Abstract
Hypertension is a leading cause of morbidity and mortality worldwide. Individuals with hypertension are at increased risk of stroke, heart disease and kidney failure. Although the etiology of essential hypertension has a genetic component, lifestyle factors such as diet play an important role. Reducing dietary salt is effective in lowering blood pressure in salt-sensitive individuals. Insulin resistance and altered glucose metabolism are common features of hypertension in humans and animal models, with or without salt sensitivity. Altered glucose metabolism leads to increased formation of advanced glycation end products. Insulin resistance is also linked to oxidative stress, and alterations in the nitric oxide pathway and renin angiotensin system. A diet rich in protein containing the semiessential amino acid, arginine, and arginine treatment, lowers blood pressure in humans and in animal models. This may be due to the ability of arginine to improve insulin resistance, decrease advanced glycation end products formation, increase nitric oxide, and decrease levels of angiotensin II and oxidative stress, with improved endothelial cell function and decreased peripheral vascular resistance. The Dietary Approaches to Stop Hypertension (DASH) study demonstrated that the DASH diet, rich in vegetables, fruits and low-fat dairy products; low in fat; and including whole grains, poultry, fish and nuts, lowered blood pressures even more than a typical North American diet with similar reduced sodium content. The DASH diet is rich in protein; the blood pressure-lowering effect of the DASH diet may be due to its higher arginine-containing protein, higher antioxidants and low salt content.
Keywords: Advanced glycation end products, Arginine, Hypertension, Insulin resistance, Nitric oxide, Oxidative stress, Renin angiotensin system
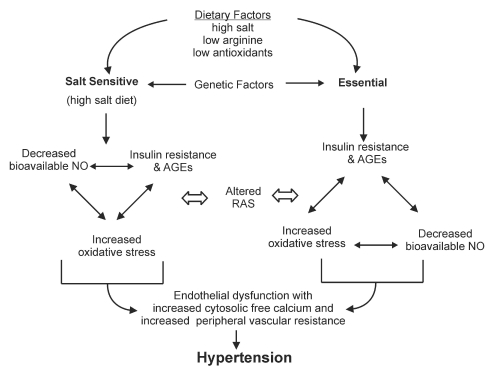
Hypertension is a leading cause of morbidity and mortality affecting over 600 million people worldwide (1). Greater than 95% of individuals with hypertension are classified as having ‘essential’ hypertension, meaning that the exact cause is unknown. However, essential hypertension is associated with insulin resistance and altered glucose metabolism, oxidative stress, and changes to vascular reactivity and kidney morphology (2–8). Both genetic and lifestyle factors may be involved in these alterations (Figure 1). Diet is one lifestyle factor that has come under much investigation. In particular, dietary salt has been acknowledged as a major culprit in increasing blood pressure in those individuals who are salt-sensitive. Because approximately one-half of hypertensive patients and one-quarter of normotensive individuals are salt sensitive (9), the impact of this one dietary additive may be quite significant.
Figure 1).
Mechanism of salt-sensitive and essential hypertension. AGEs Advanced glycation end products; NO Nitric oxide; RAS Renin angiotensin system
The effect of salt on blood pressure was demonstrated in the Dietary Approaches to Stop Hypertension (DASH) study (10,11). The DASH diet is rich in vegetables, fruits and low-fat dairy products; low in total fat, saturated fat and cholesterol; and includes whole grains, poultry, fish and nuts with only small amounts of red meat. Amounts of sweets and sugar-containing beverages were also reduced. The study showed that lowering sodium intake (100 mmol/day or less) in the control group consuming a typical North American diet lowered blood pressure significantly. However, it also revealed another interesting finding. Subjects consuming the DASH diet with a similar sodium intake had even lower blood pressures than the low sodium diet alone. This raised the question – in what way did the DASH diet differ from the control diet that could account for this added antihypertensive effect?
The DASH diet contained more protein than did the control diet (17.9% versus 13.8%) (10,12). Several other studies, including the OmniHeart (13), Multiple Risk Factor Intervention Trial (MRFIT) (14) and the INTERnational sudy of SALT and blood pressure (INTERSALT) (15) studies, have shown that increased protein intake is associated with a decrease in blood pressure. One component of protein that may explain its antihypertensive properties is arginine. Soy protein, a protein rich in arginine, is effective in lowering blood pressure (16). Arginine is a semiessential amino acid that is a necessary substrate in the nitric oxide (NO) pathway, is involved in the renin angiotensin system (RAS), and partially regulates insulin secretion. These systems play a role in blood pressure homeostasis and vascular health. Although arginine can be formed endogenously, dietary intake contributes to the body’s supply and may address deficiencies or alterations in arginine metabolism. Several animal studies and some studies in humans show that arginine supplementation, either by infusion or oral supplementation, lowers blood pressure and improves vascular function (17–27).
The present paper reviews studies on arginine and blood pressure, and examines possible antihypertensive mechanisms of arginine in salt-sensitive and essential hypertension. This information may not only characterize arginine’s potential as an antihypertensive treatment, but define its role in hypertension and add to our understanding of the mechanism of hypertension itself. We will begin by discussing some of the proposed factors which contribute to the etiology and/or development of hypertension.
MECHANISM OF HYPERTENSION
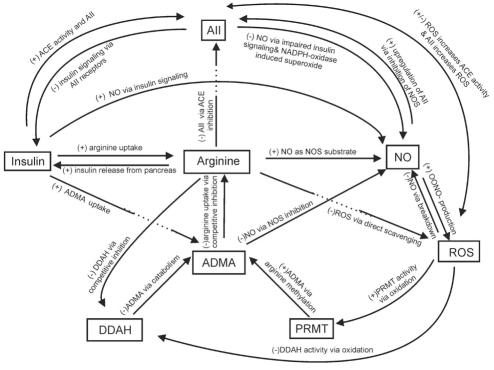
Under normal physiological conditions, the NO pathway, RAS and insulin are major factors involved in a complex biochemical network that maintains vascular homeostasis (Figure 2). Alteration to any one of these factors potentially affects all others, leading to impaired blood pressure regulation and hypertension. Insulin resistance and its downstream effects including altered glucose metabolism and advanced glycation end product (AGEs) formation, as well as loss of bioavailable NO, angiotensin II (AII)-mediated alterations and oxidative stress have all been implicated in the etiology and/or development of hypertension (2–7,28–41) (Figure 1).
Figure 2).
The interaction of various factors, including arginine, that comprise the complex biochemical network involved in blood pressure regulation. Alterations in any of these factors (eg, insulin resistance, oxidative stress, arginine deficiency) affects the system as a whole and can result in endothelial dysfunction, increased peripheral vascular resistance and hypertension. + Increase; – Decrease; AII Angiotensin II; ACE Angiotensin converting enzyme; ADMA Asymmetrical dimethyl arginine; DDAH Dimethylarginine dimethylamino-hydrolase; NO Nitric oxide; NOS NO synthase; OONO− Peroxynitrite; PRMT Protein methyltransferase; ROS Reactive oxygen species
Insulin resistance
Insulin release from the beta cells of the pancreas is triggered by a rise in blood glucose, or an increase in plasma amino acids such as arginine (Figure 2). Gastrointestinal hormones also stimulate an anticipatory increase in insulin with food intake. Insulin functions as a hormone to regulate carbohydrate and lipid metabolism, and stimulates protein synthesis. The effects of insulin are initially mediated by binding of the hormone to the insulin receptors on the cell surface. The tyrosine kinase activity of the receptor then stimulates a number of intracellular signalling cascades, known as insulin signalling. This signalling not only promotes glucose uptake but also modulates other functions involved in blood pressure regulation such as NO-mediated vasodilation (42,43), AII signalling (40,44,45) (Figure 2) and antinatriuretic activity (46). Insulin also promotes the uptake of arginine (Figure 2) into cells via chloramphenicol acetyl transferase (CAT) protein of the Y+ receptors (47,48), a step that likely supports insulin’s vasodilatory action via the NO pathway (to be discussed later). Thus, alterations to insulin, insulin receptor or insulin signalling can have an impact on the control of vascular function.
Insulin resistance is characterized by an inadequate glucose uptake with altered glucose metabolism in peripheral tissues at a given concentration of plasma insulin (2). There is increasing evidence that insulin resistance plays a key role in essential hypertension in humans (2,3,49). Abnormalities in glucose utilization are estimated to exist in 25% of the general population and in up to 80% of subjects with essential hypertension (2,3,50). In humans with essential hypertension, insulin resistance involves impairment of the nonoxidative (glycolytic) pathways of intracellular glucose metabolism (2). Insulin resistance appears to be a primary defect as it exists in normotensive individuals at risk for essential hypertension (51). In healthy normotensive offspring of patients with essential hypertension, blood pressure and plasma insulin was higher when compared with normotensive subjects with no family history of hypertension, and the number of insulin receptors in erythrocytes was reduced (52). This reduction in insulin receptors was also demonstrated in erythrocyte ghosts of patients with essential hypertension (53). Salt-sensitive essential hypertensive patients are more insulin resistant than salt-resistant individuals (54), and high salt intake exacerbates insulin resistance in salt-sensitive hypertensive subjects (55). Salt-sensitive normotensive subjects were more insulin resistant than their salt-resistant counterparts (56).
In spontaneously hypertensive rats (SHR), an animal model of human essential hypertension, insulin resistance is found at a young age (57,58), and before development of hypertension (59). In this model, there is no difference in insulin receptor number or affinity in skeletal muscle compared with normotensive Wistar-Kyoto (WKY) rats, and binding of insulin to the receptor does not seem to be impeded (58,60). However, insulin receptor messenger (m)RNA is lower in liver of SHR on low salt diet compared with WKY control rats (61). Additionally, it has been shown that insulin-stimulated phosphorylation is reduced in the insulin receptor substrate/phosphatidylinositol 3-kinase/protein kinase B (IRS/PI 3 kinase/Akt) insulin signalling pathway in skeletal muscle and the insulin receptor substrate/phosphatidylinositol 3-kinase/protein kinase B/endothelial NO synthase (IRS/PI 3 kinase/Akt/eNOS) pathway in aorta of SHR (62). Increasing salt intake from low to high exacerbated existing insulin resistance in SHRs (61). This increase in salt had no effect on density and messenger (m)RNA levels of insulin receptor in kidney of SHR compared with normal WKY rats where these parameters were downregulated in response to high salt (61). This apparent loss of ability to downregulate insulin receptor in kidney was associated with an increased anti-natriuretic response to insulin, which may explain the salt-induced increase in blood pressure.
In normotensive humans, high salt intake had no effect on blood pressure but impaired insulin sensitivity (63). Insulin resistance in Dahl salt-sensitive (DSS) rats, a model of salt sensitive hypertension, appears to be secondary to salt intake, because those given high salt diet develop insulin resistance, whereas those on normal salt diet do not (64). Insulin resistance in this model is not associated with any change in insulin receptor number or affinity in skeletal muscle or kidney, or with a decrease in receptor binding (65). Sprague Dawley (SD) rats develop mild hypertension and insulin resistance when treated with high salt (66), but this is associated with a decrease in insulin receptor number and level of insulin receptor mRNA in kidney (67). In contrast to SHR, insulin resistance in these salt-induced models is associated with enhanced insulin-stimulated phospholylation in the IRS/PI 3 kinase/Akt pathway (64,66). This indicates that the insulin-signalling step impaired by high salt intake is likely to be downstream of PI 3-kinase or Akt activation, and it may be that the enhanced activity is a compensatory response to this impairment.
NO
One of the major regulators of blood pressure is NO. The enzyme NO synthase (NOS) catalyzes the conversion of arginine to NO in the presence of cofactors tetrahydrobiopterin, flavin adenine dinucleotide (oxidized), flavin mononucleotide, heme and NADPH (Figure 2). There are three isoforms of NOS – neuronal, inducible (iNOS) and endothelial (eNOS), with eNOS being responsible for endothelial-derived NO production. Endothelium-derived NO activates guanylate cyclase to generate cyclic (c)GMP), resulting in vascular smooth muscle cell (VSMC) relaxation and vasodilation, thus contributing to the regulation of peripheral blood flow, vascular resistance and blood pressure. NO also regulates renal blood flow and acts on all tubular segments to reduce sodium reabsorption in the kidney (68). Additionally, NO maintains other aspects of vascular health by inhibiting platelet aggregation, VSMC migration and proliferation, and adhesion molecule expression and monocyte adhesion (69). Production of NO is regulated in part by insulin via insulin receptors on the cell surface (42,70,71).
