Abstract
Recent studies demonstrated that diffusion tensor imaging (DTI) can provide information regarding white matter integrity of the corpus callosum (CC). In this study, DTI parameters were compared between cocaine dependent subjects (CDs) and non-drug-using controls (NCs) in midsagittal CC. DTI images were acquired from 19 CDs and 18 age-matched NCs. The midsagittal CC was segmented into: genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium. Linear Mixed Models analyses showed that, relative to NCs, CDs had lower fractional anisotropy (FA), higher radial diffusivity (λ⊥), and higher mean diffusivity (Dav) in the isthmus; higher λ⊥ and Dav in the rostral body; and lower FA in the splenium. After including mass of lifetime alcohol use in the mixed model ANCOVA as a covariate, significant between-group differences in λ⊥ in the rostral body and isthmus remained. These results suggest that alterations in λ⊥ in the rostral body and isthmus were mainly due to cocaine use, consistent with previous studies showing that cocaine may alter myelin integrity. Between-group differences in FA in the isthmus and splenium, and Dav in the rostral body and isthmus became non-significant after inclusion of alcohol use as a covariate. This is suggestive of alcohol influencing these values, or may be related to the decreased degrees of freedom for these effects. Consistent with clinical data of greater severity of drug use in smoked versus intranasal cocaine, subjects who smoked cocaine showed lower FA and higher λ⊥ compared to intranasal CDs.
Keywords: cocaine, alcohol, white matter, corpus callosum, isthmus, route of cocaine administration, DTI
1. Introduction
Investigating cocaine-associated white matter abnormalities is an active area in substance abuse research. Using proton magnetic resonance spectroscopy (MRS), Smith and associates found increased total creatine (an indicator of energy metabolism) in the frontal white matter in the brains of children exposed to cocaine in utero when compared to age-matched normal control children (Smith et al., 2001). Using magnetic resonance imaging (MRI), Bartzokis and associates found that, unlike age-matched normal control subjects, adult cocaine dependent subjects did not demonstrate any age-related increase in white matter volume of the frontal and temporal lobes, suggesting that cocaine abuse may arrest normal white matter development in these regions (Bartzokis et al., 2002). Using voxel based morphometry (VBM), Sim and associates found that, apart from reduced gray matter volumes in cerebrum and cerebellum, cocaine dependent subjects have reduced right cerebellum white matter volume that correlated with deficits in executive function and decreased motor performance (Sim et al., 2007). Using diffusion tensor imaging (DTI), Lim and associates found reduced fractional anisotropy (FA) on DTI in inferior frontal white matter as well as a trend of reduction in frontal gray and white matter volumes in cocaine dependent subjects when compared to control subjects (Lim et al., 2008). The duration of cocaine use was also found to be associated with decreased gray and white matter volumes. These reports indicate that cocaine abuse may be associated with altered white matter structure.
The corpus callosum (CC) is a large white matter fiber network that connects the cerebral hemispheres. The CC is characterized by region-dependent fiber density and myelination levels that reflect functional specialization (Aboitiz et al., 1992). The CC has been used as a sensitive marker of normal brain development (Hasan et al., 2008a; Hasan et al., 2008b; Ota et al., 2006) and pathological white matter impairment (Chaim et al., 2007; Ewing-Cobbs et al., 2008; Haut et al., 2006; Keshavan et al., 2002; Moeller et al., 2005; Schmitt et al., 2001; Villarreal et al., 2004). The midsagittal CC is generally sub-divided into different sections for quantitative MRI studies. A commonly used scheme is that proposed by Witelson (Witelson, 1989) in which the CC is divided into seven subregions.
DTI is a potentially powerful technique for noninvasively investigating the microstructural organization of white matter (Taber et al., 2002). DTI exploits the directionality of diffusion of water molecules in tissues. The commonly used parameters derived from DTI for quantifying differences in patterns of diffusion are fractional anisotropy (FA), axial diffusivity (λ║), radial diffusivity (λ⊥), and mean diffusivity (Dav).
Using DTI, Moeller and associates found that FA in the genu and rostral body of the anterior CC in cocaine dependent subjects was significantly reduced compared to normal control subjects (Moeller et al., 2005), suggesting altered microstructure (reduced white matter integrity) in anterior CC in the cocaine dependent subjects.
In order to confirm and extend the findings of previous studies of CC integrity in cocaine dependence, we used Witelson’s CC parcellation method to compare midsagittal DTI parameters of CC between cocaine dependent subjects and controls. We also examined the area of each CC subregion and the relationship between the CC measures and the quantity of past cocaine and alcohol use.
