Abstract
Background:
Resistin-like molecule (Relm) α is a secreted protein and a hallmark signature gene for alternatively activated macrophages. Relm-α is highly induced by allergic inflammatory triggers and perceived to promote tissue repair. Yet the function of Relm-α remains unknown.
Objective:
We sough to determine the role of Relm-α in dextran sodium sulfate (DSS)–induced colonic injury.
Methods:
The cellular source of Relm-α was determined after oral DSS-induced colitis. Retnla−/− mice were generated, subjected to DSS treatment, and monitored for disease progression (clinical and histopathologic features). Cytokine production in the supernatants of ex vivo colon cultures, and of LPS-stimulated macrophages incubated with Relm-α was assessed. Relm-α was administered intraperitoneally, and the cellular recruitment to the peritoneum was assessed.
Results:
After innate intestinal stimulation with DSS, Relm-α was highly expressed by eosinophils and epithelial cells. Retnla gene–targeted mice were protected from DSS-induced colitis (eg, decreased diarrhea, rectal bleeding, colon shortening, disease score, and histopathologic changes). Relm-α coactivated IL-6 and TNF-α release and inhibited IL-10 release from LPS-activated bone marrow–derived macrophages. Consistent with these finding, colon cultures of DSS-treated Retnla−/− mice produced decreased IL-6 and increased IL-10 ex vivo. Furthermore, Retnla−/− mice had substantially decreased c-Jun N-terminal kinase phosphorylation in vivo. In vivo administration of Relm-α initiated cellular recruitment to the peritoneum, and Relm-α was able to induce eosinophil chemotaxis in vitro.
Conclusions:
These findings demonstrate a central proinflammatory role for Relm-α in colonic innate immune responses, identifying a novel pathway for regulation of macrophage activation.
Keywords: Resistin-like molecule α, colitis, macrophages, eosinophils, LPS, inflammation, inflammatory bowel disease
Immune-related diseases, such as inflammatory bowel disease (IBD), diabetes, and obesity, have become some of the fastest growing and most persistent public health problems in the Western world and are currently on the rise.1 These diseases have a shared component of inflammation involved in disease pathogenesis and complications.1 Substantial evidence suggests that macrophages have a key role in the pathogenesis of inflammatory diseases, such as IBD.2 After diverse stimuli, macrophages are capable of releasing a wide plethora of mediators, including superoxide anions, reactive nitrogen intermediates (eg, nitric oxide and peroxynitrite), bioactive lipids, and cytokines, that might lead to cell death and tissue injury.2 For example, LPS-stimulated macrophages produce TNF-α and IL-6,3 and increased levels of these cytokines and activated macrophages correlate with IBD severity.4
Resistin-like molecule (Relm) α belongs to a newly defined family of secreted proteins (Resistin, Relm-α, Relm-β, and Relm-γ) that contain highly conserved C-terminal cysteine residues, which support the assembly of disulfide-dependent multimeric units.5 Interestingly, and despite opposing physiologic effects on insulin resistance, the multimeric assembly of the resistin family is similar to that of adiponectin and suggests a functional role for Relm in the regulation of glucose.5 Relm-α, formerly known as “found in inflammatory zone 1,” is a hallmark signature gene of murine alternatively activated macrophages and is thought to be involved in processes such as tissue repair.6 Functional studies demonstrate that Relm-α stimulates α-smooth muscle actin and collagen I production by fibroblasts.7 Furthermore, other hallmark alternatively activated macrophage products, such as arginase-1, have been shown to contribute to tissue repair.8 Collectively, these data suggest an anti-inflammatory role for Relm-α. Nevertheless, the function of Relm-α in vivo and especially in intestinal inflammation is yet to be defined. Importantly, Relm-α and Relm-β are highly induced by allergic inflammatory triggers (eg, TH2 cytokines, such as IL-13) yet have differential expression patterns and pleiotropic functions. The expression of Relm-β at baseline is restricted to the colonic epithelium and regulated by commensal bacteria through Caudal type homeobox transcription factor 2 (Cdx2), whereas Relm-α is mainly expressed by hematopoietic cells and adipose tissue and is more similar to the expression pattern of resistin.9
In this study we aimed to determine the cellular source and role of Relm-α in vivo. Herein, we demonstrate that after innate intestinal stimulation with dextran sodium sulfate (DSS), Relm-α is highly expressed by eosinophils and colonic epithelial cells. Furthermore, Relm-α deficiency protects against experimental colitis, and recombinant Relm-α had potent proinflammatory effects on macrophages, identifying a key role for Relm-α in colonic inflammation.
