Abstract
Current thought regarding the progression of calcific aortic stenosis (AS) is presented. After summarizing contemporary ideas about AS pathogenesis, the present article examines the factors that may affect disease progression. Data indicate that this process may be accelerated by aortic valve structure, degree of valvular calcification, chronic renal insufficiency and cardiovascular risk factors such as diabetes and dyslipidemia. Finally, the present review discusses potential therapeutic targets to slow AS progression.
Keywords: Calcific aortic stenosis, Progression of aortic stenosis, Prophylaxis, Valvular heart disease
Calcific aortic stenosis (AS) is the most common valvular lesion in the elderly and results in more than 50,000 aortic valve replacements each year in the United States (1,2). Advancing from the base of the cusps of the aortic valve to the leaflets, this slowly progressive disease eventually reduces leaflet motion and valve area (3). Associated with a life expectancy of less than five years, patients with severe, symptomatic AS generally have poor outcomes, and surgical correction is the only recommended treatment (3,4). Meanwhile, recommended treatment for asymptomatic AS patients is frequent monitoring for disease progression and symptom development, given the highly variable rate of AS progression (3). While the average rate of reduction of aortic valve area (AVA) in AS patients is approximately 0.10 cm2 per year, the reduction in AVA may be as great as 1.0 cm2 per year (5,6). The pathological processes leading to AS and AS progression have similarities to the atherosclerotic process and are potentially modifiable. After discussing AS pathogenesis, the present article explores the variables affecting the progression of calcific AS and highlights potential therapies and management issues.
AS PATHOGENESIS
Analysis of potential secondary prevention strategies requires a basic overview of AS pathogenesis, an area in which medical thought has rapidly evolved. The ‘wear and tear’ theory, in which repeated mechanical stress and hemodynamic forces purportedly lead to valve injury and passive calcification, served as the conventional explanation for AS (7–9). While evidence of more rapid progression of disease in patients with bicuspid valves than those with tricuspid valves suggests that increased mechanical stress may serve as an initiating factor, current theories suggest that AS pathogenesis involves active cellular and inflammatory processes, a notion justified by the histological similarities of AS to coronary artery disease (CAD) (10,11).
Pathogenesis of calcific AS involves a multistage process in which inflammatory tissue milieu, atherosclerotic-like lesions, osteoblastic transformations and calcification processes each play roles in disease development (12–15). Cardiovascular risk factors, genetic characteristics and proinflammatory cytokines may help produce an inflammatory tissue environment that disrupts the endothelial layer of the aortic leaflets in a mechanism similar to that in atherosclerosis (14). These sclerotic valvular lesions present with a chronic inflammatory cell infiltrate, which can include macrophages, T-lymphocytes, alpha-actin-expressing cells, and the deposition of apolipoproteins B, (a) and E2. After being taken up by macrophages, oxidatively modified low-density lipoproteins (LDLs) have been found to become foam cells, which act in ways similar to atherosclerotic lesions (16). The fact that angiotensin-converting enzymes (ACEs) and angiotensin type-1 receptors have been found in sclerotic lesions, along with evidence that sclerotic aortic valve tissue upregulates ACE expression, suggests that ACE is active in AS lesions (17,18). Leukocytes may then induce myofibroblast activation and cellular proliferation through the release of pro-inflammatory cytokines (19,20). Bone-associated cytokines and matrix metalloproteinases may then trigger the osteoblastic transformation of valvular tissue, neoangiogenesis and calcification (14). Lrp5, a coreceptor of the family of LDL receptors, has been found to regulate cellular proliferation and bone formation in the aortic valve (21,22). Genetic bone markers have been found to be important in the development of osteoblast bone formation, a process that precedes calcification (23).
Given these discoveries, the next phase of research involves further clarification of the signalling mechanisms involved in progressive valvular calcification, a major aspect of AS pathology (24). Studies have thus far shown relationships between osteopontin messenger RNA expression and valvular calcification (25,26). Patients with abnormal mineral metabolism, such as those with Paget’s disease, have been found to have more rapid disease progression (27). Genetic factors, such as those related to vitamin D receptors, interleukin-10, connective tissue growth factor and chemokine receptor-5 appear to impact the process of calcification (28,29). Advances that clarify our understanding of these pathogenetic processes will facilitate the development of secondary prevention strategies.
FACTORS ASSOCIATED WITH THE PROGRESSION OF CALCIFIC AS
AS pathogenesis contextualizes our discussion of AS progression because of the similarities between the AS and atherosclerotic disease processes, which are features that may explain why AS shares many risk factors with CAD. For instance, the development of AS has been associated with age, sex, hypertension, smoking, diabetes mellitus and dyslipidemia, including elevated total cholesterol, elevated LDL, elevated triglycerides and elevated lipoprotein(a) (3,30–42). These factors may have an effect on the progression of calcific AS as well. Our goal is to provide a thorough analysis of the studies regarding AS progression so that the correlations between this pathology and atherosclerosis can be better explored. Table 1 presents the factors associated with the progression of calcific AS and the literature relevant to each.
TABLE 1.