A decrease in NO bioavailability may occur in several ways. A lack of arginine or a decrease in its cellular uptake (72,73), a deficiency of NOS cofactors (74,75), an alteration in signalling pathways (62), or inhibition or uncoupling of NOS (76) will result in reduced NO production, while presence of reactive oxygen species (ROS) will breakdown the NO that is formed (77). In hypertension, any combination of these may be occurring. Where there is a deficiency of cofactors or exposure to ROS, NOS has been shown to ‘uncouple’, disabling the enzyme and producing superoxide radical (O2−) (76,78). Also, oxidation of NO gives rise to the potent nitrogen radical peroxynitrite (ONOO−) (79). Thus, these malfunctions of the NO pathway can serve to increase oxidative stress and perpetuate the loss of bioavailable NO.
Salt-sensitive and essential hypertension are characterized by impaired endothelial function, suggesting that a process that reduces bioavailable NO may contribute to elevated blood pressure (69,80). Tetrahydrobiopterin infusion improved endothelium-dependent vasodilation in humans with essential hypertension, suggesting oxidative uncoupling of NOS in endothelial dysfunction (81). Further, the antioxidant, vitamin C, improves endothelial dysfunction and this improvement is reversed using a NOS inhibitor, implicating oxidative breakdown of NO in endothelial dysfunction in essential hypertensives (7). In SHRs, treatment with a NOS inhibitor diminished ROS production, implicating NOS uncoupling as the source of oxidative stress (82). NOS activity in aorta and plasma nitrate+nitrite (NOx) was decreased, and lipid peroxides were increased in SHRs compared with normotensive WKY controls (83). Treatment with vitamin E significantly improved these alterations, suggesting that oxidative stress was involved in the decreased bioavailability of NO in this animal model (83). Zalba et al (77) have shown that in SHRs diminished availability of NO is secondary to an increase in NADH/NAPDH-oxidase-mediated superoxide production, suggesting the possible involvement of AII. Treatment with an AII type I receptor (AT1R) blocker decreased the NADH/NADPH-oxidase activity and normalized altered endothelium-dependent vasodilation, substantiating the involvement of AII (77). Binding of insulin to insulin receptors on the cell surface initiates a phosphorylation cascade along the IRS/PI 3 kinase/Akt/eNOS pathway, stimulating formation of eNOS and subsequent production of NO (71). As already discussed, phosphorylation is reduced in this insulin-signalling pathway in aorta of SHR (62), which would result in a decrease in NO production.
Salt-sensitive human hypertensive individuals had lower levels of NO metabolites in urine and plasma compared with controls when exposed to high salt diet, indicating that they failed to maintain NO production during salt loading (84,85). Similarly, Bayorh et al (86) demonstrated a decrease in plasma NO levels when DSS rats were given a high salt diet. This was associated with a reduced antioxidant capacity suggesting a role for oxidative stress in the loss of NO. In DSS rats, production of NO by neuronal NOS in response to salt was blunted (87), and high salt decreased the level of iNOS in kidney (88). Ni et al (89) also showed a decrease in eNOS in the renal cortex and medulla of kidney, and a downregulation of iNOS in aorta and kidney of DSS rats.
The RAS
The RAS plays an important role in regulating blood volume and arterial pressure (90). Angiotensinogen is abundant in many tissues and in circulating blood. The enzyme renin, produced by the kidneys, acts on angiotensinogen to produce angiotensin I, which is subsequently converted to AII by angiotensin converting enzyme (ACE). AII acts on AT1R in vascular tissue, causing vasoconstriction. AII also triggers the release of aldosterone to increase renal sodium and water retention.
AII infusion in animal models has been shown to increase blood pressure and cause an oxidative stress-related insulin resistance (40). In a murine model, Crowley et al (41) demonstrated that AII causes hypertension and cardiac hypertrophy primarily through its effects on AT1R in kidney. AII increases the production of O2− by the enzyme NADPH oxidase (Figure 2) in VSMCs in culture (91), causes release of inflammatory factors from vascular endothelial cells (92) and results in hypertrophy in proximal tubular cells (93). Binding of AII to angiotensin receptors also inhibits insulin signalling, decreasing the insulin-induced phosphorylation of the insulin receptor substrate-1 and p85 binding to insulin receptor substrate-1 (37) (Figure 2). ACE inhibitors and AII receptor blockers, commonly used antihypertensive therapies, are effective in lowering blood pressure and providing cardiovascular and renal protection (94). In humans with mild essential hypertension, a combination of these therapies decreased levels of plasma asymmetrical dimethyl arginine (ADMA), a competitive inhibitor of arginine uptake. This may implicate AII in limiting substrate availability for NO production (95). High salt diet given to DSS rats increased kidney angiotensinogen levels (96). On the other hand, both DSS and DSR show a downregulation of AT1R in aorta and kidney when exposed to high salt, indicating that this is not a factor in salt-sensitive hypertension in this model (97). Although a conflicting report showed that DSS on low salt had increased AT1R in kidney compared with their salt-resistant controls, that was further increased by high salt intake (98). In individuals with essential hypertension, high plasma AII levels in relation to sodium excretion was positively associated with cardiac hypertrophy (39). Collectively, these data seem to indicate that AII has a role in hypertension. However, there does not appear to be strong evidence implicating defects in the RAS as a primary etiological mechanism of hypertension.
It may be that the role of AII in hypertension is secondary to other agents that are involved in the etiology or development of hypertension. Studies show that blockade of NO production (99), oxidative stress (100), moderate to high insulin concentrations (101) and AGEs (102) increase AII production or AII-mediated alterations. This idea is strengthened by evidence that there is cross talk between insulin, NO and the RAS (45,70,71,103,104). It may also be that when the balance between AII and other vasoactive systems is disturbed, allowing AII to act with limited opposition, its effects are enhanced, and likely exacerbate and perpetuate hypertension (38). This may explain the antihypertensive effect of ACE inhibitors and AII receptor blockers in hypertensive patients and animal models where the RAS is not overactive.
AGE formation
Many studies show that AGEs formed from glucose and aldehydes are likely to be involved in diabetic complications (105–108). Additionally, some research suggests that increased aldehydes and AGEs also play a role in the development of hypertension (28–36,109). Increased formation of AGEs is a downstream effect of insulin resistance and oxidative stress. Insulin resistance leads to altered glucose metabolism, resulting in an increase in aldehydes including methylglyoxal (32,110,111). Oxidative stress, as seen in hypertension, increases the formation of glyoxal via lipid peroxidation and autooxidation of glucose (112). Methylglyoxal, glyoxal and other reactive aldehydes react with free sulfhydryl and amino groups of proteins to form AGEs (113–115). This structural change in proteins also alters protein function (116–120). AGEs may also influence protein function indirectly by reacting with receptors of AGEs (RAGEs) and scavenger receptors to stimulate intracellular changes (121,122).
Methylglyoxal given in diet to WKY rats increases tissue AGEs, cytosolic free calcium and blood pressure (28). As well, methylglyoxal and AGEs have been shown to be elevated in hypertensive rat models (29–31,34,36). AGEs affect the functioning of various tissue proteins and enzymes in ways that may result in hypertension. For example, sulfhydryl groups of vascular calcium channels are susceptible to inactivation by aldehydes (123,124), which may lead to increased cytosolic free calcium and abnormal contractile activity, increased resistance in peripheral vessels and hypertension. In essential human hypertensives and hypertensive animals, cytosolic free calcium levels in VSMCs are elevated (125,126). Several enzymes contain free sulfhydryl or amino groups at their active sites and AGEs alter their function. For example, alteration to antioxidant enzymes, glutathione peroxidase and glutathione reductase (116,117,127) or stimulation of superoxide-producing enzymes such as NADPH-oxidase (128), can result in increased oxidative stress (129–131). NOS and guanylate cyclase are both thiol-dependent enzymes. Alteration to these enzymes may decrease NO production, reducing vasodilatory ability and causing endothelial dysfunction (120,132). Aldehydes, including methylglyoxal, strongly bind to arginine (133), potentially contributing to a deficiency and limiting substrate availability for NO production. AGEs also influence the RAS. They act via RAGEs to increase intracellular ROS stimulating an increase AII (134). In SD rats, AGEs cause an AT1-mediated increased renal expression of angiotensinogen, ACE, renin and AT1R, increased renal ACE activity, and is associated with morphological changes in kidney (102). A rat model of AII hypertension showed increased renal AGEs and RAGEs (135).
Oxidative stress
Numerous reactive oxygen or nitrogen derived radicals are formed in the body including O2−, hydrogen peroxide (H2O2), hydroxyl radical, ONOO− and peroxynitrous acid (ONOOH). Under normal circumstances, ROS are generated from various sources and play a role in normal physiological functions (Figure 2). The enzyme NADPH-oxidase is associated with membranes of cells including endothelial and VSMCs and catalyzes the production of O2−. H2O2, is produced from dismutation of O2−. NOS can become uncoupled resulting in the production of O2− (76,78) and NO itself can react with O2− to form the reactive radicals, ONOO− and ONOOH (79,131). ONOOH is very unstable at physiological pH and rapidly decomposes to hydroxyl radical (79). ROS are controlled in part by enzymes such as superoxide dismutase (SOD), glutathione peroxidase and glutathione reductase. Alternatively, antioxidant vitamins and other reducing compounds such as lipoic acid, glutathione and cysteine reduce radicals nonenzymatically. Where there is an increased generation of ROS or a decreased antioxidant capacity (or both), the resulting imbalance is referred to as oxidative stress (131).
Although oxidative stress is strongly associated with hypertension (Figure 1) (4,5,131), it is still not clear whether it is a causative agent or results from some other coexisting pathology in hypertension. Oxidative stress alters the function of antioxidant enzymes (136), thus reducing antioxidant defence and perpetuating oxidative stress. Oxidative stress decreases the activity of glycolytic enzymes (137), likely exacerbating altered glucose metabolism and AGE formation. It also stimulates ACE activity (100), thereby increasing AII production. ROS alters cell signalling (138,139), increases intracellular calcium levels (124,140) and reduces the level of bioavailable NO (76–78). In in vitro studies, both H2O2 and O2− have been shown to produce contraction of isolated rabbit aorta, suggesting modulation of vascular tone by oxidative stress (141,142).
Humans with essential hypertension show an elevation in O2− and H2O2 production by polymorphonuclear cells (4,143), an increase in lipid peroxidation parameters in serum or plasma (143–145), and a decline in antioxidant capacity (5,144,145). In SHRs, evidence of oxidative stress appears very early, and occurs prior to blood pressure increases or inflammatory renal damage (146). In older SHRs, aortic NADPH-oxidase activity is greater when compared with control rats (77). Shear stress, AII and AGEs stimulate O2− production by this enzyme (91,128,147). Support for the role of oxidative stress in the mechanism of hypertension in SHRs comes from a study by Schnackenberg et al (148) that showed Tempol (4-hydroxy-2,2,6,6-tetamethyl-1-piperidine 1-oxy), a SOD mimetic, significantly reduced blood pressure and normalized renal vascular resistance. Use of a NOS inhibitor counteracted this effect of Tempol suggesting that its effect is due to protection of NO destruction by O2− (148). In SHRs, treatment with a NOS inhibitor diminished aortic ROS production implicating NOS uncoupling as the source of oxidative stress (82). Studies of antioxidants, including vitamins C and E, lipoic acid and N-acetyl cysteine, show that they lower blood pressure in humans and animal models of essential hypertension. This also supports a role for oxidative stress in hypertension (29,149–151).
High salt increased aortic O2− production and plasma 8-isoprostane levels, a measure of lipid peroxidation, in both DSS rats and their resistant controls (86). However, plasma and kidney reduced glutathione (GSH), and the ratio of reduced/oxidized glutathione (GSH/GSSG) in blood were lower in the salt sensitive animals. In a temporal study in DSS rats, high salt diet increased blood pressure within 1 week, followed a week later by an increase in renal and plasma oxidative stress and a decrease in NO production (152). Fujii et al (23) showed that in DSS rats, high salt treatment increased membrane NADPH-oxidase activity and expression of its subunits, p47phox and gp91phox in renal cortex. Additionally, it has also been shown that the p22phox subunit of NADPH-oxidase is increased, and Cu/Zn-SOD abundance is decreased, in kidney of DSS rats on a high salt diet (98). SD rats on high salt showed an increase in urinary lipid peroxidation products coincident with an increase NADPH-oxidase activity in kidney (153). Treatment with antioxidants, such as vitamin E and lipoic acid, attenuates but does not normalize blood pressure in DSS rats (151,154,155). Taken together, these findings in salt-sensitive hypertension suggest that oxidative stress is secondary to increased salt intake, likely via NADPH-oxidase, and contributes to elevated blood pressure.
