Abstract
Superior vena cava (SVC) syndrome was originally described as being secondary to an infection. Currently, it is almost exclusively secondary to malignancy. A case of SVC syndrome presenting with dyspnea, facial swelling, neck distension and cough developed over a period of 10 days is reported. The approach included imaging studies and tissue diagnosis. Computed tomography scan of the chest revealed a lobulated mass on the right upper chest invading the mediastinum, and cytology obtained from bronchoscopy revealed squamous cell carcinoma. The etiology, diagnosis and treatment modalities of the SVC syndrome are discussed.
Keywords: Carcinoma, Catheter, Chemotherapy, CT scan, Superior vena cava syndrome, Thrombosis
Superior vena cava (SVC) syndrome occurs in approximately 15,000 people in the United States each year (1). The syndrome was originally described as being secondary to an infection, such as tuberculosis, or a syphilitic aortic aneurysm (2–4). Currently, SVC syndrome is generally due to cancer or thrombotic events.
Most SVC syndromes are associated with advanced malignant diseases that cause invasion of the venous intima or an extrinsic mass effect. Lung, breast and mediastinal neoplasms are common causes of SVC syndrome (1,3,5–7), with adenocarcinoma of the lung being the most common cause (2,3,8) (Table 1).
Table 1.
Common causes of superior vena cava syndrome
| Malignant (>85%) | Benign (3% to 15%) |
|---|---|
| Lung cancer | Indwelling catheters |
| Lymphoma | Thymoma |
| Breast cancer | Cystic hygroma |
| Tuberculosis | |
| Histoplasmosis | |
| Thyroid goiter | |
| Aortic aneurysm |
Thrombotic causes of SVC syndrome are increasing because of the rise in the use of pacemakers and central venous catheters for access or treatment purposes (1–3,9). SVC syndrome has also been reported secondary to endocardial defibrillator and pacemaker leads (9,10), and dialysis catheters (11,12). The latter can be aggravated by the placement of an upper extremity arteriovenous graft (11). The frequent use of central venous cannulations and dialysis catheters has led to increased venous thrombosis with SVC and subclavian obstruction (12–14). Other cases of SVC syndrome include aortic pseudoaneurysm (5,15) and compressive mediastinal hematoma (16).
SVC syndrome is caused by gradual compression of the SVC, leading to edema and retrograde flow, but it can also be caused more abruptly in thrombotic cases (1). Symptoms may include cough, dyspnea, dysphagia, and swelling or discolouration of the neck, face and upper extremities. Often, collateral venous circulation causes distension of the superficial veins in the chest wall (1,3,14,17). Although SVC syndrome is a clinical diagnosis, plain radiography, computed tomography (CT) and venography are used for confirmation (1).
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to reduce the tumour that is causing the obstruction. However, the use of intravenous stents is becoming increasingly common (1). Tissue diagnosis (ie, sputum, cytology, thoracocentesis, bronchoscopy or needle aspiration) is often necessary to direct treatment decisions (1). Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation and elevating the head of the patient’s bed (1).
If thrombosis is found, thrombolysis and anticoagulation may be indicated (2). Fibrinolytic therapy and endovascular treatment are currently achieving good results via catheter-directed (intraclot) infusion thrombolytic therapy with urokinase (18).
In cases of compression, dilation and stenting of the SVC may be performed. In some cases, a bypass of the SVC may be indicated (2,8). Percutaneous stent placement in malignant cases of SVC syndrome is a simple, safe and effective technique to rapidly relieve SVC syndrome caused by malignant diseases (19). Alleviation of severe compressive symptoms in a patient with advanced lung carcinoma using tracheal and SVC stents has been reported (20).
With carcinoma or infection, specific drugs or radiation may be used (2). Patients with SVC syndrome usually have advanced disease and fewer than 10% survive more than 30 months after treatment (1).
