SUMMARY
Nippostrongylus brasiliensis larvae are particularly susceptible to immunological attack during the pre-lung stage of primary and secondary infections in mice. Whilst most of the common laboratory strains of mice are permissive hosts for the parasite, in this study we report for the first time, the strong resistance of naïve FVB/N mice to N. brasiliensis. Damage to larvae is evident within the first 24 h of infection and this may be critical to later larval development and reproductive success. Inflammatory responses in the skin, and larval escape from this tissue were comparable in susceptible CBA/Ca and resistant FVB/N mice, with most larvae exiting within 4 h of a primary infection. Lung larval burdens were also similar between strains, but larvae recovered from FVB/N mice were smaller and less motile. In FVB/N mice, larval colonization of the gut was impaired and worms produced very few eggs. However FVB/N mice did not show enhanced resistance to Heligmosomoides bakeri (also known as Heligmosomoides polygyrus), a nematode largely restricted to the gut. Damage done in the pre-lung or lung stages of infection with N. brasiliensis is likely to contribute to ongoing developmental and functional abnormalities, which are profoundly evident in the gut phase of infection.
Keywords: FVB/N mouse, Nippostrongylus brasiliensis, Heligmosomoides bakeri, Heligmosomoides polygyrus, primary resistance
INTRODUCTION
Parasitic helminth infections are a major problem worldwide and there are currently no vaccines available for use in humans. Tissue-invasive helminths employ immune evasion strategies that allow them to reside in the host for extended periods and, in some cases, for decades. Helminthic infections may result in a cellular response characterized by eosinophils, neutrophils and basophils (Kay et al. 1985). Eosinophils can be recruited to sites of infections in high numbers; however, the contribution they may make to an effective immune response is controversial and not well understood (Behm and Ovington, 2000; Meeusen and Balic, 2000; Lee and Lee, 2005). Eosinophils appear to be important for protection in some parasitic infections (Sasaki et al. 1993; Ovington et al. 1998; Daly et al. 1999; Knott et al. 2007), while in others, their presence may be of little consequence (Matthaei et al. 1997; Takamoto et al. 1997; Dent et al. 1999) or may even be counter-productive (McCormick et al. 1996; Dent et al. 1997a, b).
To further understand and characterize the roles of eosinophils in parasitic helminth infections, transgenic (Tg) mouse models are often used. Mice with constitutive eosinophilia due to expression of an interleukin (IL)-5 transgene (Dent et al. 1990) are highly resistant to infections with Nippostrongylus brasiliensis, and entrapment of infective larvae in the skin is associated with the rapid recruitment of eosinophils (Dent et al. 1997b; Daly, 1999; Dent et al. 1999; Giacomin et al. 2008). Other studies have shown that IL-5 Tg mice are resistant to Strong-yloides stercoralis infections and again, eosinophil recruitment into the skin is associated with the elimination of larvae (Herbert et al. 2000).
The intestinal phase of helminthic infections has been the focus of considerable research, since many species spend much of their life cycle in this organ. While the roles of IL-13, IL-4 and signal transduction and activator of transcription 6 (STAT6) in the intestinal phase of the N. brasiliensis life cycle have been well characterized (Urban et al. 1998; Fallon et al. 2002, 2006), the contributions that IL-5 and eosinophils make at this stage of infection are largely unknown. Recent data from our laboratory suggest that deletion of IL-5 may cause impairment of resistance in the intestinal phase of primary infections with N. brasiliensis, as well as the early pre-lung phase of secondary infections (Knott et al. 2007). Initially, a major objective of this study was to examine the consequences of intestine-specific expression of IL-5 (i-FABP-IL-5) or eotaxin (i-FABP-eotaxin) transgenes (Mishra et al. 2002) on helminth infections. Unexpectedly, the wildtype (WT) FVB/N animals used as controls were found to be highly resistant to primary infections with N. brasiliensis. This paper describes an investigation of the kinetics of nematode infections in FVB/N mice and the nature of the cellular inflammatory responses elicited.
MATERIALS AND METHODS
Animals
Heterozygous male and female CBA/Ca mice with approximately 49 copies (Tg5C2, abbreviated IL-5 Tg) of the IL-5 transgene (Dent et al. 1990) and their WT littermates were bred in the Medical School Animal House of the University of Adelaide. WT FVB/N male and female mice were bred by Laboratory Animal Services, University of Adelaide. Intestinal fatty acid binding protein (i-FABP)-IL-5 and i-FABP-eotaxin FVB/N Tg mice (Mishra et al. 2002) were bred at the John Curtin School of Medical Research, Australian National University. All mice were closely age-matched within experiments. Hooded Wistar rats used for passage of N. brasiliensis were obtained from Laboratory Animal Services at the University of Adelaide. All animals were handled according to the guidelines of the University of Adelaide Animal Ethics Committee.
Parasitological techniques
Parasite maintenance
Infective N. brasiliensis third-stage larvae (L3) were obtained by the faecal culture method after passage through Hooded Wistar rats as described previously (Daly, 1999), washed twice in MilliQ water and once in mouse osmolality phosphate-buffered saline (MPBS) (Sheridan and Finlay-Jones, 1977). Each mouse received 500 larvae for air pouch injections and 750 or 1000 larvae for subcutaneous (s.c.) injections. L3 were injected at the base of the neck or into air pouches in the middorsal skin using a 19-gauge needle. Mice in secondary infection groups were injected s.c. with 750 L3 and 3 weeks later, after clearance of the primary infection, mice were challenged with 750 L3 and lung larval numbers enumerated.
Infective Heligmosomoides bakeri (H. polygyrus) (Cable et al. 2006) L3 were also obtained by the faecal culture method, but passage was performed in BALB/c mice. Larvae were stored at 4 °C until used, at which time the concentration was adjusted to 2000 L3/ml and each experimental animal received 200 L3 by oral gavage.
Parasite recovery from tissues
For N. brasiliensis experiments, lung larvae (L4) were recovered from animals on days 1 and/or 2 post-injection (p.i.) as previously described (Knott et al. 2007). The motility of larvae in minced lung preparations was assessed after 2 h of incubation at 37 °C by recording the number of major movements by individual larvae over 30 sec. Larvae were recovered from 3 mice/group and 30 larvae were examined/mouse. Motility data for larvae recovered from individual mice were combined to yield n=90 larvae/group. Faecal egg counts (day 6 p.i. for N. brasiliensis and various time-points from 13–116 days p.i. for H. bakeri) and intestinal worm burdens (day 7 p.i. for N. brasiliensis and days 41 and 117 p.i. for H. bakeri) were also performed as previously described (Giacomin et al. 2005; Knott et al. 2007).
Measurement of parasite length
Intestinal worms (day 7 p.i.) or lung larvae (day 2 p.i.) were collected from WT FVB/N and WT CBA/Ca mice and photographed using Konica Centuria ASA 400 film, a dissecting microscope (Olympus) and an Olympus c-35AD-4 camera. 6–19 larvae or worms were photographed from lungs or intestines at 56× magnification for each of 3–4 mice/group. The lengths of larvae and worms were determined from photographs using a cartographer's wheel (Kartenmesser, Germany).
Generation of skin air pouches
Air pouches were generated as previously described (Daly et al. 1999). Briefly, the shaved dorsal skin of anaesthetized mice was inflated subcutaneously with 2.5 ml of filtered air and 3 days later, pouches were re-inflated with 2 ml of sterile air and larvae were injected (500 L3/mouse) after a further 2 days. Air pouches were lavaged 4 or 5 times with 2 ml of MPBS, 0 and 4 h after injection of larvae. The viable cells and larvae recovered were enumerated and differential leukocyte counts were estimated from cytocentrifuged samples, with a minimum of 200 cells counted per sample using oil immersion light microscopy.
Histology
Lungs were removed on day 2 p.i. and the post-caval lobe (right lung) was taken for histology. Tissues were fixed in 10% buffered formalin and embedded in paraffin. Five μm sections were cut and stained with haematoxylin & eosin (H&E) and photographed at 20× or 40× objective magnification.