In summary, insulin, NO and angiotensin II interact with each other to control vascular tone and blood pressure. Abnormalities in one or all of these factors, along with an increase in oxidative stress and AGEs, can increase peripheral vascular resistance and cause hypertension (Figure 1). Arginine is involved in the modulating the action of each of these (Figure 2). The next section will look at this amino acid, its normal physiological role, and its potential as a supplement to improve insulin resistance and decrease AGEs, ameliorate alterations in the nitric oxide pathway and RAS, and decrease oxidative stress to prevent hypertension.
ARGININE
Normal physiological functions
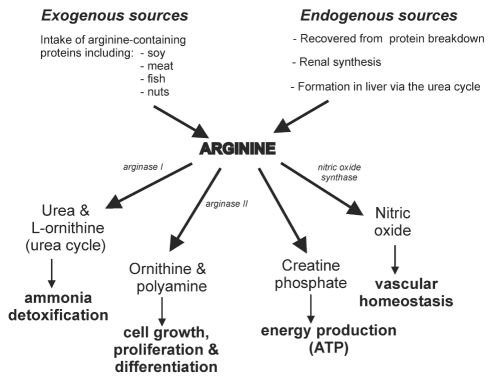
The semi-essential amino acid, arginine, can be obtained exogenously from dietary sources including meat, fish, soy, beans, lentils, whole grains and nuts (156). It is produced endogenously in kidney, and in the liver via the urea cycle, and is also recovered through normal turnover of body proteins (157,158) (Figure 3).
Figure 3).
Sources and physiological functions of arginine. ATP Adenosine triphosphate
Arginine is a key player in a set of physiological functions that interact to maintain vascular health and homeostasis (Figure 3). Arginine is used in the formation of creatine which is subsequently converted to creatine phosphate, a major source of ATP, the energy source for muscle contraction and other energy demanding processes such as cellular membrane pumps. The urea cycle is essential for conversion of toxic ammonia to urea which can be safely excreted by the kidney. In the final step of this cycle, the enzyme arginase converts arginine to urea and L-ornithine. Circulating levels of urea may affect cellular uptake of arginine (159,160). L-ornithine is used in polyamine production, which is involved in cell growth and proliferation processes, and in proline synthesis, which plays a role in production of collagen and wound healing.
Because the substrate for NOS in the formation of the potent vasodilator NO, arginine is essential in the NO pathway. Although arginine can be synthesized intracellularly, it is predominantly transported from outside the cell via receptor systems such as the Y+ and Y+L transport systems (158). This uptake of arginine may be regulated by the arginine analogue, asymmetrical dimethyl arginine (ADMA) (73,161). Other amino acids, such as lysine, also compete for these receptors (73). Another means by which arginine influences vasodilation is via insulin. Arginine promotes insulin release from the beta cells of the pancreas (162,163), and in turn, insulin decreases plasma ADMA concentrations (164) and stimulates cellular uptake of arginine (47,48). Binding of insulin to insulin receptors stimulates the production of NO via activation of an insulin-signaling pathway (71) resulting in an insulin-mediated vasodilation (42).
Arginine also modulates the RAS. It inhibits ACE activity (165), thus decreasing AII and its downstream effects. Arginine’s effect on the RAS may be insulin-mediated. Low to moderate concentrations of insulin have been shown to decrease the expression of angiotensinogen and AT1Rs in endothelial cells, while high concentrations upregulate ACE (44).
Arginine has been shown to have antioxidant activities (166,167), which may help regulate redox-sensitive proteins and may lower blood pressure.
Effect of arginine supplementation on blood pressure
Arginine is involved in several important physiological processes, many of which impact vascular function. Arginine deficiency or lack of availability, and changes in arginine metabolism, have the potential to contribute to increased blood pressure and endothelial cell dysfunction. Thus the question arises – is arginine supplementation effective in preventing or treating hypertension, and if so, what is the mechanism? This section is a review of the studies concerning the effect of arginine on blood pressure in humans and animals. We have limited our discussion to studies that looked at essential hypertension (without consideration of salt sensitivity) and those that specifically looked at salt sensitive hypertension. The term ‘arginine’ refers to L-arginine.
Salt sensitivity in humans has been defined differently by various researchers (9,55,80,85,168) but in all cases, the term essentially refers to individuals who have a significantly greater blood pressure response to changes in salt intake than others. The terms essential and salt sensitive are not mutually exclusive because 51% of those subjects classified as essential hypertensives are also salt sensitive (9). This implies that high salt is not a primary etiological agent in these subjects, but rather, further increases their blood pressure. There may be a genetic component involved in salt sensitivity. African Americans with hypertension show greater frequency of salt sensitivity versus their Caucasian counterparts (9). In a Japanese population, a positive family history of hypertension was associated with an increase in salt sensitivity (169).
Studies that investigated the effect of arginine in salt-sensitive humans are scanty. A study in humans investigated the acute effects of intravenous (iv) arginine (500 mg/kg for 30 mins) in mild to moderate hypertensive Japanese patients on a high salt diet. Results showed that although mean arterial pressure (MAP) was higher in salt-sensitive patients, arginine infusion decreased MAP by the same amount in salt-sensitive and salt-resistant patients (168). In contrast to this, a study in African Americans (27) (normotensive controls, salt-sensitive hypertensives and salt-resistant hypertensives) on high salt diet, showed that iv arginine (500 mg/kg over 30 mins) lowered MAP in all subjects, but the decrease was greater in subjects who were salt sensitive.
The majority of studies in animal models of salt sensitivity show that arginine supplementation is effective in decreasing blood pressure. Daily intraperitoneal (ip) administration of arginine (100 mg/kg or 250 mg/kg body weight for two weeks) (18) or 300 mg/kg body weight for three weeks (17) in DSS rats on a high salt diet prevented the increase in MAP. Chen et al (19) showed that oral arginine (1.25 g/L in drinking water for four weeks) prevented hypertension and nephrosclerosis in these animals. In DSS rats pretreated with high salt for two weeks, arginine was able to reverse hypertension, but was not effective in animals pretreated with high salt for three weeks (19). This suggests a ‘point of no return’ where supplemental arginine is not able to overcome the effects of some other pathological change, possibly renal or vascular damage arising from increased blood pressure or oxidative stress. In another study, oral arginine (15 g/L in drinking water for four weeks) attenuated, but did not prevent, the increase in arterial pressure in DSS rats on a high salt diet (20). Daily intramedullary infusion of arginine (300 μg/kg/min for 1 week) prevented an increase in MAP in DSS rats treated with high salt, while an iv dose given to achieve the same rise in plasma arginine levels, did not prevent the increase in blood pressure (21). Oral arginine (20 g/L in drinking water for four weeks) prevented an increase in systolic and MAP in high salt-treated DSS rats (22). Fujii et al (23) also demonstrated that oral arginine (20 g/L in drinking water for four weeks) attenuated the increase in systolic pressure in DSS rats fed a high salt diet.
Contrary to these reports, a few studies in salt-sensitive models have shown no antihypertensive effect of arginine. A study in DSS rats given oral arginine (1.25 g/L in drinking water for four weeks) showed no effect on systolic blood pressure (170). Arginine (250 mg/kg body weight/day ip for two weeks) did not prevent hypertension in SHRs given high salt diet (18).
There are a few studies of arginine supplementation in humans and animal models of essential hypertension, and the results are equivocal. Schlaich et al (171) compared blood pressure response to an intra-arterial infusion of arginine in normotensive humans with and without a family history of hypertension, and in essential hypertensives, and found that there was no effect on blood pressure in either group. In hypertensives who had failed to achieve full blood pressure control while taking an ACE inhibitor plus diuretic for three months, addition of oral arginine (6 g/day) lowered systolic and diastolic blood pressures (26). Oral arginine (2 g/L in drinking water for three weeks) had no effect on MAP but reduced nephrosclerotic changes in mature SHRs (172). A higher dose of oral arginine (7.5 g/L in drinking water for 12 weeks) also failed to lower MAP in SHRs (173). In a rat model of type 1 diabetes, oral arginine (1.25 g/L in drinking water) effectively lowered elevated systolic blood pressure to normal levels after four weeks of treatment (24). In a fructose-induced model of hypertension, oral arginine (1 g/L in drinking water for eight weeks) prevented the development of hypertension (25).
It appears that supplemental arginine is effective in lowering blood pressure in salt sensitive hypertension, but is less effective in essential hypertension. Do these findings imply anything about arginine’s potential as therapeutic agent? How does arginine produce its antihypertensive action, and what does this tell us about the pathology behind salt sensitive and essential hypertension? The following section will shed some light on these aspects.
MECHANISM OF ARGININE ACTION (FIGURE 4)
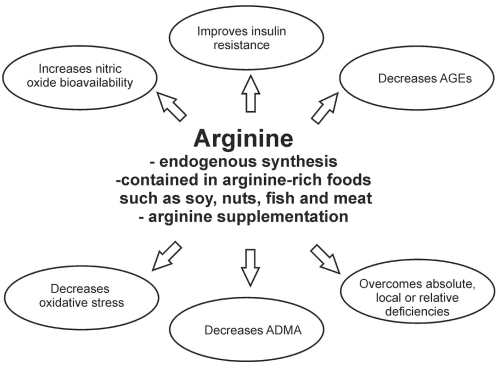
Figure 4).
Mechanism of antihypertensive action of arginine. Arginine improves insulin resistance and decreases advanced glycation end product (AGE) formation, increases nitric oxide production, decreases angiotensin II levels and reduces oxidative stress, resulting in improved endothelial function, and decreased peripheral vascular resistance and blood pressure. ADMA Asymmetrical dimethyl arginine
The term ‘arginine paradox’ was coined to explain the following phenomenon. Despite the fact that at physiological concentrations of arginine there is sufficient substrate to saturate NOS, addition of arginine increases NO production and lowers blood pressure in certain cases (174). Additionally, because arginine can be produced endogenously and most diets appear to contain sufficient arginine to meet requirements, it seems unlikely that a true arginine deficiency exists. Therefore, why does arginine supplementation work? The probable explanations for this paradox are discussed below.
Arginine overcomes absolute, local or relative deficiency
Absolute deficiency:
Under normal circumstances, the net amount of amino acids available from catabolism of body proteins is relatively constant. The balance to requirement is obtained from either dietary intake or, as in the case of arginine, from endogenous production. An abnormality that limits arginine production or re-routes arginine to a non-hemodynamic pathway over a long period of time could result in an absolute deficiency of arginine. This could also be compounded by an inadequate arginine intake.
In DSS rats, plasma arginine levels remain unchanged in response to high salt treatment compared to salt-resistant animals whose arginine levels increase (175), suggesting an inability of DSS rats to produce adequate arginine in response to high salt. The enzyme arginase competes with NOS for arginine and can thereby limit NO production. Coronary arterioles from hypertensive pigs showed impaired endothelium-dependent vasodilation with an increase in arginase I expression and arginase activity, and a decrease in eNOS protein expression (176). A study in SHRs showed initially elevated and increasing levels of vascular arginase I and II expression from five weeks of age onwards, followed by low levels of plasma arginine at 19 and 26 weeks of age (177). This may suggest an early metabolic defect shunting arginine to a catabolic pathway leading to a systemic arginine deficiency at a later age. A study in 10 week-old DSS rats also implicated over-activity of arginase in impaired availability of arginine. High salt intake increased the arteriolar expression of arginase I and II in DSS rats, and in vitro pretreatment of vessels with an arginase inhibitor or supplemental arginine restored impaired endothelium-dependent vasodilation (178).
