Abstract
Proteins of the Bcl-2 family are critical regulators of apoptosis, but how its BH3-only members activate the essential effectors Bax and Bak remains controversial. The indirect activation model suggests that they simply must neutralize all of the prosurvival Bcl-2 family members, whereas the direct activation model proposes that Bim and Bid must activate Bax and Bak directly. As numerous in vitro studies have not resolved this issue, we have investigated Bim's activity in vivo by a genetic approach. Because the BH3 domain determines binding specificity for Bcl-2 relatives, we generated mice having the Bim BH3 domain replaced by that of Bad, Noxa, or Puma. The mutants bound the expected subsets of prosurvival relatives but lost interaction with Bax. Analysis of the mice showed that Bim's proapoptotic activity is not solely caused by its ability to engage its prosurvival relatives or solely to its binding to Bax. Thus, initiation of apoptosis in vivo appears to require features of both models.
Introduction
A pivotal step in apoptosis is activation of Bax and Bak. It leads to mitochondrial outer membrane permeabilization (MOMP) and the release to the cytosol of apoptogenic molecules such as cytochrome c, which provoke the proteolytic caspase cascade that dismantles the cell (Adams and Cory, 2007). Prosurvival members of the Bcl-2 family (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1) prevent Bax/Bak activation and thereby preclude MOMP and apoptosis. The BH3-only proteins (e.g., Bim, Bid, Puma, Bad, and Noxa) promote cell death, but how they do so remains vigorously debated (Adams and Cory, 2007; Chipuk and Green, 2008). BH3-only proteins bind tightly and neutralize distinct subsets of prosurvival relatives (Fig. 1 A; Chen et al., 2005; Kuwana et al., 2005; Certo et al., 2006), and the indirect activation model suggests that engaging all of them is necessary and seemingly sufficient to activate Bax and Bak (Chen et al., 2005; Willis et al., 2005, 2007). In this model, the prosurvival Bcl-2 family members hold subpopulations of Bax and Bak molecules in check until BH3-only proteins free them. However, in cell-free studies, BH3 peptides from Bim or Bid and the active form of Bid (truncated Bid [tBid]) can activate Bax or Bak directly and cause MOMP (Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006; Walensky et al., 2006; Gavathiotis et al., 2008; Lovell et al., 2008). The direct activation model posits that this interaction is critical; it suggests that the other BH3-only proteins, termed “sensitizers” (e.g., Bad and Noxa), function simply by binding the prosurvival proteins to free any sequestered Bim or tBid. Although the Puma BH3 has also been proposed to bind and activate Bax (Cartron et al., 2004; Kim et al., 2006; Gallenne et al., 2009), other findings argue against that (Kuwana et al., 2005; Certo et al., 2006; Willis et al., 2007; Chipuk et al., 2008; Jabbour et al., 2009).
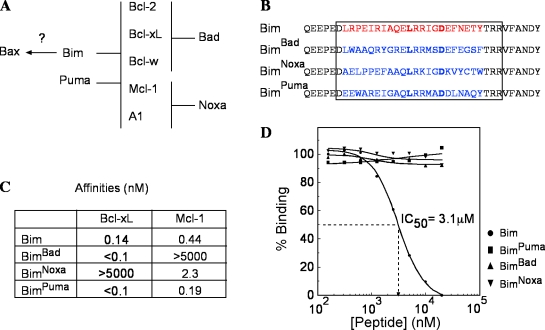
Figure 1.
BH3 replacements in Bim alter its binding profile. (A) Interactions between Bcl-2 family members show the rationale of our experiments. Bad binds Bcl-2, Bcl-xL, and Bcl-w, whereas Noxa binds only Mcl-1 and A1. Bim and Puma bind all of their prosurvival relatives, but Bim (the BimS isoform) binds Bax, whereas Puma does not. (B) Amino acids 140–161 of mouse BimEL (red) were replaced by the BH3 sequences of mouse Bad, NoxaA, or Puma. Boxed sequences indicate the 26-mer peptides used to measure the affinities of the mutant BH3 peptides for mouse Bcl-xL, Mcl-1, and Bax. (C) Nanomolar affinities of the BH3 peptides for GST–Bcl-xL and GST–Mcl-1 as measured on an S51 biosensor. (D) Tests for binding of the same peptides to Bax. The dashed line corresponds to the method used to determine the IC50 value. Starting from the left side, 50% binding of Bim to Bax occurs when a concentration of 3.1 µM of peptide is present in the system. The horizontal dashed line is for the 50%, the place where it intersects with the curve determines the position of the vertical line, and the reading is made on the peptide concentration scale.