Statistical analysis
Parasite burdens and size and inflammatory leukocyte numbers were compared using the non-parametric Kruskal-Wallis (KW) test, followed by Mann-Whitney U (MW) post-hoc testing or the parametric two-way ANOVA followed by Bonferroni post-hoc testing in Prism Version 5.01 (GraphPad Software Inc., La Jolla, CA, USA). Worm length was assessed in a linear mixed effects model followed by Bonferroni post-hoc testing with SAS Version 9.1 (SAS Institute Inc., Cary, NC, USA) software by Mr Thomas Sullivan (Statistician, Department of Public Health, University of Adelaide). In all tests P<0.05 was considered significant.
RESULTS
Intestinal phase of infection
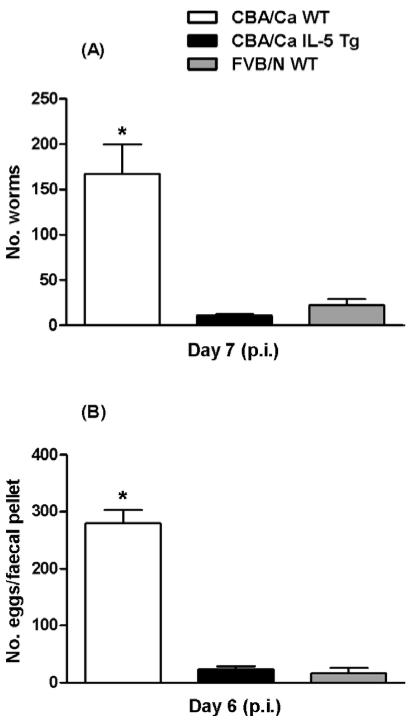
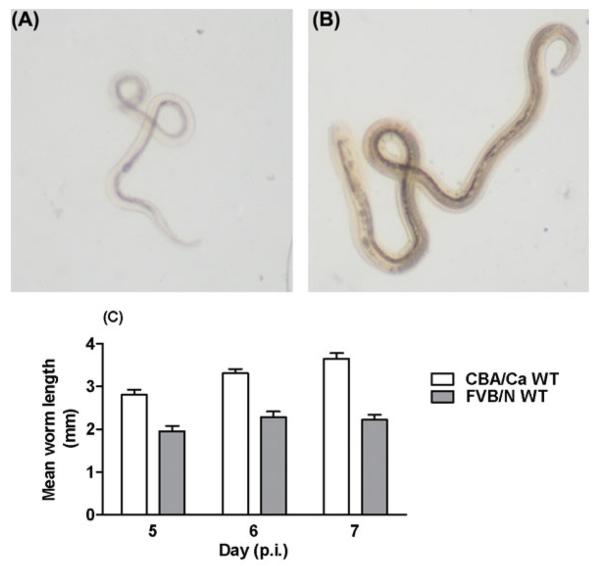
In initial experiments with N. brasiliensis infections in i-FABP-IL-5 Tg and i-FABP-eotaxin Tg FVB/N mice, few worms were present in the small intestines of the control WT FVB/N mice compared with WT CBA/Ca mice. We further investigated the observation to explore the cause of this potent resistance to N. brasiliensis. Using WT CBA/Ca mice as a comparison strain, which have similar susceptibility to other commonly used mouse strains such as BALB/c (Knott et al. 2007) and C57BL/6 (Giacomin et al. 2005, 2008), we confirmed that fewer worms were present in the intestines of WT FVB/N mice (Fig. 1A) and fewer eggs were found (Fig. 1B). The numbers of both worms and eggs in WT FVB/N mice were comparable to those seen during primary infections in the highly resistant IL-5 Tg CBA/Ca mice (Fig. 1). We also observed that the few worms that were present in the intestines of WT FVB/N mice appeared to be smaller and much paler in colour (Fig. 2A) than worms found in the intestines of WT CBA/Ca mice (Fig. 2B). To test for differences in worm length for hosts from each of the WT CBA/Ca and FVB/N strains on day 5, 6 and 7 p.i., a linear mixed effects model was used. In the model, strain, day and the interaction between strain and day were fitted as fixed effects. A random mouse ID effect was also fitted in the model to account for dependence in repeated observations for individual worms from the same mouse. There were 228 observations in total and the linear mixed effects model used 24 degrees of freedom to estimate the fixed and random effects. The results of this test showed that the interaction effect between strain and day was not statistically significant. Thus there is no evidence to suggest that the difference in average worm length between the strains varied according to the day of measurement. In order to accurately interpret the main effects of strain and day, the linear mixed effects model was rerun but the interaction term was excluded. The results of the model showed a significant difference in worm lengths between the strains and over time. The model estimates of the mean worm length for each strain and for each day were then calculated. These estimates were adjusted for dependence within each mouse. Worm lengths were higher in the CBA/Ca strain and increased over the successive days. The increase in mean worm length from WT FVB/N hosts over 3 days was modest (12%), relative to that seen in worms recovered from WT CBA/Ca mice over the same period (23%).
Fig. 1.
Nippostrongylus brasiliensis intestinal worm burdens on day 7 (A) and faecal egg production on day 6 (B) in WT CBA/Ca, IL-5 Tg CBA/Ca and WT FVB/N mice after infection. 750 L3/mouse. Mean+s.e.m., n=4 mice for all groups. Significant effect between strains (KW test: for (A) H=8·192, P=0·0166 and (B) H=7·731, P=0·0210). * Significantly greater than other groups (MW post-hoc tests, P<0·05). Data representative of 5 experiments.
Fig. 2.
Representative photomicrographs (56× magnification) of Nippostrongylus brasiliensis worms recovered on day 6 p.i. from WT FVB/N mice (A) and WT CBA/Ca mice (B). Intestinal worms recovered 5–7 days p.i. from WT FVB/N mice were paler (A) and smaller (C) than those found in WT CBA/Ca hosts. Worm length (mm) for 4 mice/group/day, with 6–12 worms measured/mouse, n=32–43 worms/group/day (C). A random mouse identity effect was fitted in the model to account for the dependence in repeated observations (worms) from the same mouse. The interaction effect between strain and day was not statistically significant (linear mixed effects model F2, 204=1·26, P=0·2871). There was a significant difference in lengths of worms recovered from the two host strains and over time (linear mixed effects model; F1, 204=52·39, P<0·0001 for strain and F2, 204=5·21, P=0·0062 for day). On average, worm lengths were greater in the CBA/Ca strain (difference in means=1·0929, P<0·0001) and increased from day 5 to day 7 (P=0·0062). The mean worm length was greater at day 7 than at day 5 (Bonferroni post-hoc test, t=3·13, P=0·0060). There was no difference in mean worm length between days 5 and 6 and days 6 and 7 (Bonferroni post-hoc test, t=2·29, P=0·0699 and t=0·85, P=1·1913 respectively).
The numbers of leukocytes in the small intestine were examined on days 6 and 7 p.i. Eosinophil numbers in the intestines of WT CBA/Ca and WT FVB/N mice both increased from day 6 to day 7, but there were no significant differences in the number of eosinophils between the 2 strains (data not shown). There were no other overt differences in the nature of the cellular inflammatory responses seen in the gut in the two strains.