Factors associated with calcific aortic stenosis progression with supporting literature
| Clinical factor | References |
|---|---|
| Age | 43–47 |
| Aortic valve area | 6,48,59,67 |
| Aortic valve calcification | 48,56 |
| C-reactive protein | 49,70–74 |
| Coronary artery disease | 34,43,55,57 |
| Calcium: Elevated serum values, supplementation | 6,47,58,62 |
| Diabetes mellitus | 34,35,50 |
| Dyslipidemia | 6,34,39,40,42,50,57,64,65 |
| Hypertension | 50,53–54 |
| Left ventricular mass index | 48 |
| Left ventricular outflow tract velocity | 6,51,66 |
| Mitral annular calcification | 44,63 |
| Obesity | 55 |
| Renal failure and elevated serum creatinine | 6,47,58–61 |
| Sex (male) | 44,47–52 |
| Smoking | 6,32,34,50,55 |
| Valve structure | 11,68,69 |
Age and sex
Older AS patients have a faster rate of disease progression than their younger counterparts. One of the earlier studies on this subject, by Peter et al (43), found that approximately one-half of the patients with initially mild or moderate AS had an increase of the pressure gradient across the valve of 10 mmHg or greater. Progression was faster in older populations (P<0.01). While conflicting data exist, other studies have shown that older age is associated with faster disease progression in patients with congenital AS, mild or moderate AS, as well as chronic renal failure (44–47).
Studies have provided strong data to suggest that the rate of AS progression is faster among men than among women. Rapidly progressing AS populations have been found to have larger proportions of men (P<0.01), and decreases in AVA per year being found to be significantly greater in men 60 to 74 years of age than in women in the same age group (P=0.025) (44,48). Others have come to similar conclusions, and this result has been reproduced in hemodialysis patients (47,49–50). Although two studies have found that sex was not a predictor of progression to a level of statistical significance, in general the data suggest otherwise (51,52).
Atherosclerotic risk factors and smoking
Many atherosclerotic risk factors are also risk factors for AS progression. Researchers have identified diabetes mellitus as a risk factor for the progression of AS, and some have postulated that the condition may play a causative role in pathogenesis and progression (34,35,50). Several studies have found that systemic hypertension is associated with AS progression, with findings supporting a causal relationship between the two (50,53–54). Obesity has also been found to be an independent risk factor for AS progression (55).
Studies have consistently found that smoking is a risk factor for the progression of calcific AS. In 1991, Mohler et al (34) found that smoking was a statistically significant risk factor for the development and progression of what was then labelled degenerative AS. Stewart et al (32) subsequently found that smoking was associated with a 35% increase in risk for calcific aortic valve disease. In 2000, Palta et al (6) found that absolute and percentage reduction in AVA per year in those with AS was accelerated in the presence of smoking, a result that has also been confirmed by subsequent studies (50,55).
Aortic valve calcification
The degree of aortic valve calcification has been correlated with AS progression. Bahler et al (48) found more rapid disease progression in patients with more marked leaflet calcification. Moreover, Rosenhek et al (56) concluded that moderate or severe valvular calcification, when taken with a rapid increase in aortic-jet velocity, identified those with poor prognoses. Hence, it has been suggested these patients, even when asymptomatic, be considered for early valve replacement.
Disease states: CAD, chronic renal failure
CAD also appears to be a risk factor for AS progression. In 1993, Peter et al (43) found the presence of CAD was more prevalent in a subgroup of patients with rapid AS disease progression (P=0.01). Although this was a small study, others have produced similar results (34,57). Although Ngo et al (55) have produced conflicting evidence, this finding has raised questions about the management of patients with CAD undergoing coronary artery bypass graft surgery who also have mild or incidental AS.
Patients with chronic renal failure, those on dialysis and those with elevated creatinine are strongly associated with rapid AS progression. In 1999, in a study of 110 patients on hemodialysis, Urena et al (47) found significant AS in 16% of patients and that AS is generally associated with poor outcomes in hemodialysis patients. Palta et al (6) demonstrated that elevated serum creatinine levels, even when found within the normal range, accelerate AS progression. Subsequent studies have also consistently found that AS progresses more rapidly in renal failure patients on dialysis as well as in those with elevated serum creatinine (58–61). Because risk factors in these populations sometimes included higher calcium-phosphate product and elevated vitamin D3 levels, there is the possible role of dystrophic and metastatic calcification. Other factors, such as homocysteine or humoral levels, may also be operative in these patients. While pathophysiological mechanisms remain unclear, it is possible that creatinine, or some of the other biochemical factors that accumulate in the blood with a fall in the glomerular filtration rate, may act as catalysts in AS progression.
Elevated serum calcium and mitral annular calcification
Elevated serum calcium levels are associated with more rapid progression of AS. Palta et al (6) found that elevated, yet normal, serum calcium levels were associated with more rapid AS progression. In patient populations on dialysis, there is a relationship between the calcium-phosphate product – as well as vitamin D3 – and AS progression (47). There may also be a relationship between hyperparathyroidism and rapid AS progression (62). Moreover, calcium supplementation has also been associated with accelerated AS progression (58). This raises questions that we will discuss later regarding the safety of calcium and vitamin D supplements administered for the purpose of preventing osteoporosis.