METHODS
Mice
Male and female, 8- to 12-week-old Retnla−/− mice (backcrossed to c57BL/6 or BALB/c background for at least 7 and 10 generations, respectively) were generated by using Velocigene technology (Regeneron Pharmaceuticals, Inc., Tarrytown, NY).10 Mice were genotyped by means of PCR analysis with the following primers: Relm-α forward, GTCAGCAATCCCATGGCGTA; Relm-α reverse, ACTTCCCTACCCACCCATTCC; and Lac-Z, GTCTGTCCTAGCTTCCTCACTG. This yielded an 800-bp band for wild-type mice and a 400-bp band for gene–targeted mice. Wild-type mice (4-5 weeks old) were obtained from Taconic Laboratories (Hudson, NY) and environmentally matched with the Retnla−/− mice for 2 to 3 weeks. All mice were housed under specific pathogen-free conditions and treated according to institutional guidelines.
DSS-induced colonic injury and histopathologic examination
DSS (average molecular weight of 41 kd; ICN Biomedical, Inc, Costa, Mesa, Calif) was administered in the drinking water as a 2.5% (wt/vol) solution for up to 10 days, and disease monitoring and histopathologic changes in the colon were scored as previously described.10
Cell activation
Bone marrow–derived macrophages (2 × 105 cells) were incubated for 24 hours with recombinant Relm-α (kind gift of Peprotech, Rocky Hill, NJ) or LPS (Sigma-Aldrich, St Louis, Mo). Thereafter, the supernatant was collected and stored at −70°C until assessed for cytokines.
Immunofluorescence
Fixed frozen sections were treated with 100% acetone and blocked with 3% goat serum in PBS. Slides were incubated with isotype controls (Rat IgG1 and Rabbit IgG; Vector, Burlingame, Calif), anti–Mac-3 (BD PharMingen, San Jose, Calif), anti-major basic protein (anti-MBP; kindly provided by James Lee, Mayo Clinic, Rochester, Minn), anti–phospho c-Jun N-terminal kinase (anti-pJNK; Cell Signaling, Danvers, Mass), and anti–Relm-α and anti–Relm-β (kind gift of Peprotech, overnight at 4°C), followed by goat anti-rabbit Alexa 488 and donkey anti-rat Alexa 494 (Invitrogen, Carlsbad, Calif) and counterstaining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)/Supermount G solution (Fluoromount-G; Southern Biotech, Birmingham, Ala). Images were captured with a Zeiss microscope and Axioviewer image analysis software (Deutsland; Carl Zeiss Corp, Jena, Germany).
Western blotting
Whole colon lysates were loaded to 4% to 12% Bis-Tris Gels and transferred to a nitrocellulose membrane (Invitrogen). Arginase and YM1 were detected by using rabbit anti–arginase-1 (Abcam, Cambridge, Mass) and anti-YM1 (StemCell technologies, Vancouver, British Columbia, Canada) antibodies followed by anti-rabbit peroxidase-conjugated antibody (Cell Signaling) and ECL-plus detection reagents (GE Healthcare, Buckinghamshire, United Kingdom). Anti-actin (Santa Cruz Biotechnology, Santa Cruz, Calif) was used as loading control.
ELISA
Cytokine levels were measured in the supernatants of LPS-activated (Sigma-Aldrich) and Relm-α–activated (Peprotech) bone marrow–derived macrophages or supernatants of colon punch biopsy specimens with a commercially available ELISA Duo-Set kit, according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn). Lower detection limits for IL-6, TNF-α, and IL-10 were 15.6, 32.25, and 15.6 pg/mL, respectively. For detection of serum Relm-α, purified anti–Relm-α and biotinylated anti–Relm-α (Peprotech) were used according to a protocol provided by the manufacturer. The lower detection limit for Relm-α was 15.62 pg/mL.
Relm-α administration
Recombinant Relm-α (2 and 20 μg per mouse) was injected intraperitoneally in 200 μL of saline. Twenty-four and 48 hours after injection, the mice were killed, and peritoneal lavage and cell counts were performed.
Chemotaxis assay
Chemotaxis of eosinophils obtained from the spleens of CD2-IL-5Tg mice toward Relm-α was assessed as previously described.11
Statistical analysis
Data were analyzed by means of ANOVA, followed by the Tukey post-hoc test with GraphPad Prism 4 (San Diego, Calif). Data are presented as the mean ± SD. P values of less than .05 were considered statistically significant.