SVC syndrome can lead to the formation of downhill esophageal varices (11) and pleural effusion (21). Numerous case reports have described pleural effusions in conjunction with the SVC syndrome. These effusions occur in 60% of SVC syndrome cases (21). The effusions are small, usually occupying less than one-half of the affected hemithorax, and occur approximately equally on either side or bilaterally. Although previously thought to be largely transudates, a large case series (21) found that 18% of the effusions were chylous, with the remainder being exudates. None of the effusions sampled in the series were transudates. Occluded lymphatic flow from increased hydrostatic pressure in the SVC and left brachiocephalic vein probably contributes to the development of chylous pleural fluid. The pathophysiology of the exudative effusions, however, remains unknown. Many factors, including diuresis, small pulmonary emboli, and the underlying inflammatory or malignant condition all likely contribute. Chylous or exudative pleural effusions occur in most patients with SVC syndrome. The effusions are usually small and resolve upon correction of the underlying SVC obstruction (21).
CASE PRESENTATION
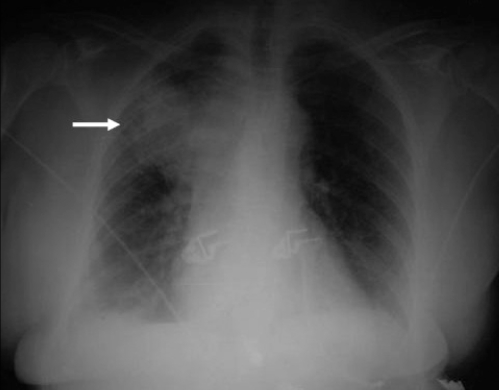
A 74-year-old Hispanic woman with a medical history of coronary artery disease (CAD) status postmyocardial infarction (MI) (twice), severely depressed systolic function (ejection fraction 25%), ex-chronic smoker and carcinoma of the lung diagnosed in the Dominican Republic in May 2007 presented to the emergency department with the chief complaint of neck swelling for a week and a half, a dry cough and intermittent, nonradiating, epigastric pain lasting 3 min that worsened with exertion and was relieved by rest. Other associated symptoms were weight loss (7 kg in two months), anorexia, weakness, fatigue and early satiety. The patient had had similar symptoms previously, at which time she was found to have a lung mass on chest x-ray (Figure 1). On the present occasion, the symptoms were worsening and accompanied by bilateral neck swelling.
Figure 1).
Chest x-ray showing right apical pleural thickening, a large right upper lobe opacity (arrow) and minimal right basilar atelectasis/interstitial
In the emergency room, the patient’s vital signs were assessed as blood pressure 137/79 mmHg, heart rate 82 beats/min and temperature 36.0°C, and the patient was alert, oriented to person, place and time, and in no distress.
The patient was initially admitted to telemetry under the diagnosis of abnormal electrocardiogram to rule out myocardial infarction.
The patient’s surgical history was notable for an appendectomy, and her obstetrical and gynecological history included four pregnancies and four deliveries. She was postmenopausal. A review of her family history revealed her mother had had a cerebrovascular accident.
The review of systems confirmed weakness, weight loss, neck swelling and dyspnea. On physical examination, her pupils were equal, round and reactive to light and accomodation. She had bilateral neck swelling, more so on left than on the right, and no facial plethora. On auscultation, the patient had bilaterally good air entry and bilateral rales, as well as S1/S2, regular rate and rhythm, and no murmur.
Her abdomen was soft, nontender and not distended. There was no edema of the extremities, and pulses were present.
Laboratory workup revealed white blood cell count 12×109/L; hemoglobin and hematocrit 13.5 g/L and 42.9%, respectively; platelet count 392×109/L; sodium 140 mmol/L; potassium 4.7 mmol/L; chloride 102 mmol/L; bicarbonate 27 mmol/L; blood urea nitrogen 7 mmol/L; and creatinine 88.4 μmol/L.