Mitral annular calcification has been associated with more rapid AS progression (44). Cosmi et al (63) conducted a study using 2131 patients with aortic valve thickening and at least one year of echocardiographic follow-up. AS developed in 15.9% of patients, and multivariate analysis revealed that mitral annular calcification was independently and significantly associated with progression to AS. Associated with age, aortic valve calcification and CAD, this condition may also reflect an abnormal biochemical process involving calcium-phosphorus metabolism or the patient’s tendency for dystrophic calcification.
Dyslipidemia
Dyslipidemia is strongly associated with the rapid progression of AS (64). This holds true for definitions of dyslipidemia including a serum LDL cholesterol level greater than 3.1 mmol/L and a high-density lipoprotein cholesterol level lower than 0.9 mmol/L, total triglyceride levels, total serum cholestrol levels, LDL cholesterol level and the total cholesterol/high-density lipoprotein cholesterol level ratio (34,50,57,65). Rapid in patients with homozygous hypercholesterolemia, AS progression can occur at an early age with concomitant premature CAD and other vascular diseases (42). It has also been shown that patients undergoing aortic valve replacement for AS (with or without coronary artery bypass graft surgery) have higher serum cholesterol, triglyceride and LDL levels compared with those undergoing bypass surgery alone (39). In our study (6) of 170 AS patients, the rate of AS progression was twice as high in the patient population with a serum cholesterol level greater than 5.0 mmol/L. Curiously, the effect of cholesterol on AS progression appears to be greater in patients with tricuspid aortic valves compared with those with bicuspid aortic valves (40).
Valvular function and structure
A higher left ventricular (LV) outflow tract velocity, a characteristic associated with greater cardiac output, is correlated with more rapid AS progression (6). Not only is peak jet velocity an independent predictor of outcome but, in asymptomatic AS patients, the rate of progression and clinical outcome are also predicted by jet velocity and the rate of change in jet velocity (51,66). This raises the possibility that, just as in the genesis of CAD, mechanical factors may initiate inflammatory processes. It is interesting that the rate of progression is slower than in patients with more severe AS (6). This may be due to the stretching effect of a greater gradient or the nature of valve pathology, due to fibrous tissue content, lipid content and inflammatory components, when AS is more severe.
A patient’s AVA has been associated with AS progression. Researchers using multivariate linear regression analysis found that initial AVA was an independent predictor of disease progression in dialysis patients, which is a result that has been shown to be generally true in AS patients (6,48,59). Moreover, the rate of AVA change during the cardiac cycle has been found to predict the hemodynamic progression of AS. Lester et al (67) found that rapid progression was significantly associated with an AVA ratio of 1.25 or greater.
Higher LV mass indexes have been found to be associated with more rapid AS progression (48). This may be due to changes in mechanical stress on the valve, changes in biochemical milieu produced by altered hemodynamics, or the effect of factors responsible for LV hypertrophy or remodelling in the first place.
Valve structure and AS etiology may also play roles in disease progression. In their study of 646 patients, Passik et al (68) found that in patients younger than 70 years of age who received aortic valve replacement surgery for AS, 50% had bicuspid aortic valves. Among those 322 patients older than 70 years, 48% had degenerative calcification. Secondly, Roberts et al (11) found that the relative distribution of patients with either bicuspid or tricuspid valves varied with age among those receiving aortic valve replacement surgery for isolated AS, with the frequency of tricuspid valves increasing with age. Finally, Fernandes et al (69) found that the morphology of the bicuspid aortic valve is predictive of clinically important end points, with the fusion of right and noncoronary leaflets leading to more rapid progression of AS and regurgitation in young patients. These all suggest that mechanical stress associated with abnormal valve structures influences the progression of AS.
C-reactive protein
While there is conflicting data regarding the correlation of AS progression with C-reactive protein (CRP), a marker for inflammation, it appears at this point that no such association exists. Early studies, such as those by Galante et al (70) and Gerber et al (71), found strong associations between elevated CRP levels and the presence of AS. Extending this connection to AS progression, some started to advocate use of CRP levels as a useful value in monitoring AS progression (72,73). However, in 2007, two studies undermined the validity of this conclusion. Examining data from the 5621 participants in the Cardiovascular Health Study, Novaro et al (49) found no association between CRP levels and the presence of calcific or incident AS and, as such, they determined that CRP was a poor predictor of subclinical calcific aortic valve disease. Moreover, Jeevanantham et al (74) concluded that, while high-sensitivity CRP was associated with calcific AS in early stages, there was not sufficient evidence to suggest use of this protein as a marker for progression.
Given the pathogenesis of AS and the similarities between risk factors, the connection between AS and atherosclerosis appears quite clear. However, there are other noteworthy aspects of AS pathology that generate many unanswered questions. For instance, biochemical factors, such as those related to calcium, appear uniquely related to AS progression. Moreover, valve structure, hemodynamics and renal failure also seem to affect disease progression. Along with those processes related to atherosclerosis, consideration of these aspects should guide research aimed at the clarification of pathology and strategies for secondary prevention.
