in place by a set screw. The mouse’s body is covered with a loose fitting plastic half-tube (5 cm diameter, 10 cm long) to limit body movements. The half-tube is held in place with adhesive tape. A water spout is placed within tongue-reach (4–6 mm) in front of the mouse’s mouth. A multiple-electrode microdrive with integrated pre-amplifiers is shown with the electrode guiding tubes inserted into the recording chamber.
A cylindrical plastic recording chamber (0.45 cm diameter and 8 mm height) was fashioned from a drinking straw. Small curved scissors were used to shape the bottom of the chamber to fit the curvature of the bone. The chamber was treated with 100% ETOH, dried, and placed over the skull opening. A few drops of Vetbond tissue adhesive (3 M St. Paul, MN) were applied to where the bottom rim of the chamber touched the skull bone. This served to hold the chamber in place until it would eventually be embedded in acrylic cement. The chamber was then filled with Triple Antibiotic Ointment, which would seal the chamber, keep the dura moist and prevent infections. The Triple Antibiotic Ointment had to be removed for each recording session and was replaced with new ointment upon the end of each session.
A stereotaxic manipulator was used to place a custom-made aluminum headpost (Fig. 2D) in a stereotaxically defined position, relative to Bregma. The right caudal corner of the post was placed at Bregma as shown in Fig. 1. Thus, the corresponding corner on the upper end of the headpost served as a stereotaxic reference for electrode placements during awake recordings. Finally, the chamber and headpost were secured into place with dental acrylic (Teets methyl methacrylate denture material; CoOral-Lite Mfg. Co., Diamond Springs, CA, USA). At the end of surgery, mice were subcutaneously (SC) injected with 8 mg/g of the analgesic butorphanol tartrate (Torbugesic, Fort Dodge, USA) to alleviate pain. Body fluid was also supplemented towards the end of the surgery with subcutaneous injections of 0.5 ml of lactated Ringer’s solution. For the first hour after surgery mice were kept underneath a 250 W infrared heating lamp (at a distance of 50 cm) to prevent a drop in body temperature during the recovery period. Butorphanol tartrate injections were repeated the next morning. Following surgery, mice were individually housed.
Fig. 2.
Arrangement of headpost and recording chamber on the mouse’s skull and detailed photographs of the headpost and headpost clamp. All drawings are true to scale. (A) Side view of the skull with mountings. The headpost and recording chamber are embedded in acrylic cement, which is anchored to the skull with three screws, two of which are visible in this side view (black arrowheads). A stereotaxic micromanipulator was used to position the caudo-medial corner of the headpost on top of bregma. The vertical dashed line indicates the anterior–posterior coordinates of bregma. The recording chamber was fashioned from a drinking straw and the bottom was shaped with small scissors to match the curvature of the skull bone. (B) Top view of the skull showing the location of all three skull screws in relation to the headpost and recording chamber. This view illustrates that the headpost cannot be mounted more rostral and thus, that the distance between the recording chamber and the post cannot be further increased. The bend in the post increased the space between the recording chamber and the headpost clamp to allow unhindered access with recording equipment (see Fig. 1). The skull bone at the bottom of the recording chamber was removed to provide access to the underlying brain area (cerebellum shown here) through the intact dura. (C) Bottom view and (D) side view of the headpost clamp. The metal plate at the wide end of the fixture was used to attach the clamp to stationary metal post, which was mounted onto the surface of the experimental table. In (D) the headpost is maximally inserted into the opening as it would be during an experiment. The bend serves as a mechanical stop and determines how deep the post can be maximally inserted. This allows the reliable reproduction of z-axis coordinates across experiments. (E) Enlarged top view of the headpost clamp with headpost (post contour marked with dotted line) held in the lower right corner by the set screw. (F) Enlarged view of the headpost, which was cut out of a 3 mm thick aluminum sheet. The bend added three millimeters of distance between the headpost clamp and the recording chamber and also determined how deep the post could be inserted into the clamping fixture. (G) A true-to-scale drawing of the headpost clamp and set screw (dashed outlines) and a mouse’s skull with headpost and chamber. The 5–7 mm distance between the posterior edge of the clamping fixture and the recording chamber were sufficient for accessing the recording chamber with an electrode microdrive.
2.2. Training and experimental procedures
After a 3–4-day recovery period mice were adapted to the head-restrained experimental situation during 2 sessions of head fixation of 15 and 30 min duration performed on the same day at 8 a.m. (15 min session) and 4 p.m. (30 min session). During these sessions the head was held fixed and the body was covered with a loose fitting plastic half-tube (5 cm diameter, 10 cm long) to limit body movements (Fig. 1). Mice typically adapted to the head fixation within 2–3 sessions as judged from observations of markedly reduced walking and running movements during the third session compared to the first or second. After completing the adaptation procedure, access to water in the home cages was restricted until the next day, when the first experiment was performed. Controlling access to water was necessary in this paradigm to ensure that the mice would lick water during the experimental sessions. Mice consumed water during the experiments and during an hour of free access to water after each experiment. During the 5 days of the week water access was thus controlled. Over the weekend, animals had ad libitum access to water. Access to food was not restricted at any time. Body weight was constantly monitored to ensure that no weight loss greater than 10% of the initial body weight occurred.