Lung larval recovery, cellular response and migration to the small intestine
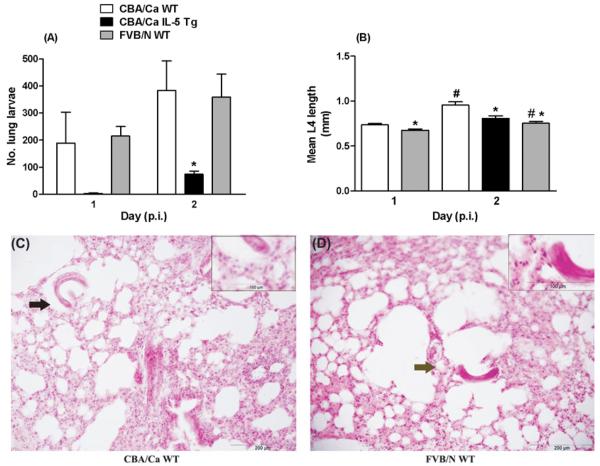
Under normal conditions, N. brasiliensis larvae migrate from the skin to the lungs and then to the small intestine. Since larval numbers and maturation were impaired at the intestinal phase of the infection in WT FVB/N mice, we next quantified the number and the size of larvae in the lungs, and assessed the accompanying cellular response. Lungs were removed from mice 1 and 2 days after injection of 750 L3 and lung-stage larvae were enumerated. We have previously found that in naïve IL-5 Tg CBA/Ca mice, the majority of larvae are trapped in the skin and pre-lung phase (Daly et al. 1999). WT CBA/Ca and WT FVB/N mice had higher lung larval burdens than the IL-5 Tg CBA/Ca mice, with values reaching statistical significance on day 2 p.i. (Fig. 3A). Interestingly, the numbers of lung larvae found in WT CBA/Ca and FVB/N mice were comparable on each of days 1 and 2 (Fig. 3A). However, on day 2 p.i., larvae recovered from WT FVB/N animals were less motile than larvae recovered from the lungs of WT CBA/Ca mice (mean±s.e.m.: 0·57±0·23 and 2·11± 0·47 movements/30 sec respectively, n=30 larvae/group). The lengths of L4 were calculated to determine if impaired larval development or damage in the pre-lung or lung phases of infection in WT FVB/N mice might restrict migration to the intestines. On days 1 and 2 p.i., the mean lengths of lung larvae recovered from WT FVB/N mice were significantly less than for larvae recovered from WT CBA/Ca mice on the same day (Fig. 3B) and on day 2, comparable to those few recovered from IL-5 Tg CBA/Ca mice. Larvae from WT FVB/N mice increased in size from day 1 to day 2 p.i. but growth was more substantial in parasites recovered from WT CBA/Ca mice (11% vs 29% increase in mean length respectively). This suggests that larvae migrating through WT FVB/N hosts may be damaged or suffer from impaired development prior to or upon arrival in the lungs. Histological analysis of parasite-infected lungs revealed no major inflammatory response in either strain of mice (Fig. 3C and D). Leukocytes were not present in high numbers in the tissue or lumen of the lungs and were not attached to and were rarely seen in close proximity to larvae. This is typical of the early lung inflammatory response in other strains of mice (Knott et al. 2007).
Fig. 3.
Number (A) and length (B) of Nippostrongylus brasiliensis larvae recovered from the lungs of WT CBA/Ca, IL-5 Tg CBA/Ca and WT FVB/N mice 1 and 2 days p.i. and lung histology for WT CBA/Ca (C) and WT FVB/N mice (D) 2 days p.i. 750 L3/mouse. (A) Mean number of lung larvae recovered+s.e.m., n=3–4 mice/group. There was a significant difference between strains on day 2 p.i. (KW test, H=7·053, P=0·0294). * Values for larvae from IL-5 Tg CBA/Ca mice were significantly less than those from WT CBA/Ca and FVB/N hosts on day 2 p.i. (MW post-hoc test, P=0·0286 and 0·0143 respectively). (B) Mean length (mm) of lung larvae+s.e.m. from 3 mice/group for 10–19 larvae/mouse and n=34–45 larvae/group/day. Insufficient larvae recovered from IL-5 Tg mice to provide reliable data on day 1. Day 1: * significantly different from WT CBA/Ca values (MW test, P=0·0067). Day 2: significant difference between strains (KW test H=23·97, P<0·0001), * significantly different from WT CBA/Ca values (MW post-hoc test, P=0·0013 for WT CBA/Ca vs IL-5 Tg CBA/Ca; P<0·0001 for WT CBA/Ca vs WT FVB/N). (C and D) Lung sections stained with H&E, 150× magnification, inset 260× magnification and scale bars represent 200 μm and 100 μm respectively. *, lung larvae. ➡, area shown in inset.
Since larvae or worms were not detected in the intestines of WT FVB/N mice in large numbers, the movement of parasites from the lungs to the intestine was assessed to determine if larvae were completing this phase of the migration. We enumerated larval or worm numbers in the lungs, trachea, oesophagus, stomach, small intestine and colon over days 2–6 p.i. (Table 1). As expected, larval numbers in the lungs were comparable between WT CBA/Ca and WT FVB/N mice on day 2 p.i. and numbers declined rapidly thereafter. Larvae were not found in high numbers when sampling the trachea, oesophagus or stomach in either strain (mean <10 larvae/site), but the detection of larvae from days 2–3 p.i. in most of these tissues suggests that larvae were migrating via this route from the lungs to the small intestine. On almost all days sampled, 2–3 times more larvae or worms were found in the small intestines of WT CBA/Ca mice than in WT FVB/N animals. The lower numbers of worms in WT FVB/N animals suggests a failure to colonize this compartment. No worms were seen in the colon in either strain on days 2–4, and only a small number (mean <10 worms/mouse) were found in the colon in WT CBA/Ca mice on days 5 and 6, suggesting that N. brasiliensis larvae and worms were rapidly lost from the entire length of the small intestine in WT FVB/N mice.
Table 1.
Nippostmngylus brasiliensis recovery from infected tissues of CBA/Ca WT and FVB/N WT mice (Larvae and worms were enumerated after s.c. infection with 750 N. brasiliensis L3. Mean±s.e.m. for 3 mice/group, ND=not done.)
| Day 2 (p.i.) | Day 3 (p.i.) | Day 4 (p.i.) | Day 5 (p.i.) | Day 6 (p.i.) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | CBA/Ca WT | FVB/N WT | CBA/Ca WT | FVB/N WT | CBA/Ca WT | FVB/N WT | CBA/Ca WT | FVB/N WT | CBA/Ca WT | FVB/N WT |
| Lungs | 135·3±14·6 | 185·0+58·2 | 12·3±5·8 | 46·3±13·7 | 2·3±0·3 | 16·0±4·0 | 0·7±0·3 | 5·3±4·3 | ND | ND |
| Trachea | 9·3±3·3 | 6·0±2·6 | 0·3±0·3 | 1·3±0·81 | 0 | 0 | ND | ND | ND | ND |
| Oesophagus | 0·3±0·3 | 0·3±0·3 | 0 | 0 | 0 | 0 | ND | ND | ND | ND |
| Stomach | 0 | 5·7±1·7 | 0·7±0·7 | 0·3±0·3 | 0 | 0 | ND | ND | ND | ND |
| Small intestine | 23·0+11·4 | 41·0+14·2 | 191·3±87·2 | 86·0±21·6 | 221·3±106·5 | 63·0+1·5 | 119·3±10·1 | 43·7±41·2 | 102·0±8·0 | 57·0±20·0 |
| Colon | 0 | 0 | 0 | 0 | 0 | 0 | 4·67±4·67 | 0 | 6·0+5·0 | 0 |
Larval retention and leukocyte recruitment in skin air pouches
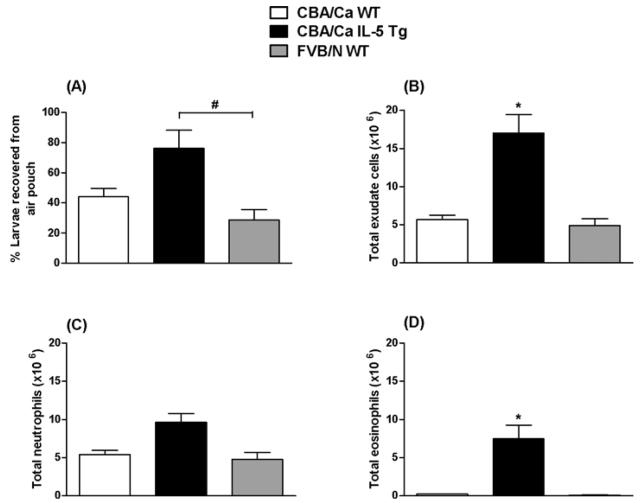
In IL-5 Tg mice, low intestinal worm loads and poor fecundity may, in large part, be attributed to innate immune responses in the skin that are initiated within an hour of infection (Daly et al. 1999; Giacomin et al. 2008). To determine if this also applied in WT FVB/N mice, the inflammatory response to larvae injected into dorsal skin air pouches was assessed in WT FVB/N, WT CBA/Ca mice and IL-5 Tg CBA/Ca animals 0 and 4 h after infection. As expected, the total number of cells recovered from each group at 0 h was minimal and, as is typically the case, approximately 100% of larvae were recovered (data not shown). Larval retention in IL-5 Tg CBA/Ca mice at 4 h p.i. (Fig. 4A) was significantly higher than in the WT FVB/N group. There was no significant difference in the number of larvae recovered from air pouches in WT CBA/Ca and WT FVB/N mice. The strength and the composition of the cellular immune response were comparable in the two WT groups and significantly more cells were recovered from infected IL-5 Tg CBA/Ca mice (Fig. 4B). By 4 h p.i. neutrophils (Fig. 4C) were the dominant leukocyte subset, though eosinophils (Fig. 4D) were also present in very large numbers in IL-5 Tg mice. Lymphocytes and macrophages were not numerous in any strain (data not shown). These data suggest that impaired development of larvae and worms that can be detected in the lungs and gut in WT FVB/N mice is not due to obvious quantitative or qualitative enhancement of the cellular inflammatory response induced in the skin soon after infection.