2. Methods
2.1. Subjects
This study was approved by the Committee for the Protection of Human Subjects (the institutional review board (IRB) at the University of Texas Health Science Center at Houston), and was performed in accordance with the Declaration of Helsinki. The consent process and all procedures were reviewed and approved prior to initiating studies. Subjects with current cocaine dependence and non-drug using normal controls were recruited through advertisements for research volunteers. Informed consent was obtained from all subjects by investigators and research staff. All subjects were screened for psychiatric and nonpsychiatric medical disorders using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996). All subjects underwent physical examination and their medical history was obtained. Prior to MRI scan, urine drug screening was performed on all subjects using an immunochromatographic assay for THC, opiates, cocaine (benzoylecgonine), amphetamines, and benzodiazepines (Syva Company). Subjects also underwent the Addiction Severity Index (McLellan et al, 1992) to document lifetime drug and alcohol use. All female subjects underwent a urine pregnancy test. Subjects were excluded if they had any current or past DSM-IV Axis I disorders other than substance abuse or substance dependence. Subjects were excluded who had medical disorders that may affect the central nervous system. Subjects were also excluded if they were not free of alcohol at the time of scanning as determined by an Intoximeter Alcosensor III breathalyzer (Intoximeters, Inc., St Louis MO). We excluded subjects who had abnormal Fluid-Attenuated Inversion Recovery (FLAIR) MRI scans, since one of the aims of our research involving DTI was to determine whether DTI can detect subtle white matter changes that are not detectable on conventional MRI such as FLAIR. Two control subjects and one cocaine subject were excluded because of definitely abnormal FLAIR scans as read by a clinical radiologist (LAK). Four control subjects and five cocaine subjects who were included in the study had FLAIR scans with a few small white matter hyperintensities that were judged to be clinically insignificant. All of the other control and cocaine subjects had no brain abnormalities on FLAIR scans. Control subjects were excluded if they had a positive urine drug screen, or if they met current or lifetime psychiatric, substance abuse, or substance dependence criteria. Female subjects were excluded if they had a positive pregnancy test result. A total of 19 cocaine dependent subjects and 18 control subjects met these criteria. All cocaine dependent subjects were referred for treatment of cocaine dependence at the end of the study.
2.2. Brain Image Acquisition and Processing
Whole brain DTI data were acquired on a Philips 3.0 T Intera system with a six channel receive head coil (Philips Medical Systems, Best, Netherlands). DTI images were acquired in the transverse plane using a single shot spin-echo diffusion sensitized echo-planar imaging (EPI) sequence (b-factor= 1000 s/mm−2, repetition time or TR=6100 ms, echo time or TE=84 ms, 44 contiguous axial slices, field-of-view or FOV = 240 mm × 240 mm, 112 × 112 acquisition matrix, 256 × 256 reconstructed matrix, 0.9375 mm × 0.9375 mm reconstructed in-plane resolution, slice thickness = 3 mm, no interslice gap). The diffusion tensor encoding scheme is based on the uniformly distributed and balanced rotationally invariant Icosa21 tensor-encoding set (Hasan and Narayana, 2003). A SENSE acceleration factor or k-space undersampling R=2 was used to help reduce EPI image distortions. The diffusion-encoded volumes were acquired with fat suppression. The DTI acquisition time was approximately 7 min and resulted in signal to noise ratio independent DTI-metric estimation (Hasan, 2007). The DTI-derived rotationally-invariant metrics (FA, λ║, λ⊥, and Dav) were computed using the method as described elsewhere (Hasan and Narayana, 2006). FA measures deviation from isotropy, and reflects the degree of alignment of cellular structures within fiber tracts, as well as their structural integrity (Cercignani et al., 2001). Dav measures average water molecular motion independent of any tissue directionality, and is affected by cellular size and integrity (Cercignani et al., 2001). Thus, Dav measures magnitude of diffusion, whereas FA reflects the anisotropy (or directionality) of water diffusion (Hasan and Narayana, 2006). We also computed λ║ and λ⊥ because there is considerable experimental evidence that these two measures improve pathological specificity compared to FA and Dav (Hasan and Narayana, 2006). Higher λ⊥ is indicative of greater diffusion perpendicular to the fiber tracts and has been associated with impairment in myelin (Deo et al., 2006; Gulani et al., 2001; Herrera et al., 2008; Song et al., 2002). Lower λ║ may indicate less diffusion of water in the direction parallel to the fiber tract and may be associated with axonal damage (Song et al., 2002).