RESULTS
Relm-α expression in experimental colitis
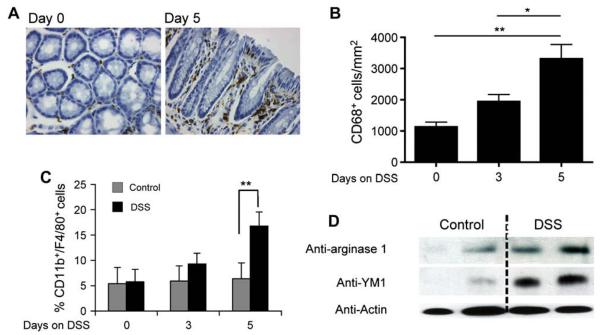
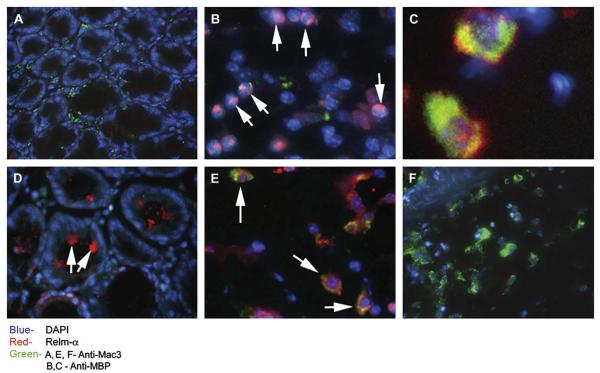
We first aimed to determine the cellular source of Relm-α in the colon during a DSS-induced model of experimental colitis. This model directly damages the intestinal epithelial barrier, resulting in activation of innate immune cells (predominantly tissue macrophages) and recruitment of additional macrophages, neutrophils, and eosinophils.2 After DSS treatment, CD68+ myeloid cells accumulated in the colon (see Fig E1 in this article's Online Repository at www.jacionline.org). Phenotyping of these cells by means of flow cytometry revealed a substantial increase (from 4.82% ± 1.33% to 16.23% ± 2.88%) of F4/80+/CD11b+/FSChigh cells in the colon (see Fig E1). Western blot analysis of whole colon lysates from control or DSS-treated mice showed a significant increase in expression of the alternatively activated macrophage markers arginase-1 and YM1 (see Fig E1).12 Immunofluorescence staining of colon samples of DSS-treated mice revealed a marked increase in Relm-α+ cells, whereas Relm-α was hardly expressed in the normal colon (Fig 1, A). Unexpectedly, analysis of the cellular source of Relm-α expression identified that the majority of Relm-α+ cells were not macrophages but rather eosinophils (MBP+ cells; Fig 1, B and C) and epithelial cell subsets (Fig 1, D). Supporting our immunofluorescence findings, quantitative PCR analysis of Relm-α expression failed to detect upregulation of Relm-α in purified DSS-treated colonic macrophages (data not shown). Further characterization of Relm-α expression in eosinophils showed that Relm-α was found to colocalize with MBP in eosinophil granules (Fig 1, C). In fact, isolated eosinophils expressed 0.25 pg of Relm-α per cell at baseline (data not shown). Although occasional Mac-3+/Relm-α+ cells corresponding with macrophage morphology were observed (Fig 1, E), they were less than 5% of the macrophage population, and the majority of the macrophages were Relm-α− (Fig 1, F). Collectively, these data demonstrate that eosinophils are a chief source of Relm-α after DSS.
FIG E1.
Macrophage accumulationin the colon and arginase-1 and YM1 expression after DSS treatment. Wild-type mice were treated with DSS for up to 8 days. At the indicated time points (days 0, 3, and 5), the colon was excised and either frozen for slide and protein lysate preparation (A, B, and D) or enzymatically digested for differential cell analysis (C). Representative microphotographs of anti-CD68–stained slides are shown (Fig E1, A). Anti-CD68+ cells were quantified by using digital morphometric analysis (Fig E1, B). Single-cell suspensions were stained with anti-CD11b and anti-F4/80 and analyzed by means of FlowJo software (Tree Star, Inc, Ashland, Ore) for CD11b+/F4/80+ cells in the colon (Fig E1, C). Total protein lysates of whole colon specimens were subjected to Western blot analysis and probed with anti-arginase, anti-YM1, and anti-actin as loading control. A representative Western blot is shown in which each lane represents the colon from 1 mouse (n = 4; Fig E1, D).
FIG 1.
Expression of Relm-α in DSS-induced colitis. Wild-type mice were treated with DSS for up to 8 days. Representative immunofluorescence microphotographs of Relm-α expression in the colons of wild-type control mice (A) and DSS-treated wild-type mice (B-F) are shown. Frozen sections were stained with anti–Relm-α (red; Fig 1, A-F), DAPI (blue; Fig 1, A-F), and either anti–Mac-3 (green; Fig 1, A, E, and F) or anti-MBP (green; Fig 1, B and C). A high-resolution image of double-stained MBP+/Relm-α+ cells is shown (Fig 1, C). Arrows indicate Relm-α+ cells (Fig 1, B-E).