The total body requirement for arginine will vary depending on metabolic demand. The kidney, the primary source for endogenously produced arginine, makes approximately 2 g/day. The degree of synthesis appears to be independent of arginine or protein content of the diet (157), but is regulated by citrulline delivery from the intestine. However, arginine in diet is required to maintain normal metabolism as demonstrated by models of dietary arginine deficiency (179). Considering these, it is difficult to establish a recommended daily intake for arginine. Although, as Visek (180) suggests, strictly consuming only the amount needed to meet body demands might deprive individuals of the beneficial effects that may be obtained from increased arginine intake.
The amount and type of protein included in diet, determines the intake of arginine. Foods rich in arginine include meat, fish, soy, beans, lentils, whole grains, and nuts. Recent data on dietary intake of arginine in general population is scanty. A report published in 1986 and often referenced since then (180), estimated that the arginine intake in the average US diet was 5.4 g/day, based on protein consumption (100 g/day) and composition data provide by other authors (180). Another study, the Third National Health Nutrition and Examination Survey (NHANES III), collected physical, biochemical and nutritional data from approximately 40,000 randomly selected residents in 89 communities across the United States from 1989 to 1994. Wells et al (181) used diet recall data from those participants 25 years of age and older and showed that the median arginine intake was 3.8 g/day and only about 30% had intakes higher than 5.0 g/day. Although the current North American diet generally contains a high proportion of processed and fast foods that are carbohydrate-and fat-dense, there does not appear to be any evidence that this diet is protein deficient. However, it may be possible that proteins rich in arginine are lacking. Data on arginine intake in other countries is also limited, making it is difficult to comment on the contribution of diet to potential arginine deficiency. One study using a cohort of 878 elderly men from the Zutphen study (Zutphen, The Netherlands), showed a mean arginine intake of 4.35 g/day, with meat comprising the main source of arginine (182).
A few studies have measured plasma arginine in an attempt to establish whether an absolute deficiency exists in hypertension, but information is scanty and inconclusive. One study showed that there was no difference in plasma arginine levels among normotensive individuals, normotensive individuals with a family history of hypertension, and individuals with essential hypertension (171). In contrast, two other studies reported that plasma arginine was actually higher in essential hypertensive humans (72,183).
If an absolute arginine deficiency exists, arginine supplementation would likely increase plasma levels. No data on the effect of supplementation on plasma arginine levels in salt sensitive or essential hypertensive humans are available; however, there are some data in subjects with other conditions. Oral arginine supplementation (3 g/day for three weeks) in preeclamptic women decreased blood pressure but had no effect on plasma arginine levels (184). In healthy subjects, oral supplementation with either 9 g or 21 g of arginine/day for one week increased serum arginine levels to the same extent (185). Also, three week’s of oral arginine, 12 g/day, increased plasma arginine in hypercholesterolemic men (186). Another study in hypercholesterolemic individuals showed that an oral dose of arginine (7 g/day for two weeks) increased plasma arginine by 60% (187). In DSS rats, acute (2 h) and chronic (three days) renal medullary interstitial infusion and iv infusion of arginine increased plasma arginine levels (21).
Relative deficiency:
The enzyme protein methyltransferase (PRMT) catalyzes the dimethylation of arginine contained in proteins (Figure 2). When these proteins are broken down, one of the products released is asymmetrical dimethyl arginine (ADMA), an arginine analogue. ADMA is excreted by the kidney, or broken down in liver and kidney by the redox sensitive enzyme dimethylarginine dimethylamino-hydrolase (DDAH) (Figure 2) into L-citrulline and dimethylamine. The biological activity of arginine and ADMA appear to be linked. Arginine regulates ADMA levels by inhibiting DDAH activity (188), and via insulin, may mediate a decrease in ADMA (164,189) (Figure 2). ADMA is preferentially taken up by endothelial cells (190), and this competitive action may regulate cellular uptake of arginine and NO production (Figure 2).
ADMA given intravenously to normal volunteers increased plasma ADMA levels and mean blood pressure (191). Arginine uptake has been shown to be inhibited by ADMA in platelets of normal controls and this effect is more pronounced in individuals with hypertension (161). In untreated essential hypertensive patients, plasma ADMA was elevated and the plasma arginine-to-ADMA ratio was reduced (192). In another study of untreated essential hypertensive subjects, despite baseline elevated plasma arginine and ADMA levels, intra-arterial arginine infusion enhanced endothelium-dependent vasodilation (183). In individuals with essential hypertension, elevated plasma ADMA was positively correlated to insulin resistance (189). ROS inhibit DDAH activity and increase PRMT expression (193). This would slow catabolism and promote formation of ADMA. Normotensive salt-sensitive individuals and patients with essential hypertension show an increase in plasma ADMA when exposed to high salt (85,194). In DSS rats, urinary excretion of ADMA increased in response to a high salt diet, and was positively correlated to MAP (195). These data would suggest that it is the amount of arginine relative to ADMA that determines the uptake of arginine and its availability as a substrate for NO production.
A deficiency in arginine synthesis, or a dietary intake favouring lysine-rich proteins at the expense of those containing arginine, may result in a deficiency of arginine relative to lysine. Lysine competes with arginine for cellular uptake via amino acid transport systems and may thereby interfere with arginine uptake and bioavailability (48,73). In perfused lung of lipopolysaccharide-treated neonatal pigs, arginine increased NO production, while lysine inhibited arginine uptake, reduced NO production and increased peripheral vascular resistance (196).
It may be that the amount of arginine relative to ADMA or lysine, but not its absolute amount, determines its bioactivity, and that arginine supplementation exerts its antihypertensive effects by overcoming a relative deficiency.
Local deficiency:
The concentration of arginine in specific cellular locations may be the key to its appropriate bioactivity, and localized deficiencies may be implicated in the pathology of hypertension. Arginine is present in both plasma and intracellularly. It may also be compartmentalized within the cell and may exist in higher concentrations in certain organs such as the kidney where it is involved with natriuretic functions. So, although plasma arginine concentrations may indicate sufficient or abundant levels of arginine, and arginine supplementation may increase plasma concentrations, it is not known whether this translates to appropriate concentrations of arginine where it is required.
Despite the fact that arginine can be synthesized intracellularly, arginine is predominantly transported from outside the cell via the Y+ and Y+L transport systems (72,73,158). In endothelial cells, the CAT 1 protein of the Y+ transport system accounts for 60% to 80% of total carrier mediated uptake (73,197). One study showed that arginine uptake into peripheral blood mononuclear cells was reduced in untreated essential hypertensive subjects and normotensive individuals with a family history of hypertension compared to normotensive controls subjects, and this was related to impaired endothelium-dependent vasodilation (171). However, there was no difference between groups in the endothelial mRNA or expression of CAT protein and the impaired arginine uptake was not due to an increase in plasma ADMA. Similarly, red blood cells and platelets from a group of essential hypertensive subjects showed reduced arginine influx via the Y+L system when compared with normotensive controls (72). However, there was no difference in transport via the Y+ system. Further, these authors demonstrated that Y+L transport of arginine was also diminished in red blood cells of SHRs compared with that of normotensive WKY rats.
Caveolae are invaginations of the plasma membrane where molecules involved in intracellular signalling are located (48). Colocalization of molecules such as CAT proteins and eNOS in caveolae may indicate that these sites are important to NO production (197). If arginine is not readily available at these sites, it may result in an impaired NO production.
Kidney is a critical organ in arginine metabolism and is also an essential regulator of blood volume and pressure. It is possible that renal deficiency of arginine results in defective NO synthesis in kidney leading to elevated blood pressure. In DSS rats, blood flow in the kidneys was decreased compared with resistant rats, while there was no difference in blood flow of brain, heart lung, spleen, intestine, skeletal muscle or skin. Oral arginine supplementation (15 g/L in drinking water for four weeks) normalized renal blood flow (198). Daily renal intramedullary infusion of arginine (300 μg/kg/min for one week) prevented an increase in MAP in DSS rats treated with high salt, while an iv dose of arginine, given to achieve a similar plasma arginine level, did not prevent the blood pressure increase. This suggests a renal deficiency of arginine in hypertension in this model (21). In salt-sensitive hypertensive patients, the systemic vascular response to iv arginine infusion (500 mg/kg body weight for 30 mins) was greater compared to salt resistant and normotensive controls, whereas the renal response was blunted (27). This implies that systemically administered arginine may not be reaching hemodynamically functional sites in kidney at effective concentrations or that it is not being utilized effectively at these sites in salt sensitive hypertension.
Arginine improves insulin resistance
There is little information available on the effects of arginine supplementation on insulin resistance in hypertension. In one study of SD rats with fructose-induced hypertension, oral arginine (1 g/L in drinking water for eight weeks) prevented an increase in glucose and insulin concentrations in response to an oral glucose challenge, and prevented a decrease in insulin sensitivity (25). In normal subjects, arginine infusion (30 g in 30 min) did not induce any changes in insulin receptor affinity or density in monocytes (199). In other insulin-resistant conditions, arginine appears to increase insulin sensitivity. For example, in a study of healthy controls, patients with obesity and individuals with type 2 diabetes, iv arginine infusion (0.52 mg/kg/min body weight) improved insulin sensitivity in all subjects, and restored impaired insulin-mediated vasodilation in the obese and diabetic subjects (200). Another double-blind study in type 2 diabetic patients showed that oral arginine (9 g/day for one month) improved peripheral and hepatic insulin sensitivity (201). A concurrent normalization of plasma cGMP and an increase in forearm blood flow suggests that the improvement in insulin resistance is associated with an increase in NO production and improved endothelial function (201).
A study of normotensive and hypertensive subjects demonstrated that plasma ADMA levels were positively correlated to an impairment in insulin-mediated glucose disposal in normotensive individuals, and treatment with an insulin sensitizing agent reduced ADMA levels (189). This lends support to the concept of a relative arginine deficiency, and suggests that supplementation of arginine might improve insulin resistance by restoring the arginine/ADMA ratio.
Arginine decreases AGEs
Arginine may act indirectly to limit AGEs formation by improving insulin resistance and reducing oxidative stress, thereby decreasing production of methylglyoxal and other aldehydes. Arginine may also act directly by binding to aldehydes (133), forming free glycation adducts that are excreted in urine (114,202). There is no evidence that free glycation adducts themselves cause any untoward effects. Rather, the act of scavenging aldehydes may competitively inhibit the formation of AGEs. For example, in an in vitro study (203), arginine inhibited AGE formation in human serum albumin incubated with glucose for two weeks. One in vivo study in diabetic mice (204) showed that oral arginine (50 mg/kg body weight/day) reduced the formation of the AGEs in glomerular basement membrane collagen. The enzyme DDAH has a reactive cysteine residue at its active site (205) making it susceptible to AGE modification. Increasing arginine intake may thereby protect DDAH modification and its inhibition, and thus prevent an increase in ADMA. Also, as DDAH activity is inhibited and PRMT expression increased by ROS (193), and AGEs are known to increase oxidative stress, the lowering of AGEs might also prevent these oxidative changes that lead to elevated ADMA.
Arginine improves NO bioavailability
Some research shows that arginine improves NO production and this may account for its antihypertensive properties. ADMA inhibited eNOS in vitro, and inhibited eNOS-derived NO generation in endothelial cells in culture. Addition of arginine diminished this effect (190). In individuals newly diagnosed with essential hypertension, plasma arginine and ADMA levels were elevated. However, despite having no effect on blood pressure, intra-arterial infusion of arginine in these patients enhanced the impaired endothelium-dependent vasodilation (183). Similarly, in a randomized, double-blind trial of a single oral dose of arginine (6 g) in subjects with essential hypertension, arginine had no effect on blood pressure but improved impaired endothelium-dependent flow-mediated dilation (206). A study of oral arginine (7.5 g/L in drinking water for 12 weeks) in SHRs showed an increase in cardiac cGMP and NOx content, indirect measures of NO production, associated with an attenuation of cardiac hypertrophy with no effect on blood pressure (173). In contrast to these findings, a study by Panza et al (6) in essential hypertensives, showed no effect of intra-arterial infusion of arginine on endothelium-dependent vasodilation suggesting that a decreased availability of arginine was not involved in the impaired endothelial response.
The results of these studies of essential hypertension predominantly suggest that arginine increases bioavailable NO thereby improving endothelial function but not blood pressure. This implies the following: that defects in peripheral NO and endothelial dysfunction do not play a major role in increasing blood pressure in essential hypertension; that the routes of arginine administration or doses used in these studies are inappropriate to supply arginine in sufficient quantities and/or to critical sites of action (ie, kidney) to influence blood pressure; or that any positive effect of increased NO production on blood pressure is being masked or counteracted by other forces such as oxidative stress or alterations in the RAS.