2.3. DTI Based Segmentation of the Corpus Callosum
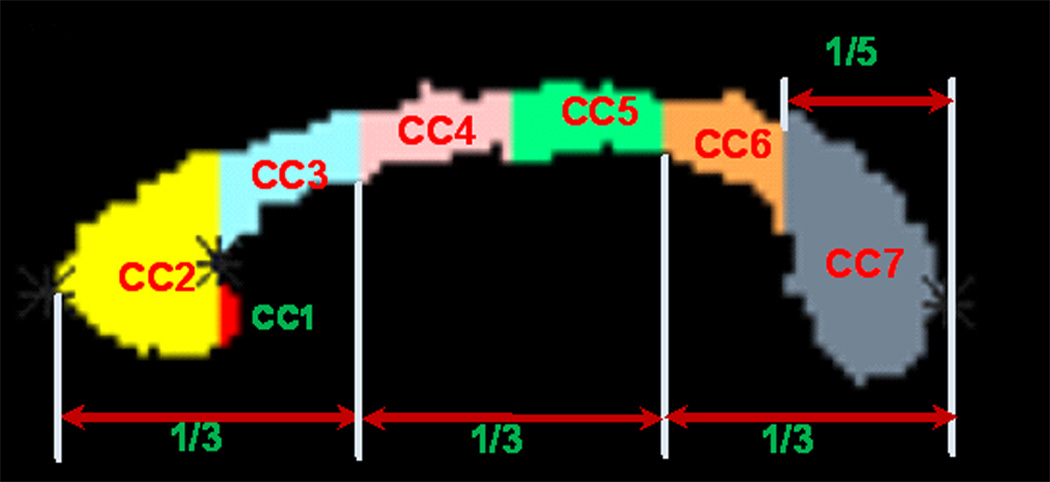
The midsagittal CC was identified based on the appearance of the interthalamic mass and the fornix on the isotropically interpolated DTI maps (Hasan et al., 2008b). The CC was then parcellated on the midsagittal slice using the semi-automated method described in (Hasan et al., 2008b) and the references therein. The procedure parcellated the CC into seven parts: CC1-rostrum, CC2-genu, CC3-rostral body, CC4-anterior midbody, CC5-posterior midbody, CC6-isthmus and CC7-splenium (Figure 1), from anterior to posterior (Aboitiz et al., 1992; Witelson, 1989). CC1 is not consistently visualized in all the images probably due to partial volume effects resulting from the small size of this region and the resolution of the DTI scans. Because CC1 measures were less reliable, we report findings for CC2 through CC7. A detailed description of the validation of the DTI-based CC parcellation using manually delineated high resolution anatomical MRI data is provided in (Hasan et al., 2008a). The mean values of the DTI measures (that survived a threshold of FA ≥ 0.2) and areas in each of the 6 CC segments were computed.
Fig. 1.
Illustration of the DTI-based parcellation of the 7 subregions of the human corpus callosum. The magnified seven subregions of the midsagittal CC were based on the semi-automated DTI implementation of the Witelson (1989) geometric CC subdivisions. CC1 = rostrum; CC2 = genu; CC3 = rostral body; CC4 = anterior midbody; CC5 = posterior midbody; CC6 = isthmus; CC7 = splenium.
Since the area of the midsagittal CC is associated with the brain volume (Jancke et al., 1997; Leonard et al., 2008; Smith, 2005), apart from the raw CC area, we also considered the CC area normalized to brain volume (Leonard et al., 2008; Smith, 2005). Because the cross-sectional area is proportional to the square of length, while the brain volume is proportional to the cube of length, we computed the normalized CC area as the ratio of the CC area and the 2/3 power of the brain volume (Jancke et al., 1997). The brain volume was computed using the method described in (Hasan et al., 2007).
2.4. Statistical Analyses
Student’s t-test and Pearson χ2 analysis were used to evaluate group differences on continuous demographic variables and categorical demographic variables respectively. Group comparisons on DTI measures or areas of the six CC subregions were performed using SPSS Version 16 (SPSS Inc, Chicago, Illinois) for Windows. Linear Mixed Models analyses, with a first order autoregressive (AR1) model of the covariance between CC subregions for DTI measures, or a diagonal (DIAG) model for areas (assumes no between-regions covariance for areas), were used. The Linear Mixed Models analysis included one between-subject factor (group) with two levels, and one within-subject factor (subregion) with six levels based on the six CC subregions. In the SPSS Linear Mixed Models procedure, the degrees of freedom of the F test is obtained by the Satterthwaite approximation, which typically results in non-integer values for the degrees of freedom. If the group × subregion interaction effect was statistically significant, then post hoc simple main effects were examined that compared group differences for each CC subregion using the Bonferroni correction for multiple comparisons. The main effect of alcohol use was analyzed by using an SPSS mixed model analysis of covariance (ANCOVA), if a preliminary complete factorial analysis (analysis of slopes model) revealed that the criterion of homogeneity of within-class regression coefficients (i.e., parallel slopes) was met (Winer et al., 1991; pp. 764–768). Pearson correlations were used to examine the relationship between normally distributed variables, and Spearman correlations were used to examine relationships between non-normally distributed variables. Difference between correlation coefficients was evaluated by using the Fisher Z-transform.