MEDICAL MANAGEMENT
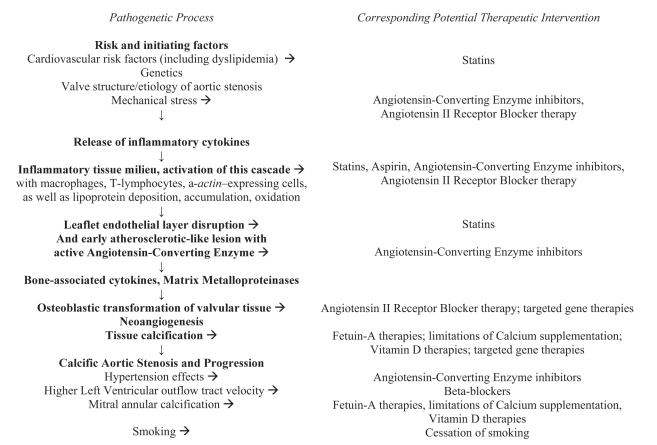
With our understanding of AS steadily evolving, one would hope that therapeutic strategies advance at a similar pace. However, current guidelines do not recommend medical therapy for asymptomatic AS patients because medical treatments have not been proven to prevent the onset or delay the progression of AS disease process (3). However, given our deepening understanding of AS pathogenesis, many therapies appear to have the potential of playing a role in secondary prevention. Figure 1 presents the potential therapeutic targets for AS pathogenesis.
Figure 1).
Potential therapeutic targets for aortic stenosis pathogenesis
Given the roles of atherosclerotic processes in AS pathology, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors appear as one type of therapy. In addition to statins’ lipid-lowering actions, other mechanisms of the drug class may help slow AS progression, such as the modification of endothelial function, inflammatory responses and thrombus formation (75). While retrospective studies consistently found that statin therapy in AS patients was associated with a reduced rate of disease progression, the first prospective study on this matter, the Scottish Aortic Stenosis and Lipid Lowering Trial Impact on Regression (SALTIRE) study, found that intensive atorvastatin therapy did not halt AS progression or induce its regression (50,76–78). Not only has this study and conclusion been criticized for methodological reasons, but the more recent Rosuvastatin Affecting Aortic Valve Endothelium (RAAVE) study found that prospective treatment of AS with rosuvastatin slowed the hemodynamic progression of AS (79,80). The results of upcoming, large, randomized clinical trials, such as the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study, should give more definitive guidance regarding this matter (81).
ACE inhibitors show tremendous promise as well. While these drugs were once contraindicted for AS patients, short-term treatment with ACE inhibitors has been shown to be well tolerated in patients with mild to moderate AS and preserved LV function (82). ACE inhibitors may help slow AS progression for a number of reasons. Not only does sclerotic aortic valve tissue upregulate the expression of ACE and another angiotensin II-forming enzyme, but ACE inhibitors may prevent LV hypertrophy, preserve LV function and reduce frequency of arrhythmias (18,83). Secondly, the blood pressure-lowering effect of ACE inhibitors reduces the mechanical stress and strain on the aortic valve (84). Finally, ACE inhibitors may play a role in slowing AS progression by reducing inflammation, stabilizing plaque formation and mitigating the fibrotic process.
However, clinical studies have yet to conclusively demonstrate such results in practice. The first large retrospective study (77) on the effect of ACE inhibitor therapy found the drug class was not associated with a significant difference in AS progression. However, others, such as O’Brien et al (85), later found an association between ACE inhibition and lower calcium accumulation rates in the aortic valve. Others have found that ACE inhibitors favourably affect stress hemodynamic functions in hypertensive AS patients (86). Despite some of these promising results, it is too early to make definitive conclusions regarding the effect of ACE inhibitors on the progression of AS. The efficacy of this drug may depend on use in particular patient populations. As such, large long-term prospective trials are warranted.
Therapies involving fetuin-A also show potential in inhibiting the calcification processes of progressive AS. Fetuin-A is a multifunctional hepatic secretory protein that inhibits dystrophic vascular and valvular calcification. Among patients with CAD, Ix et al (87) found an inverse relationship between fetuin-A and AS in patients without diabetes. Kaden et al (88) determined that serum fetuin-A levels were lower in patients with calcific AS than in the control group. These studies suggest that calcium homeostasis plays a role in the progression of calcific AS. Fetuin-A therapies may slow disease progression in patients with milder forms of AS but this has yet to be explored in a trial environment.
Older patients at risk for AS progression may be considering the use of a calcium supplement for primary or secondary prevention of osteoporosis. However, Wongpraparut et al (58) found that calcium supplementation was a predictor of accelerated AS progression. Because calcium-phosphorus product is inversely related to AS severity, the importance of keeping serum calcium-phosphate levels within normal range is quite clear (89). More data are needed to support this interesting observation and raise any questions regarding the safety of calcium supplementation, a strategy of proven benefit in osteoporosis.