Generation of Retnla−/− mice
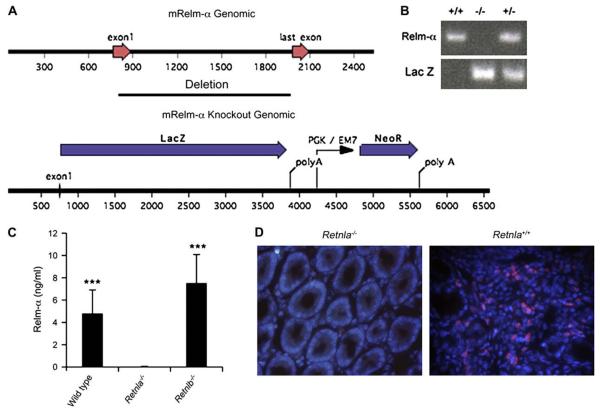
To define the role of Relm-α in vivo, we used the Velocigene technology to delete genomic Retnla by means of replacement with a LacZ neoexpression cassette (Fig 2, A).10 The specific genetic ablation of Retnla (Fig 2, B) was confirmed by assessing serum levels of Relm-α in wild-type, Retnla−/−, and Retnlb−/− mice (Fig 2, C). Indeed, Relm-α was not detected in Retnla−/− mice, whereas it was readily detected in the sera of wild-type and Retnlb−/− mice. Retnla−/− mice were fertile and produced offspring at predicted mendelian inheritance patterns, with no gross abnormalities observed. Analysis of peripheral blood cell counts did not show any differences in differential cell numbers (data not shown). Further validation of the gene deletion and anti–Relm-α antibody specificity was obtained by demonstrating that Retnla−/− mice exhibited minimal reactivity with the anti–Relm-α antibody in DSS-treated colon samples (Fig 2, D).
FIG 2.
Generation of Retnla−/− mice. The Retnla gene was replaced by a reporter-selection cassette, which consists of β-galactosidase and a neomycin resistance gene. The diagram shows the wild-type murine Retnla gene locus and the gene-targeted locus. The construct deletes all 4 exons of the Retnla gene (A). The mice were identified as heterozygotes and homozygotes by means of the Taqman assay with probes for LacZ genes and the Retnla loss-of-allele probes. Each lane represents a separate animal (B). Sera of wild-type, Retnla−/−, and Retnlb−/− mice were subjected to ELISA (C). ***P < .001 when comparing with Retnla−/− mice (n = 8-10 mice). Validation of anti–Relm-α antibody specificity and Retnla deficiency was obtained by means of immunofluorescent staining of a DSS-treated colon sections of Retnla−/− mice (D). mRelm-α, Murine Relm-α.
Retnla−/− mice are protected from DSS-induced experimental colitis
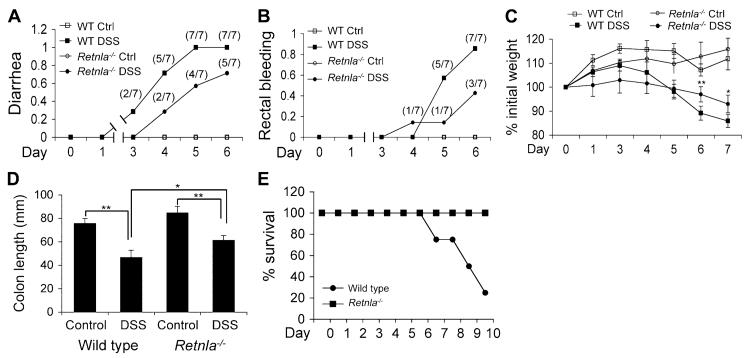
Because Relm-α expression was induced during DSS-triggered colitis, we aimed to determine the role of Relm-α in intestinal inflammation. Retnla−/− mice were markedly protected against DSS-induced colitis (Fig 3) in comparison with wild-type mice. Retnla−/− mice displayed delayed development of diarrhea (Fig 3, A), substantially less rectal bleeding (Fig 3, B), and weight loss (Fig 3, C). In addition, Retnla−/− mice displayed decreased colon shortening as a result of the DSS insult (Fig 3, D). Notably, Retnla−/− mice were protected from DSS-induced death (Fig 3, E). For example, by day 10, 80% of wild-type mice were dead, and 100% of Retnla−/− mice were still viable.
FIG 3.