3. Results
3.1. Demographics
A total of 19 cocaine dependent subjects (39.1±7.8 (mean±SD) years old; 13 males, 6 females; 16 right-handed, 3 left-handed) and 18 controls (35.1±10.7 years old; 9 males, 9 females; 15 right-handed, 3 left-handed) were included in this study. There was no significant difference in age between groups (t=1.31, df=35, p=0.1997). Gender (χ2=2.78, df=1, p=0.0954) and handedness (χ2=0.07, df=1, p=0.7913) did not differ statistically between groups.
3.2. Substance Abuse or Dependence
The DSM-IV diagnoses were summarized in Table 1. All of the cocaine dependent subjects had one or more DSM-IV diagnoses of both current and past cocaine dependence. None of the control subjects had DSM-IV diagnosis of any drug abuse or dependence. Nine of the cocaine subjects had one substance related disorder diagnosis; one cocaine subject had two diagnoses, and nine had more than two diagnoses due to overlap. Cocaine dependent subjects reported lifetime cocaine use duration for an average of (10.7±6.7) years, ranging from 2 to 23 years; and past 30-day cocaine use prior to scanning of (17±7.8) days, ranging from 2 to 30 days.
Table 1.
Number of DSM-IV substance abuse and dependence diagnoses (other than cocaine) for cocaine and control subjects.
| DSM-IV diagnosis | Number of diagnoses | |
|---|---|---|
| Cocaine | Control | |
| Past sedative abuse | 1 | 0 |
| Past opiate abuse | 2 | 0 |
| Current cannabis abuse | 1 | 0 |
| Past cannabis abuse | 6 | 0 |
| Past cannabis dependence | 1 | 0 |
| Current alcohol abuse | 2 | 0 |
| Current alcohol dependence | 1 | 0 |
| Past alcohol abuse | 2 | 0 |
| Past alcohol dependence | 4 | 0 |
| Past hallucinogen abuse | 1 | 0 |
| Past PCP (pentachlorophenol) dependence | 1 | 0 |
| Past MDMA (ecstasy) dependence | 1 | 0 |
| Past stimulant abuse (the subject used another stimulant drug in addition to cocaine) |
1 | 0 |
| Past stimulant dependence (the subject used another stimulant drug in addition to cocaine) |
3 | 0 |
3.3. Group Comparison
There were statistically significant interaction effects of group × subregion for FA (F=2.49; df=5, 148.93; P=0.034), λ⊥ (F=3.46; df=5, 150.92; P=0.006), and Dav (F=2.99; df=5, 151.29; P=0.013). Post hoc comparisons on FA for the CC subregions found significant group differences only in the isthmus (F=8.06; df=1, 152.94; P=0.005) and splenium (F=4.04; df=1, 152.94; P=0.046), with cocaine dependent subjects showing lower FA in both of these subregions compared to controls. Post hoc comparisons of λ⊥ for the CC subregions found significant group differences only in the rostral body (F=12.53; df=1, 175.07; P=0.001) and isthmus (F=10.74; df=1, 175.07; P=0.001), with cocaine dependent subjects showing higher λ⊥ in both of these subregions compared to controls. Post hoc comparisons of Dav for the CC subregions found significant group differences only in the rostral body (F=11.88; df=1, 155.97; P=0.001) and isthmus (F=6.14; df=1, 155.97; P=0.014), with cocaine dependent subjects showing higher Dav in both of these subregions compared to controls. Table 2 lists mean and standard derivation values of FA, λ⊥, and Dav by the six CC subregions and the two subject groups. There were no significant interaction effects of group × subregion for CC area, normalized CC area, and λ║.
Table 2.
Mean and standard deviation values of DTI measurements by region and group (cocaine vs. controls). The selected DTI measurements are those for CC subregions that were significantly different between subject groups. Significantly lower mean values are highlighted by bold font.