In contrast, oral arginine, given to salt-sensitive rat models, appears to be effective in controlling blood pressure and improving endothelial dysfunction by increasing NO. DSS rats on high salt diet, oral arginine (20 g/L in drinking water for four weeks) lowered blood pressure, increased urinary cGMP and NOx, and improved impaired endothelium-dependent vasodilation (22). Similarly, oral arginine (1.25 g/L in drinking water for two weeks) increased urinary excretion of cGMP and prevented hypertension in DSS rats (18). Tomohiro et al (198) studied regional blood flow in DSS rats on high salt diet and showed that blood flow to kidney, but not other tissues, was impaired. Furthermore, oral arginine (15 g/L in drinking water for four weeks) improved renal blood flow and prevented hypertension, implicating abnormalities in renal NO production in increased blood pressure in this model of salt sensitive hypertension.
Renal medullary NO production plays an important role in regulation of natriuresis and blood pressure control (68), which may make it particularly critical in salt-sensitive hypertension. Renal medullary interstitial infusion of arginine in DSS rats on high salt increased medullary blood flow, a functional indicator of NO production, and prevented hypertension. To assess whether the arginine administered in the kidney was escaping and acting systemically to lower blood pressure, a dose designed to give an equivalent increase in plasma arginine was given intravenously. This did not result in prevention of hypertension, indicating that arginine, at this dose, was acting locally in kidney, not systemically, to lower blood pressure in this model (21). An arginine-induced (500 mg/kg body weight iv over 30 min) increase in renal blood flow and plasma cGMP was reduced by salt loading in salt-sensitive hypertensive patients (168), suggesting that high salt either reduces the ability of arginine to produce NO in the endothelium of renal vasculature, or that some condition exists in salt sensitive patients (eg. oxidative stress) that disables the NO that is produced before it can act. Campese et al (27) demonstrated that iv infusion of arginine improved renal plasma flow in all subjects on high salt, but was less effective in salt-sensitive hypertensive patients compared with controls and salt-resistant individuals, suggesting an impairment of renal NO that is to some degree compensated for by supplementation of substrate.
Arginine modulates the RAS
Arginine supplementation has been shown to inhibit ACE activity (165), and this would decrease production of AII and limit its downstream effects. The effect of arginine may also be mediated by insulin. This hormone has been shown to decrease the expression of angiotensinogen and AT1 receptors in endothelial cells (44,207). In hypertension, the effects of AII may be pronounced as a result of a functional imbalance arising from a decrease in opposing forces (insulin and NO). If arginine improves insulin resistance and NO production, this imbalance will be corrected, restoring vascular homeostasis.
Arginine lowers oxidative stress
Arginine has been shown to have antioxidant properties. This may be an indirect action achieved by ensuring normal function of the nitric oxide pathway and the RAS, thus minimizing radicals (O2−, ONOO−) generated by these systems. In a more direct antioxidant action, arginine has been shown to react nonenzymatically with H2O2 to form NO in vitro (208). This would not only increase levels of the vasodilator, but decrease a potentially harmful reactive oxygen species. In an in vitro study of vascular endothelial cells in culture, arginine diminished superoxide generation in a concentration dependent manner, and protected extracellularly applied NO from degradation (167). In diabetic patients, oral arginine (2 g/day for three months) decreased urine malondialdehyde levels, a measure of lipid peroxidation (166), and in diabetic hypertensive rats, oral arginine (1.25 g/L for four weeks) normalized plasma malondialdehyde levels (24).
The antioxidant ability of arginine may help regulate redox-sensitive proteins. For example, the enzyme DDAH which controls the degradation of ADMA is a redox-sensitive enzyme that is inhibited by ROS (193). By preventing this oxidation, arginine may increase breakdown of ADMA thereby reducing competitive inhibition of arginine uptake. In DSS rats, high salt increased NADPH oxidase activity and expression of p47phox and gp91phox, subunits of NADPH-oxidase in the membrane fraction of the renal cortex. Treatment with oral arginine counteracted these changes (23). It has been suggested that the balance of NO and O2− regulates normal kidney function (209). If alterations in NO bioavailability in kidney stem from an imbalance favoring O2−, then the antihypertensive effects of arginine may be due at least in part to its antioxidant effects.
Arginine as a therapeutic agent
Is there sufficient evidence to suggest that arginine could be used as a therapeutic agent? Supplemental arginine has the ability to increase absolute and relative levels of plasma arginine, improve insulin resistance and decrease AGE formation, increase NO production, decrease AII production, and reduce oxidative stress. Any of these actions may result in a decrease in blood pressure and attenuation of hypertensive complications, possibly justifying arginine as a therapeutic agent.
A review of arginine studies indicates that it is effective in preventing an increase in blood pressure in salt-sensitive hypertension. As there is a dearth of studies in salt sensitive humans, this conclusion is based mostly on results from studies in animal models of salt sensitivity. There is a limitation of using animal models to study human diseases. However, specific models do generally represent disease conditions and are most convenient for studying in vivo mechanism of disease and response to treatments. If the results of the studies on arginine supplementation in salt sensitive models apply to human populations, arginine could have significant potential as an antihypertensive treatment as approximately half of individuals with hypertension are salt sensitive. As demographics or family history (9,169) appears to predispose certain groups to salt sensitivity, arginine may represent a potential preventative measure in these individuals. In humans and animals with essential hypertension, arginine appears to be less effective in lowering blood pressure although it does improve endothelial dysfunction. The inconsistency of effect in salt sensitive and essential hypertensives may reflect the varying degree to which arginine deficiency or altered metabolism, insulin resistance, alterations in the NO pathway and RAS, or oxidative stress, are involved in these forms of hypertension. Whatever the case, arginine supplementation appears to improve endothelium-dependent vasodilation in hypertension.
Of the studies reviewed, many showed the effectiveness of ip, iv or intrarenal administration of arginine on blood pressure, but these routes of administration are impractical in the day-to-day treatment of humans. Several other studies also reported the ability of oral doses of arginine to lower blood pressure. In rat studies, oral arginine was given in drinking water without providing water consumption data making it difficult to establish the effective daily dose. In a study designed to investigate the pharmacokinetics and safety of arginine, using both oral and iv applications, Wu et al (210) studied its effects in pigs, rats and sheep. These authors concluded that a 70 kg human subject should be able to tolerate long-term parenteral and enteral supplementation doses of 6 g/day and 15 g/day, respectively, in addition to arginine obtained in normal diet of 4 g/day to 6 g/day. Additionally, several studies in hypercholesterolemic humans have shown that daily oral doses of arginine of 7 g/day to 21 g/day for up to 12 weeks (186,187,211–213) are effective in lowering blood pressure (186), increasing plasma arginine levels (187), normalizing plasma arginine/ADMA ratio (213), attenuating mononuclear cell adhesiveness ((187,213) and platelet aggregation (212), and improving endothelium-dependent vasodilation (211). These doses have been well tolerated, with few gastrointestinal side effects usually only seen at the highest dosage.
A few studies have suggested that arginine supplementation may be detrimental to vascular health. In apoE/iNOS double knockout mice fed a typical western-type diet, oral arginine supplementation (25 g/L in drinking water for 24 weeks) negated the protective effect of iNOS deficiency causing increased atherosclerotic lesion size and oxidative stress (214). Loscalzo (215) suggested in a commentary editorial, that by providing substrate that increases NO production in an oxidative environment, the nitrogen radical, peroxynitrite, may be overproduced resulting in local vascular oxidative stress. This author also suggested that increasing arginine might result in elevations in homocysteine, ADMA and ROS (215). A later study by Handy et al (216) did show that arginine supplementation in this same model increased plasma homocysteine by 37%. The dose used in these studies was higher than that used in many arginine studies. This may have resulted in an amino acid imbalance which could have had some impact on the findings. However, in a study of hypercholesterolemic rabbits, a similar dose was shown to block progression of atherosclerotic lesions (217).
Arginine supplementation has been suggested for use as a prophylactic treatment in diabetics to prevent AGE formation and diabetic complications. Tsai et al (218) performed an in vitro study designed to test for potential adverse effects of arginine for this purpose. They added methylglyoxal (1.56 μM) to arginine (2.0 mM to 8.0 mM) and showed that this reaction produced superoxide radical in a dose-dependent manner. Additionally, serum from diabetic patients treated with arginine (8.0 mM) also showed significantly more superoxide production than serum of control subjects. They suggested arginine should be used with caution due to this ability to produce harmful radicals. Taking arginine in combination with an antioxidant may overcome this potential to increase oxidative stress and allow arginine to work effectively without untoward effect. A study of pregnant SHR and WKY rats and their offspring showed that intake of arginine and antioxidants combined for two weeks before, and four and eight weeks after birth, reduced spontaneous increases in blood pressure in SHR and age-related increases in WKY rats. This treatment also provided a persistent reduction in blood pressure and renal protection in SHR even after supplementation was withdrawn (219).
CONCLUSION
Essential hypertension develops due to an interaction of genetic traits and lifestyle factors such as diet. Increased dietary salt is one lifestyle factor that causes an increase in blood pressure, and approximately one-half of individuals with hypertension are salt sensitive. Both salt-sensitive and essential hypertension are associated with insulin resistance and its downstream effects, including altered glucose metabolism and AGEs formation. Hypertension is also associated with alterations in the NO pathway and RAS, and with oxidative stress. As arginine modulates insulin, NO and AII, it is reasonable to assume that it may address these abnormalities that lead to hypertension. Studies show that arginine attenuates insulin resistance and decreases AGE formation, increases NO production, decreases AII levels, reduces oxidative stress and improves endothelial function. Additionally, animal studies provide substantial evidence that arginine is an effective anti-hypertensive agent in salt-sensitive models due, primarily, to its ability to improve NO bioavailability, particularly in the kidney. In models of essential hypertension, where loss of bioavailable NO appears a result of increased oxidative breakdown, arginine combined with an antioxidant may be more effective in lowering blood pressure. The paucity of human studies in salt-sensitive and essential hypertension, combined with the slight possibility of adverse effects, indicates that more studies in humans need to be done before supplementary arginine can be recommended broadly for treatment of hypertension. It may be beneficial for individuals with, or at risk for hypertension, particularly salt-sensitive hypertension, to limit foods high in salt and to increase their intake of foods containing arginine. Ideally, a lifestyle change to include a balanced diet such as the DASH diet, that is low in salt, and rich in antioxidants and arginine-containing proteins such as meat, fish, soy, nuts, whole grains, lentils and beans, may be advisable to obtain the most benefit from nutrients with antihypertensive properties.
Acknowledgments
We would like to thank the Canadian Institutes of Health Research Regional Partnership Program and the Discipline of Medicine, Memorial University, St John’s, Newfoundland, for financial support.