While attention has mostly been given to the aforementioned issues, there are many other management issues worth considering. Considering the pathogenetic similarities of AS to atherosclerosis, it may be worth investigating the potential role of anti-inflammatory agents such as acetylsalicylic acid in the attenuation of AS progression. Because AS progression is associated with higher LV outflow tract velocity, (6) it is attractive to speculate whether the use of beta-blockers in this patient population will help slow disease progression. Given that smoking is associated with the progression of AS (6), the cessation of smoking may assist with secondary prevention of disease, a hypothesis yet to be confirmed. The evidence that angiotensin receptor blocker therapy inhibits the development of atherosclerosis as well as myofibroblast and osteoblast trans-differentiation in rabbit aortic valves suggests this drug class may be helpful in slowing AS progression (90). Because it is not clear how renal failure accelerates AS progression, the identification of the biochemical and cytokine mediators associated with the high-normal serum creatinine levels that accelerate AS progression may shed light on other therapeutic options. Given the association with the B allele of the vitamin D receptor and calcific AS, as well as the induction of peripheral atherosclerosis and aortic valve leaflet thickening in rabbits through the feeding of a cholesterol-rich diet supplemented with vitamin D2, further research regarding the effect of eliminating vitamin D administration on AS progression may be warranted (28,91). Given the dearth of data on these matters, researchers should consider integrating some of these aspects into their study designs.
The fact that the clinical management of AS patients contains many unanswered questions should not be cause for pessimism. Not only do current cardiovascular medications appear to have the potential to slow AS progression, but other factors influencing AS pathophysiology, such as heart rate and serum creatinine values, may eventually become part of the therapeutic equation as well. Studies addressing these questions will facilitate the development of therapeutic strategies tailored to the appropriate stage of disease and, if applicable, the level of valvular calcification.
THE SURGICAL MANAGEMENT OF AS PATIENTS
American College of Cardiology and American Heart Association guidelines (3) may need to account for AS patients at particularly high risk for rapidly progressing AS. They currently recommend aortic valve replacement surgery for patients with severe symptomatic AS or LV dysfunction and for those patients with severe AS who are undergoing coronary artery bypass graft or cardiac valvular surgery. As such, they do not currently integrate the specific nature of the patient populations into clinical decision-making processes. For instance, studies (59–60,92) have consistently demonstrated that AS patients on chronic dialysis are strongly associated with rapid disease progression. As such, this particular patient population may require more frequent monitoring. Given the improvement in surgical mortality rates, it has been reasoned that the presence of moderate or severe valvular calcification, together with a rapid increase in aortic-jet velocity or positive exercise tests, identifies patients with poor prognoses who may benefit from early valve replacement before development of symptoms (56,93). Considering the malignant natural history of asymptomatic severe AS and improving surgical techniques, it needs to be determined whether the benefits of earlier surgical intervention, such as preclusion of disease progression and further hypertrophy of the left ventricle, outweigh the risks of surgery (94). As further clinical evidence is collected and changes to professional guidelines are considered, this line of reasoning may need to be applied to the aforementioned clinical variables as well as others.
CONCLUSION
There is a great need for a multifaceted approach to the investigation and treatment of calcific AS. While AS is the most common reason for valve replacement in the United States, aortic valve replacement surgery remains the only established treatment for symptomatic AS patients (3,95). Roles of atherosclerotic, chondrification and ossification processes in AS progression have been elucidated (96–98). Given the fact that most patients with asymptomatic, hemodynamically significant AS will develop symptoms within five years, researchers should now pay close attention to issues of medical management, secondary prevention and surgical correction (99). These efforts should examine the effects of cardiovascular medications, such as statins and ACE inhibitors, on AS progression and the potential to tailor therapeutic strategies for rapidly progressing populations. Guided by advances in basic science, clinical researchers should help develop treatments that consider the specific medical context of the patient at hand and address the progression of AS at the appropriate stage of disease.