As the central issue of how the BH3-only proteins trigger apoptosis has not been resolved by the many experiments with overexpressed proteins in cell lines or the use of BH3 peptides in cell-free systems, we have devised a genetic approach to investigate this important question in vivo. We have focused on the Bim locus because, unlike Bid, Puma, Bad, or Noxa (Strasser, 2005), its disruption markedly increases hematopoietic cell numbers (Bouillet et al., 1999). Bim loss also prevents the fatal polycystic kidney disease (PKD) and other degenerative disorders provoked by the abnormal cell attrition in the absence of Bcl-2 (Bouillet et al., 2001). Bim is an unstructured polypeptide (Hinds et al., 2007), and its binding specificity, like that of other BH3-only proteins, appears to be defined entirely by its BH3 domain (Chen et al., 2005). We have altered its specificity by engineering knockin mouse strains having its BH3 domain replaced by that of Bad (BimBad), Noxa (BimNoxa), or Puma (BimPuma). The Bim BH3 can bind to all of its prosurvival relatives and weakly to Bax; the Puma BH3 also engages all of the prosurvival proteins, whereas the Bad and Noxa BH3 bind only subsets of them and not Bax (Fig. 1 A).
These mice allow tests within the physiological setting of whether Bim's proapoptotic function relies upon engaging Bax or all of its prosurvival relatives. Surprisingly, the observed phenotypes of the Bim BH3 mutant mice in both the wild-type (WT) and sensitized Bcl-2–deficient background do not entirely fit with either model and suggest that aspects of each must hold to fully account for Bim's ability to initiate programmed cell death.
Results and discussion
Generation of mice with altered Bim specificities
Fig. 1 B depicts the Bim mutations generated. Because the binding affinity of a BH3-only protein determines its proapoptotic potential (Chen et al., 2005; Kuwana et al., 2005; Certo et al., 2006; Lee et al., 2008), we determined the affinities of 26-mer peptides spanning the mutant BH3 domains for Bcl-xL and Mcl-1 (Fig. 1 C). The BimBad and BimPuma peptides bound at least as tightly as the WT Bim peptide to Bcl-xL, as did the BimPuma peptide to Mcl-1. The BimNoxa peptide bound Mcl-1 approximately fivefold less tightly than its WT Bim counterpart, which is in keeping with the lower affinity of Noxa than Bim for Mcl-1 (Chen et al., 2005). Similar tests of the binding of the peptides to Bax (Fig. 1 D) showed that WT Bim peptide bound full-length Bax, albeit much more weakly (IC50 = 3.1 µM) than the low nanomolar binding to its prosurvival relatives, but neither the BimBad, BimNoxa, nor BimPuma peptide bound Bax detectably (IC50 > 20 µM; Fig. 1 D).
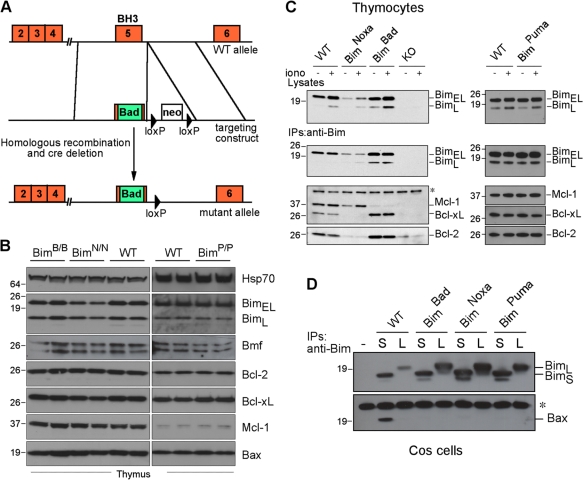
Mice homozygous for each mutation (designated BimB/B, BimN/N, and BimP/P) were outwardly indistinguishable from WT littermates. The knockin mutations do not alter the exon structure, splicing sites, or any Bim motif (e.g., phosphorylation or ubiquitination sites) implicated in regulating its level or activity (Fig. 2 A). Accordingly, Western blots of thymocyte lysates revealed that the BimBad and BimPuma mice had WT Bim levels, and those in BimNoxa mice appeared only slightly lower (Fig. 2 B). The levels of other family members (Bcl-2, Bcl-xL, Mcl-1, and Bax) were unaffected (Fig. 2 B). As expected, ionomycin increased Bim expression comparably in both the mutant and WT thymocytes (Fig. 2 C).
Figure 2.
BH3 replacements in Bim change its binding profile. (A) Knockin strategy for the BH3 replacements (not to scale). Note that the introduced sequences do not overlap the splice sites. (B) Normal expression of the Bim mutant proteins and Bcl-2 family members in the thymi of 6–8-wk-old knockin animals. Triton X-100 lysates of 4 × 106 cells were loaded per lane. The Western blots show samples from two animals per genotype. Hsp70 provided a loading control. (C) Immunoprecipitations (IPs) from thymocyte extracts of the indicated genotypes reveal the binding of WT Bim and the mutants to prosurvival relatives. (D) Test for binding of WT Bim and the mutants to Bax. HA-tagged BimL (L) and BimS (S) isoforms of each protein were overexpressed in COS-7 cells, lysates were prepared in buffer containing Triton X-100, which promotes heterodimerization, and association with endogenous Bax was tested using an anti-HA antibody–coupled affinity resin. Only WT BimS showed significant binding to Bax. (C and D) The asterisks indicate nonspecific bands caused by the secondary antibody. (B–D) Molecular mass is shown in kilodaltons.