During experiments the mouse’s head was immobilized by affixing the headpost to a custom-made aluminum headpost clamp (Fig. 2), which was attached to a metal stand. The metal stand was attached to the surface of a vibration isolation table with 4 machine screws. Fig. 2 shows photomicrographs of the headpost clamp from underside (Fig. 2A), from the side (Fig. 2B) and of the headpost in the headpost clamp held in place by the diagonally arranged screw (Fig. 2C). The curved or bent aluminum headpost was cut out of a 3 mm thick aluminum sheet (Fig. 2D). The bend in the post served two purposes. First, it increased the distance between the head-post clamp and the recording chamber to provide sufficient room to access the chamber with a multi-electrode drive. Secondly, it served as a mechanical stop when inserting the post into the head-post clamp, thus keeping the z-axis coordinate constant between experiments. All components of the head-fixation assembly were custom built by the machine shop of the Department for Biomedical Engineering at the University of Tennessee Health Science Center. Inserting the headpost of a mouse could be performed by hand for a mouse that was accustomed to the experimental sessions, which occurred after several sessions. Initially, the use of forceps greatly aids the process. These mice are generally adapted to the experimental session within 1 day. We judged the improved adaptation or reduced stress level by the duration and amount of “escape attempts” in form or running movements performed while under head fixation. These occurrences of such movements are greatly reduced after 1 day of adaptation and decreases more during subsequent experimental sessions.
After the headpost was fixed, the mouse’s body was covered with a plastic half-tube (5 cm diameter, 10 cm long). The triple antibiotic filling was removed from the recording chamber. Then, the chamber was rinsed and filled with Ringer’s solution. Recording electrodes (glass insulated tungsten/platinum, 80 μm OD, impedance: 2–12 MΩ) were advanced through the intact dura into the brain using a computer controlled microdrive (MiniMatrix, Thomas Recording, Germany). Purkinje cells were identified based on their location and firing characteristics such as the presence of complex spikes as well as sustained high frequency simple spike firing rates (Thach, 1970). The raw spike signals were high-pass filtered (300 Hz to 8 kHz) and amplified 230 × (FA32 filter-amplifier, Multichannel Systems, Germany). Filtered and amplified spike signals were digitized and stored on a computer hard disk (16 bit A/D converter, sampling rate 25 kHz) using a CED power1401 and the Spike2 software (both Cambridge Electronic Design, UK). Licking behavior was monitored using a junction potential based measuring device (Hayar et al., 2006). Licking signals were neither amplified nor filtered, digitized at 2 kHz and stored in the same file as the spike signals.
After a sufficient number of licks were performed (n > 50), the water spout was manually removed to interrupt the licking behavior. Then a new unit was sought by either advancing the electrode or by retracting it from the tissue and moving to a different site on the cerebellar hemisphere. Upon completion of each recording session, the Ringer’s solution was removed and the recording chamber was again filled with triple antibiotic. To remove the mouse from the head-restrained position, the screw that held the headpost firmly in place was loosened. Usually the mouse would remove the post from the holder by lowering its head but the process could be aided by using forceps to push the headpost out of the holder. Mice were then returned to their home cages. Each animal typically participated in experiments for 3–6 weeks, reliably performing licking behavior during each recording session. Within this time-frame, triple antibiotic maintained the accessibility of the tissue by keeping the dura soft and free of infection.
During the last experimental session, two or three small electrolytic lesions (10 μA, 10 s) were set in stereotaxically defined locations using the headpost as a reference. The lesion sites were later used as reference points for mapping of the recording site coordinates onto the cerebellar cortex. Mice were euthanized with 2.5 g/kg urethane and perfused with 3.7% formaldehyde. Following fixation, the brain was cut into 40 μm coronal sections using a Vibratome Series 3000 Plus (Vibratome, St. Louis, MO), which were mounted and stained with cresyl violet.