REFERENCES
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;2:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, Otto CM. Targeted therapy to prevent progression of calcific aortic stenosis. Circulation. 2004;110:1180–2. doi: 10.1161/01.CIR.0000140722.85490.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease) Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 4.Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–7. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 5.Brener SJ, Duffy CI, Thomas JD, Stewart WJ. Progression of aortic stenosis in 394 patients: Relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol. 1995;25:305–10. doi: 10.1016/0735-1097(94)00406-g. [DOI] [PubMed] [Google Scholar]
- 6.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: Implications for secondary prevention. Circulation. 2000;101:2497–502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- 7.Pomerance A. Aging changes in human heart valves. Br Heart J. 1967;29:222–31. doi: 10.1136/hrt.29.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomerance A. Pathogenesis of aortic stenosis and its relation to age. Br Heart J. 1972;34:569–74. doi: 10.1136/hrt.34.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58:304–8. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 10.Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol. 1993;71:322–7. doi: 10.1016/0002-9149(93)90799-i. [DOI] [PubMed] [Google Scholar]
- 11.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–5. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 12.Faggiano P, Aurigemma GP, Rusconi C, Gaasch WH. Progression of valvular aortic stenosis in adults: Literature review and clinical implications. Am Heart J. 1996;132:408–17. doi: 10.1016/s0002-8703(96)90440-8. [DOI] [PubMed] [Google Scholar]
- 13.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 14.Liebe V, Brueckmann M, Borggrefe M, Kaden JJ. Statin therapy of calcific aortic stenosis: Hype or hope? Eur Heart J. 2006;27:773–8. doi: 10.1093/eurheartj/ehi697. [DOI] [PubMed] [Google Scholar]
- 15.Novaro GM, Griffin BP. Calcific aortic stenosis: Another face of atherosclerosis? Cleve Clin J Med. 2003;70:471–7. doi: 10.3949/ccjm.70.5.471. [DOI] [PubMed] [Google Scholar]
- 16.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–22. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien KD, Shavelle DM, Caulfield MT, et al. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–30. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 18.Helske S, Lindstedt KA, Laine M, et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–66. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Kaden JJ, Dempfle CE, Grobholz R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–7. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Kaden JJ, Dempfle CE, Grobholz R, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–11. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 21.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesteremia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:1229–34. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajamannan NM. Calcific aortic stenosis: A disease ready for prime time. Circulation. 2006;114:2007–9. doi: 10.1161/CIRCULATIONAHA.106.657759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien KD, Kuusisto J, Reichenbach DD, et al. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–8. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 26.Mohler ER, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–52. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 27.Strickberger SA, Schulman SP, Hutchins GM. Association of Paget’s disease of bone with calcific aortic valve disease. Am J Med. 1987;82:953–6. doi: 10.1016/0002-9343(87)90157-4. [DOI] [PubMed] [Google Scholar]
- 28.Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P. The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart. 2001;85:635–8. doi: 10.1136/heart.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortlepp JR, Schmitz F, Mevissen V, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur Heart J. 2004;25:514–22. doi: 10.1016/j.ehj.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–9. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 31.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkilä J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–70. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 32.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 33.Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: Characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:4724. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: Atherosclerosis risk factors – a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–9. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 35.Deutscher S, Rockette HE, Krishnaswami V. Diabetes and hypercholesterolemia among patients with calcific aortic stenosis. J Chronic Dis. 1984;37:407–15. doi: 10.1016/0021-9681(84)90108-5. [DOI] [PubMed] [Google Scholar]
- 36.Mautner GC, Mautner SL, Cannon RO, III, Hunsberger SA, Roberts WC. Clinical factors useful in predicting aortic valve structure in patients > 40 years of age with isolated valvular aortic stenosis. Am J Cardiol. 1993;72:194–8. doi: 10.1016/0002-9149(93)90159-a. [DOI] [PubMed] [Google Scholar]
- 37.Gotoh T, Kuroda T, Yamasawa M, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study) Am J Cardiol. 1995;76:928–32. doi: 10.1016/s0002-9149(99)80263-x. [DOI] [PubMed] [Google Scholar]
- 38.Wilmshurst PT, Stevenson RN, Griffiths H, Lord JR. A case-control investigation of the relation between hyperlipidaemia and calcific aortic valve stenosis. Heart. 1997;78:475–9. doi: 10.1136/hrt.78.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novaro GM, Pearce GL, Sprecher DL, Griffin BP. Comparison of cardiovascular risk and lipid profiles in patients undergoing aortic valve surgery versus those undergoing coronary artery bypass grafting. J Heart Valve Dis. 2001;10:19–24. [PubMed] [Google Scholar]