We verified that the mutant Bim proteins had the expected binding profiles (Fig. 1 A) by coimmunoprecipitation from thymocyte lysates. Indeed, BimBad bound Bcl-2 and Bcl-xL but not Mcl-1, and BimNoxa bound Mcl-1 but not Bcl-2 or Bcl-xL, whereas BimPuma, like WT Bim, bound all of these prosurvival proteins (Fig. 2 C). Because the only mouse Bim isoform that binds Bax detectably (in Triton X-100–containing buffers) is BimS (Willis et al., 2007), the rarest of the three main Bim isoforms in all tissues (O'Connor et al., 1998), we overexpressed the L and S isoforms of WT Bim and each Bim BH3 mutant in COS cells and tested their binding to endogenous Bax. As expected, WT BimS but not WT BimL bound some Bax, but neither form of BimBad, BimNoxa, or BimPuma bound detectable Bax (Fig. 2 D), which is in accord with the BH3 peptide–binding data (Fig. 1 D). Thus, none of the Bim mutants should have direct activator function for Bax. No binding of any Bim isoform to Bak has been detected (Willis et al., 2007).
Engaging all prosurvival relatives is necessary but not sufficient for full Bim proapoptotic function
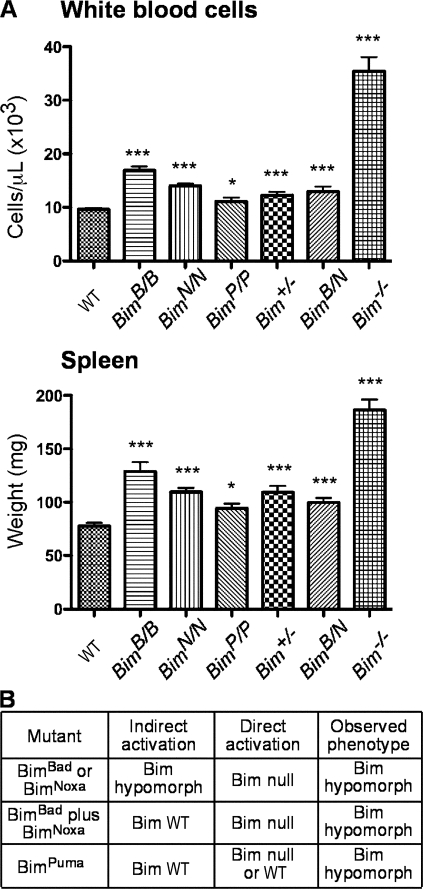
As Bim plays major roles in hemopoietic homeostasis, Bim-null mice accumulate excess white blood cells (WBCs) and splenocytes (Bouillet et al., 1999). To determine whether the BH3 replacement mutants retain all, some or none of Bim's proapoptotic activity, we compared their WBC numbers and spleen weight and those of mice bred to bear both a BimBad and a BimNoxa allele (BimB/N animals) with the levels in WT, Bim+/−, and Bim−/− mice (Fig. 3 A). For all genotypes, WBC numbers mirrored spleen weight, as expected. Fig. 3 B compares the observed phenotypes with those predicted by the indirect and direct activation models.
Figure 3.
BH3 replacements in Bim restrict its function. (A) WBC numbers in at least 16 mice of each genotype were determined on an ADVIA blood analyzer. Spleens of at least eight mice per genotype were weighed. Means ± SEM are shown, and the significance of differences from WT cells was determined by two-tailed t tests (*, P < 0.05; ***, P < 0.001). (B) The phenotypes of the BH3 substitution mutants predicted by the indirect and direct activation models and the observed phenotype. The prediction by the direct activation model for BimPuma depends on whether Puma is an activator, which remains in dispute (see Introduction).
Consistent with the inability of BimBad or BimNoxa to bind certain prosurvival relatives (Figs. 1 and 2), each had reduced proapoptotic function: both BimB/B and BimN/N mice had significantly more WBCs and greater spleen weight than WT mice (Fig. 3 A). Thus, BimBad and BimNoxa are hypomorphs, as expected from the indirect activation model (Fig. 3 B). Comparison with the Bim−/− mice clearly shows that each retained substantial proapoptotic activity, indeed more than half of the WT Bim activity (Fig. 3 A). As neither BimBad nor BimNoxa binds Bax (Figs. 1 D and 2 D), this conflicts with the direct activation model (Fig. 3 B). Overall, these results suggest that neutralization of any of the prosurvival proteins increases the odds that many cells will undergo apoptosis. Surprisingly, BimPuma also proved to be a hypomorph because the BimP/P mice had significant albeit smaller increases in their WBCs (P = 0.02; n = 39) and spleen weight (P = 0.019; n = 13) over WT mice (Fig. 3 A). Thus, although BimPuma and WT Bim bind all of the prosurvival members with comparable high affinity (Figs. 1 C and 2 C), BimPuma is somewhat less potent than WT Bim, a finding which is discordant with both models (Fig. 3 B).