REFERENCES
- 1.World Health Organization . Global strategy on diet, physical activity, and health: Chronic disease risk factors. < www.who.int/dietphysicalactivity/publications/facts/riskfactors/en> (Version current at November 28, 2007). [Google Scholar]
- 2.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–7. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Insulin resistance, hyperinsulinemia, and hypertriglyceridemia in the etiology and clinical course of hypertension. Am J Med. 1991;90:7S–12S. doi: 10.1016/0002-9343(91)90028-v. [DOI] [PubMed] [Google Scholar]
- 4.Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK. Oxygen free radicals in essential hypertension. Mol Cell Biochem. 1992;111:103–8. doi: 10.1007/BF00229580. [DOI] [PubMed] [Google Scholar]
- 5.Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- 6.Panza JA, Casino PR, Badar DM, Quyyumi AA. Effect of increased availability of endothelium-derived nitric oxide precursor on endothelium-dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation. 1993;87:1475–81. doi: 10.1161/01.cir.87.5.1475. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–9. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 8.Hill GS, Heudes D, Jacquot C, Gauthier E, Bariety J. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int. 2006;69:823–31. doi: 10.1038/sj.ki.5000163. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–34. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 12.Elliott P. Protein intake and blood pressure in cardiovascular disease. Proc Nutr Soc. 2003;62:495–504. doi: 10.1079/pns2003266. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Sacks FM, Carey VJ, et al. OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 14.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT) Circulation. 1996;94:2417–23. doi: 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 15.Stamler J, Elliott P, Kesteloot H, et al. Inverse relation of dietary protein markers with blood pressure. Findings for 10,020 men and women in the INTERSALT study. INTERSALT Cooperative Research Group. INTERnational study of SALT and blood pressure. Circulation. 1996;94:1629–34. doi: 10.1161/01.cir.94.7.1629. [DOI] [PubMed] [Google Scholar]
- 16.Vasdev S, Gill V. Effect of soybean in diet on blood pressure. Hypertens Canada. 2006;88:3–5. [Google Scholar]
- 17.Patel A, Layne S, Watts D, Kirchner KA. L-arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension. 1993;22:863–9. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]
- 18.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–67. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PY, St John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest. 1993;68:174–84. [PubMed] [Google Scholar]
- 20.He H, Kimura S, Fujisawa Y, et al. Dietary L-arginine supplementation normalizes regional blood flow in Dahl-Iwai salt-sensitive rats. Am J Hypertens. 1997;10:89S–93S. [PubMed] [Google Scholar]
- 21.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr Renal medullary interstitial infusion of L-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol. 1998;275:R1667–73. doi: 10.1152/ajpregu.1998.275.5.R1667. [DOI] [PubMed] [Google Scholar]
- 22.Zhou MS, Kosaka H, Tian RX, et al. L-arginine improves endothelial function in renal artery of hypertensive Dahl rats. J Hypertens. 2001;19:421–9. doi: 10.1097/00004872-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Zhang L, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42:1014–20. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- 24.Ozcelikay AT, Tay A, Guner S, et al. Reversal effects of L-arginine treatment on blood pressure and vascular responsiveness of streptozotocin-diabetic rats. Pharmacol Res. 2000;41:201–9. doi: 10.1006/phrs.1999.0576. [DOI] [PubMed] [Google Scholar]
- 25.Tay A, Ozcelikay AT, Altan VM. Effects of L-arginine on blood pressure and metabolic changes in fructose-hypertensive rats. Am J Hypertens. 2002;15:72–7. doi: 10.1016/s0895-7061(01)02231-2. [DOI] [PubMed] [Google Scholar]
- 26.Pezza V, Bernardini F, Pezza E, Pezza B, Curione M. Study of supplemental oral l-arginine in hypertensives treated with enalapril + hydrochlorothiazide. Am J Hypertens. 1998;11:1267–70. doi: 10.1016/s0895-7061(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 27.Campese VM, Amar M, Anjali C, Medhat T, Wurgaft A. Effect of L-arginine on systemic and renal haemodynamics in salt-sensitive patients with essential hypertension. J Hum Hypertens. 1997;11:527–32. doi: 10.1038/sj.jhh.1000485. [DOI] [PubMed] [Google Scholar]
- 28.Vasdev S, Ford CA, Longerich L, Parai S, Gadag V. Aldehyde induced hypertension in rats: Prevention by N-acetylcysteine. Artery. 1998;23:10–36. [PubMed] [Google Scholar]
- 29.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:567–73. doi: 10.1097/00004872-200018050-00009. [DOI] [PubMed] [Google Scholar]
- 30.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr Metab Cardiovasc Dis. 2000;10:339–46. [PubMed] [Google Scholar]
- 31.Vasdev S, Gill V, Longerich L, Parai S, Gadag V. Salt-induced hypertension in WKY rats: Prevention by alpha-lipoic acid supplementation. Mol Cell Biochem. 2003;254:319–26. doi: 10.1023/a:1027354005498. [DOI] [PubMed] [Google Scholar]
- 32.Vasdev S, Gill V, Singal P. Role of advanced glycation end products in hypertension and atherosclerosis: Therapeutic implications. Cell Biochem Biophys. 2007;49:48–63. doi: 10.1007/s12013-007-0039-0. [DOI] [PubMed] [Google Scholar]
- 33.Wu L. Is methylglyoxal a causative factor for hypertension development? Can J Physiol Pharmacol. 2006;84:129–39. doi: 10.1139/Y05-137. [DOI] [PubMed] [Google Scholar]
- 34.Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16:173–9. doi: 10.1016/s0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Desai K, Chang T, Wu L. Vascular methylglyoxal metabolism and the development of hypertension. J Hypertens. 2005;23:1565–73. doi: 10.1097/01.hjh.0000173778.85233.1b. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Desai K, Clausen JT, Wu L. Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney Int. 2004;66:2315–21. doi: 10.1111/j.1523-1755.2004.66034.x. [DOI] [PubMed] [Google Scholar]
- 37.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–69. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulman IH, Zhou MS, Raij L. Nitric oxide, angiotensin II, and reactive oxygen species in hypertension and atherogenesis. Curr Hypertens Rep. 2005;7:61–7. doi: 10.1007/s11906-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 39.Schmieder RE, Langenfeld MR, Friedrich A, Schobel HP, Gatzka CD, Weihprecht H. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation. 1996;94:1304–9. doi: 10.1161/01.cir.94.6.1304. [DOI] [PubMed] [Google Scholar]
- 40.Ogihara T, Asano T, Ando K, et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40:872–9. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 41.Crowley SD, Gurley SB, Herrera MJ, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–90. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin’s vascular effects in humans. J Clin Invest. 1994;94:2511–5. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–42. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 44.Kamide K, Rakugi H, Nagai M, et al. Insulin-mediated regulation of the endothelial renin-angiotensin system and vascular cell growth. J Hypertens. 2004;22:121–7. doi: 10.1097/00004872-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Folli F, Saad MJ, Velloso L, et al. Crosstalk between insulin and angiotensin II signalling systems. Exp Clin Endocrinol Diabetes. 1999;107:133–9. doi: 10.1055/s-0029-1212088. [DOI] [PubMed] [Google Scholar]
- 46.Tiwari S, Riazi S, Ecelbarger CA. Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol. 2007;293:F974–84. doi: 10.1152/ajprenal.00149.2007. [DOI] [PubMed] [Google Scholar]
- 47.Sobrevia L, Nadal A, Yudilevich DL, Mann GE. Activation of L-arginine transport (system y+) and nitric oxide synthase by elevated glucose and insulin in human endothelial cells. J Physiol. 1996;490:775–81. doi: 10.1113/jphysiol.1996.sp021185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 49.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 50.Levy J, Zemel MB, Sowers JR. Role of cellular calcium metabolism in abnormal glucose metabolism and diabetic hypertension. Am J Med. 1989;87:7S–16S. doi: 10.1016/0002-9343(89)90489-0. [DOI] [PubMed] [Google Scholar]
- 51.Beatty OL, Harper R, Sheridan B, Atkinson AB, Bell PM. Insulin resistance in offspring of hypertensive parents. BMJ. 1993;307:92–6. doi: 10.1136/bmj.307.6896.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makris TA, Paizis I, Krespi PG, et al. Insulin receptor number is reduced in healthy offspring of patients with essential hypertension. Am J Hypertens. 2004;17:911–4. doi: 10.1016/j.amjhyper.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Margalet V, Valle M, Lobon JA, et al. Diminished insulin receptors on erythrocyte ghosts in nonobese patients with essential hypertension independent of hyperinsulinemia. J Cardiovasc Pharmacol. 1994;24:74–7. doi: 10.1097/00005344-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Giner V, Coca A, de la Sierra A. Increased insulin resistance in salt sensitive essential hypertension. J Hum Hypertens. 2001;15:481–5. doi: 10.1038/sj.jhh.1001216. [DOI] [PubMed] [Google Scholar]
- 55.Fuenmayor N, Moreira E, Cubeddu LX. Salt sensitivity is associated with insulin resistance in essential hypertension. Am J Hypertens. 1998;11:397–402. doi: 10.1016/s0895-7061(97)00490-1. [DOI] [PubMed] [Google Scholar]
- 56.Sharma AM, Schorr U, Distler A. Insulin resistance in young salt-sensitive normotensive subjects. Hypertension. 1993;21:273–9. doi: 10.1161/01.hyp.21.3.273. [DOI] [PubMed] [Google Scholar]
- 57.Hulman S, Falkner B, Freyvogel N. Insulin resistance in the conscious spontaneously hypertensive rat: Euglycemic hyperinsulinemic clamp study. Metabolism. 1993;42:14–8. doi: 10.1016/0026-0495(93)90165-k. [DOI] [PubMed] [Google Scholar]
- 58.Mondon CE, Reaven GM, Azhar S, Lee CM, Rabkin R. Abnormal insulin metabolism by specific organs from rats with spontaneous hypertension. Am J Physiol. 1989;257:E491–8. doi: 10.1152/ajpendo.1989.257.4.E491. [DOI] [PubMed] [Google Scholar]
- 59.Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Hypertension. 1995;26:290–3. doi: 10.1161/01.hyp.26.2.290. [DOI] [PubMed] [Google Scholar]
- 60.Kahn CR, Saad MJ. Alterations in insulin receptor and substrate phosphorylation in hypertensive rats. J Am Soc Nephrol. 1992;3:S69–77. doi: 10.1681/ASN.V34s69. [DOI] [PubMed] [Google Scholar]
- 61.Sechi LA, Griffin CA, Giacchetti G, et al. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertension. 1996;27:955–61. doi: 10.1161/01.hyp.27.4.955. [DOI] [PubMed] [Google Scholar]
- 62.Zecchin HG, Bezerra RM, Carvalheira JB, et al. Insulin signalling pathways in aorta and muscle from two animal models of insulin resistance – the obese middle-aged and the spontaneously hypertensive rats. Diabetologia. 2003;46:479–91. doi: 10.1007/s00125-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 63.Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol. 1993;264:E730–4. doi: 10.1152/ajpendo.1993.264.5.E730. [DOI] [PubMed] [Google Scholar]
- 64.Ogihara T, Asano T, Ando K, et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension. 2002;40:83–9. doi: 10.1161/01.hyp.0000022880.45113.c9. [DOI] [PubMed] [Google Scholar]
- 65.Sechi LA, Griffin CA, Zingaro L, et al. Glucose metabolism and insulin receptor binding and mRNA levels in tissues of Dahl hypertensive rats. Am J Hypertens. 1997;10:1223–30. doi: 10.1016/s0895-7061(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 66.Ogihara T, Asano T, Ando K, et al. Insulin resistance with enhanced insulin signaling in high-salt diet-fed rats. Diabetes. 2001;50:573–83. doi: 10.2337/diabetes.50.3.573. [DOI] [PubMed] [Google Scholar]
- 67.Sechi LA, Griffin CA, Schambelan M. Effect of dietary sodium chloride on insulin receptor number and mRNA levels in rat kidney. Am J Physiol. 1994;266:F31–8. doi: 10.1152/ajprenal.1994.266.1.F31. [DOI] [PubMed] [Google Scholar]
- 68.Cowley AW, Jr, Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–69. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 69.Taddei S, Ghiadoni L, Virdis A, Versari D, Salvetti A. Mechanisms of endothelial dysfunction: Clinical significance and preventive non-pharmacological therapeutic strategies. Curr Pharm Des. 2003;9:2385–402. doi: 10.2174/1381612033453866. [DOI] [PubMed] [Google Scholar]
- 70.Kahn NN, Acharya K, Bhattacharya S, et al. Nitric oxide: The “second messenger” of insulin. IUBMB Life. 2000;49:441–50. doi: 10.1080/152165400410308. [DOI] [PubMed] [Google Scholar]
- 71.Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–45. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 72.Moss MB, Brunini TM, Soares De Moura R, et al. Diminished L-arginine bioavailability in hypertension. Clin Sci (Lond) 2004;107:391–7. doi: 10.1042/CS20030412. [DOI] [PubMed] [Google Scholar]