- 40.Chui MC, Newby DE, Panarelli M, Bloomfield P, Boon NA. Association between calcific aortic stenosis and hypercholesterolemia: Is there a need for a randomized controlled trial of cholesterol-lowering therapy? Clin Cardiol. 2001;24:52–5. doi: 10.1002/clc.4960240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rallidis L, Naoumova RP, Thompson GR, Nihoyannopoulos P. Extent and severity of atherosclerotic involvement of the aortic valve and root in familial hypercholesterolaemia. Heart. 1998;80:583–90. doi: 10.1136/hrt.80.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprecher DL, Schaefer EJ, Kent KM, et al. Cardiovascular features of homozygous familial hypercholesterolemia: Analysis of 16 patients. Am J Cardiol. 1984;54:20–30. doi: 10.1016/0002-9149(84)90298-4. [DOI] [PubMed] [Google Scholar]
- 43.Peter M, Hoffmann A, Parker C, Luscher T, Burckhardt D. Progression of aortic stenosis. Role of age and concomitant coronary artery disease. Chest. 1993;103:1715–9. doi: 10.1378/chest.103.6.1715. [DOI] [PubMed] [Google Scholar]
- 44.Nassimiha D, Aronow WS, Ahn C, Goldman ME. Rate of progression of valvular aortic stenosis in patients > or = 60 years of age. Am J Cardiol. 2001;87:807–9. doi: 10.1016/s0002-9149(00)01513-7. [DOI] [PubMed] [Google Scholar]
- 45.Yap SC, Kouwenhoven GC, Takkenberg JJ, et al. Congenital aortic stenosis in adults: Rate of progression and predictors of clinical outcome. Int J Cardiol. 2007;122:224–31. doi: 10.1016/j.ijcard.2006.11.092. [DOI] [PubMed] [Google Scholar]
- 46.Kume T, Kawamoto T, Okura H, et al. Rapid progression of mild to moderate aortic stenosis in patients older than 80 years. J Am Soc Echocardiogr. 2007;20:1243–6. doi: 10.1016/j.echo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Urena P, Malergue MC, Goldfarb B, Prieur P, Guedon-Rapoud C, Petrover M. Evolutive aortic stenosis in hemodialysis patients: Analysis of risk factors. Nephrologie. 1999;20:217–25. [PubMed] [Google Scholar]
- 48.Bahler RC, Desser DR, Finkelhor RS, Brener SJ, Youssefi M. Factors leading to progression of valvular aortic stenosis. Am J Cardiol. 1999;84:1044–8. doi: 10.1016/s0002-9149(99)00496-8. [DOI] [PubMed] [Google Scholar]
- 49.Novaro GM, Katz R, Aviles RJ, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: The Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–8. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 50.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–5. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 51.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–70. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 52.Roger VL, Tajik AJ, Bailey KR, Oh JK, Taylor CL, Seward JB. Progression of aortic stenosis in adults: New appraisal using Doppler echocardiography. Am Heart J. 1990;119:331–8. doi: 10.1016/s0002-8703(05)80024-9. [DOI] [PubMed] [Google Scholar]
- 53.Pate GE. Association between aortic stenosis and hypertension. J Heart Valve Dis. 2002;11:612–4. [PubMed] [Google Scholar]
- 54.Cuniberti LA, Stutzbach PG, Guevara E, Yannarelli GG, Laguens RP, Favaloro RR. Development of mild aortic valve stenosis in a rabbit model of hypertension. J Am Coll Cardiol. 2006;47:2303–9. doi: 10.1016/j.jacc.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 55.Ngo MV, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ. Smoking and obesity are associated with the progression of aortic stenosis. A J Geriatr Cardiol. 2001;10:86–90. doi: 10.1111/j.1076-7460.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 56.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcomes in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–7. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 57.Yilmaz MB, Guray U, Guray Y, et al. Lipid profile of patients with aortic stenosis might be predictive of rate of progression. Am Heart J. 2004;147:915–8. doi: 10.1016/j.ahj.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Wongpraparut N, Apiyasawat S, Crespo G, Yazdani K, Jacobs LE, Kotler MN. Determinants of progression of aortic stenosis in patients aged > or = 40 years. Am J Cardiol. 2002;89:350–2. doi: 10.1016/s0002-9149(01)02241-x. [DOI] [PubMed] [Google Scholar]
- 59.Perkovic V, Hunt D, Griffin SV, du Plessis M, Becker GJ. Accelerated progression of calcific aortic stenosis in dialysis patients. Nephron Clin Pract. 2003;94:40–5. doi: 10.1159/000071280. [DOI] [PubMed] [Google Scholar]
- 60.Ohara T, Hashimoto Y, Matsumura A, Suzuki M, Isobe M. Accelerated progression and morbidity in patients with aortic stenosis on chronic dialysis. Circulation. 2005;69:1535–9. doi: 10.1253/circj.69.1535. [DOI] [PubMed] [Google Scholar]
- 61.Kume T, Kawamoto T, Akasaka T, et al. Rate of progression of valvular aortic stenosis in patients undergoing dialysis. J Am Soc Echocardiogr. 2006;19:914–8. doi: 10.1016/j.echo.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Fujise K, Amerling R, Sherman W. Rapid progression of mitral and aortic stenosis in a patient with secondary hyperparathyroidism. Br Heart J. 1993;70:282–4. doi: 10.1136/hrt.70.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cosmi JE, Kort S, Tunick PA, et al. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–7. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 64.Nassimiha D, Aronow WS, Ahn C, Goldman ME. Association of coronary risk factors with progression of valvular aortic stenosis in older persons. Am J Cardiol. 2001;87:1313–4. doi: 10.1016/s0002-9149(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 65.Pohle K, Mäffert R, Ropers D, et al. Progression of aortic valve calcification: Association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–32. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- 66.Rosenhek R, Klaar U, Schemper M, et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:185–7. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Lester SJ, McElhinney DB, Miller JP, Lutz JT, Otto CM, Redberg RF. Rate of change in aortic valve area during a cardiac cycle can predict the rate of hemodynamic progression of aortic stenosis. Circulation. 2000;101:1947–52. doi: 10.1161/01.cir.101.16.1947. [DOI] [PubMed] [Google Scholar]