| Region | FA (× 1000) | λ⊥(10−6 mm2/sec) | Dav (10−6 mm2/sec) | |||
|---|---|---|---|---|---|---|
| Cocaine | Control | Cocaine | Control | Cocaine | Control | |
| Genu | 555±35 | 550±32 | 579±56 | 583±55 | 905±75 | 905±70 |
| Rostral body | 418±34 | 435±24 | 795±82 | 712±53 | 1071±114 | 974±61 |
| Anterior midbody | 419±31 | 428±24 | 751±72 | 709±72 | 1012±98 | 962±84 |
| Posterior midbody | 430±49 | 428±34 | 741±100 | 725±77 | 1008±105 | 983±81 |
| Isthmus | 433±47 | 466±38 | 814±97 | 738±66 | 1110±102 | 1040±74 |
| Splenium | 585±39 | 608±26 | 608±56 | 572±51 | 990±81 | 962±76 |
Most subjects (except for 2 cocaine dependent subjects and 2 controls) reported some current or past alcohol use. Cocaine dependent subjects reported drinking alcohol for an average of (13.8±10.3) years, ranging from 0 years to 30 years; and lifetime mass of consumed alcohol was (103.2±118.3) kg, ranging from 0 kg to 389.7 kg. Control subjects reported drinking alcohol for an average of (15.5±12.3) years, ranging from 0 years to 43.5 years and alcohol mass of (13.9±20.1) kg, ranging from 0 kg to 70.2 kg. There was no significant difference in the duration of alcohol use between groups (t=−0.46, df=35, p=0.6505). However, there was a significant difference in the mass of lifetime alcohol use between groups (t=3.16, df=35, p=0.0033), with cocaine dependent subjects consuming significantly more alcohol than control subjects. In a preliminary analysis, we included the mass of lifetime alcohol use as a factor in an SPSS Linear Mixed Model complete factorial analysis (analysis of slopes model), along with factors for group (cocaine users vs. non-users), CC subregion, and the two-way and three-way interactions among the factors. A separate analysis was conducted for CC area, normalized CC area, and each DTI measure as the dependent variable. There were no significant interaction effects of alcohol with the other factors (group and CC subregions) in any of these analyses.
Since the above preliminary analyses revealed that the prerequisite criterion for ANCOVA of homogeneity of within class regression coefficients (i.e., parallel slopes) was met (Winer et al., 1991; pp. 764–768), we then conducted an SPSS mixed model analysis of covariance (ANCOVA) that included the mass of lifetime alcohol use as a covariate without alcohol interaction terms. For the CC area as dependent variable, there was a significant main effect of group, with cocaine subjects showing lower CC area compared to control subjects (F=14.53; df=1, 125.13; P<0.0005), and a significant main effect of alcohol (F=5.41; df=33, 83.88; P<0.0005), with increased mass of lifetime alcohol use associated with decreased CC area. For the normalized CC area as a dependent variable, there also was a significant main effect of group, with cocaine subjects showing lower normalized CC area compared to control subjects (F=9.45; df=1, 91.43; P=0.003), and a significant main effect of alcohol (F=5.61; df=33, 59.15; P<0.0005), with increased mass of lifetime alcohol use associated with decreased normalized CC area.
For the mixed model ANCOVA conducted separately for each DTI measure as a dependent variable and with mass of lifetime alcohol use as covariate, there were no significant main effects of group or alcohol. In addition, there were no significant interaction effects of group × CC subregion for λ║. However, there were significant interaction effects of group × CC subregion for FA (F=2.50; df=5, 110.87; P=0.034), λ⊥ (F=3.29; df=5, 112.86; P=0.008), and Dav (F=2.94; df=5, 111.59; P=0.016). Post hoc comparisons, derived from the above mixed model with mass of lifetime alcohol use as covariate, on FA for the CC subregions found only a trend of significant group difference in the isthmus (F=3.21; df=1, 27.69; P=0.084), with cocaine dependent subjects showing lower FA compared to controls. Post hoc comparisons on λ⊥ for the CC subregions, with mass of lifetime alcohol as covariate, found significant group differences in the rostral body (F=5.42; df=1, 41.65; P=0.025) and isthmus (F=4.74; df=1, 41.65; P=0.035), with cocaine dependent subjects showing higher λ⊥ in both of these subregions compared to controls. Post hoc comparisons on Dav for the CC subregions, with mass of lifetime alcohol as covariate, found only a trend of significant group difference in the rostral body (F=3.67; df=1, 26.80; P=0.066), with cocaine dependent subjects showing lower Dav compared to controls. Because of the fact that the influence of lifetime mass of alcohol use on the DTI results was analyzed in the ANCOVA, which examined the main effects as well as interaction effects of alcohol, a separate simple correlation analysis of the effects of lifetime mass of alcohol would yield less specific information than the ANCOVA and thus is not warranted.
The cocaine dependent subjects consumed cocaine either by smoking (“crack”) or intranasal route. 14 subjects reported smoking cocaine and 5 reported intranasal cocaine use. There were significant main effects of route for FA (F=6.01; df=1, 22.52; P=0.022) and λ⊥ (F=5.55; df=1, 31.64; P=0.025), with smoking subjects showing lower FA and higher λ⊥ compared to intranasal subjects. There were no significant main effects of route for Dav. There were no significant interaction effects of route × CC subregion for any of the DTI measures.