- 73.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 74.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–20. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 75.Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Diez J. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol Dial Transplant. 2001;16(Suppl 1):2–5. doi: 10.1093/ndt/16.suppl_1.2. [DOI] [PubMed] [Google Scholar]
- 78.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–8. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 79.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–8. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 81.Higashi Y, Sasaki S, Nakagawa K, Fukuda, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–32. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 82.Li H, Witte K, August M, Brausch I, et al. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–44. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 83.Newaz MA, Nawal NN, Rohaizan CH, Muslim N, Gapor A. Alpha-tocopherol increased nitric oxide synthase activity in blood vessels of spontaneously hypertensive rats. Am J Hypertens. 1999;12:839–44. doi: 10.1016/s0895-7061(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 84.Cubeddu LX, Alfieri AB, Hoffmann IS, et al. Nitric oxide and salt sensitivity. Am J Hypertens. 2000;13:973–9. doi: 10.1016/s0895-7061(00)00283-1. [DOI] [PubMed] [Google Scholar]
- 85.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: Modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–61. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 86.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–6. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in dahl rats. Am J Physiol. 1995;268:H2375–83. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 88.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW., Jr Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens. 2000;18:1497–505. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 89.Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension. 1999;34:552–7. doi: 10.1161/01.hyp.34.4.552. [DOI] [PubMed] [Google Scholar]
- 90.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 91.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 92.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 93.Wolf G, Jablonski K, Schroeder R, Reinking R, Shankland SJ, Stahl RA. Angiotensin II-induced hypertrophy of proximal tubular cells requires p27Kip1. Kidney Int. 2003;64:71–81. doi: 10.1046/j.1523-1755.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 94.Stojiljkovic L, Behnia R. Role of renin angiotensin system inhibitors in cardiovascular and renal protection: A lesson from clinical trials. Curr Pharm Des. 2007;13:1335–45. doi: 10.2174/138161207780618768. [DOI] [PubMed] [Google Scholar]
- 95.Delles C, Schneider MP, John S, Gekle M, Schmieder RE. Angiotensin converting enzyme inhibition and angiotensin II AT1-receptor blockade reduce the levels of asymmetrical N(G), N(G)-dimethylarginine in human essential hypertension. Am J Hypertens. 2002;15:590–3. doi: 10.1016/s0895-7061(02)02278-1. [DOI] [PubMed] [Google Scholar]
- 96.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strehlow K, Nickenig G, Roeling J, et al. AT(1) receptor regulation in salt-sensitive hypertension. Am J Physiol. 1999;277:H1701–7. doi: 10.1152/ajpheart.1999.277.5.H1701. [DOI] [PubMed] [Google Scholar]
- 98.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28:158–67. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 99.Luvara G, Pueyo ME, Philippe M, et al. Chronic blockade of NO synthase activity induces a proinflammatory phenotype in the arterial wall: Prevention by angiotensin II antagonism. Arterioscler Thromb Vasc Biol. 1998;18:1408–16. doi: 10.1161/01.atv.18.9.1408. [DOI] [PubMed] [Google Scholar]
- 100.Ikemoto F, Song G, Tominaga M, Yamamoto K. Oxidation-induced increase in activity of angiotensin converting enzyme in the rat kidney. Biochem Biophys Res Commun. 1988;153:1032–7. doi: 10.1016/s0006-291x(88)81332-9. [DOI] [PubMed] [Google Scholar]
- 101.Tuck ML, Bounoua F, Eslami P, Nyby MD, Eggena P, Corry DB. Insulin stimulates endogenous angiotensin II production via a mitogen-activated protein kinase pathway in vascular smooth muscle cells. J Hypertens. 2004;22:1779–85. doi: 10.1097/00004872-200409000-00023. [DOI] [PubMed] [Google Scholar]
- 102.Thomas MC, Tikellis C, Burns WM, et al. Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol. 2005;16:2976–84. doi: 10.1681/ASN.2005010013. [DOI] [PubMed] [Google Scholar]
- 103.Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211–8. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 104.Horiuchi M, Mogi M, Iwai M. Signaling crosstalk angiotensin II receptor subtypes and insulin. Endocr J. 2006;53:1–5. doi: 10.1507/endocrj.53.1. [DOI] [PubMed] [Google Scholar]
- 105.Beisswenger PJ, Makita Z, Curphey TJ, et al. Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes. 1995;44:824–9. doi: 10.2337/diab.44.7.824. [DOI] [PubMed] [Google Scholar]
- 106.Aso Y, Inukai T, Tayama K, Takemura Y. Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol. 2000;37:87–92. doi: 10.1007/s005920070025. [DOI] [PubMed] [Google Scholar]
- 107.Meerwaldt R, Links TP, Graaff R, et al. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia. 2005;48:1637–44. doi: 10.1007/s00125-005-1828-x. [DOI] [PubMed] [Google Scholar]
- 108.Mostafa AA, Randell EW, Vasdev SC, et al. Plasma protein advanced glycation end products, carboxymethyl cysteine, and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol Cell Biochem. 2007;302:35–42. doi: 10.1007/s11010-007-9422-9. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, Chang T, Jiang B, Desai K, Wu L. Attenuation of hypertension development by aminoguanidine in spontaneously hypertensive rats: Role of methylglyoxal. Am J Hypertens. 2007;20:629–36. doi: 10.1016/j.amjhyper.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- 111.Beisswenger PJ, Howell SK, Smith K, Szwergold BS. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim Biophys Acta. 2003;1637:98–106. doi: 10.1016/s09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 112.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–62. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 113.Zeng J, Davies MJ. Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of alpha-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chem Res Toxicol. 2005;18:1232–41. doi: 10.1021/tx050074u. [DOI] [PubMed] [Google Scholar]
- 114.Thornalley PJ, Battah S, Ahmed N, et al. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581–92. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 116.Morgan PE, Dean RT, Davies MJ. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch Biochem Biophys. 2002;403:259–69. doi: 10.1016/s0003-9861(02)00222-9. [DOI] [PubMed] [Google Scholar]
- 117.Park YS, Koh YH, Takahashi M, Miyamoto Y, et al. Identification of the binding site of methylglyoxal on glutathione peroxidase: Methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites arg 184 and 185. Free Radic Res. 2003;37:205–11. doi: 10.1080/1071576021000041005. [DOI] [PubMed] [Google Scholar]
- 118.Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289–99. doi: 10.2337/db05-0857. [DOI] [PubMed] [Google Scholar]
- 119.Jia X, Olson DJ, Ross AR, Wu L. Structural and functional changes in human insulin induced by methylglyoxal. FASEB J. 2006;20:1555–7. doi: 10.1096/fj.05-5478fje. [DOI] [PubMed] [Google Scholar]
- 120.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;17:1289–91. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- 121.Collison KS, Parhar RS, Saleh SS, et al. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs) J Leukoc Biol. 2002;71:433–44. [PubMed] [Google Scholar]
- 122.Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283–92. doi: 10.1007/s00726-003-0029-5. [DOI] [PubMed] [Google Scholar]
- 123.Schauenstein E, Esterbauer H, Zollner H. Aldehydes in biological systems. In: Lagnado JR, editor. Aldehydes in Biological Systems, their Natural Occurrence and Biological Activities. London: Pion Limited; 1977. pp. 1–7. [Google Scholar]
- 124.Zaidi NF, Lagenaur CF, Abramson JJ, Pessah I, Salama G. Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. J Biol Chem. 1989;264:21725–36. [PubMed] [Google Scholar]
- 125.Aoki K, Kawaguchi Y, Sato K, Kondo S, Yamamoto M. Clinical and pharmacological properties of calcium antagonists in essential hypertensionin humans and spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1982;4:S298–S302. [PubMed] [Google Scholar]
- 126.Robinson BF. Altered caclium handling as a cause of primary hypertension. J Hypertens. 1984;2:435–60. doi: 10.1097/00004872-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 127.Stadtman TC. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–27. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- 128.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 129.Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–14. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 130.Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-beta(II) in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676–83. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 131.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 132.Brandwein HJ, Lewicki JA, Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem. 1981;256:2958–62. [PubMed] [Google Scholar]
- 133.Selwood T, Thornalley PJ. Binding of methylglyoxal to albumin and formation of fluorescent adducts. inhibition by arginine, N-alpha-acetylarginine and aminoguanidine. Biochem Soc Trans. 1993;21:170S. doi: 10.1042/bst021170s. [DOI] [PubMed] [Google Scholar]
- 134.Fukami K, Ueda S, Yamagishi S, Kato S, et al. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137–47. doi: 10.1111/j.1523-1755.2004.66004.x. [DOI] [PubMed] [Google Scholar]
- 135.Bohlender J, Franke S, Sommer M, Stein G. Advanced glycation end products: A possible link to angiotensin in an animal model. Ann N Y Acad Sci. 2005;1043:681–4. doi: 10.1196/annals.1333.078. [DOI] [PubMed] [Google Scholar]
- 136.Tabatabaie T, Floyd RA. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch Biochem Biophys. 1994;314:112–9. doi: 10.1006/abbi.1994.1418. [DOI] [PubMed] [Google Scholar]
- 137.Tsuchiya Y, Yamaguchi M, Chikuma T, Hojo H. Degradation of glyceraldehyde-3-phosphate dehydrogenase triggered by 4-hydroxy-2-nonenal and 4-hydroxy-2-hexenal. Arch Biochem Biophys. 2005;438:217–22. doi: 10.1016/j.abb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 138.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–6. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 139.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–14. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 140.Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–8. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 141.Bharadwaj L, Prasad K. Mediation of H2O2-induced vascular relaxation by endothelium-derived relaxing factor. Mol Cell Biochem. 1995:149–150. 267–70. doi: 10.1007/BF01076587. [DOI] [PubMed] [Google Scholar]
- 142.Bharadwaj L, Prasad K. Mechanism of superoxide anion-induced modulation of vascular tone. Int J Angiol. 2002;11:23–9. [Google Scholar]
- 143.Prabha PS, Das UN, Koratkar R, Sagar PS, Ramesh G. Free radical generation, lipid peroxidation and essential fatty acids in uncontrolled essential hypertension. Prostaglandins Leukot Essent Fatty Acids. 1990;41:27–33. doi: 10.1016/0952-3278(90)90127-7. [DOI] [PubMed] [Google Scholar]
- 144.Parik T, Allikmets K, Teesalu R, Zilmer M. Evidence for oxidative stress in essential hypertension: Perspective for antioxidant therapy. J Cardiovasc Risk. 1996;3:49–54. [PubMed] [Google Scholar]
- 145.Tandon R, Sinha MK, Garg H, Khanna R, Khanna HD. Oxidative stress in patients with essential hypertension. Natl Med J India. 2005;18:297–9. [PubMed] [Google Scholar]
- 146.Biswas SK, de Faria JB. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic Res. 2007;41:216–24. doi: 10.1080/10715760601059672. [DOI] [PubMed] [Google Scholar]
- 147.Fortuno A, Olivan S, Beloqui O, et al. Association of increased phagocytic NADPH oxidase-dependent superoxide production with diminished nitric oxide generation in essential hypertension. J Hypertens. 2004;22:2169–75. doi: 10.1097/00004872-200411000-00020. [DOI] [PubMed] [Google Scholar]
- 148.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: Role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 149.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2001;218:97–103. doi: 10.1023/a:1007234027421. [DOI] [PubMed] [Google Scholar]
- 150.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2002;238:111–7. doi: 10.1023/a:1019915306581. [DOI] [PubMed] [Google Scholar]
- 151.Vasdev S, Gill V. Antioxidants in the treatment of hypertension. Int J Angiol. 2005;14:60–73. [Google Scholar]
- 152.Trolliet MR, Rudd MA, Loscalzo J. Oxidative stress and renal dysfunction in salt-sensitive hypertension. Kidney Blood Press Res. 2001;24:116–23. doi: 10.1159/000054217. [DOI] [PubMed] [Google Scholar]
- 153.Kitiyakara C, Chabrashvili T, Chen Y, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–82. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 154.Vasdev S, Gill V, Parai S, Gadag V. Dietary vitamin e supplementation attenuates hypertension in Dahl salt-sensitive rats. J Cardiovasc Pharmacol Ther. 2005;10:103–111. doi: 10.1177/107424840501000204. [DOI] [PubMed] [Google Scholar]