- 68.Passik CS, Ackermann DM, Pluth JR, Edwards WD. Temporal changes in the causes of aortic stenosis: A surgical pathologic study of 646 cases. Mayo Clin Proc. 1987;62:119–23. doi: 10.1016/s0025-6196(12)61880-1. [DOI] [PubMed] [Google Scholar]
- 69.Fernandes SM, Khairy P, Sanders SP, Colan SD. Bicuspid aortic valve morphology and interventions in the young. J Am Coll Cardiol. 2007;49:2211–4. doi: 10.1016/j.jacc.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 70.Galante A, Pietroiusti A, Vellini M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. 2001;38:1078–82. doi: 10.1016/s0735-1097(01)01484-x. [DOI] [PubMed] [Google Scholar]
- 71.Gerber IL, Stewart RA, Hammett CJ, et al. Effect of aortic valve replacement on C-reactive protein in nonrheumatic aortic stenosis. Am J Cardiol. 2003;92:1129–32. doi: 10.1016/j.amjcard.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Sánchez PL, Santos JL, Kaski JC, et al. Grupo AORTICA (Grupo de Estudio de la Estenosis Aórtica) Relation of circulating C-reactive protein to progression of aortic valve stenosis. Am J Cardiol. 2006;97:90–3. doi: 10.1016/j.amjcard.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 73.Sánchez PL, Mazzone AM. C-reactive protein in aortic valve disease. Cardiovasc Ultrasound. 2006;4:24–7. doi: 10.1186/1476-7120-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Jeevanantham V, Singh N, Izuora K, D’Souza JP, Hsi DH. Correlation of high sensitivity C-reactive protein and calcific aortic valve disease. Mayo Clin Proc. 2007;82:171–4. doi: 10.4065/82.2.171. [DOI] [PubMed] [Google Scholar]
- 75.Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: From the bench to the bedside-emerging clinical and cellular concepts. Heart. 2003;89:801–5. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher D, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–9. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 77.Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–5. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 78.Cowell SJ, Newby DE, Prescott RJ, et al. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 79.Rosenhek R. Statins for aortic stenosis. N Engl J Med. 2005;352:2441–3. doi: 10.1056/NEJMe058070. [DOI] [PubMed] [Google Scholar]
- 80.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–61. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossebø AB, Pedersen TR, Allen C, et al. Design and baseline characteristics of the simvastatin and ezetimibe in aortic stenosis (SEAS) study. Am J Cardiol. 2007;99:970–3. doi: 10.1016/j.amjcard.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 82.O’Brien KD, Zhao XQ, Shavelle DM, et al. Hemodynamic effects of the angiotensin-converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Investig Med. 2004;52:185–91. doi: 10.1136/jim-52-03-33. [DOI] [PubMed] [Google Scholar]
- 83.Routledge HC, Townend JN. ACE inhibition in aortic stenosis: Dangerous medicine or golden opportunity? J Hum Hypertens. 2001;15:659–67. doi: 10.1038/sj.jhh.1001260. [DOI] [PubMed] [Google Scholar]
- 84.Newby DE, Cowell SJ, Boon NA. Emerging medical treatments for aortic stenosis: Statins, angiotensin converting enzyme inhibitors, or both? Heart. 2006;92:729–34. doi: 10.1136/hrt.2005.066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Brien KD, Probstfield JL, Caulfield MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–62. doi: 10.1001/archinte.165.8.858. [DOI] [PubMed] [Google Scholar]
- 86.Jimenez-Candil J, Bermejo J, Yotti R, et al. Effects of angiotensin converting enzyme inhibitors in hypertensive patients with aortic valve stenosis: A drug withdrawal study. Heart. 2005;91:1311–8. doi: 10.1136/hrt.2004.047233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: Data from the Heart and Soul Study. Circulation. 2007;115:2533–9. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaden JJ, Reinöhl JO, Blesch B, et al. Systemic and local levels of fetuin-A in calcific aortic valve stenosis. Int J Mol Med. 2007;20:193–7. [PubMed] [Google Scholar]
- 89.Mills WR, Einstadter D, Finkelhor RS. Relation of calcium-phosphorus product to the severity of aortic stenosis in patients with normal renal function. Am J Cardiol. 2004;94:1196–8. doi: 10.1016/j.amjcard.2004.07.095. [DOI] [PubMed] [Google Scholar]
- 90.Arishiro K, Hoshiga M, Negoro N, et al. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol. 2007;49:1482–9. doi: 10.1016/j.jacc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 91.Drolet M, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol. 2003;41:1211–7. doi: 10.1016/s0735-1097(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 92.Malergue MC, Urena P, Prieur P, Guedon-Rapoud C, Petrover M. Incidence and development of aortic stenosis in chronic hemodialysis. An ultrasonographic and biological study of 112 patients. Arch Mal Coeur Vaiss. 1997;90:1595–601. [PubMed] [Google Scholar]
- 93.Chaliki HP, Brown ML, Sundt TM, Tajik AJ. Timing of operation in asymptomatic severe aortic stenosis. Expert Rev Cardiovasc Ther. 2007;5:1065–71. doi: 10.1586/14779072.5.6.1065. [DOI] [PubMed] [Google Scholar]
- 94.Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: Benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–22. doi: 10.1016/j.athoracsur.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 95.Carabello BA, Crawford FA., Jr Valvular heart disease. N Engl J Med. 1997;337:32–41. doi: 10.1056/NEJM199707033370107. [DOI] [PubMed] [Google Scholar]
- 96.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 97.Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pai RG. Degenerative valve disease. J Am Coll Cardiol. 2006;48:2601. doi: 10.1016/j.jacc.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 99.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–5. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]