The effects of Relm-α on DSS-induced colitis. Wild-type (WT) and Retnla−/− mice were exposed to DSS for the indicated time points and analyzed for the clinical disease features diarrhea (A), rectal bleeding (B), and weight loss (C). The colon length of wild-type and Retnla−/− mice after DSS treatment was assessed (D). Mice were also monitored for survival after 3.5% DSS treatment (E). In Fig 3, A and B, the numbers in parentheses represent the actual number of mice that displayed clinical symptoms of disease development. Data are for a representative experiment of 4 (6-8 mice per experimental group). *P < .05, **P < .01, ***P < .001. Ctrl, Control.
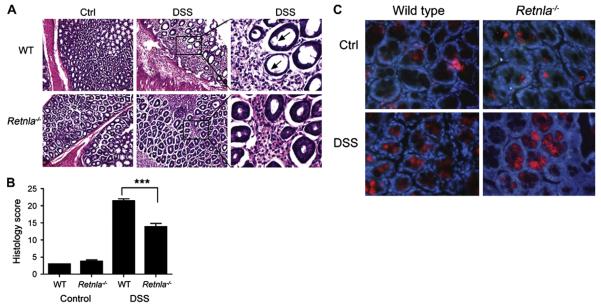
Analysis of the histopathology induced by DSS revealed that Retnla−/− mice had significantly less erosion and damage of the epithelial layer (Fig 4, A, right panel) and substantially decreased submucosal inflammation compared with that seen in wild-type mice (Fig 4, A, middle and right panels). Quantitation of the histology score revealed significant reduction in the histologic features of DSS-induced disease in Retnla−/− mice (Fig 4, B). Importantly, all of the aforementioned parameters were independent of mouse strain because both c57BL/6 and BALB/c mice (treated with 5% DSS) were protected similarly (data not shown). It is notable that Retnlb−/− mice are also protected from DSS-induced colitis.10,13 Therefore we examined Relm-β expression in Retnla−/− mice. Notably, Retnla−/− mice expressed Relm-β; Relm-β was readily detected in colonic epithelial cells and upregulated in the colons of DSS-treated Retnla−/− mice similar to that seen in wild-type mice (Fig 4, C, and data not shown).
FIG 4.
The effects of Relm-α on DSS-induced colitis. Wild-type (WT) and Retnla−/− mice were subjected to 2.5% DSS in drinking water for up to 8 days. At day 7, colons were excised, fixed, paraffin embedded, and stained with hematoxylin and eosin. A representative microphotograph of control (Ctrl) and DSS-treated colons is shown. Arrows indicate sites of epithelial erosion (A, left and middle). In addition, high-resolution images of the DSS-treated colons of wild-type and Retnla−/− mice are shown (Fig 4, A; right column; magnification ×10, left and middle panels; magnification ×40, right panel). Digital morphometric analysis of the histologic score is shown. B, Data represent n = 4 (6-8 mice per experimental group). ***P<.001. Representative immunofluorescent photomicrographs of Relm-β expression in the colons of control (C, upper panels) and DSS-treated (Fig 4, C; lower panels) wild-type (Fig 4, C; left panels) or Retnla−/− (Fig 4, C; right panels) mice is shown. Frozen sections were stained with anti–Relm-β (red) and DAPI (blue; Fig 4, C). A high-resolution image (magnification ×40) is shown.
The proinflammatory role of Relm-α
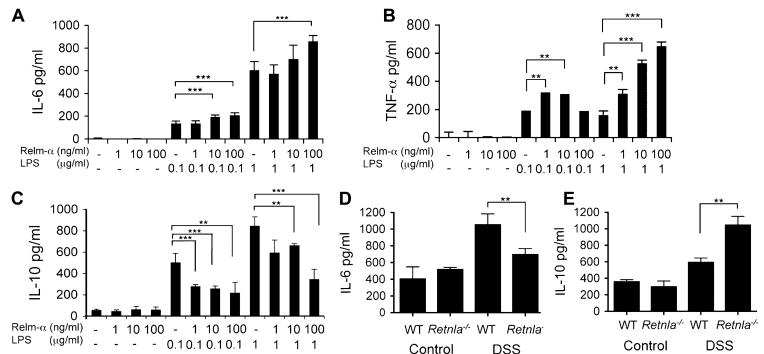
We hypothesized that Relm-α can induce its effects by altering the balance of proinflammatory (ie, IL-6) and anti-inflammatory (ie, IL-10) cytokine production.2,3 Thus we subsequently examined the effects of recombinant Relm-α on macrophage activation in vitro. Activation of bone marrow–derived macrophages with Relm-α in combination with LPS significantly increased induction of IL-6 and TNF-α secretion compared with LPS alone, whereas Relm-α alone did not induce any cytokine production (Fig 5, A-C). Moreover, Relm-α markedly inhibited LPS-induced IL-10 secretion (Fig 5, C). These results highlight that Relm-α acts as a proinflammatory cofactor that regulates macrophage activation and consequent cytokine production.