Within the cocaine group, 8 subjects were diagnosed to have current cannabis abuse or dependence (Table 1). There were no significant main effects of subgroup (cannabis vs. non-cannabis) in any of the DTI measures. There were no significant interaction effects of subgroup × CC subregion for any of the DTI measures.
4. Discussion
Significant alterations in white matter microstructure were found in both anterior and posterior CC, as revealed by higher λ⊥, and higher Dav in the rostral body; lower FA, higher λ⊥, and higher Dav in the isthmus; and lower FA in the splenium, in the cocaine group compared to the control group, indicating compromised fiber, myelin, and/or cellular integrities in these CC subregions in the cocaine dependent subjects. The fiber, myelin, and cellular integrities were all lower in the isthmus in the cocaine dependent subjects compared to the control subjects. This is the first study to show microstructural differences in the isthmus of the CC between cocaine dependent subjects and non drug-using control subjects. The alterations in the microstructure of the anterior CC (the rostral body) are consistent with previous findings in an entirely different group of cocaine dependent subjects using a 1.5 T GE scanner (Moeller et al., 2005; Moeller et al., 2007). The alterations in the microstructure of the posterior CC (the splenium) are consistent with the finding by Lim et al., (2008). These results are also consistent with a recent animal study showing lower FA and higher λ⊥ in CC in chronically cocaine administered rats compared to saline controlled rats (Narayana et al., 2009).
The microstructural alterations in the isthmus were not detected in previous studies (Lim et al., 2008; Moeller et al., 2005; Moeller et al., 2007). Possible reasons for differences between the findings in this study and previous studies include the fact that previous studies (Moeller et al., 2005; Moeller et al., 2007) used a scanner with lower magnetic field strength (1.5 T). Based on the whole-brain histogram analysis and ROI analysis, Fushimi and associates have shown that there exist significant difference in FA between DTI data acquired from 3 T and 1.5 T scanners (Fushimi et al., 2007). Another important difference in the DTI data between the present study and our previous studies is the slice thickness: 3 mm for the present study, and 4 mm for our previous studies. Differences in slice thickness may be especially important for the isthmus, which is a relatively small region in the midsagittal CC. These differences in DTI acquisition may account for the lack of group differences in the isthmus in previous studies. Previous studies (Moeller et al., 2005; Moeller et al., 2007) found differences between cocaine users and controls on FA or λ⊥ in the genu of the CC, however the present study did not find a significant difference between groups in this region. One possible explanation for the lack of differences between groups is related to aging. The mean ages of both cocaine and control subjects in the present study were greater than those in the previous studies (Moeller et al., 2005; Moeller et al., 2007). Hasan and associates have shown that the microstructure of genu is sensitive to normal aging (Hasan et al., 2008a; Hasan et al., 2008b).
Cocaine subjects consumed significantly more alcohol (in terms of mass of lifetime alcohol use) than the control subjects. Alcohol consumption per se can induce neuroanatomical changes in human CC (O'Neill et al., 2001; Venkatasubramanian et al., 2007). However, in the mixed model ANCOVA conducted separately for each DTI measure as dependent variable and with mass of lifetime alcohol use as covariate, we did not find any significant main effects or interaction effects of alcohol, suggesting that alcohol use was unlikely to be a major influence in the current DTI results.
After including the mass of lifetime alcohol use in the mixed model ANCOVA as a covariate, we found that the significant between-group differences in λ⊥ in the rostral body and isthmus were still significant. These results suggest that alterations in λ⊥ in the rostral body and isthmus were mainly due to cocaine use rather than alcohol use. This finding is consistent with previous studies showing that cocaine use may alter myelin integrity (Albertson et al., 2004; Bannon et al., 2005; Feng, 2008; Kristiansen et al., 2009). We also found that the between-group differences in FA in the isthmus and splenium, and the between-group differences in Dav in the rostral body and isthmus became non-significant after inclusion of lifetime quantity of alcohol use as a covariate. The reduction in significance level of the between-groups differences in FA and Dav after controlling for alcohol use suggests that alcohol use may have influenced these values in both anterior and posterior CC subregions. Alternatively, the reduction in significance level of the between-groups differences in FA and Dav after including alcohol use as a covariate may be related to the decreased degrees of freedom for these effects because of the presence of the covariate in the model.