Fig. 4.
Larvae (A), total leukocytes (B), neutrophils (C) and eosinophils (D) recovered by lavage of air pouches in WT CBA/Ca, IL-5 Tg CBA/Ca and WT FVB/N mice 4 h after injection of 500 Nippostrongylus brasiliensis L3/mouse. Data in A represent larvae recovered as percentage of inoculum+s.e.m. Data in B, C and D are mean number of leukocytes (106)+s.e.m., n=4 mice for all groups. Significant effect between strains for A, B and D (KW test (A) H=6·615, P=0·0366, (B) H=7·538, P=0·0231, (D) H=9·846, P=0·0073). No significant effect between strains in C (KW test H=5·808, P=0·0548). * Significantly greater than WT CBA/Ca and FVB/N values (MW post-hoc test, P<0·05). # Significantly greater than WT FVB/N value (MW post-hoc test, P<0·05). Representative of 3 experiments.
CBA/Ca×FVB/N F1 offspring
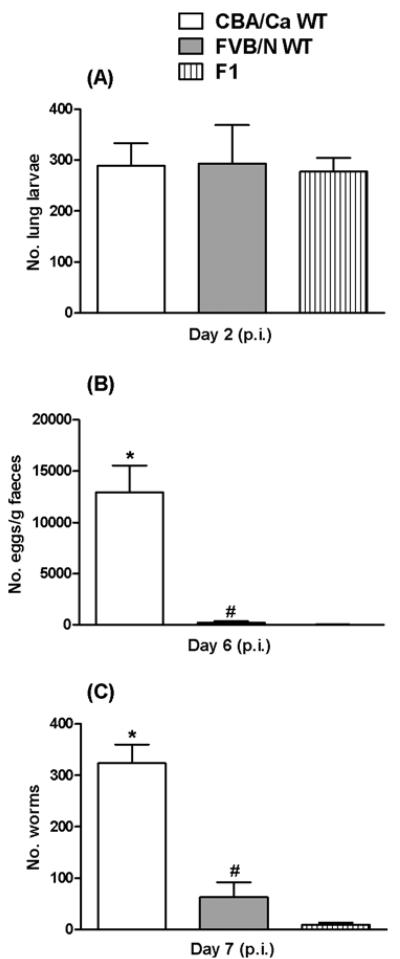
It would appear that in WT FVB/N hosts, larvae were either being damaged prior to the intestinal phase of infection, resulting in impaired development, migration or colonization, or that factors required for these processes were not sufficient. F1 hybrids of WT CBA/Ca mice and WT FVB/N mice were infected to determine if components required for larval development that were lacking in FVB/N mice could be restored by introducing the susceptible CBA/Ca genotype. F1 mice showed the same pattern of infection as the parental WT FVB/N strain (Fig. 5). Lung larval numbers in the F1 crosses were similar to both parental strains (Fig. 5A). Lung larvae recovered from both the parental WT FVB/N strain and the F1 mice were significantly smaller in length, and were less motile than those recovered from parental WT CBA/Ca mice (data not shown). Relative to infections in parental WT CBA/Ca mice, significantly fewer eggs (Fig. 5B) and worms (Fig. 5C) were found in both the parental FVB/N and F1 mice.
Fig. 5.
Recovery of Nippostrongylus brasiliensis larvae from the lungs (A) of WT CBA/Ca, WT FVB/N and WT FVB/N × CBA/Ca F1 hybrid mice 2 days p.i. Faecal eggs (B) and intestinal worms (C) for day 6 and 7 p.i. respectively. 750 L3/mouse. Data represent mean number of larvae, eggs/g faeces or worms recovered+s.e.m., n=4–6 mice/group. Significant effect between strains in B and C (KW test (B) H=12·60, P=0·0018 (C) H=12·38, P=0·0021). * Significantly greater than WT FVB/N and F1 mice (MW post-hoc test, P<0·05). # Significantly greater than F1 mice (MW post-hoc test, P<0·05). Representative of 2 experiments.
Primary versus secondary infections
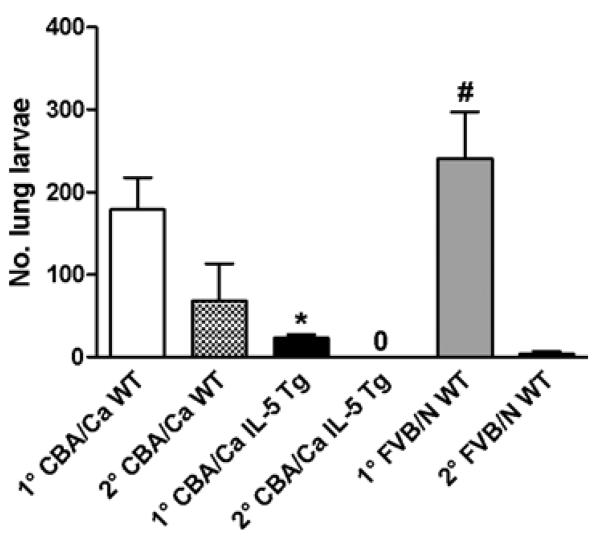
To determine if secondary immune responses to N. brasiliensis were enhanced in WT FVB/N mice compared with WT CBA/Ca mice, L3 were injected into animals 3 weeks after a primary infection and lung larval burdens were determined on day 2 of the challenge. The numbers of larvae recovered from the lungs after secondary infection of WT FVB/N mice were significantly lower than those recovered during primary infection (Fig. 6). The numbers of larvae recovered from the lungs of WT FVB/N and WT CBA/Ca mice during the secondary infection were not significantly different.
Fig. 6.
Recovery of Nippostrongylus brasiliensis lung larvae during primary (1°) and secondary (2°) infections of WT CBA/Ca, IL-5 Tg CBA/Ca and WT FVB/N mice on day 2 p.i. 750 L3/mouse for 1° and 2° infections. 2° challenges were delivered 3 weeks after the 1° infection. Mean number of larvae recovered/mouse+s.e.m., n=3–4 mice/group. 0=no lung larvae detected. The interaction between strain and infection (1° or 2°) was significant (2-way ANOVA, F2,16=3·98, P=0·0397), indicating that the difference in L4 numbers between the strains depended on infection (1° or 2°). * 1° IL-5 Tg CBA/Ca values significantly less than in 1° infections of WT CBA/Ca and FVB/N groups (Bonferroni post-hoc test, WT CBA/Ca vs IL-5 Tg CBA/Ca t=2·852, P<0·05; WT FVB/N vs IL-5 Tg CBA/Ca t=3·99, P<0·01). # 1° WT FVB/N values significantly greater than 2° infection of the same genotype (Bonferroni post-hoc test, t=4·688, P<0·001).