FIG 5.
Relm-α induces LPS- and DSS-induced IL-6 and TNF-α expression and inhibits IL-10 expression. Bone marrow–derived macrophages were obtained from wild-type (WT) or Retnla−/− mice and stimulated with LPS, Relm-α, or both at the indicated concentrations for 24 hours (A-C). IL-6 (Fig 5, A), TNF-α (Fig 5, B), and IL-10 (Fig 5, C) concentrations in the cell supernatant were assessed by using a commercially available ELISA (n = 3). **P < .01; ***P < .001. The concentrations of IL-6 (D) and IL-10 (E) in the culture supernatants of colon biopsy specimens taken from the distal part of control and DSS-treated wild-type and Retnla−/− mice are shown (Fig 5, D and E; n = 6). **P < .01.
We aimed to determine whether these mechanistic findings might be responsible for the in vivo phenotype observed in Retnla−/− mice. Accordingly, colon punch biopsy specimens were obtained from control and DSS-treated wild-type and Retnla−/− mice and assessed for IL-6 and IL-10 production ex vivo. Consistent with the observed proinflammatory role of Relm-α in vitro, colonic organ cultures obtained from DSS-treated Retnla−/− mice displayed marked reduction in the levels of IL-6 and a substantial increase in IL-10 levels (Fig 5, D and E).
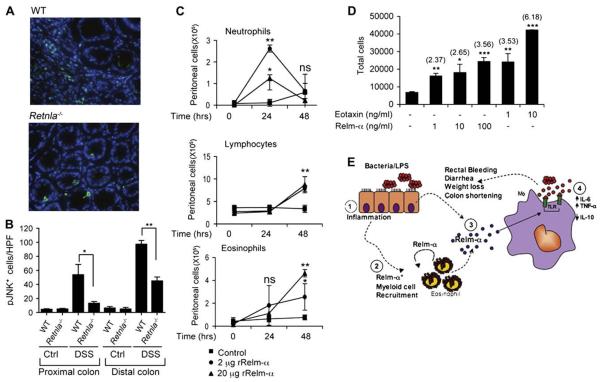
Consistent with these findings, DSS-treated Retnla−/− mice displayed a marked reduction in JNK phosphorylation in vivo in comparison with that seen in wild-type mice (Fig 6, A and B). Of note, the proximal colons of DSS-treated Retnla−/− mice showed a marked protection, and pJNK+ cell levels were similar to those seen in control mice, whereas in the distal colon pJNK+ cells were observed, albeit in significantly lower numbers. Nevertheless, the expression pattern was similar between proximal and distal colon segments and correlated with the overall protection that was observed in the Retnla−/− mice. Interestingly, the cellular source for pJNK+ cells in the DSS-treated Retnla−/− mice was different than that of wild-type mice. In the wild-type mice pJNK+ cells were mainly localized to the lamina propria and corresponded with infiltrating cells, whereas in Retnla−/− mice most of the pJNK+ cells were epithelial cells similar to those seen in control mice, and infiltrating pJNK+ immunoreactive cells were observed only in the distal colon (Fig 6, A and B, and data not shown).
FIG 6.
The proinflammatory effects of Relm-α. Frozen sections of DSS-treated wild-type (WT) and Retnla−/− mice were stained with anti-pJNK and DAPI (A and B). A representative photomicrograph of pJNK+ cells in the proximal colon is shown (Fig 6, A). Computerized morphometric analysis and quantitation of pJNK+ cells per high-power field is shown (Fig 6, B; n = 6-8). **P < .01. The indicated concentrations of Relm-α (C; bottom left) were injected intraperitoneally to wild-type mice. Thereafter, peritoneal lavage followed by differential cell analysis was performed (Fig 6, C; n = 4). **P < .01. ns, Nonsignificant. Chemotaxis of CD2-IL5Tg eosinophils was assessed toward Relm-α and eotaxin (D). The numbers in parentheses indicate the average fold increase (n = 3). *P < .05, **P < .01, ***P < .001. A schematic model for Relm-α activity in colonic inflammation is shown (E). The colonic luminal content, including bacteria and LPS, triggers an inflammatory response (1) involving recruitment of Relm-α+ myeloid cells, mainly eosinophils, as well as induction of Relm-α expression by epithelial cells (2 and 3). On its release, Relm-α synergizes with LPS-induced signaling to amplify a proinflammatory response characterized by increased colonic IL-6 and TNF-α and decreased IL-10 production from macrophages (4). In addition, Relm-α can promote further eosinophil accumulation, acting in an autocrine fashion. Ctrl, Control.