Cerebral ischemia is a possible factor that can cause the alterations of CC microstructure. Using DTI, Nagy and associates found that the adolescents with moderate hypoxic-ischemic encephalopathy (HIE) had significantly lower FA values in the posterior CC including the isthmus and the splenium (Nagy et al., 2005). Cerebral ischemia is frequently observed in cocaine dependent subjects (Kosten, 1998). In a recent perfusion functional MRI (fMRI) study, Rao and associates observed that the decrease of cerebral blood flow for in-utero-cocaine-exposed adolescents was primarily in the posterior brain regions (Rao et al., 2007). Thus, the effect of cocaine on the posterior CC (i.e., the isthmus) microstructure may be related to cocaine-associated ischemia, which is more potent in posterior than in anterior brain in cocaine dependent subjects (Rao et al., 2007). Further studies using perfusion fMRI and DTI are needed to test this hypothesis. Another possible reason for the findings is cocaine-related changes in myelin, either directly due to effects of cocaine or through chronic effects on reduced perfusion. In a recent study in rodents, rats chronically treated with cocaine had significantly lower FA in the splenium of the CC and a significant reduction in myelin basic protein (Narayana et al., 2009). Recently it has been suggested that chronic cocaine may alter myelin through epigenetic mechanisms (Feng, 2008; Sokolov, 2007).
The rostral body contains crossing fibers from the prefrontal and frontal cortex (Barbas and Pandya, 1984); thus the alterations in the rostral body may result in deficits in executive function of these areas, which are frequently observed in cocaine dependent subjects (Fein et al., 2002; Lane et al., 2007; Woicik et al., In Press). The isthmus contains transcallosal fibers from the parietal and temporal areas (Witelson, 1989), therefore the alterations in the isthmus may affect the normal function of these regions. Cocaine-associated functional differences in the parietal and temporal cortices have been reported (Sim et al., 2007; Strickland et al., 1993; Tomasi et al., 2007b).
Recently several studies have been published indicating that the callosal motor fibers in humans run in the posterior CC (Hofer and Frahm, 2006; Meyer et al., 1998; Park et al., 2008; Wahl et al., 2007; Zarei et al., 2006). Using whole brain gray matter parcellation and DTI-based fiber tracking, Park and associates (Park et al., 2008) found that fibers arising from the sensorimotor cortex pass through the isthmus in the normal subjects. Based on the work of Park and associates (Park et al., 2008), it can be speculated that the alterations in the isthmus may also result in decreased performance of the sensorimotor system. Recent studies in animal models (Porrino et al., 2004; Willuhn and Steiner, 2006) and human subjects (Anderson et al., 2006; Browndyke et al., 2004; Tomasi et al., 2007a) indicate that the sensorimotor system may be affected by cocaine abuse.
The group comparison on the DTI measures between the smoking route and intranasal route showed significant differences. There was no significant interaction between the route and the CC subregions, suggesting that the route of cocaine administration affects entire CC rather than the specific CC subregions. The subjects who consumed cocaine by smoking showed a lower FA and higher λ⊥ relative to the intranasal cocaine using subjects. These results are consistent with the literature showing more severe cocaine addiction in cocaine smokers (Jeffcoat et al., 1989).
SPSS Linear Mixed Model group analysis did not show significant difference in CC area or normalized CC area between groups. However, after including the mass of lifetime alcohol use as a covariate in the models, we found significant main effects of group, with cocaine dependent subjects showing lower CC area or normalized CC area; and significant main effects of alcohol, with increasing mass of lifetime alcohol use associated with decreased CC area or normalized CC area. This result is consistent with the finding by Venkatasubramanian and associates, who found that CC area is susceptible to alcohol use (Venkatasubramanian et al., 2007).
One previous study found that heavy marijuana use was associated with structural damage in CC as measured by DTI (Arnone et al., 2008). It is unlikely that marijuana use was a factor in the current study, since the subgroup comparison of cannabis users and non-users found neither significant subgroup main effects nor significant interactions of subgroup × CC subregion. These results suggest that, in this study, the cannabis use was unlikely to be a major influence in the results.
Because subjects were scanned only after they had already developed cocaine dependence, an alternate explanation of the results of this study is that the alterations in CC subregions preceded the onset of cocaine dependence. However, at least one previous study in rodents found that chronic cocaine administration produced similar white matter changes on DTI measures (Narayana et al., 2009), supporting the link between cocaine use and white matter pathology.