3. Results
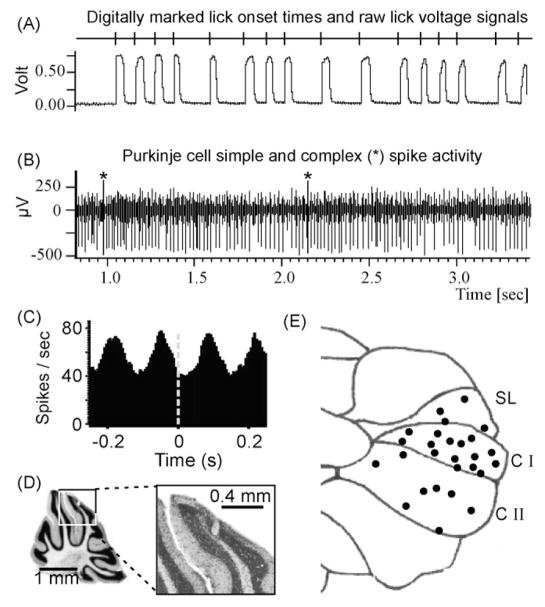
We recorded Purkinje cell simple and complex spike activity in the cerebellar cortex of awake mice during licking behavior. Licking behavior was registered in the form of positive junction potentials, which lasted for the duration of each tongue-to-waterspout contact (Fig. 3A). Fig. 3B shows an example of single unit Purkinje cell activity recorded during licking. Stable extracellular recordings of single unit Purkinje cell activity were readily obtained and remained stable during licking behavior. A threshold algorithm was used to detect and mark the ascending slope of the lick junction potentials (vertical lines in top trace of Fig. 3A), which were used as temporal markers for a cross correlation analysis of lick times with simple spike activity. Purkinje cell simple and complex spikes were extracted from the raw signals using a semi-automatic spike sorting procedure implemented in the Spike2 software (CED, Cambridge, UK). Cross-correlation analysis revealed that a large number of Purkinje cells exhibited rhythmically modulated simple spike activity, phase-locked to the lick rhythm (Fig. 3C). Lick-related Purkinje cells were recorded in the simple lobule, Crus I, and Crus II of the mouse cerebellar cortex (Fig. 3E). During 3–6 weeks of recordings the coordinates of each recoding site relative to the caudolateral top-end corner of the headpost were documented. The electrolytic lesions used to define borders of the recording zones were later identified in a series of 40 μm thick Nissl stained slices (Fig. 3D) and their locations relative to bregma were verified using a stereotaxic mouse brain atlas (Paxinos and Franklin, 2001). The locations of all other recording sites were determined based on their headpost related coordinates and in relation to the locations of the lesions. In Fig. 3E the locations of recordings sites where lick-related Purkinje cell simple spikes were recorded are shown as dots on the schematic drawing of the right cerebellar hemisphere. No correlation between single unit complex spikes and licking behavior was observed. This was consistent with previous reports demonstrating that lick timing is represented in complex spike population activity but not in individual units (Welsh et al., 1995).
Fig. 3.
Representation of licking behavior in single unit Purkinje cell activity. (A) Recording of licking behavior: vertical lines in the top trace mark the ascending slopes of the lick junction potential, which correspond to the onsets of tongue-to-waterspout contacts. The trace below is the lick junction potential raw signal. Whenever the tongue touched the waterspout a positive junction potential lasting for the duration of the contact could be recorded. Junction potential onset times (ascending slopes) were detected with a threshold algorithm and used as temporal aligns for the lick-triggered correlation analysis of neuronal spike activity. (B) Single unit Purkinje cell simple and complex spike activity recorded simultaneously with the licking signals in (A). Asterisks mark complex spikes. We were able to obtain stable recordings from well-isolated single units for up to 20 min. (C) Cross-correlation analysis of simple spike activity with licking revealed a rhythmic phase-locked modulation of simple spike activity with rhythmic fluid licking. (D) Nissl stained parasagittal slice of a mouse cerebellum showing an electrolytic lesion at a recording site. The lesion was placed at stereotaxic coordinates relative to the headpost and thus relative to bregma. The framed part of the left image is shown enlarged on the right. (E) Schematic drawing of the right cerebellar hemisphere seen from the top. Black dots mark locations where single unit Purkinje cells with lick-locked rhythmically modulated simple spike activity were recorded. SL = simple lobule, C I, C II = Crus I and II.
4. Discussion
We have described a method for acute stereotaxic recordings of neuronal activity from awake mice in a head-restrained paradigm. We demonstrated the ability to maintain stable single unit recordings of neuronal activity of Purkinje cells in the mouse cerebellum during rhythmic fluid licking behavior. Fluid licking is a natural and spontaneous behavior in rodents and is for several reasons ideally suited for neurophysiological investigations. It requires no training, can be readily quantified and mice generate large numbers of licks per experiment. Several aspects of fluid licking are under genetic control (Boughter et al., 2007) and fluid licking can be a sensitive phenotyping tool for the detection of subtle genetic differences (Heck et al., 2008). An important feature of acute stereotaxic recordings in head-restrained mice is that large numbers of different neurons can be tested and spatial maps of behavior related neuronal activity can be generated. The described technique can readily be adapted for recordings from other brain areas, like the barrel cortex (Poulet and Petersen, 2008). We have used the technique to successfully record from the deep cerebellar nuclei and from the brain stem (data not shown).