In addition, intraperitoneal injection of Relm-α induced a local inflammatory response. Kinetic analysis of peritoneal cell populations after Relm-α administration revealed that Relm-α initiated neutrophil accumulation that culminated after 24 hours, and a significant increase in eosinophils and lymphocytes was observed after 48 hours (Fig 6, C). Indeed, in vitro chemotaxis assays revealed that Relm-α is capable of inducing up to a 3-fold increase in eosinophil chemotaxis (Fig 6, D).
DISCUSSION
Effector molecules involved in the regulation of gastrointestinal inflammation have been an area of intense research in the last decade.2 Of even greater interest are molecular pathways that might be shared between several immune-related diseases, such as asthma, obesity, and IBD.1,14-16 The role of Relm-α, a highly conserved Relm family member with a unique expression pattern mainly in alternatively activated macrophages, was unknown before our study.9,12 Herein we show that the major Relm-α+ population after DSS treatment is eosinophils. By generating Retnla−/− mice, we define a critical role for this protein in mediating the cardinal features of experimental colitis and demonstrate a potent proinflammatory role for Relm-α through multiple independent approaches. Collectively, our data demonstrate a key role for Relm-α in colonic inflammation and a novel regulatory role for this molecule in the promotion of inflammation (Fig 6, E). Given the unique function of Relm family members in experimental colitis, these results highlight that targeting Relm family members might be beneficial for immune modulation in patients with IBD. Nevertheless, given the potential metabolic roles of Relm family members, the exact settings in which such therapeutic intervention is desirable should be carefully addressed.
Our data demonstrate that Relm-α expression in the inflamed colon is markedly eosinophil derived. The differential expression pattern of Relm-α in myeloid cells compared with Relm-β, which is mainly expressed by epithelial cells,10,17 might explain the non-redundant roles of the Relm family members. Although Relm-β alone was sufficient only to induce TNF-α and IL-15 release from bone marrow–derived macrophages (of 22 measured cytokines),13 Relm-α did not induce cytokine secretion by itself but rather acted as a coactivator to amplify LPS-induced IL-6 and TNF-α secretion and regulate IL-10 levels. Thus Relm-α might regulate inflammatory processes by polarizing a proinflammatory rather than an anti-inflammatory response.
Recent findings demonstrate a proinflammatory role for eosinophils in DSS-induced colitis. In fact, eosinophil peroxidase has been shown to promote the pathogenesis of ulcerative colitis.18 Interestingly, eosinophil peroxidase can synergize with macrophage reactive oxygen species to kill tumor cells or potentially catalyze the oxidation of nitrite to generate additional cytotoxic radicals.19,20 Recent evidence suggests an active cross-talk between eosinophils and macrophages. Alternatively activated macrophages have a role in recruiting eosinophils after Nippostrongylus brasiliensis infection, and we have recently detected eotaxin in human macrophages from patients with ulcerative colitis and in murine intestinal macrophages after DSS treatment.21,22 Furthermore, macrophages are a major source of TNF-α that can induce eosinophil activation and survival.23 Thus it is likely that eosinophil recruitment by macrophages can initiate a self-perpetuating cross-talk in the inflamed tissue in which macrophages are activated by eosinophil-derived products and vice versa. However, similar to macrophages, eosinophils share the potential to promote tissue repair through multiple yet distinct pathways.24 For example, activated eosinophil numbers are higher in the quiescent phase of ulcerative colitis than in the active phase, and eosinophils have been linked to activation of fibroblasts to explain the phenomenon of fibrosis and stricture formation in Crohn disease.25 Taken together, our results highlight a new pathway in which eosinophils can amplify macrophage activation and perpetuate colonic inflammation.18,26 Future studies are required to shed more light on the eosinophil and macrophage interaction.
Notably, Retnlb−/− mice are also protected against DSS-induced colitis.10,13 Nevertheless, examination of Relm-β expression in Retnla−/− mice revealed that Relm-β levels were intact at baseline and after DSS treatment. These data demonstrate that Relm-α and Relm-β independently and through distinct cell types contribute to eliciting intestinal inflammation (in the DSS-induced model). In agreement with this hypothesis and supporting a proinflammatory role for resistin and the Relm protein family are recent findings showing that resistin increases the production of TNF, IL-1, IL-6, and IL-12 and induces the activation of p38, extracellular signal-regulated kinase, and phosphatidylinositol 3-kinase.27,28
Resistin and Relm-β have been attributed important roles in glucose metabolism. It has been recently reported that transgenic mice overexpressing resistin exhibit impaired insulin-mediated glucose transport.29 Indeed, mice lacking resistin exhibit low blood glucose levels because of reduced hepatic glucose production.30 Furthermore, lack of resistin diminishes the increases in glucose levels that are associated with increased weight, suggesting a role for resistin in mediating hyperglycemia associated with obesity.30 Moreover, infusion of Relm-β (or resistin) into rats decreased hepatic insulin sensitivity and was not attributed to peripheral insulin resistance.31 It is thus likely that Relm-α will possess similar activities as well.