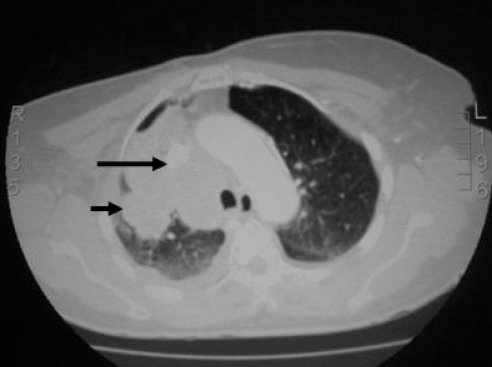
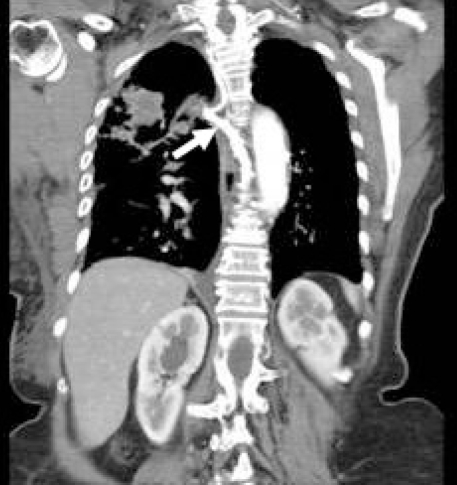
An electrocardiogram showed normal sinus rhythm, anterior Q wave and inverted T wave in leads V3 to V6. Myocardial infarction was ruled out by telemetry in conjunction with two sets of negative cardiac enzymes, and the attending cardiologist referred the patient for a work-up for lung mass, and to rule out SVC syndrome. Abdominal and pelvis CT scans were negative for metastases (Figures 2 and 3).
Figure 2).
Computed tomography of the chest with contrast showing a large lobulated mass on the right upper chest (large arrow) measuring 7.8 cm × 6 cm × 5.8 cm invading the mediastinum and extending to the apex and lateral chest wall (small arrow)
Figure 3).
Computed tomography reconstruction (coronal plane) showing dilated azygos vein (arrow)
Under the medical service, patient was treated for congestive heart failure with furosemide, carvedilol, a angiotensin-converting enzyme inhibitor, spironolactone and acetylsalicylic acid.
After pulmonary consultation, the patient was diagnosed with lung cancer, unknown type, and pulmonary function tests and results of a previous biopsy from the Dominican Republic were requested.
Oncology service was called for possible radiotherapy, and dexamethasone 2 mg by mouth every 6 h was prescribed.
The report from a previous biopsy was suggestive of squamous cell carcinoma. Bronchoscopy and biopsy were repeated, and bronchial wash cytology was positive for squamous cell carcinoma.
The patient’s neck swelling and other symptoms improved, and she was discharged home with an appointment for radio-therapy, and appointments in the cardiology, pulmonary and oncology clinics.
DISCUSSION
SVC syndrome was first described by William Hunter in 1757 in a patient with a large syphilitic aortic aneurysm compressing the SVC. It occurs in approximately 15,000 persons in the United States each year (1). SVC syndrome is the clinical manifestation of SVC obstruction and occurs through external compression, thrombosis or invasion of the vein. SVC syndrome is now almost exclusively (more than 90%) secondary to malignancy. The most common malignant cause is non-small cell lung cancer in approximately 50% of patients, and is the cause of SVC obstruction in the present case review.
Our patient presented with the classic findings of dyspnea (which is the most commonly reported symptom in literature), facial swelling, head fullness, neck distension (exacerbated by bending forward or lying down) and cough (secondary to functional compromise of the upper airways).
To understand the clinical manifestations of the syndrome, an appreciation of the regional anatomy is necessary. Because the venous drainage from the upper extremities, upper thorax and head is obstructed, SVC syndrome presents with symptoms related to engorgement of these areas. Both the degree of SVC compromise and the extent of collateral veins determine the varied clinical presentation, which can be as mild as slight facial and upper extremity edema or as dire as intracranial swelling, seizures, hemodynamic instability and tracheal obstruction. The rapidity of onset of symptoms and signs from SVC obstruction is dependent upon the rate at which complete obstruction of the SVC occurs in relation to the recruitment of venous collaterals. Our patient developed symptoms within 10 days, which favours a diagnosis of malignancy because the rapidity of tumour growth does not allow adequate time to develop collateral flow.