Resistance to N. brasiliensis in i-FABP-IL-5 and i-FABP-eotaxin Tg mice
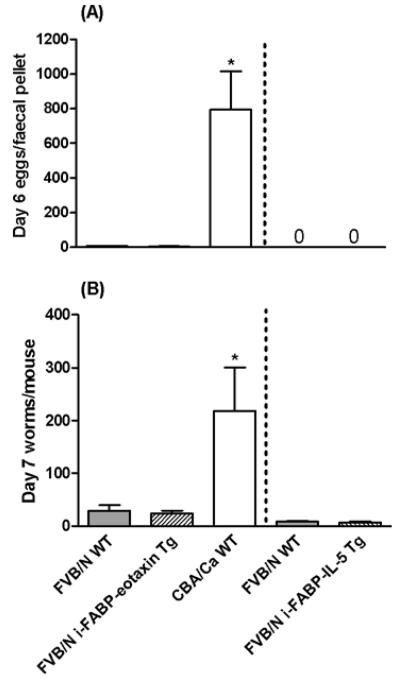
To investigate the role of eosinophils in the small intestines during infections with N. brasiliensis, FVB/N mice with intestine-specific expression of either IL-5 or eotaxin transgenes were used. Faecal egg (Fig. 7A) and intestinal worm (Fig. 7B) burdens were very low in primary infections of both of these Tg lines, but not significantly different to those recorded for WT FVB/N mice. Thus, expression of intestine-specific IL-5 or eotaxin transgenes, at least on this genetic background, did not provide additional resistance to N. brasiliensis.
Fig. 7.
Egg (A) and intestinal worm (B) numbers from WT FVB/N, i-FABP-Eotaxin Tg FVB/N, i-FABP-IL-5 Tg FVB/N and WT CBA/Ca mice. 1000 Nippostrongylus brasiliensis L3/mouse. Mean+s.e.m. for eggs enumerated on day 6 and worms recovered on day 7 p.i., n=4–8 mice/group. Separate experiments delineated by dashed line. 0=no eggs detected. Representative of 2–3 experiments for each of the transgenic lines. Significant effect between groups to the left of the dashed line (KW test (A) H=9·770, P=0·0076 (B) H=8·779, P=0·0124). * Significantly greater than other groups in the same experiment (MW post-hoc test, P<0·05).
H. bakeri infections in FVB/N mice
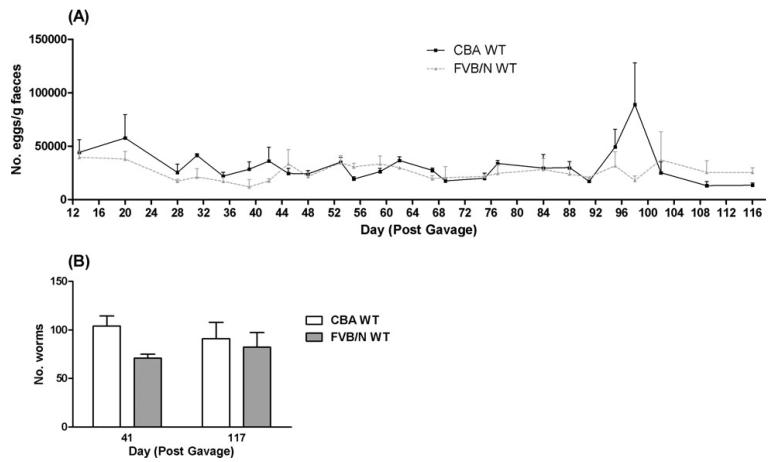
Since WT FVB/N mice were highly resistant to N. brasiliensis, these animals were assessed for resistance to primary infection with H. bakeri, another intestinal nematode. In WT CBA/Ca and WT FVB/N mice there were no significant differences in parasite egg production over days 13–116 p.i. (Fig. 8A) or worm numbers on days 41 and 117 p.i. (Fig. 8B). This suggests that the mechanisms that protect WT FVB/N mice against tissue-penetrating N. brasiliensis do not also provide effective resistance against a long-term parasitic nematode that is restricted to the gut.
Fig. 8.
Egg (A) and intestinal worm (B) numbers for WT CBA/Ca and WT FVB/N mice infected by oral gavage with 200 Heligmosomoides bakeri L3. Mean+s.e.m., n=3–7 mice/group.
DISCUSSION
When available in sufficient numbers, eosinophils are capable of providing potent resistance to primary infections with N. brasiliensis. IL-5 Tg mice with constitutive eosinophilia are highly resistant to this parasite and the majority of larvae are trapped in the skin, with eosinophils rapidly recruited in large numbers (Daly et al. 1999). Eosinophils are also necessary for protection in the early phase of secondary infections with N. brasiliensis. IL-5−/− and ΔDdblGATA (Yu et al. 2002) mice, both of which are deficient in eosinophils, have comparable lung larval burdens to those seen in primary infections (Knott et al. 2007). In the present study we have determined that WT FVB/N mice are also highly resistant to primary infections with N. brasiliensis, but not to H. bakeri. As with IL-5 Tg mice infected with N. brasiliensis, few worms were found in the small intestines of WT FVB/N mice and egg production was minimal. While resistance to N. brasiliensis in WT FVB/N mice may be initiated in the pre-lung stage of infection as it is in IL-5 Tg mice, inflammation and larval retention in the skin was similar to that in WT CBA/Ca animals and eosinophils were not a particularly prominent subset within the leukocytes recruited. Inhibition of development at this early stage of infection may adversely affect the capacity of larvae to develop and colonize the gut.
IL-5 is important for eosinophil growth and development (Lopez et al. 1986; Enokihara et al. 1988; Yamaguchi et al. 1988a, b; Dent et al. 1990). Eotaxin is a chemotactic factor for eosinophils (Jose et al. 1994) and under normal conditions is expressed throughout the length of the gastrointestinal tract (Rothenberg et al. 1995). It would seem therefore that these factors are likely to be important in intestinal homeostasis and may play a significant role in producing and recruiting eosinophils into the gut during helminth infections. This is seen during infections with N. brasiliensis, where eosinophil numbers increase in the gut from days 6–11 in both WT and IL-5 Tg mice (Dent et al. 1999). The mechanisms responsible for this tissue-specific recruitment and the roles that eosinophils play in host protection during the gut phase of infection are not well understood, although eosinophilia may assist worm expulsion (Knott et al. 2007). Our initial studies were therefore designed to investigate the importance of both IL-5 and eotaxin for protection against N. brasiliensis at the level of the small intestine. In recent years, studies of immune responses to parasites have been aided by technology that permits selective over-expression of genes in mice. Pro-nuclear injections are often performed in FVB/N mice because fertilized eggs from this strain have prominent nuclei and this facilitates injection of DNA constructs (Taketo et al. 1991). While investigating infections in i-FABP-IL-5 and i-FABP-eotaxin Tg FVB/N mice (Mishra et al. 2002), we established that WT FVB/N mice had potent natural resistance to N. brasiliensis and the expression of either transgene did not further enhance this resistance to the parasite.
FVB/N mice have most frequently been cited in cancer, behavioural and neurological studies and are known to be resistant to atherosclerosis and collagen-induced arthritis. The immunobiology of FVB/N mice has not been extensively reported, but some minor abnormalities in thymus development have been noted (Nabarra et al. 2001). FVB/N mice share the Hc0 allele with strains such as DBA/2, A/J, AKR and SWR and are deficient in the complement protein C5. Mice described as FVB or FVB/N have been compared to other laboratory mouse strains in several parasite studies. FVB mice eliminate H. bakeri faster than the C57BL/6 strain (Donskow-Schmelter et al. 2007), but FVB/N mice are more susceptible to Clonorchis sinensis than BALB/c, severe combined immunodeficiency (SCID) and athymic mice (Yoon et al. 2001). BALB/c, C57BL/6 and FVB mice are of comparable susceptibility to the fluke Echinostoma hortense and clear infections more rapidly than C3H/HeN and ICR mice (Lee et al. 2004).