We reasoned that if antagonizing prosurvival Bcl-2 family members was the sole function of Bim, combining BimBad and BimNoxa should reconstitute its full function (Fig. 1 A), and thus, the BimB/N mice might have a WT phenotype (Fig. 3 B). However, they also presented with significantly greater WBC numbers and spleen weights (P < 0.0001) than WT mice (Fig. 3 A), which is in conflict with indirect activation. A caveat is that these mice possess only a single allele of each Bim BH3 mutant; thus, any cells relying entirely on Bcl-2/Bcl-xL/Bcl-w or entirely on Mcl-1/A1 for their survival would have an advantage because they would behave as if they had only half a dose of Bim. In any case, the retention of substantial proapoptotic function by Bim mutants that do not bind Bax argues that direct activation of Bax cannot be the sole essential proapoptotic function of Bim (Fig. 3 B).
Unlike the long-term effects on cell death in vivo in Fig. 3 A, in vitro survival assays with several cytotoxic stimuli on sorted thymocyte and B cell populations from mice of all of the genotypes (illustrated for CD4+8+ thymocytes in Fig. S1) revealed no significant differences between the death rates of BimB/B, BimN/N, BimB/N, BimP/P, and WT cells. The comparable killing by mutant and WT Bim with certain stimuli in ex vivo assays (Fig. S1) suggests that distinguishing subtle differences in their apoptotic potential requires analysis of long-term effects in vivo. The discrepancy between the in vivo and in vitro results probably reflects both the substantial activity of the mutants and the difficulty of fully replicating in vivo stresses in vitro.
The Bim mutants ameliorate or prevent the PKD and lymphopenia provoked by Bcl-2 deficiency
In the absence of Bcl-2, Bim drives the attrition of several tissues (Bouillet et al., 2001). On the C57BL/6 background, Bcl-2–deficient mice are runty (Fig. 4 A), profoundly lymphopenic, and usually die by 6–7 wk of age from the renal failure induced by PKD, but all of these degenerative disorders are prevented by loss of Bim, and even loss of a single Bim allele precludes the PKD and renders the mice as healthy as WT or Bcl-2−/−Bim−/− animals (Bouillet et al., 2001).
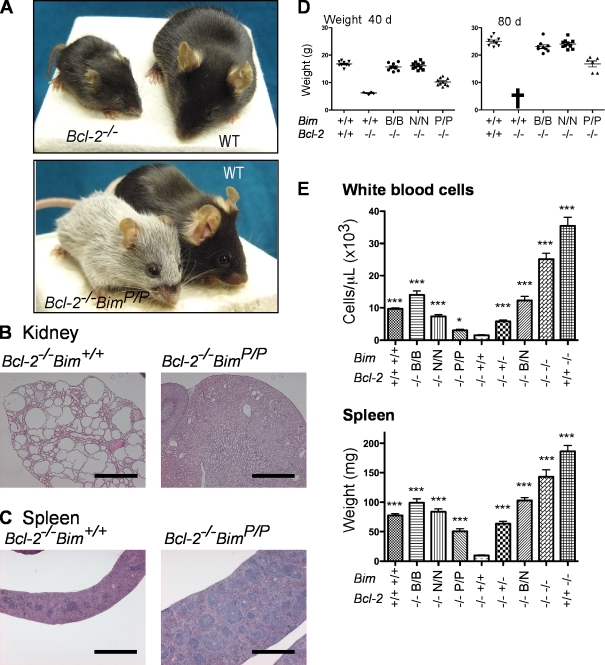
Figure 4.
BH3 mutations in Bim ameliorate the degenerative disorders of Bcl-2–deficient mice. (A) A 40-d-old Bcl-2−/−Bim+/+ mouse and its WT littermate are shown above a 120-d-old Bcl-2−/−BimP/P mouse and a WT mouse of that age. (B) Kidneys of Bcl-2−/−BimP/P mice show only mild signs of PKD compared with Bcl-2−/−Bim+/+ kidneys. (C) Normal spleen structure in Bcl-2−/−BimP/P mice versus the atrophied Bcl-2−/−Bim+/+ spleen. (D) Weights of WT and Bcl-2–null mice at 40 and 80 d showing that the Bcl-2–null BimBad and BimNoxa animals grow normally, whereas growth of the BimPuma mice is retarded (P < 0.0001) but much less so than the Bcl-2−/−Bim+/+ animals, which have all died by 80 d (cross). (E) Blood composition for at least 10 mice per genotype was analyzed on an ADVIA blood analyzer, except for the Bcl-2−/−Bim+/+ animals (n = 4). Spleens from at least eight mice per genotype were weighed, except for the Bcl-2−/−Bim+/+ animals (n = 5). Means ± SEM and the significance of differences (by two-tailed t test) from the values in Bcl-2−/−Bim+/+ animals (*, P < 0.05; ***, P < 0.001) are shown. Bars, 1 mm.