Genetically altered mice have become an important animal model for the investigation of genetic brain disorders. The development of techniques to record neuronal activity from awake and behaving mice, as described here, is a significant methodological advancement. It provides unique new opportunities for the investigation of normal and diseased brain function, currently not available in any other animal species.
Acknowledgments
We would like to thank Bob Gallik and Michael Nguyen from the UTHSC Department of Biomedical Instrumentation for outstanding technical support and creative suggestions on the design of the head-fixation assembly. This work was supported in part by a grant from the National Institute of child health and human development (1R03HD057244-01), a grant from the National Institute of Mental Health (5R01MH068433-02) and an award from the American Psychological Association’s Diversity Program in Neuroscience (APA Fellowship R079008117) to JLB.
References
- Bengtsson F, Jorntell H. Ketamine and xylazine depress sensory-evoked parallel fiber and climbing fiber responses. J Neurophysiol. 2007;98:1697–705. doi: 10.1152/jn.00057.2007. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Baird JD, Bryant JL, St John SJ, Heck DH. C57BL/6J and DBA/2J Mice Vary in Lick rate and ingestive microstructure. genes Brain Behav. 2007;6:619–27. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Cheron G, Gall D, Servais L, Dan B, Maex R, Schiffmann SN. Inactivation of calcium-binding protein genes induces 160 Hz oscillations in the cerebellar cortex of alert mice. J Neurosci. 2004;24:434–41. doi: 10.1523/JNEUROSCI.3197-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Bliss TV, Dutrieux G, Laroche S, Errington ML. Induction and duration of long-term potentiation in the hippocampus of the freely moving mouse. J Neurosci Methods. 1997;75:75–80. doi: 10.1016/s0165-0270(97)00053-8. [DOI] [PubMed] [Google Scholar]
- Errington ML, Bliss TV, Morris RJ, Laroche S, Davis S. Long-term potentiation in awake mutant mice. Nature. 1997;387:666–7. doi: 10.1038/42625. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–23. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Genn RF, Higgs S, Cooper SJ. The effects of 7-OH-DPAT, quinpirole and raclopride on licking for sucrose solutions in the non-deprived rat. Behav Pharmacol. 2003;14:609–17. doi: 10.1097/00008877-200312000-00005. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Hoebeek FE, Van Alphen AM, Van Der SJ, Stahl JS, De Zeeuw CI, et al. Simple spike and complex spike activity of floccular Purkinje cells during the optokinetic reflex in mice lacking cerebellar long-term depression. Eur J Neurosci. 2004;19:687–97. doi: 10.1111/j.0953-816x.2003.03173.x. [DOI] [PubMed] [Google Scholar]
- Hayar A, Bryant JL, Boughter JD, Heck DH. A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. J Neurosci Methods. 2006;2:203–7. doi: 10.1016/j.jneumeth.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Kuemmell F, Thach WT, Aertsen A. Dynamic correlation of neuronal activity in rat cerebellar cortex modulated by behavior. Ann NY Acad Sci. 2002;978:156. doi: 10.1111/j.1749-6632.2002.tb07563.x. [DOI] [PubMed] [Google Scholar]
- Heck DH, Thach WT, Keating JG. On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. Proc Natl Acad Sci. 2007;104:7658–63. doi: 10.1073/pnas.0609966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Zhau Y, Roy S, LeDoux MS, Reiter LT. Analysis of cerebellar function in Ube3a deficient mice reveals novel genotype specific behaviors. Hum Mol Genet. 2008;17:2181–9. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz GP, Stephan FK, Smith JC, Whitney G. Genetic and environmental variability in lick rates of mice. Physiol Behav. 1977;19:493–6. doi: 10.1016/0031-9384(77)90224-4. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Spencer R. Analysis of licking responses in rats: effects of cholecystokinin and bombesin. Behav Neurosci. 1983;97:234–45. doi: 10.1037//0735-7044.97.2.234. [DOI] [PubMed] [Google Scholar]
- Jones MW, Peckham HM, Errington ML, Bliss TV, Routtenberg A. Synaptic plasticity in the hippocampus of awake C57BL/6 and DBA/2 mice: interstrain differences and parallels with behavior. Hippocampus. 2001;11:391–6. doi: 10.1002/hipo.1053. [DOI] [PubMed] [Google Scholar]
- Koranda JL, Masino SA, Blaise JH. Bidirectional synaptic plasticity in the dentate gyrus of the awake freely behaving mouse. J Neurosci Methods. 2008;167:160–6. doi: 10.1016/j.jneumeth.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–56. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–5. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Khosrovani S, Winkelman BH, Hoebeek FE, De Jeu MT, Larsen IM, et al. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–61. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970;33:537–47. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Sugihara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]