In summary, this study demonstrated alterations in the microstructure of the midsagittal CC in the cocaine dependent subjects in both the anterior and posterior subregions. These findings lend support to the growing body of evidence of subtle white matter pathology in cocaine dependent subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J. Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict. Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp. Brain Res. 1984;55:187–191. doi: 10.1007/BF00240516. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol. Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Browndyke JN, Tucker KA, Woods SP, Beauvals J, Cohen RA, Gottschalk PC, Kosten TR. Examining the effect of cerebral perfusion abnormality magnitude on cognitive performance in recently abstinent chronic cocaine abusers. J. Neuroimaging. 2004;14:162–169. [PubMed] [Google Scholar]
- Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 2001;22:952–958. [PMC free article] [PubMed] [Google Scholar]
- Chaim TM, Duran FL, Uchida RR, Perico CA, de Castro CC, Busatto GF. Volumetric reduction of the corpus callosum in Alzheimer's disease in vivo as assessed with voxel-based morphometry. Psychiatry Res. 2007;154:59–68. doi: 10.1016/j.pscychresns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Deo AA, Grill RJ, Hasan KM, Narayana PA. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J. Neurosci. Res. 2006;83:801–810. doi: 10.1002/jnr.20783. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochem. Res. 2008;33:1940–1949. doi: 10.1007/s11064-008-9693-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York: New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IV Axis I DisordersFPatient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Fushimi Y, Miki Y, Okada T, Yamamoto A, Mori N, Hanakawa T, Urayama S, Aso T, Fukuyama H, Kikuta K, Togashi K. Fractional anisotropy and mean diffusivity: comparison between 3.0-T and 1.5-T diffusion tensor imaging with parallel imaging using histogram and region of interest analysis. NMR Biomed. 2007;20:743–748. doi: 10.1002/nbm.1139. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn. Reson. Med. 2001;45:191–195. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hasan KM. A framework for quality control and parameter optimization in diffusion tensor imaging: theoretical analysis and validation. Magn. Reson. Imaging. 2007;25:1196–1202. doi: 10.1016/j.mri.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Ewing-Cobbs L, Kramer LA, Fletcher JM, Narayana PA. Diffusion tensor quantification of the macrostructure and microstructure of human midsagittal corpus callosum across the lifespan. NMR Biomed. 2008a;21:1094–1101. doi: 10.1002/nbm.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, Ewing-Cobbs L, Fletcher JM. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. Neuroimage. 2007;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Kramer LA, Papnicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008b;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magn. Reson. Med. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn. Reson. Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Haut MW, Kuwabara H, Ducatman AM, Hatfield G, Parsons MW, Scott A, Parsons E, Morrow LA. Corpus callosum volume in railroad workers with chronic exposure to solvents. J. Occup. Environ. Med. 2006;48:615–624. doi: 10.1097/01.jom.0000205211.67120.23. [DOI] [PubMed] [Google Scholar]
- Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J. Neurosci. Res. 2008;86:443–447. doi: 10.1002/jnr.21481. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb. Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jeffcoat AR, Perez-Reyes M, Hill JM, Sadler BM, Cook CE. Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking. Drug Metab. Dispos. 1989;17:153–159. [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J. Neurol. Neurosurg. Psychiatry. 2002;72:757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR. Pharmacotherapy of cerebral ischemia in cocaine dependence. Drug Alcohol Depend. 1998;49:133–144. doi: 10.1016/s0376-8716(97)00158-0. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Bannon MJ, Meador-Woodruff JH. Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem. Res. 2009;34:46–54. doi: 10.1007/s11064-008-9655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am. J. Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb. Cortex. 2008;18:2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Woiciechowsky C. Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann. Neurol. 1998;43:360–369. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Lindstrom K, Westerberg H, Skare S, Andersson J, Hallberg B, Lilja A, Flodmark O, Lagercrantz H, Klingberg T, Fernell E. Diffusion tensor imaging on teenagers, born at term with moderate hypoxic-ischemic encephalopathy. Pediatr. Res. 2005;58:936–940. doi: 10.1203/01.pdr.0000186516.85702.61. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J. Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eliez S, Warsofsky IS, Bellugi U, Reiss AL. Corpus callosum morphology of Williams syndrome: relation to genetics and behavior. Dev. Med. Child. Neurol. 2001;43:155–159. [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ. Relative size versus controlling for size. Interpretation of ratios in research on sexual dimorphism in the human corpus callosum. Curr. Anthropol. 2005;46:249–273. [Google Scholar]
- Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int. J. Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J. Neuropsychiatry Clin. Neurosci. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Taber KH, Pierpaoli C, Rose SE, Rugg-Gunn FJ, Chalk JB, Jones DK, Hurley RA. The future for diffusion tensor imaging in neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 2002;14:1–5. doi: 10.1176/jnp.14.1.1. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Anthony G, Reddy US, Reddy VV, Jayakumar PN, Benegal V. Corpus callosum abnormalities associated with greater externalizing behaviors in subjects at high risk for alcohol dependence. Psychiatry Res. 2007;156:209–215. doi: 10.1016/j.pscychresns.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, Brooks WM. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J. Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Steiner H. Motor-skill learning-associated gene regulation in the striatum: effects of cocaine. Neuropsychopharmacology. 2006;31:2669–2682. doi: 10.1038/sj.npp.1300995. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. 3rd ed. New York: McGraw-Hill, Inc; 1991. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The Neuropsychology of Cocaine Addiction: Recent Cocaine Use Masks Impairment. Neuropsychopharmacology. doi: 10.1038/npp.2008.60. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J. Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]