In the past, SVC syndrome due to malignant disease was considered a potentially life-threatening medical emergency requiring immediate radiation therapy. However, in the present case, we took a less aggressive approach. Symptomatic obstruction is often a prolonged process developing over a period of weeks before clinical presentation; therefore, deferring therapy until a full diagnostic work-up has been completed does not pose a hazard for most patients, provided the evaluation is efficient and the patient is clinically stable. Furthermore, prebiopsy radiation may obscure the histological diagnosis.
Most cases of SVC syndrome are diagnosed readily on clinical examination alone, but several diagnostic tests and procedures may be useful. When a patient presents with suspected SVC syndrome, the first step is to obtain an imaging study to both confirm the diagnosis and assist in treatment decisions. Magnetic resonance imaging, contrast-enhanced CT scanning, radionuclide flow studies and traditional venography are all adequate modalities, but CT scanning is the most readily available technology in most centres. CT scans and magnetic resonance imaging also provide information regarding possible etiologies and, thus, can direct the approach to a tissue diagnosis. The approach to establishing a tissue diagnosis is defined by both the patient’s clinical stability and the findings on clinical examination and radiographic studies. Tissue diagnoses are important because they guide treatment; specifically, they identify patients for whom SVC syndrome should be treated with combination chemotherapy rather than with local measures such as radiation therapy or percutaneous vascular procedures.
Treatment of SVC syndrome is divided into supportive and definitive therapy. An obvious therapeutic manoeuvre is to elevate the patient’s head to decrease the hydrostatic pressure and thereby the edema. There are no data documenting the effectiveness of this manoeuvre, but it is simple and without risk. Glucocorticoid therapy (dexamethasone, 4 mg every 6 h) is commonly prescribed, although its effects have not been formally well studied. Loop diuretics are also commonly used, but it is unclear whether venous pressure distal to the obstruction is affected by small changes in right atrial pressure. SVC obstruction is a strong predictor of poor prognosis in patients with non-small cell lung cancer. Many clinical trials show no significant difference in the rate of relief from the SVC syndrome whether chemotherapy, radiotherapy, or chemotherapy with radiotherapy was used.
CONCLUSIONS
SVC syndrome is most frequently encountered in patients with malignancies (especially in patients with lung cancer). Patients typically present with shortness of breath along with facial and upper extremity edema. We recommend obtaining a histological diagnosis before initiating treatment. Radiation therapy with or without chemotherapy is the mainstay of treatment for most patients. Intravascular stents are proven to be safe and effective and allow the most rapid resolution of symptoms. Because the overall prognosis of the majority of patients with malignancy is poor, palliation is often the focus of treatment.
REFERENCES
- 1.Higdon ML, Higdon JA. Treatment of oncologic emergencies. Am Fam Physician. 2006;74:1873–80. [PubMed] [Google Scholar]
- 2.Nunnelee JD. Superior vena cava syndrome. J Vasc Nurs. 2007;25:2–5. doi: 10.1016/j.jvn.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: Clinical characteristics and evolving etiology. Medicine. 2006;85:37–42. doi: 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 4.Zisis C, Skevis K, Kefaloyannis E, Avgoustou K, Bellenis I. Mediastinal tuberculous lymphadenitis presenting as superior vena cava syndrome. J Thorac Cardiovasc Surg. 2006;131:e11–2. doi: 10.1016/j.jtcvs.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Osawa S, Sakamoto A, Iwasaki H, et al. Superior vena cava syndrome associated with the metastasis of gastric adenocarcinoma to cervical lymph nodes. Dig Dis Sci. 2007;52:3343–5. doi: 10.1007/s10620-007-8101-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M, Yoshino I, Osoegawa A, et al. Inflammatory myofibroblastic tumor of the mediastinum presenting as superior vena cava syndrome. J Thorac Cardiovasc Surg. 2003;126:870–2. doi: 10.1016/s0022-5223(03)00611-1. [DOI] [PubMed] [Google Scholar]