Although N. brasiliensis did successfully migrate to the lungs in WT FVB/N mice, there was evidence of developmental retardation, since the larvae recovered were smaller and less motile than those in the susceptible WT CBA/Ca mice. This was apparent even in lung larvae recovered 24 h post-primary infection and so the damage may have been inflicted in the pre-lung stage. There was also evidence of impaired development in the few larvae that belatedly migrated to the lungs of IL-5 Tg mice on day 2 p.i. However, unlike infections in mice expressing the IL-5 transgene on CBA/Ca (Daly et al. 1999 and this study), BALB/c (Knott et al. 2007) and C57BL/6 (Giacomin et al. 2008) genetic backgrounds, larvae were not trapped in the skin in significant numbers and eosinophils were only a small part of the inflammatory infiltrate recruited into the skin. Should larvae be trapped in the lungs of WT FVB/N mice, a strong cellular inflammatory response might be expected. However, as in other mouse strains (Daly et al. 1999; Reece et al. 2006; Knott et al. 2007), virtually no cellular infiltrate was seen in the lungs or around larvae during the period in which the majority of parasites were present (1–2 days p.i.), suggesting that if larvae are being damaged at this site, cellular inflammatory mechanisms are not likely to be responsible. It is also important to note that relatively few larvae were actually trapped in the lungs of any of the mice used. While the majority of larvae have left the site of injection by 2–4 h p.i., few appear in the lungs before 16 h p.i. (Daly et al. 1999) and during the intervening period, the parasite is essentially invisible. It has long been assumed that during this time larvae are migrating via the lymphatics and/or blood vessels. However, given the likely significance of a pre-lung stage for innate resistance, it will be important to identify more accurately where the larvae are located during this period. In this way cellular and/or soluble resistance mechanisms are more likely to be identified.
Our investigations of the gut phase of the infection revealed that the few worms that were present in the small intestines of WT FVB/N mice were much paler in colour and significantly smaller in size than those recovered from WT CBA/Ca mice. It is feasible that damage acquired by N. brasiliensis larvae during migration from the skin, may be largely responsible for the ultimate failure of the parasite to feed and mature and to colonize the gut. Resistance mechanisms initiated in the pre-gut phase of infection may also be replicated and expanded upon in the gut. However, FVB/N mice are as susceptible as CBA/Ca mice to the nematode H. bakeri, which resides primarily in the gut. H. bakeri either suppresses or is resistant to the factors that protect FVB/N mice against N. brasiliensis or it is not exposed to them because it does not have a tissue migratory phase. Since H. bakeri is known to be immunosuppressive (Pritchard et al. 1984; Pritchard and Behnke, 1985; Crawford et al. 1989), it would be useful to explore the resistance of FVB/N mice to other intestinal parasites such as Trichinella spiralis and Trichuris muris, to determine if these parasites can be damaged in the gut in this host strain.
Eosinophils play a protective role in the gut in infections with Strongyloides venezuelensis (El-Malky et al. 2003) and N. brasiliensis (Knott et al. 2007), but as in the skin, these and other leukocytes were found in similar numbers in the intestines of infected WT FVB/N and WT CBA/Ca mice. We have yet to do an extensive analysis of leukocyte subsets in either the skin or gut in FVB/N mice during infection, but any differences to those in WT CBA/Ca mice were not overt. Although over-expression of IL-5 and eotaxin in the gut did not confer additional protection against N. brasiliensis on the naturally resistant FVB/N genetic background, it will be important to test the effect of these transgenes again on a more permissive strain. This will help to address the contribution that eosinophils can make to elimination of N. brasiliensis and other intestinal helminths from the gut.
The major histocompatibility complex (MHC) appears to be implicated in differential patterns of resistance to a number of parasitic helminths of sheep, cattle and mice (Else and Wakelin, 1989; Stear et al. 1990; Wassom and Kelly, 1990; Behnke and Wahid, 1991; Outteridge et al. 1996; Su and Dobson, 1997). However, as the resistance seen in WT FVB/N mice occurs within the first 2–3 days of primary infection, it is most likely due to innate rather than adaptive immune mechanisms and would therefore be less dependent on allelic differences in the MHC.
Many factors could contribute to resistance to tissue-invasive intestinal helminthic parasites. Although FVB/N mice are deficient in C5 and we have found complement and the C5a receptor to be important in WT and IL-5 Tg C57BL/6 mice in the first 2–3 h of infection with N. brasiliensis, complement deficiency does not ultimately cause major changes in lung or intestinal parasite burdens (Giacomin et al. 2008). Elements that influence expulsion of N. brasiliensis include STAT6, the IL-4 Receptor (R)a and IL-13 (Urban et al. 1998, 2001; Zhao et al. 2003). Similarly, the IL-4Rα and STAT6 are required to expel adult T. spiralis worms (Urban et al. 2000).
Amphiregulin, a member of the epidermal growth factor family that is expressed by activated Th2 cells, is required for rapid expulsion of T. muris (Zaiss et al. 2006). Resistin-like molecules (RELM) are upregulated in inflammatory cells, including macrophages (Loke et al. 2002), and in the lungs and gut during T. muris, N. brasiliensis and S. stercoralis infections (Artis et al. 2004; Voehringer et al. 2004; Wang et al. 2005). RELM may contribute to both the pre-intestinal and intestinal phases of resistance to N. brasiliensis in WT FVB/N mice, since RELM-β binds to T. muris and may aid in expulsion of the parasite from the gut (Artis et al. 2004). Both RELM-α and -β may affect gastrointestinal function through actions on nerve and goblet cells (Artis et al. 2004; Wang et al. 2005; Nair et al. 2006). 129/SvJ mice are naturally resistant to S. venezuelensis and it is thought that higher concentrations of chondroitin sulphate in the intestine of infected mice may be responsible for this (Nakamura-Uchiyama et al. 2001). Relative to C57BL/6J mice, the CBA/Ca strain is highly resistant to primary infections with Ascaris suum and it is proposed that the parasites are most susceptible to damage during migration from the liver to the lungs (Lewis et al. 2006, 2007). The mechanisms responsible for this resistance in CBA/Ca mice have not yet been determined, but since the effect seems to be on migration within the first few days of infection, innate factors are likely to make major contributions.
These examples clearly demonstrate that the ways in which different parasites are eliminated from the host vary and are not necessarily directly associated with immunological mechanisms. Factors or pathways responsible for the resistance of WT FVB/N mice to N. brasiliensis may not be immunological in nature or at least not dependent on a cellular inflammatory response. Products of intestinal epithelial cells or other soluble factors found in the lungs and/or gut may play a crucial role. It is also possible that FVB/N mice lack essential nutrient/s required by N. brasiliensis larvae for development and colonization, but this seems less likely, given our observation that CBA/Ca×FVB/N F1 mice are also resistant. Our data lead us to propose that resistance factors may be differentially expressed in the pre-lung phase of infection in WT FVB/N mice.