Because BimBad, BimNoxa, and BimPuma all behaved as hypomorphs (Fig. 3), we reasoned that they might ameliorate or even prevent some of the disorders in Bcl-2–deficient mice. Indeed, breeding Bcl-2–null animals expressing each mutant revealed that no Bcl-2−/−BimB/B or Bcl-2−/−BimN/N mice developed PKD. These animals grew normally (Fig. 4 D) and had normal kidney histology (Fig. S2). They differed from Bcl-2–proficient littermates only by turning gray at 5–7 wk of age, as expected if some Bim function remains in melanocytes (Bouillet et al., 2001). Thus, BimBad and BimNoxa also retained partial Bim function in a nonhemopoietic cell type, which is in accord with indirect but not direct activation (Fig. 3 B).
Remarkably, although the growth of Bcl-2−/−BimP/P mice was retarded (Fig. 4, A and D), most of these animals (n = 24) became healthy adults. These mice were smaller than WT mice, and their kidneys showed mild signs of PKD (Fig. 4 B and Fig. S2 A), but it progressed slowly, and they could live up to 300 d, whereas all 35–40-d-old Bcl-2−/−Bim+/+ mice exhibited large kidney cysts (Fig. 4 B) and died by 50 d. The less severe PKD in Bcl-2−/−BimP/P mice than in Bcl-2−/−Bim+/+ mice confirms that BimPuma is less potent than WT Bim in these cells.
Bcl-2−/−Bim+/+ mice have very small spleens (5–10 mg vs. 60–100 mg in WT) with very few lymphoid follicles (white pulp) but many apoptotic cells (Fig. 4, C and E; and Fig. S2). However, splenic structure was normal in Bcl-2−/−BimB/B, Bcl-2−/−BimN/N, and Bcl-2−/−BimB/N mice and, remarkably, even in Bcl-2−/−BimP/P animals (Fig. 4 C and Fig. S3). As in the Bcl-2–proficient animals (Fig. 2), spleen weights and WBC numbers varied in parallel with genotype, and all of the mutants behaved as hypomorphic Bim alleles (Fig. 4 E). Also, the Bcl-2−/−BimB/N spleens were much heavier than those in Bcl-2−/−Bim+/+ mice (P < 0.0001) or even Bcl-2−/−Bim+/− animals (P < 0.0001), and WBCs were approximately sixfold more abundant in Bcl-2−/−BimB/N than in Bcl-2−/−Bim+/+ mice (P = 0.0003). The greater potency of WT Bim or even of a single WT Bim allele than the combination of BimBad plus BimNoxa conflicts with the indirect activation model (Fig. 3 B).
Similarly, the spleens of Bcl-2−/−BimP/P mice were heavier than the Bcl-2−/−Bim+/+ spleens (Fig. 4 E) and displayed less apoptosis (Fig. S3). The Bcl-2−/−BimP/P mice also had more WBCs than the Bcl-2−/−Bim+/+ mice (P = 0.018) but decidedly fewer WBCs and lower spleen weights than the Bcl-2−/−BimB/B or Bcl-2−/−BimN/N animals (Fig. 4 E). Thus, BimPuma again is the mutant most similar to WT Bim, which is in accord with its ability to engage all of the prosurvival proteins (Fig. 1 A), emphasizing this as a critical part of Bim function. Nevertheless, BimPuma is less potent than WT Bim despite binding to the prosurvival proteins with similar affinity (Fig. 1 C). Thus, WT Bim has a function beyond engaging all of its prosurvival relatives.
Aspects of both indirect and direct activation appear to contribute to programmed cell death in vivo
Our interpretation of these findings on the role of Bim in programmed cell death within the intact animal is depicted in Fig. 5. They show first that restricting Bim's binding spectrum for Bcl-2–like prosurvival proteins indeed limits its proapoptotic potential, which is consistent with the indirect model for Bax/Bak activation (Chen et al., 2005; Willis et al., 2007). Thus, both BimBad and BimNoxa, which each bind only a subset of the prosurvival proteins (Figs. 1 and 4 B), were less potent than WT Bim, as shown both by elevated hematopoietic cell numbers (Figs. 3 and 4 E) and their prevention of the PKD and lymphopenia provoked by Bcl-2 loss (Fig. 4 and Fig. S2). As neither BimBad nor BimNoxa binds all of the prosurvival proteins, inactivation of any of them must increase the probability that a cell will undergo apoptosis (Fig. 5 B). Notably, although neither the Bad nor the Noxa BH3 binds to Bax (Figs. 1 D and 2 D), both BimBad and BimNoxa retained substantial proapoptotic activity, at least in hematopoietic cells and melanocytes, indicating that engagement of Bax is not essential for Bim function (Fig. 3 B).