- 7.Kalra M, Gloviczki P, Andrews JC, et al. Open surgical and endovascular treatment of superior vena cava syndrome caused by nonmalignant disease. J Vasc Surg. 2003;38:215–23. doi: 10.1016/s0741-5214(03)00331-8. [DOI] [PubMed] [Google Scholar]
- 8.Krayem HK, Bowles A, Hanbali A. Mediastinal hematoma with SVC syndrome from a central venous catheter insertion: A case report. Chest. 2003;124:314S. (Abst). [Google Scholar]
- 9.Aryana A, Sobota KD, Esterbrooks DJ, Gelbman AI. Superior vena cava syndrome induced by endocardial defibrillator and pacemaker leads. Am J Cardiol. 2007;99:1765–7. doi: 10.1016/j.amjcard.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Batts TC, Campbell CL, Roman-Gonzalez J. Superior vena cava syndrome associated with implantable cardioverter defibrillator (ICD): An uncommon complication of an increasingly common procedure: CAR-4. South Med J. 2006;99:1042. (Abst) [Google Scholar]
- 11.Greenwell MW, Basye SL, Dhawan SS, Parks FD, Acchiardo SR. Dialysis catheter-induced superior vena cava syndrome and downhill esophageal varices. Clin Nephrol. 2007;67:325–30. doi: 10.5414/cnp67325. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal SK, McCauley W. Tunnelled central venous catheter-induced thrombosis: a rare case of superior vena cava syndrome. Can J Emerg Med. 2005;7:273–7. doi: 10.1017/s1481803500014433. [DOI] [PubMed] [Google Scholar]
- 13.Ansari MJ, Syed A, Wongba W, et al. Superior vena cava obstruction presenting as a complication of repeated central venous cannulations. Compr Ther. 2006;32:189–91. doi: 10.1007/s12019-006-0011-8. [DOI] [PubMed] [Google Scholar]
- 14.Khalili B, Boggs PB, Bahna SL, Marsala AJ, Tapolyai M, Bryn RO. Superior vena cava syndrome: A masquerader of anaphylaxis. J Allergy Clin Immunol. 2007;119(Supple 1):S29. (Abst) [Google Scholar]
- 15.Baldari D, Chiu S, Salciccioli L. Aortic pseudoaneurysm as a rare cause of superior vena cava syndrome – a case report. Angiology. 2006;57:363–6. doi: 10.1177/000331970605700313. [DOI] [PubMed] [Google Scholar]
- 16.Szokol JW, Alspach D, Mehta MK, Parilla BV, Liptay MJ. Intermittent airway obstruction and superior vena cava syndrome in a patient with an undiagnosed mediastinal mass after cesarean delivery. Anesth Analg. 2003;97:883–4. doi: 10.1213/01.ANE.0000076143.59737.32. [DOI] [PubMed] [Google Scholar]
- 17.Gaslin M, Seymour P, Willcox TO. Superior vena cava syndrome presenting as serous otitis media. Otolaryngol Head Neck Surg. 2005;133(Suppl 1):P243. (Abst) [Google Scholar]
- 18.Guijarro Escribano JF, Anton RF, Colmenarejo Rubio A, Saenz Cascos L, Sainz Gonzalez F, Alguacil Rodríguez R. Superior vena cava syndrome with central venous catheter for chemotherapy treated successfully with fibrinolysis. Clin Transl Oncol. 2007;9:198–200. doi: 10.1007/s12094-007-0036-1. [DOI] [PubMed] [Google Scholar]
- 19.Baltayiannis N, Magoulas D, Anagnostopoulos D, et al. Percutaneous stent placement in malignant cases of superior vena cava syndrome. J BUON. 2005;10:377–80. [PubMed] [Google Scholar]
- 20.Morales JP, Sabharwal T, Man-Harun S, Adam A. Alleviation of severe compressive symptoms in a patient with advanced lung carcinoma using tracheal and superior vena cava stents. J Palliat Med. 2007;10:24–9. doi: 10.1089/jpm.2006.0175. [DOI] [PubMed] [Google Scholar]
- 21.Rice TW. Pleural effusions in superior vena cava syndrome: Prevalence, characteristics, and proposed pathophysiology. Curr Opin Pulm Med. 2007;13:324–7. doi: 10.1097/MCP.0b013e32812144aa. [DOI] [PubMed] [Google Scholar]