- 155.Vasdev S, Gill V, Parai S, Gadag V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol Cell Biochem. 2005;275:135–141. doi: 10.1007/s11010-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 156.Pennington JAT. Supplementary tables – amino acids. In: Allen A, editor. Bowes & Church’s Food Values of Portions Commonly Used. 16th edn. Philadelphia: JB Lippincott Company; 1994. pp. A325–77. [Google Scholar]
- 157.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134:2791S–5S. doi: 10.1093/jn/134.10.2791S. [DOI] [PubMed] [Google Scholar]
- 158.Tong BC, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4:823–32. doi: 10.2174/1389557043403305. [DOI] [PubMed] [Google Scholar]
- 159.Xiao S, Wagner L, Mahaney J, Baylis C. Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol. 2001;280:F989–95. doi: 10.1152/ajprenal.2001.280.6.F989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209–20. doi: 10.1038/ncpneph0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brunini T, Moss M, Siqueira M, et al. Inhibition of l-arginine transport in platelets by asymmetric dimethylarginine and N-monomethyl-l-arginine: Effects of arterial hypertension. Clin Exp Pharmacol Physiol. 2004;31:738–40. doi: 10.1111/j.1440-1681.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 162.Fajans SS, Floyd JC, Jr, Knopf RF, Conn FW. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–62. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- 163.Sener A, Best LC, Yates AP, et al. Stimulus-secretion coupling of arginine-induced insulin release: Comparison between the cationic amino acid and its methyl ester. Endocrine. 2000;13:329–40. doi: 10.1385/ENDO:13:3:329. [DOI] [PubMed] [Google Scholar]
- 164.Eid HM, Reims H, Arnesen H, Kjeldsen SE, Lyberg T, Seljeflot I. Decreased levels of asymmetric dimethylarginine during acute hyperinsulinemia. Metabolism. 2007;56:464–9. doi: 10.1016/j.metabol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Higashi Y, Oshima T, Ono N, et al. Intravenous administration of L-arginine inhibits angiotensin-converting enzyme in humans. J Clin Endocrinol Metab. 1995;80:2198–202. doi: 10.1210/jcem.80.7.7608279. [DOI] [PubMed] [Google Scholar]
- 166.Lubec B, Hayn M, Kitzmuller E, Vierhapper H, Lubec G. L-arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Radic Biol Med. 1997;22:355–7. doi: 10.1016/s0891-5849(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 167.Wascher TC, Posch K, Wallner S, Hermetter A, Kostner GM, Graier WF. Vascular effects of L-arginine: Anything beyond a substrate for the NO-synthase? Biochem Biophys Res Commun. 1997;234:35–8. doi: 10.1006/bbrc.1997.9994. [DOI] [PubMed] [Google Scholar]
- 168.Higashi Y, Oshima T, Watanabe M, Matsuura H, Kajiyama G. Renal response to L-arginine in salt-sensitive patients with essential hypertension. Hypertension. 1996;27:643–8. doi: 10.1161/01.hyp.27.3.643. [DOI] [PubMed] [Google Scholar]
- 169.Murakami K, Kojima S, Kimura G, et al. The association between salt sensitivity of blood pressure and family history of hypertension. Clin Exp Pharmacol Physiol Suppl. 1992;20:61–3. [PubMed] [Google Scholar]
- 170.Artigues C, Richard V, Roussel C, Lallemand F, Henry JP, Thuillez C. Increased endothelium--monocyte interactions in salt-sensitive hypertension: Effect of L-arginine. J Cardiovasc Pharmacol. 2000;35:468–73. doi: 10.1097/00005344-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 171.Schlaich MP, Parnell MM, Ahlers BA, Finch, et al. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 172.Ono H, Ono Y, Frohlich ED. L-arginine reverses severe nephrosclerosis in aged spontaneously hypertensive rats. J Hypertens. 1999;17:121–8. doi: 10.1097/00004872-199917010-00018. [DOI] [PubMed] [Google Scholar]
- 173.Matsuoka H, Nakata M, Kohno K, et al. Chronic L-arginine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 1996;27:14–8. doi: 10.1161/01.hyp.27.1.14. [DOI] [PubMed] [Google Scholar]
- 174.Vukosavljevic N, Jaron D, Barbee KA, Buerk DG. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 175.Wood PA, Hamm DA, Chen PY, Sanders PW. Studies of arginine metabolism and salt sensitivity in the Dahl/Rapp rat models of hypertension. Mol Genet Metab. 1998;64:80–3. doi: 10.1006/mgme.1998.2696. [DOI] [PubMed] [Google Scholar]
- 176.Zhang C, Hein TW, Wang W, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–43. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- 177.Demougeot C, Prigent-Tessier A, Bagnost T, et al. Time course of vascular arginase expression and activity in spontaneously hypertensive rats. Life Sci. 2007;80:1128–34. doi: 10.1016/j.lfs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 178.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–62. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- 179.Milner JA. Metabolic aberrations associated with arginine deficiency. J Nutr. 1985;115:516–23. doi: 10.1093/jn/115.4.516. [DOI] [PubMed] [Google Scholar]
- 180.Visek WJ. Arginine needs, physiological state and usual diets. A reevaluation. J Nutr. 1986;116:36–46. doi: 10.1093/jn/116.1.36. [DOI] [PubMed] [Google Scholar]
- 181.Wells BJ, Mainous AG, 3rd, Everett CJ. Association between dietary arginine and C-reactive protein. Nutrition. 2005;21:125–30. doi: 10.1016/j.nut.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 182.Oomen CM, van Erk MJ, Feskens EJ, Kok FJ, Kromhout D. Arginine intake and risk of coronary heart disease mortality in elderly men. Arterioscler Thromb Vasc Biol. 2000;20:2134–9. doi: 10.1161/01.atv.20.9.2134. [DOI] [PubMed] [Google Scholar]
- 183.Perticone F, Sciacqua A, Maio R, et al. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–23. doi: 10.1016/j.jacc.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 184.Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Invest. 2005;35:32–7. doi: 10.1111/j.1365-2362.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 185.Evans RW, Fernstrom JD, Thompson J, Morris SM, Jr, Kuller LH. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J Nutr Biochem. 2004;15:534–9. doi: 10.1016/j.jnutbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 186.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212–7. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 187.Theilmeier G, Chan JR, Zalpour C, et al. Adhesiveness of mononuclear cells in hypercholesterolemic humans is normalized by dietary L-arginine. Arterioscler Thromb Vasc Biol. 1997;17:3557–64. doi: 10.1161/01.atv.17.12.3557. [DOI] [PubMed] [Google Scholar]
- 188.Wang J, Sim AS, Wang XL, Wilcken DE. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell Mol Life Sci. 2006;63:2838–46. doi: 10.1007/s00018-006-6271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stuhlinger MC, Abbasi F, Chu JW, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287:1420–6. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 190.Cardounel AJ, Cui H, Samouilov A, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 191.Achan V, Broadhead M, Malaki M, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–9. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 192.Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33:652–8. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 193.Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW, Li YJ. Lysophosphatidylcholine-induced elevation of asymmetric dimethylarginine level by the NADPH oxidase pathway in endothelial cells. Vascul Pharmacol. 2006;44:143–8. doi: 10.1016/j.vph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 194.Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive asians. Hypertension. 2006;48:724–9. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 195.Matsuoka H, Itoh S, Kimoto M, et al. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension. 1997;29:242–7. doi: 10.1161/01.hyp.29.1.242. [DOI] [PubMed] [Google Scholar]
- 196.Carter BW, Jr, Chicoine LG, Nelin LD. L-lysine decreases nitric oxide production and increases vascular resistance in lungs isolated from lipopolysaccharide-treated neonatal pigs. Pediatr Res. 2004;55:979–87. doi: 10.1203/01.pdr.0000127722.55965.b3. [DOI] [PubMed] [Google Scholar]
- 197.Yang Z, Kaye DM. Endothelial dysfunction and impaired L-arginine transport in hypertension and genetically predisposed normotensive subjects. Trends Cardiovasc Med. 2006;16:118–24. doi: 10.1016/j.tcm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 198.Tomohiro A, Kimura S, He H, et al. Regional blood flow in Dahl-Iwai salt-sensitive rats and the effects of dietary L-arginine supplementation. Am J Physiol. 1997;272:R1013–9. doi: 10.1152/ajpregu.1997.272.4.R1013. [DOI] [PubMed] [Google Scholar]
- 199.De Pirro R, Tamburrano G, Fusco A, Lauro R. Arginine does not influence insulin binding on circulating monocytes. 1980;75:243–6. [PubMed] [Google Scholar]
- 200.Wascher TC, Graier WF, Dittrich P, et al. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27:690–5. doi: 10.1046/j.1365-2362.1997.1730718.x. [DOI] [PubMed] [Google Scholar]
- 201.Piatti PM, Monti LD, Valsecchi G, et al. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24:875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 202.Ahmed N, Thornalley PJ. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 203.Servetncik DA, Bryant D, Wells-knetch KJ, Wiesenfeld PL. L-arginine inhibits in vitro nonenzymatic glycation and advanced glycosylated end product formation of human serum albumin. 1996;11:69–81. doi: 10.1007/BF00805722. [DOI] [PubMed] [Google Scholar]
- 204.Weninger M, Xi Z, Lubec B, Szalay S, Hoger H, Lubec G. L-arginine reduces glomerular basement membrane collagen N epsilon-carboxymethyllysine in the diabetic db/db mouse. Nephron. 1992;62:80–3. doi: 10.1159/000187000. [DOI] [PubMed] [Google Scholar]
- 205.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: Further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA. 2002;99:13527–32. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lekakis JP, Papathanassiou S, Papaioannou TG, et al. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int J Cardiol. 2002;86:317–23. doi: 10.1016/s0167-5273(02)00413-8. [DOI] [PubMed] [Google Scholar]
- 207.Hodroj W, Legedz L, Foudi N, et al. Increased insulin-stimulated expression of arterial angiotensinogen and angiotensin type 1 receptor in patients with type 2 diabetes mellitus and atheroma. Arterioscler Thromb Vasc Biol. 2007;27:525–31. doi: 10.1161/01.ATV.0000254814.63768.3b. [DOI] [PubMed] [Google Scholar]
- 208.Nagase S, Takemura K, Ueda A, Hirayama, et al. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D- or L-arginine. Biochem Biophys Res Commun. 1997;233:150–3. doi: 10.1006/bbrc.1997.6428. [DOI] [PubMed] [Google Scholar]
- 209.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–52. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 210.Wu G, Bazer FW, Cudd TA, et al. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr. 2007;137:1673S–80S. doi: 10.1093/jn/137.6.1673S. [DOI] [PubMed] [Google Scholar]
- 211.Clarkson P, Adams MR, Powe AJ, et al. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97:1989–94. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wolf A, Zalpour C, Theilmeier G, et al. Dietary L-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol. 1997;29:479–85. doi: 10.1016/s0735-1097(97)00523-8. [DOI] [PubMed] [Google Scholar]
- 213.Chan JR, Boger RH, Bode-Boger SM, et al. Asymmetric dimethylarginine increases mononuclear cell adhesiveness in hypercholesterolemic humans. Arterioscler Thromb Vasc Biol. 2000;20:1040–6. doi: 10.1161/01.atv.20.4.1040. [DOI] [PubMed] [Google Scholar]
- 214.Chen J, Kuhlencordt P, Urano F, Ichinose H, Astern J, Huang PL. Effects of chronic treatment with L-arginine on atherosclerosis in apoE knockout and apoE/inducible NO synthase double-knockout mice. Arterioscler Thromb Vasc Biol. 2003;23:97–103. doi: 10.1161/01.atv.0000040223.74255.5a. [DOI] [PubMed] [Google Scholar]
- 215.Loscalzo J. Adverse effects of supplemental L-arginine in atherosclerosis: Consequences of methylation stress in a complex catabolism? Arterioscler Thromb Vasc Biol. 2003;23:3–5. doi: 10.1161/01.atv.0000040860.71626.9d. [DOI] [PubMed] [Google Scholar]
- 216.Handy DE, Scolaro J, Chen J, Huang P, Loscalzo J. L-arginine increases plasma homocysteine in apoE−/−/iNOS−/− double knockout mice. Cell Mol Biol (Noisy-le-grand) 2004;50:903–9. [PubMed] [Google Scholar]
- 217.Boger RH, Bode-Boger SM, Brandes RP, et al. Dietary L-arginine reduces the progression of atherosclerosis in cholesterol-fed rabbits: Comparison with lovastatin. Circulation. 1997;96:1282–90. doi: 10.1161/01.cir.96.4.1282. [DOI] [PubMed] [Google Scholar]
- 218.Tsai CH, Pan TL, Lee YS, Tai YK, Liu TZ. Evidence that high-dose L-arginine may be inappropriate for use by diabetic patients as a prophylactic blocker of methylglyoxal glycation. J Biomed Sci. 2004;11:692–6. doi: 10.1007/BF02256135. [DOI] [PubMed] [Google Scholar]
- 219.Racasan S, Braam B, van der Giezen DM, et al. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–8. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]