Although WT FVB/N mice are highly resistant to primary infections with N. brasiliensis, in our hands this strain proved to be quite susceptible to H. bakeri and this may be due to differences in the nature and duration of the life cycles of these parasites. In most mouse strains, H. bakeri resides in the gut for months, rather than just the few days seen in primary infections with N. brasiliensis. Variation in susceptibility to H. bakeri in different strains of mice has been well documented and candidate factors responsible for resistance have been identified. Mouse strains that effectively resist H. bakeri in a repetitive infection protocol include SWR, NIH and SJL. In contrast, BALB/c, CBA, A/J, C3H and C57BL/6 strains are susceptible to the parasite under the same regime and carry heavy worm burdens for months (Behnke et al. 2006b). Mice described as FVB were reported to eliminate H. bakeri by day 74 p.i. (Donskow-Schmelter et al. 2007), an outcome that is not consistent with our study, which was performed in WT C57BL/6, BALB/c mice (data not presented) as well as the CBA/Ca and FVB/N strains. We challenged our mice with approximately 70% more larvae than Donskow-Schmelter and colleagues and our FVB/N mice may prove to be a different substrain from the FVB animals used by this group (Cywinska et al. 2004; Donskow-Schmelter et al. 2007).
Resistance, particularly in SWR mice, is associated with increases in serum tumour necrosis factor-α, intestinal goblet cells, mucosal mast cells and mucosal mast cell protease-1 levels (Behnke et al. 2003b). The killing of H. bakeri adult worms is dependent on the production of oxygen radicals such as hydrogen peroxide and acetaldehyde/xanthine oxidase (Ben-Smith et al. 2002). Furthermore, both Th1 and Th2 responses are strong in the resistant SWR and SJL strains during primary H. bakeri infections (Ben-Smith et al. 2003). Quantitative trait linkage mapping techniques have been used to extend these studies, with the identification of candidate resistance genes involved in immune regulation on chromosomes 1 and 17 (Behnke et al. 2003a; Iraqi et al. 2003; Menge et al. 2003; Behnke et al. 2006a). A similar approach may prove useful in identifying the resistance factors expressed by WT FVB/N mice infected with N. brasiliensis.
In summary, evidence of resistance to primary infections with N. brasiliensis in WT FVB/N mice is first manifested as a deleterious impact on the development and motility of lung larvae. WT FVB/N mice provide an excellent opportunity to explore potent and potentially novel mechanisms of early innate resistance to N. brasiliensis and possibly, to other tissue-invasive helminths. Further studies should include close scrutiny of the pre-lung phase of infection in this host.
Acknowledgments
This work was funded by NHMRC Program (S.H., K.M.) and Project (L.D.) grants and the University of Adelaide. We thank Dr Marc Rothenberg (Cincinnati Children's Hospital Medical Center, Cincinnati, USA) for providing founding breeding stock of the i-FABP-IL-5 and -eotaxin Tg mice and Drs Mariela Segura, Zhong Su and Mary Stevenson (McGill University, Montreal, Canada) for providing the initial source of H. bakeri. We also thank Dr Jack Da Silva (Bioinformatics and Computational Genetics, University of Adelaide) and Associate Professor Sarah Robertson (Obstetrics and Gynaecology, University of Adelaide) for their advice and guidance with statistics.
REFERENCES
- Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences, USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitology Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Iraqi F, Menge D, Baker RL, Gibson J, Wakelin D. Chasing the genes that control resistance to gastrointestinal nematodes. Journal of Helminthology. 2003a;77:99–110. doi: 10.1079/JOH2003174. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Iraqi FA, Mugambi JM, Clifford S, Nagda S, Wakelin D, Kemp SJ, Baker RL, Gibson JP. High resolution mapping of chromosomal regions controlling resistance to gastrointestinal nematode infections in an advanced intercross line of mice. Mammalian Genome. 2006a;17:584–597. doi: 10.1007/s00335-005-0174-0. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Lowe A, Clifford S, Wakelin D. Cellular and serological responses in resistant and susceptible mice exposed to repeated infection with Heligmosomoides polygyrus bakeri. Parasite Immunology. 2003b;25:333–340. doi: 10.1046/j.1365-3024.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Mugambi JM, Clifford S, Iraqi FA, Baker RL, Gibson JP, Wakelin D. Genetic variation in resistance to repeated infections with Heligmosomoides polygyrus bakeri, in inbred mouse strains selected for the mouse genome project. Parasite Immunology. 2006b;28:85–94. doi: 10.1111/j.1365-3024.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Wahid FN. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): H-2 linked genes determine worm survival. Parasitology. 1991;103:157–164. doi: 10.1017/s0031182000059400. [DOI] [PubMed] [Google Scholar]
- Ben-Smith A, Lammas DA, Behnke JM. Effect of oxygen radicals and differential expression of catalase and superoxide dismutase in adult Heligmosomoides polygyrus during primary infections in mice with differing response phenotypes. Parasite Immunology. 2002;24:119–129. doi: 10.1046/j.1365-3024.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- Ben-Smith A, Lammas DA, Behnke JM. The relative involvement of Th1 and Th2 associated immune responses in the expulsion of a primary infection of Heligmosomoides polygyrus in mice of differing response phenotype. Journal of Helminthology. 2003;77:133–146. doi: 10.1079/JOH2003173. [DOI] [PubMed] [Google Scholar]
- Cable J, Harris PD, Lewis JW, Behnke JM. Molecular evidence that Heligmosomoides polygyrus from laboratory mice and wood mice are separate species. Parasitology. 2006;133:111–122. doi: 10.1017/S0031182006000047. [DOI] [PubMed] [Google Scholar]
- Crawford C, Behnke JM, Pritchard DI. Suppression of heterologous immunity by Nematospiroides dubius antigens in vitro. International Journal for Parasitology. 1989;19:29–34. doi: 10.1016/0020-7519(89)90018-0. [DOI] [PubMed] [Google Scholar]
- Cywinska A, Czuminska K, Schollenberger A. Granulomatous inflammation during Heligmosomoides polygyrus primary infections in FVB mice. Journal of Helminthology. 2004;78:17–24. doi: 10.1079/joh2003205. [DOI] [PubMed] [Google Scholar]
- Daly CM. Immune responses to Nippostrongylus brasiliensis in interleukin-5 transgenic mice. The University of Adelaide; Australia: 1999. Ph.D. thesis. [Google Scholar]
- Daly CM, Mayrhofer G, Dent LA. Trapping and immobilization of Nippostrongylus brasiliensis larvae at the site of inoculation in primary infections of interleukin-5 transgenic mice. Infection and Immunity. 1999;67:5315–5323. doi: 10.1128/iai.67.10.5315-5323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent LA, Daly C, Geddes A, Cormie J, Finlay DA, Bignold L, Hagan P, Parkhouse RM, Garate T, Parsons J, Mayrhofer G. Immune responses of IL-5 transgenic mice to parasites and aeroallergens. Memórias do Instituto Oswaldo Cruz. 1997b;92(Suppl 2):45–54. doi: 10.1590/s0074-02761997000800008. [DOI] [PubMed] [Google Scholar]
- Dent LA, Daly CM, Mayrhofer G, Zimmerman T, Hallett A, Bignold LP, Creaney J, Parsons JC. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infection and Immunity. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent LA, Munro GH, Piper KP, Sanderson CJ, Finlay DA, Dempster RK, Bignold LP, Harkin DG, Hagan P. Eosinophilic interleukin 5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni. Parasite Immunology. 1997a;19:291–300. doi: 10.1046/j.1365-3024.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. The Journal of Experimental Medicine. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskow-Schmelter K, Doligalska M, Rzepecka J, Jedlina-Panasiuk L. Heligmosomoides polygyrus: decreased apoptosis in fast responder FVB mice during infection. Experimental Parasitology. 2007;117:149–156. doi: 10.1016/j.exppara.2007.04.001. [DOI] [PubMed] [Google Scholar]
- El-Malky M, Maruyama H, Hirabayashi Y, Shimada S, Yoshida A, Amano T, Tominaga A, Takatsu K, Ohta N. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode, Strongyloides venezuelensis in mice. Parasitology International. 2003;52:71–79. doi: 10.1016/s1383-5769(02)00086-7. [DOI] [PubMed] [Google Scholar]