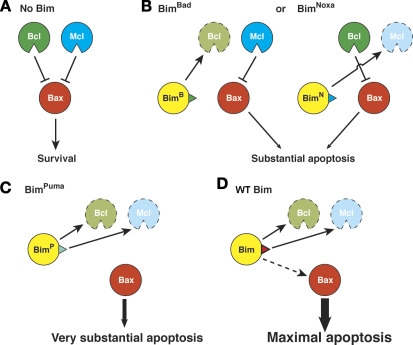
Figure 5.
Model for the initiation of apoptosis by Bim. (A) In the absence of Bim, Bax is kept in check by both subsets of its prosurvival relatives (“Bcl” represents Bcl-2, Bcl-xL, and Bcl-w; “Mcl” represents Mcl-1 and A1; Willis et al., 2007). (B) In cells expressing BimBad, the Bcl subset is neutralized, but the Mcl subset still partially restrains Bax and limits its induction of apoptosis. Conversely, in BimNoxa mice, Bax is partly restrained by the Bcl proteins. (C) In mice expressing BimPuma, all of the prosurvival proteins are neutralized, allowing the unrestrained Bax to induce very substantial apoptosis. (D) Unlike the three Bim BH3 mutants, WT Bim is proposed to also bind transiently to Bax, giving maximal activity.
Because BimPuma and the combination of BimBad plus BimNoxa can engage all of the prosurvival Bcl-2 family members (Figs. 1 and 5 C), the indirect activation model predicts that both should have full WT Bim function (Fig. 3 B). Instead, leukocytes accumulated abnormally in both BimP/P and BimB/N mice (Fig. 3 A). The far greater health of Bcl-2−/−BimP/P than Bcl-2−/−Bim+/+ mice (Fig. 4) also clearly shows that BimPuma is less potent than WT Bim. Thus, binding to prosurvival Bcl-2 family members does not fully account for Bim's proapoptotic activity (Fig. 5 D). This conclusion could only be reached by studying the long-term effects of altering Bim's specificity in a physiological context.
The most plausible additional Bim BH3 function is (transient) engagement of Bax (Fig. 5 D; Letai et al., 2002; Kuwana et al., 2005). The WT but not the mutant Bim BH3 peptide bound measurably to Bax (Fig. 1 D), and full-length BimS but not BimL coimmunoprecipitated with Bax (Fig. 2 D). Because WT BimS but neither the S nor L isoform of BimBad, BimNoxa, or BimPuma bound Bax detectably (Fig. 2 D), engagement of Bax by BimS might account for the additional Bim function (Weber et al., 2007). Alternatively, the more abundant BimL and BimEL might also do so but be more quickly displaced by the resulting Bax conformational changes so that those complexes cannot readily be detected. This conclusion is in accord with recent evidence that a stabilized Bim BH3 peptide can interact weakly with Bax and provoke its activation and oligomerization (Gavathiotis et al., 2008).
Thus, our results suggest that the ability of Bim to engage its prosurvival relatives accounts for much of its proapoptotic function in vivo (Fig. 5, B and C) but that full Bim activity requires a distinct function (Fig. 5 D), most likely its oft proposed but elusive ability to transiently engage Bax (Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006; Walensky et al., 2006; Gavathiotis et al., 2008). That ability is most likely shared by tBid (Kuwana et al., 2005; Lovell et al., 2008), although tBid is more likely to amplify rather than initiate an apoptotic response because Bid needs to be activated by caspases. The Puma BH3 probably lacks direct activator function because BimPuma proved a significantly less potent apoptosis initiator than WT Bim in both leucocytes and the kidney (Fig. 4 and Figs. S2 and S3), and neither full length BimPuma nor the corresponding BH3 peptide bound detectably to Bax (Figs. 1 D and 2 D). Thus, initiation of programmed cell death seems to require features of both the indirect and direct models. These findings will have important consequences for the design of BH3 mimetic drugs (Oltersdorf et al., 2005) of the second generation.
Materials and methods
Generation of Bim BH3 replacement knockin mice
The Bim BH3 (in exon 5) was replaced by the mouse Bad, NoxaA, or Puma BH3 domain by site-directed mutagenesis using PCR. A loxP-PGKneo-loxP cassette was inserted 840 bp downstream of exon 5 for selection of homologous recombinants. DNA constructs were electroporated into C57BL/6-derived Bruce-4 embryonic stem cells (Lemckert et al., 1997). The neo cassette was removed by crossing the mice to cre deleter transgenic mice, and the cre transgene was then eliminated by crossing to C57BL/6 mice. All of the mice analyzed were devoid of neo and cre. The Bcl-2+/− mice (Nakayama et al., 1994) and Bim−/− mice (Bouillet et al., 1999) used in this study had been serially backcrossed with C57BL/6 mice for >20 generations before being intercrossed to produce homozygotes. Peripheral blood erythrocytes and leukocytes were enumerated using an ADVIA hematology system (Bayer). All animal experiments followed the guidelines of the Melbourne Directorate Animal Ethics Committee.