In summary, our results define the involvement of Relm-α in colonic inflammation and highlight an eosinophil–Relm-α–macrophage axis. These results imply a similar role for Relm-α under other inflammatory conditions in which Relm-α and eosinophils are observed (eg, asthma). Furthermore, these results outline a novel innate immune pathway in which eosinophils have the potential to regulate immune polarization through regulating macrophage activation.
Key Messages.
Relm-α is expressed by intestinal eosinophils and epithelial cells and has a critical role in DSS-induced colitis.
Relm-α promotes macrophage activation by synergizing with LPS to induce proinflammatory cytokine release and inhibit anti-inflammatory cytokines.
Acknowledgments
We thank Dr Debroski Herbert for critical review of this manuscript, Ms Rosie Kornman for technical assistance, and Jamie Lee for anti-MBP reagent.
Supported by National Institutes of Health grants P01 HL-076383 (M.E.R.) and R01 AI057803 (M.E.R.), a fellowship award (A.M) from the Machiah Foundation, a supporting foundation of the Jewish Community Endowment Fund, the generous support of the Alexander M. and June L. Maisin Foundation, and the Kanbar Charitable Trust, the Campaign Urging Research for Eosinophilic Disorders (CURED), the Food Allergy Project, and the Buckeye Foundation.
Disclosure of potential conflict of interest: M. E. Rothenberg is on the speakers' bureau for Merck; has consulting arrangements with Merck, Ception Therapeutics, Novartis, and Nycomed; and has received research support from the National Institutes of Health, the Food Allergy and Anaphylaxis Network, and the Dana Foundation. The rest of the authors have declared that they have no conflict of interest.
Abbreviations used
- DAPI
4′,6-Diamidino-2-phenylindole dihydrochloride
- DSS
Dextran sodium sulfate
- IBD
Inflammatory bowel disease
- MBP
Major basic protein
- pJNK
Phospho–c-Jun N-terminal kinase
- Relm
Resistin-like molecule
REFERENCES
- 1.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 3.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–9. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee RR, Lazar MA. Dimerization of resistin and resistin-like molecules is determined by a single cysteine. J Biol Chem. 2001;276:25970–3. doi: 10.1074/jbc.M103109200. [DOI] [PubMed] [Google Scholar]
- 6.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh GH. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 7.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, et al. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–26. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, et al. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L34–45. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
- 9.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–9. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–68. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munitz A, McBride ML, Bernstein JS, Rothenberg ME. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood. 2008;111:5694–703. doi: 10.1182/blood-2007-12-126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–23. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abelson P, Kennedy D. The obesity epidemic. Science. 2004;304:1413. doi: 10.1126/science.304.5676.1413. [DOI] [PubMed] [Google Scholar]
- 15.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–20. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–75. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 17.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, et al. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L305–13. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 18.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–75. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 19.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–25. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF, Klebanoff SJ. Augmentation of spontaneous macrophage-mediated cytolysis by eosinophil peroxidase. J Exp Med. 1982;155:1291–308. doi: 10.1084/jem.155.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–44. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens R, Beichler M, Seidu L, Blanchard C, Carey R, Forbes E, et al. Macrophage/intestinal epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. 2008 doi: 10.4049/jimmunol.181.10.7390. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temkin V, Levi-Schaffer F. Mechanism of tumour necrosis factor alpha mediated eosinophil survival. Cytokine. 2001;15:20–6. doi: 10.1006/cyto.2001.0890. [DOI] [PubMed] [Google Scholar]
- 24.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 25.Lampinen M, Ronnblom A, Amin K, Kristjansson G, Rorsman F, Sangfelt P, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714–20. doi: 10.1136/gut.2005.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes E, Hulett M, Ahrens R, Wagner N, Smart V, Matthaei KI, et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J Leukoc Biol. 2006;80:330–41. doi: 10.1189/jlb.1105643. [DOI] [PubMed] [Google Scholar]
- 27.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 28.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–40. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 29.Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab. 2003;285:E106–15. doi: 10.1152/ajpendo.00457.2002. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–8. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 31.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–30. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]