- Else K, Wakelin D. Genetic variation in the humoral immune responses of mice to the nematode Trichuris muris. Parasite Immunology. 1989;11:77–90. doi: 10.1111/j.1365-3024.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Enokihara H, Nagashima S, Noma T, Kajitani H, Hamaguchi H, Saito K, Furusawa S, Shishido H, Honjo T. Effect of human recombinant interleukin 5 and G-CSF on eosinophil colony formation. Immunology Letters. 1988;18:73–76. doi: 10.1016/0165-2478(88)90073-9. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of Experimental Medicine. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- Giacomin PR, Gordon DL, Botto M, Daha MR, Sanderson SD, Taylor SM, Dent LA. The role of complement in innate, adaptive and eosinophil-dependent immunity to the nematode Nippostrongylus brasiliensis. Molecular Immunology. 2008;45:446–455. doi: 10.1016/j.molimm.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Giacomin PR, Wang H, Gordon DL, Botto M, Dent LA. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infection and Immunity. 2005;73:7442–7449. doi: 10.1128/IAI.73.11.7442-7449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. The Journal of Immunology. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- Iraqi FA, Behnke JM, Menge DM, Lowe AM, Teale AJ, Gibson JP, Baker LR, Wakelin DR. Chromosomal regions controlling resistance to gastro-intestinal nematode infections in mice. Mammalian Genome. 2003;14:184–191. doi: 10.1007/s00335-002-3049-7. [DOI] [PubMed] [Google Scholar]
- Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. The Journal of Experimental Medicine. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AB, Moqbel R, Durham SR, MacDonald AJ, Walsh GM, Shaw RJ, Cromwell O, Mackay J. Leucocyte activation initiated by IgE-dependent mechanisms in relation to helminthic parasitic disease and clinical models of asthma. International Archives of Allergy and Applied Immunology. 1985;77:69–72. doi: 10.1159/000233755. [DOI] [PubMed] [Google Scholar]
- Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. International Journal for Parasitology. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clinical and Experimental Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Park SK, Im JA, Kim SK, Kim GH, Kim GY, Yang EJ, Ryang YS. Susceptibility of several strains of mice to Echinostoma hortense infection. The Korean Journal of Parasitology. 2004;42:51–56. doi: 10.3347/kjp.2004.42.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R, Behnke JM, Cassidy JP, Stafford P, Murray N, Holland CV. The migration of Ascaris suum larvae, and the associated pulmonary inflammatory response in susceptible C57BL/6j and resistant CBA/Ca mice. Parasitology. 2007;134:1301–1314. doi: 10.1017/S0031182007002582. [DOI] [PubMed] [Google Scholar]
- Lewis R, Behnke JM, Stafford P, Holland CV. The development of a mouse model to explore resistance and susceptibility to early Ascaris suum infection. Parasitology. 2006;132:289–300. doi: 10.1017/S0031182005008978. [DOI] [PubMed] [Google Scholar]
- Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunology. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. The Journal of Experimental Medicine. 1986;163:1085–1099. doi: 10.1084/jem.163.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei KI, Foster P, Young IG. The role of interleukin-5 (IL-5) in vivo: studies with IL-5 deficient mice. Memórias do Instituto Oswaldo Cruz. 1997;92(Suppl 2):63–68. doi: 10.1590/s0074-02761997000800010. [DOI] [PubMed] [Google Scholar]
- McCormick ML, Metwali A, Railsback MA, Weinstock JV, Britigan BE. Eosinophils from schistosome-induced hepatic granulomas produce superoxide and hydroxyl radical. The Journal of Immunology. 1996;157:5009–5015. [PubMed] [Google Scholar]
- Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitology Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- Menge DM, Behnke JM, Lowe A, Gibson JP, Iraqi FA, Baker RL, Wakelin D. Mapping of chromosomal regions influencing immunological responses to gastrointestinal nematode infections in mice. Parasite Immunology. 2003;25:341–349. doi: 10.1046/j.1365-3024.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. The Journal of Biological Chemistry. 2002;277:4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- Nabarra B, Mulotte M, Casanova M, Godard C, London J. Ultrastructural study of the FVB/N mouse thymus: presence of an immature epithelial cell in the medulla and premature involution. Developmental and Comparative Immunology. 2001;25:231–243. doi: 10.1016/s0145-305x(00)00054-9. [DOI] [PubMed] [Google Scholar]
- Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. The Journal of Immunology. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Uchiyama F, Nagao T, Obara A, Ishiwata K, Nawa Y. Natural resistance of 129/SvJ mice to Strongyloides venezuelensis infection. Parasite Immunology. 2001;23:659–663. doi: 10.1046/j.1365-3024.2001.00425.x. [DOI] [PubMed] [Google Scholar]
- Outteridge PM, Andersson L, Douch PG, Green RS, Gwakisa PS, Hohenhaus MA, Mikko S. The PCR typing of MHC-DRB genes in the sheep using primers for an intronic microsatellite: application to nematode parasite resistance. Immunology and Cell Biology. 1996;74:330–336. doi: 10.1038/icb.1996.59. [DOI] [PubMed] [Google Scholar]
- Ovington KS, McKie K, Matthaei KI, Young IG, Behm CA. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology. 1998;95:488–493. doi: 10.1046/j.1365-2567.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DI, Ali NM, Behnke JM. Analysis of the mechanism of immunodepression following heterologous antigenic stimulation during concurrent infection with Nematospiroides dubius. Immunology. 1984;51:633–642. [PMC free article] [PubMed] [Google Scholar]
- Pritchard DI, Behnke JM. The suppression of homologous immunity by soluble adult antigens of Nematospiroides dubius. Journal of Helminthology. 1985;59:251–256. doi: 10.1017/s0022149x0000804x. [DOI] [PubMed] [Google Scholar]
- Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infection and Immunity. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Luster AD, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proceedings of the National Academy of Sciences, USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunology. 1993;15:349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Sheridan JW, Finlay-Jones JJ. Studies on a fractionated murine fibrosarcoma: a reproducible method for the cautious and a caution for the unwary. Journal of Cellular Physiology. 1977;90:535–552. doi: 10.1002/jcp.1040900316. [DOI] [PubMed] [Google Scholar]
- Stear MJ, Hetzel DJ, Brown SC, Gershwin LJ, Mackinnon MJ, Nicholas FW. The relationships among ecto- and endoparasite levels, class I antigens of the bovine major histocompatibility system, immunoglobulin E levels and weight gain. Veterinary Parasitology. 1990;34:303–321. doi: 10.1016/0304-4017(90)90077-o. [DOI] [PubMed] [Google Scholar]
- Su Z, Dobson C. H-2 genes and resistance to infection with Heligmosomoides polygyrus in selectively bred mice. International Journal for Parasitology. 1997;27:595–600. doi: 10.1016/s0020-7519(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Takamoto M, Ovington KS, Behm CA, Sugane K, Young IG, Matthaei KI. Eosinophilia, parasite burden and lung damage in Toxocara canis infection in C57Bl/6 mice genetically deficient in IL-5. Immunology. 1997;90:511–517. doi: 10.1046/j.1365-2567.1997.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proceedings of the National Academy of Sciences, USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JF, Jr., Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr., Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. The Journal of Immunology. 2001;167:6078–6081. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr., Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. The Journal of Immunology. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Wang ML, Shin ME, Knight PA, Artis D, Silberg DG, Suh E, Wu GD. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2005;288:G1074–G1083. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- Wassom DL, Kelly EA. The role of the major histocompatibility complex in resistance to parasite infections. Critical Reviews in Immunology. 1990;10:31–52. [PubMed] [Google Scholar]
- Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. The Journal of Experimental Medicine. 1988b;167:1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. The Journal of Experimental Medicine. 1988a;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BI, Choi YK, Kim DY, Hyun BH, Joo KH, Rim HJ, Lee JH. Infectivity and pathological changes in murine clonorchiasis: comparison in immunocompetent and immunodeficient mice. The Journal of Veterinary Medical Science. 2001;63:421–425. doi: 10.1292/jvms.63.421. [DOI] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. The Journal of Experimental Medicine. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- Zhao A, McDermott J, Urban JF, Jr., Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. The Journal of Immunology. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]