Affinity measurements
The affinity of 26-mer BH3 peptides for recombinant murine Bcl-xLΔC25 and murine Mcl-1ΔN151ΔC23 was determined using a biosensor (model S51; Biacore) as previously described (Lee et al., 2008). The recombinant proteins were expressed and purified as GST fusion proteins using standard techniques. Synthetic peptides (>90% purity) were purchased from Mimotopes.
Full-length Bax was expressed as a chitin-binding protein fusion and purified by chitin affinity chromatography followed by gel filtration chromatography. Binding of Bax to BH3 peptides was determined by solution competition assays using a Biacore 3000 instrument as described previously (Chen et al., 2005; Willis et al., 2005, 2007). In brief, 1 µM Bax was incubated with increasing concentrations of synthetic peptide in Biacore running buffer (10 mM Hepes, 150 mM NaCl, 3.4 mM EDTA, and 0.005% [vol/vol] Tween 20, pH 7.4) containing 1% (wt/vol) octyl-glucoside before injection onto a CM5 Biacore chip on which the Bim BH3 and the inert Bim4E peptides were immobilized by amine coupling. The specific response was determined by subtracting the response on the Bim4E channel from that on the WT Bim BH3 channel.
Immunoprecipitation and Western blotting
Cell lysates were prepared in lysis buffer (20 mM Tris, pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton X-100) supplemented with complete protease inhibitor cocktail (Roche). Immunoprecipitation was performed using a rat monoclonal antibody to Bim (3C5; Enzo Biochem, Inc.), which recognizes an N-terminal epitope present in all isoforms and mutants of Bim that were used. Proteins were resolved by SDS-PAGE and detected on Western blots with rat monoclonal antibodies to Bim (14A8 or 3C5; Enzo Biochem, Inc.) or Bmf (17A9; Enzo Biochem, Inc.), hamster monoclonal antibodies to mouse Bcl-2 (3F11; a gift from S. Korsmeyer; Dana-Farber Cancer Institute, Boston, MA), and rabbit polyclonal antibodies to Bax (Millipore), Bcl-xL (BD), or Mcl-1 (Rockland), followed by ECL (GE Healthcare).
Immunofluorescent staining, FACS analysis, cell sorting, tissue culture, and cell survival assays
Leukocyte subpopulations were identified and sorted as described previously (Bouillet et al., 1999). Cells were incubated in medium alone (no treatment; i.e., cytokine deprivation) or treated with 1 µg/ml ionomycin, 10 ng/ml PMA, or 1 µM ABT-737 (Oltersdorf et al., 2005). Cell survival was quantified daily by staining with 2 µg/ml propidium iodide and FACS analysis.
Online supplemental material
Fig. S1 shows the survival of CD4+8+ thymocytes from Bim−/−, Bim+/−, BimB/B, BimN/N, BimP/P, BimB/N, and WT mice in vitro after treatment with different apoptotic stimuli. Fig. S2 shows that BH3-replacement mutations prevent the development of PKD and spleen atrophy in Bcl-2–deficient mice. Fig. S3 shows that BH3-replacement mutations preclude the excessive apoptosis in the spleen of Bcl-2–deficient mice. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200905153/DC1.
Acknowledgments
We thank M. Robati and H. Yang for technical assistance, G. Siciliano, J. McSween, and E. Sutherland for mouse care, J. Corbin for automated blood analysis, B. Helbert and C. Young for genotyping, and Dr. S. Mihajlovic, E. Tsui, A. Hasanein, V. Babo, and K. Weston for histological sections. We are grateful to Prof. P. Colman and Dr. D.C.S. Huang for comments.
This work was supported by grants from the Australian National Health and Medical Research Council (461221, Independent Research Institutes Infrastructure Support Scheme grant 361646, and Career Development Award), the Victorian State Government (Operational Infrastructure Support grant), Cancer Council Victoria, the Leukemia and Lymphoma Society (Specialized Center of Research grant 7015), the Australian Research Council (to D. Mérino), and the Viertel Charitable Foundation (to P. Bouillet).
Footnotes
Abbreviations used in this paper: MOMP, mitochondrial outer membrane permeabilization; PKD, polycystic kidney disease; tBid, truncated Bid; WBC, white blood cell; WT, wild type.
References
- Adams J.M., Cory S. 2007. Bcl-2-regulated apoptosis: mechanism and therapeutic potential.Curr. Opin. Immunol. 19:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C.S., Tarlinton D.M., Kay T.W.H., Köntgen F., Adams J.M., Strasser A. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity.Science. 286:1735–1738 [DOI] [PubMed] [Google Scholar]
- Bouillet P., Cory S., Zhang L.-C., Strasser A., Adams J.M. 2001. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim.Dev. Cell. 1:645–653 [DOI] [PubMed] [Google Scholar]
- Cartron P.F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F.M., Juin P. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA.Mol. Cell. 16:807–818 [DOI] [PubMed] [Google Scholar]
- Certo M., Moore Vdel G., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A. 2006. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members.Cancer Cell. 9:351–365 [DOI] [PubMed] [Google Scholar]
- Chen L., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C.S. 2005. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function.Mol. Cell. 17:393–403 [DOI] [PubMed] [Google Scholar]
- Chipuk J.E., Green D.R. 2008. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 18:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk J.E., Fisher J.C., Dillon C.P., Kriwacki R.W., Kuwana T., Green D.R. 2008. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins.Proc. Natl. Acad. Sci. USA. 105:20327–20332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenne T., Gautier F., Oliver L., Hervouet E., Noel B., Hickman J.A., Geneste O., Cartron P.F., Vallette F.M., Manon S., Juin P. 2009. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members.J. Cell Biol. 185:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E., Suzuki M., Davis M.L., Pitter K., Bird G.H., Katz S.G., Tu H.C., Kim H., Cheng E.H., Tjandra N., Walensky L.D. 2008. BAX activation is initiated at a novel interaction site.Nature. 455:1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds M.G., Smits C., Fredericks-Short R., Risk J.M., Bailey M., Huang D.C., Day C.L. 2007. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets.Cell Death Differ. 14:128–136 [DOI] [PubMed] [Google Scholar]
- Jabbour A.M., Heraud J.E., Daunt C.P., Kaufmann T., Sandow J., O'Reilly L.A., Callus B.A., Lopez A., Strasser A., Vaux D.L., Ekert P.G. 2009. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim.Cell Death Differ. 16:555–563 [DOI] [PubMed] [Google Scholar]
- Kim H., Rafiuddin-Shah M., Tu H.C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H. 2006. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.Nat. Cell Biol. 8:1348–1358 [DOI] [PubMed] [Google Scholar]
- Kuwana T., Bouchier-Hayes L., Chipuk J.E., Bonzon C., Sullivan B.A., Green D.R., Newmeyer D.D. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly.Mol. Cell. 17:525–535 [DOI] [PubMed] [Google Scholar]
- Lee E.F., Chen L., Yang H., Colman P.M., Huang D.C., Fairlie W.D. 2008. EGL-1 BH3 mutants reveal the importance of protein levels and target affinity for cell-killing potency.Cell Death Differ. 15:1609–1618 [DOI] [PubMed] [Google Scholar]
- Lemckert F.A., Sedgwick J.D., Korner H. 1997. Gene targeting in C57BL/6 ES cells. Successful germ line transmission using recipient BALB/c blastocysts developmentally matured in vitro.Nucleic Acids Res. 25:917–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Bassik M., Walensky L., Sorcinelli M., Weiler S., Korsmeyer S. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics.Cancer Cell. 2:183–192 [DOI] [PubMed] [Google Scholar]
- Lovell J.F., Billen L.P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D.W. 2008. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax.Cell. 135:1074–1084 [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nakayama K.-I., Negishi I., Kuida K., Sawa H., Loh D.Y. 1994. Targeted disruption of bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia.Proc. Natl. Acad. Sci. USA. 91:3700–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L., Strasser A., O'Reilly L.A., Hausmann G., Adams J.M., Cory S., Huang D.C.S. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis.EMBO J. 17:384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T., Elmore S.W., Shoemaker A.R., Armstrong R.C., Augeri D.J., Belli B.A., Bruncko M., Deckwerth T.L., Dinges J., Hajduk P.J., et al. 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours.Nature. 435:677–681 [DOI] [PubMed] [Google Scholar]
- Strasser A. 2005. The role of BH3-only proteins in the immune system.Nat. Rev. Immunol. 5:189–200 [DOI] [PubMed] [Google Scholar]
- Walensky L.D., Pitter K., Morash J., Oh K.J., Barbuto S., Fisher J., Smith E., Verdine G.L., Korsmeyer S.J. 2006. A stapled BID BH3 helix directly binds and activates BAX.Mol. Cell. 24:199–210 [DOI] [PubMed] [Google Scholar]
- Weber A., Paschen S.A., Heger K., Wilfling F., Frankenberg T., Bauerschmitt H., Seiffert B.M., Kirschnek S., Wagner H., Hacker G. 2007. BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptotic Bcl-2 proteins.J. Cell Biol. 177:625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Chen L., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Huang D.C. 2005. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins.Genes Dev. 19:1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak.Science. 315:856–859 [DOI] [PubMed] [Google Scholar]